ABSTRACT
Background
There is an ongoing debate on whether fructose plays a role in the development of nonalcoholic fatty liver disease.
Objectives
The aim of this study was to investigate the effects of fructose restriction on intrahepatic lipid (IHL) content in a double-blind randomized controlled trial using an isocaloric comparator.
Methods
Between March 2017 and October 2019, 44 adult overweight individuals with a fatty liver index ≥ 60 consumed a 6-wk fructose-restricted diet (<7.5 g/meal and <10 g/d) and were randomly assigned to supplementation with sachets of glucose (= intervention group) or fructose (= control group) 3 times daily. Participants and assessors were blinded to the allocation. IHL content, assessed by proton magnetic resonance spectroscopy, was the primary outcome and glucose tolerance and serum lipids were the secondary outcomes. All measurements were conducted in Maastricht University Medical Center.
Results
Thirty-seven participants completed the study protocol. After 6 wk of fructose restriction, dietary fructose intake and urinary fructose excretion were significantly lower in the intervention group (difference: −57.0 g/d; 95% CI: −77.9, −39.5 g/d; and −38.8 μmol/d; 95% CI: −91.2, −10.7 μmol/d, respectively). Although IHL content decreased in both the intervention and control groups (P < 0.001 and P = 0.003, respectively), the change in IHL content was more pronounced in the intervention group (difference: −0.7% point, 95% CI: −2.0, −0.03% point). The changes in glucose tolerance and serum lipids were not significantly different between groups.
Conclusions
Six weeks of fructose restriction per se led to a small, but statistically significant, decrease in IHL content in comparison with an isocaloric control group.
This trial was registered at clinicaltrials.gov as NCT03067428.
Keywords: nonalcoholic fatty liver disease, hepatic steatosis, intrahepatic lipid, fructose, nutrition, dietary intervention, randomized controlled trial, glucose metabolism
Introduction
The drastic increase in fructose consumption since the Industrial Revolution has paralleled the current epidemic of obesity and its cardiometabolic complications, such as dyslipidemia, type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), and cardiovascular disease (1–4). Furthermore, fructose overfeeding trials have convincingly been shown to increase the accumulation of fat in the liver (5), the principal organ involved in the metabolism of fructose (6).
There is, however, an ongoing debate on whether fructose is more detrimental for liver health than other simple sugars, such as glucose (6, 7). Whereas there is abundant evidence from animal studies on the prominent role of fructose in, and the putative mechanism by which fructose causes, IHL accumulation (8–12), a previous meta-analysis of controlled trials in humans did not show any effects of isocaloric fructose exchange for other carbohydrates on intrahepatic lipid (IHL) content or serum alanine aminotransferase concentrations (5). On the other hand, Schwarz et al. (13) more recently demonstrated that a 9-d isocaloric fructose-restricted diet successfully reduced IHL content in obese children with high habitual sugar intake, which was mainly attributed to a decrease in de novo lipogenesis.
The discrepancies between these studies may be attributed to the amount of fructose that was consumed, i.e., either moderate to high (5) or very low (13). Fructose can serve as a substrate for de novo lipogenesis and stimulate de novo lipogenesis via upregulation of transcription factors that enable the expression of genes involved in de novo lipogenesis, such as sterol regulatory element binding protein 1 (SREBP1), carbohydrate response element binding protein (ChREBP), and liver X receptor (LXR) (14, 15). On the other hand, even at small (“catalytic”) amounts, fructose can also increase hepatic glucose disposal (16, 17).
Because the interpretation of the intervention study by Schwarz et al. was limited by its single-arm design and, hence, the lack of a control group, the aim of the current study (“eFfects of fRUctose restrIcTion on LivEr SteatosiS”—FRUITLESS) was to investigate the effects of fructose restriction on IHL content in a double-blind randomized controlled trial, using an isocaloric comparator.
Methods
Study population
Between March 2017 and October 2019, individuals were recruited via advertisements or contacted directly if they had participated in scientific research before and agreed to be contacted for future studies. Inclusion criteria for participation were age ≥ 18 y, a high prior chance of having an increased IHL content [i.e., a BMI (in kg/m2) ≥ 28 and a fatty liver index (FLI) ≥ 60 (18, 19)], and a daily fructose intake above the Dutch average [i.e., ≥ 45 g/d (20) according to a 3-d food journal]. If an individual did not meet the inclusion criterion of BMI ≥ 28, but had an elevated FLI, he or she was still considered eligible to participate. Owing to a slow recruitment rate, the inclusion criterion of fructose intake ≥ 45 g/d was abandoned (protocol amendment 18 July, 2018). Individuals were excluded from participation in case of (history of) liver disease, (history of) excessive alcohol consumption (i.e., > 3 and > 2 units/d for men and women, respectively), major change in weight (i.e., > 5%) and/or physical activity pattern in the 3 mo before the study, use of glucose-lowering drugs, recent illness, pregnancy and/or lactation, contraindications for MRI, and inability to give informed consent. Eligibility assessment was performed by the clinical researcher of the study (NS).
All participants gave written informed consent before inclusion in the study. The study was performed according to the Declaration of Helsinki (21) and approved by the medical ethical committee of Maastricht University Medical Center. The full trial protocol can be accessed via the corresponding author or clinicaltrials.gov (NCT03067428).
Dietary intervention
Because restriction of 1 nutrient (in this case fructose) without affecting other components of the diet is practically impossible, both groups were asked to follow a 6-wk fructose-restricted diet. In addition to this diet, the control group was supplemented with fructose powder aimed at achieving a fructose intake similar to baseline, whereas the intervention group remained fructose-restricted and received glucose powder to allow an isocaloric comparison.
At screening, baseline, and completion of the study, dietary intake was assessed with a 3-d food journal along with a personal interview by the clinical researcher (NS) who was blinded to the intervention assignment. Weight of the food products was provided by the participant or estimated using average quantities per portion. Macro- and micronutrient composition and caloric content of the diet were calculated using the Dutch food composition table (22). Average fructose intake was calculated with an extensive database of food products from Wageningen University (20). This database was also used for the composition of the fructose-restricted diet throughout the study. If the fructose content of a specific product was not available in the database, a comparable food product or the sucrose content (= 50% fructose) of the specific product was used.
All participants received extensive counseling on the fructose-restricted diet by the clinical researcher of the study (NS) under the supervision of an experienced metabolic dietitian (EMCvdP), both of whom were blinded to the intervention assignment. Participants were allowed to have an ad libitum food intake, as long as the fructose intake per meal and per day was < 7.5 g and < 10 g, respectively. For this, participants were provided a list of permitted and prohibited food products as well as examples of fructose-restricted meals. Furthermore, participants received dietary counseling and diets were adjusted on a weekly basis according to the fructose intake that was assessed by personal interviews. Substantial weight loss/gain was checked every week and (if necessary) corrected with dietary advice. Random assignment to either glucose or fructose supplementation was computer-generated using block sizes of 4 and performed by an independent researcher (MDGVdE). The allocation sequence was concealed in an opaque, sealed envelope and remained blinded to the participants and assessors (NS, PIHGS, MCGJB, CDAS, NCS, PV, and VBS-H) upon completion of all analyses. Supplementation of either glucose or fructose equaled the amount of fructose that was restricted from the diet. In case of a baseline fructose intake below the Dutch average (i.e., 45 g/d), glucose or fructose supplementation was set at 45 g/d. The glucose and fructose powders (indistinguishable in terms of color and odor) were prepacked in identical sachets by an independent researcher (MDGVdE) and distributed to the participants on a weekly basis. Participants were instructed to dissolve the glucose or fructose supplementation in water or food (e.g., yogurt or cottage cheese) for consumption during the 3 main meals. If participants preferred to dissolve the glucose or fructose powder in water, they were urged to consume the supplementation solely during or directly after finishing the meal. Because the 6-wk fructose-restricted diet is devoid of fruits and vegetables, all participants received vitamin C supplementation (70 mg/d) throughout the entire study to prevent deficiency. No additional requirements were imposed in terms of food/liquid intake or medication use. Participants were asked not to alter their physical activity throughout the study.
Primary outcome measure
Participants were asked to visit the research ward of Maastricht University Medical Center after an 8-h fast and to refrain from alcohol for 3 d before the measurements. Proton magnetic resonance spectroscopy (1H-MRS) was performed to determine IHL content at baseline and completion. All magnetic resonance (MR) measurements were performed on a 3T MR system (Achieva 3T-X, Philips Healthcare) using a 32-channel sense cardiac/torso coil (Philips Healthcare). Owing to morbid (abdominal) obesity, 5 participants were scanned on a wide-bore 1.5 T MR system (Ingenia, Philips Healthcare) at both time points (i.e., baseline and follow-up measurement). A T2-weighted turbo spin echo MR image was acquired in 3 (axial, coronal, and transversal) orientations and a 20 × 20 × 20-mm voxel was placed in the right hepatic lobe, avoiding vascular structures and edges of the liver, diaphragm, and biliary structures. Special care was taken to ensure that the placement of the voxel was in the same position at both time points. Hepatic lipid spectra were acquired using a PRESS sequence with water suppression (frequency-selective prepulses) using the following MR parameters: repetition time (TR)/echo time (TE) = 4000/32.5 ms, 32 acquisitions with 2 signal averages in each, bandwidth = 2000 Hz, and data points = 2048. In addition, spectra without water suppression were acquired (8 acquisitions with 2 signal averages in each) as an internal reference. The long TR was chosen to let the participant breathe in the rhythm of the measurement, and MR acquisition was performed at the end of expiration. A pressure-sensitive probe was placed on the abdomen of the participant to monitor the breathing pattern throughout the measurement. All MR spectra obtained were individually frequency-aligned and -phased, and lipid-CH2 and water peaks were fitted to the respective spectrum as described before (23), using a custom-built MATLAB script (MATLAB R2017b, Mathworks). The signal intensities obtained were corrected for T2 decay using the literature T2 values of 3T (26.3 ms, 59.1 ms) (24) and 1.5 T (50 ms, 60 ms) (25) for water and lipid, respectively. Finally, the IHL percentage was represented as the area ratio of the CH2:H2O peak using the T2-corrected signal intensities of lipid-CH2 and water. Because of technical reasons, MRS was not available in 1 participant. The IHL content at both time points was subsequently assessed using mDIXON imaging, which was validated against 1H-MRS in a former study [intraclass correlation coefficient (ICC)agreement = 0.82; P < 0.001 (26)].
Secondary outcome measures
On the same day as the 1H-MRS measurements, blood was drawn for the determination of serum insulin and lipids (i.e., total, HDL, and LDL cholesterol and triglycerides) and a 2-h 75-g oral-glucose-tolerance test (OGTT) was performed, all exactly as described previously (27). The AUC was calculated as a measure of glucose tolerance. Insulin resistance was estimated with the homeostasis model assessment of insulin resistance (HOMA2-IR) calculator (www.dtu.ox.ac.uk).
Other measurements
All participants filled in a health questionnaire concerning smoking, habitual alcohol consumption, and medical history. Weight was measured in solely underwear, height was assessed with a stadiometer, and waist and hip circumference were determined with a measuring tape at the level of the umbilicus and trochanter major, respectively. Twenty-four-hour urine was collected in preacidified plastic containers at T = 0, 2, 4, and 6 wk for measurement of urinary fructose, an objective biomarker of fructose consumption (28), with ultra-performance LC–tandem MS [variation coefficient (VC%) 6.6].
Statistical analysis
For this double-blind randomized controlled trial, sample size calculations showed that 19 individuals/group were required to detect a mean difference of 3% (σ = 3.2%) in IHL content (α = 0.05, β = 0.20, allocation ratio nintervention group:ncontrol group = 1:1). To account for dropout (15%), the number of individuals was increased to 22/group.
Dichotomous data are expressed as frequencies. Continuous data are presented as median [IQR]. Changes from baseline within and between groups were analyzed with Wilcoxon's Signed Rank test and a Mann–Whitney U test, respectively. The 95% CIs for these changes were calculated according to the Hodges–Lehmann method.
Sensitivity analyses were conducted 1) to assess the influence of unbalanced randomization, if present, on the primary outcome measure — for this, a 1-way ANCOVA was conducted with the unbalanced variable as a covariate; 2) to determine the effect of the protocol amendment (i.e., the omission of the fructose intake ≥ 45 g/d inclusion criterion) on the primary outcome measure; 3) to assess the effect of the degree of IHL content at baseline [i.e., above or below the 5.56% cutoff value (25)] on the primary outcome measure — for both analyses, interaction terms were tested in a 1-way ANCOVA; and 4) to explore a dose–response relation by testing the association between the change in fructose intake and the change in IHL content and by testing for an interaction between the intervention and the change in fructose intake on the change in IHL content (1-way ANCOVA).
A P value < 0.05 was considered statistically significant. All analyses were carried out with IBM SPSS version 25 for Windows (SPSS Inc.).
Results
Randomization and follow-up
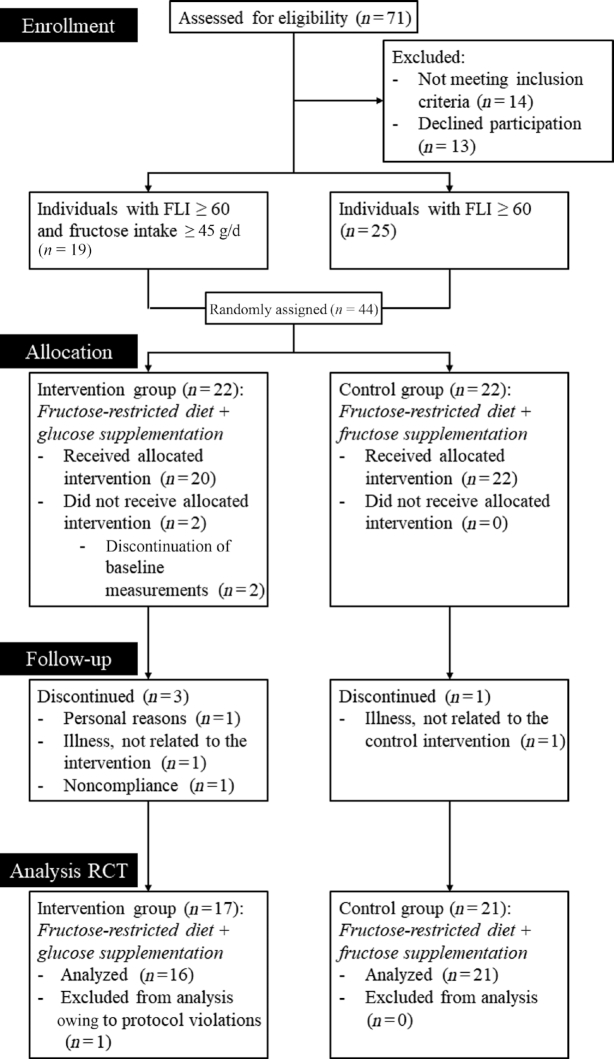
Forty-four participants were randomly assigned to the intervention group (fructose-restricted diet plus glucose supplementation) or control group (fructose-restricted diet plus fructose supplementation) (see Figure 1 for a flowchart). Six participants discontinued participation because of various reasons [i.e., claustrophobia during MR measurements (n = 2), nonadherence to the diet (as indicated by the participant) (n = 1), immobility due to ankle distortion (n = 1), symptoms related to irritable bowel syndrome (before start of the diet) (n = 1), and personal reasons (n = 1)]. Upon completion of the study, 1 participant did not appear to meet the inclusion criteria (i.e., FLI ≥ 60) and was therefore excluded from the analyses. The individualized supplementation was successfully supplied to and ingested by all participants. Because follow-up of the primary outcome could not be acquired for the 6 individuals who discontinued participation, the intention-to-treat analyses did not differ from the per-protocol analyses, which were carried out with 16 and 21 participants in the intervention group and the control group, respectively. Table 1 displays baseline characteristics.
FIGURE 1.
Flowchart of study. Seventy-one individuals were assessed for eligibility. Forty-four participants were randomly assigned to the intervention group (fructose-restricted diet plus glucose supplementation) or control group (fructose-restricted diet plus fructose supplementation), of whom 19 were assigned before and 25 after the protocol amendment (i.e., abandonment of the fructose intake ≥ 45 g/d inclusion criterion). Six participants discontinued participation because of various reasons. At completion of the study, 1 participant did not appear to meet the inclusion criteria (i.e., FLI ≥ 60) and was therefore excluded. Per-protocol analyses were carried out with 16 and 21 participants in the intervention and the control group, respectively. FLI, fatty liver index; RCT, randomized controlled trial.
TABLE 1.
General baseline characteristics of the study population1
| Characteristic | Intervention group (n = 16) | Control group (n = 21) |
|---|---|---|
| Age, y | 55 [35–62] | 52 [38–62] |
| Sex, n (M/F) | 6/10 | 6/15 |
| Smoking, % yes | 12.5 | 14.3 |
| Alcohol intake, units/wk | 2 [1–5] | 3 [0–5] |
| BMI, kg/m2 | 34.1 [28.8–37.3] | 31.1 [30.2–35.6] |
| Waist circumference, cm | 117.9 [106.5–128.4] | 110.0 [104.3–113.6] |
| HOMA2-IR | 0.84 [0.50–1.37] | 0.86 [0.73–1.14] |
| Serum total cholesterol, mmol/L | 5.2 [4.3–5.6] | 5.2 [4.2–6.0] |
| Serum HDL cholesterol, mmol/L | 1.2 [1.1–1.3] | 1.3 [1.0–1.5] |
| Serum LDL cholesterol, mmol/L | 3.1 [2.3–3.8] | 2.7 [2.3–3.8] |
| Serum triglycerides, mmol/L | 1.6 [1.2–1.9] | 1.3 [0.9–1.7] |
| Dietary fructose intake, g/d | 42.1 [20.3–73.4] | 36.9 [27.1–54.6] |
| Fatty liver index | 94 [74–96] | 84 [64–91] |
| Intrahepatic lipid content, % | 4.9 [2.3–10.3] | 2.1 [0.9–7.7] |
1Values are medians [IQRs]. HOMA2-IR, homeostasis model assessment of insulin resistance.
Adherence and tolerability of the intervention
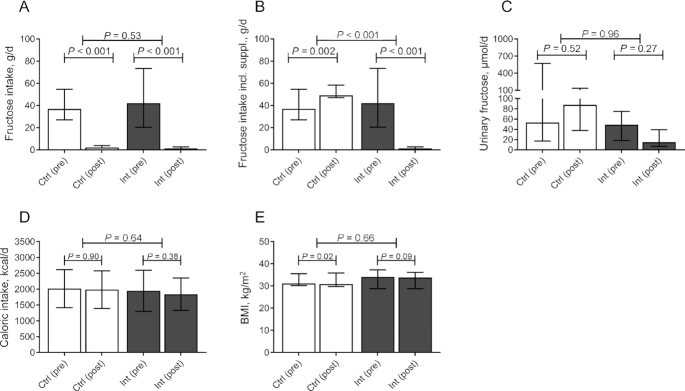
Compared with baseline, the habitual dietary intake of fructose decreased drastically in both groups (Figure 2A). The median dietary fructose intake after 6 wk of fructose restriction was 1.4 g/d in the intervention group and 2.2 g/d in the control group (change from baseline: −45.7 g/d; 95% CI: −61.4, −29.8 g/d; and −37.9 g/d, 95% CI: −48.3, −28.6 g/d, respectively). The change from baseline was not significantly different between the groups (difference between change from baseline: −6.7 g/d; 95% CI: −24.0, 13.0 g/d). When the supplementation was taken into account, the fructose intake in the control group increased to 49.1 g/d (change from baseline: +12.4 g/d; 95% CI: 6.2, 20.8 g/d). This was higher than baseline, because supplementation of fructose (and glucose) was set at 45 g/d in all participants with a baseline fructose intake below the Dutch average (see the Methods section). This change was significantly different from the intervention group (difference between change from baseline: −57.0 g/d; 95% CI: −77.9, −39.5 g/d) (Figure 2B; Supplemental Figure 1). The cumulative difference in fructose intake during the full study period — calculated as the individual difference from baseline times the number of days each participant was included in the study — was 1894 g (95% CI: 1727, 2052 g). This difference was also reflected by a significantly different urinary fructose excretion between the intervention and the control group at completion of the study (−38.8 μmol/d; 95% CI: −91.2, −10.7 μmol/d). The change from baseline between the groups was not significantly different, which was mainly attributed to the large variation in urinary fructose concentration at baseline in the control group (Figure 2C).
FIGURE 2.
Adherence to the intervention. (A) Daily fructose intake, (B) daily fructose intake including supplementation, (C) 24-h urinary fructose concentration, (D) daily caloric intake, and (E) BMI in Ctrl (white bars, n = 21) and Int (grey bars, n = 16) at baseline (pre) and after completion of the study (post). Data are expressed as median ± IQR. Differences within groups are analyzed with Wilcoxon's Signed Rank test. Differences between groups are analyzed with a Mann–Whitney U test. Ctrl, control group; IHL, intrahepatic lipid; Int, intervention group.
Caloric intake remained stable throughout the intervention as a consequence of the isocaloric supplementation and did not differ between the 2 groups (Figure 2D). Nevertheless, BMI decreased in both the intervention and control groups (change from baseline: −0.2; 95% CI: −0.5, 0.0; and −0.4; 95% CI: −0.6, −0.1, respectively), a decrease which was not significantly different between both groups (difference between change from baseline: −0.1; 95% CI: −0.3, 0.5) (Figure 2E). No statistically significant effect was observed for macronutrient composition (i.e., carbohydrate, protein, total fat, and saturated fat intake) (Supplemental Figure 2A–D). When macronutrient composition was expressed as percentage of total daily energy intake, a significant difference between change from baseline was observed for carbohydrate intake (difference between change from baseline: 7.1%, 95% CI: 0.7%, 14.3%) (Supplemental Figure 3A), which appeared to be in exchange for protein intake (difference between change from baseline: −5.9%; 95% CI: −13.3%, 0.4%) (Supplemental Figure 2B).
The intervention was generally well tolerated. Diarrhea (grade 1–2) was reported in 3 and 5 participants in the intervention and the control group, respectively. Only 1 participant in the control group experienced a serious adverse event, which occurred between the screening visit and baseline measurements.
Effect of fructose restriction on IHL content
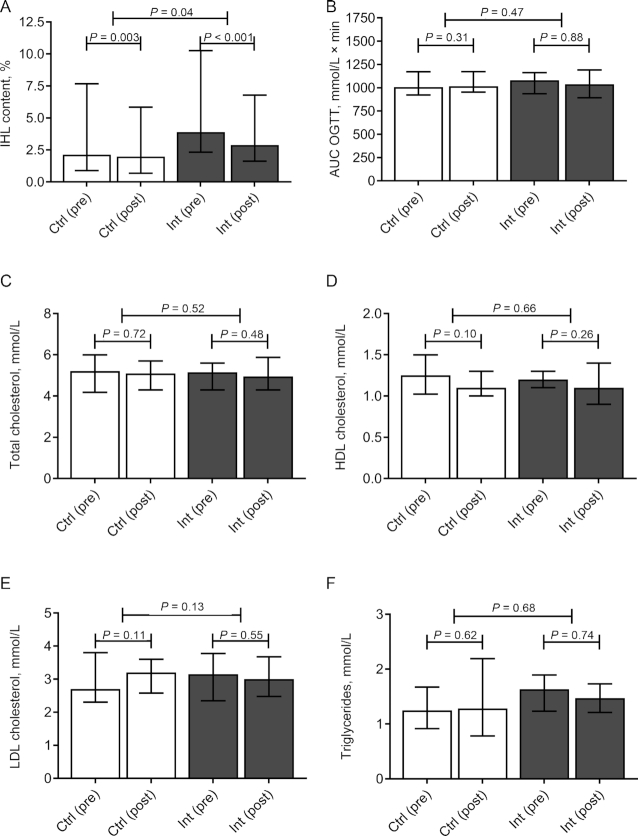
IHL content decreased in both treatment arms (Figure 3A). The reduction in IHL content was significantly greater in the intervention group, albeit small (difference between change from baseline: −0.7%, 95% CI: −2.0%, −0.03%) (Figure 3A). Because baseline BMI and IHL content tended to be higher in the intervention group (Table 1), we subsequently conducted a sensitivity analysis to assess the impact thereof on the primary outcome measure. Addition of baseline BMI and IHL content as covariates to a 1-way ANCOVA did not have substantial effects on the (log-transformed) primary outcome measure (β: −0.06; 95% CI: −0.116, 0.007; P = 0.08).
FIGURE 3.
IHL content, glucose tolerance, and serum lipid concentrations. (A) IHL content, (B) glucose AUC during an OGTT, (C) serum total cholesterol, (D) serum HDL cholesterol, (E) serum LDL cholesterol, and (F) serum triglycerides in Ctrl (white bars, n = 21) and Int (grey bars, n = 16) at baseline (pre) and after completion of the study (post). Data are expressed as median ± IQR. Differences within groups are analyzed with Wilcoxon's Signed Rank test. Differences between groups are analyzed with a Mann–Whitney U test. Ctrl, control group; IHL, intrahepatic lipid; Int, intervention group; OGTT, oral-glucose-tolerance test.
Subgroup analyses did not show significantly different effect sizes between participants with either high or low habitual fructose intake, or high or low baseline IHL content (P-interaction: 0.63 and 0.75, respectively) (Supplemental Figure 4). Finally, the change in fructose intake was not associated with the change in IHL content (P = 0.34), nor was there an interaction between the intervention and the change in fructose intake on the change in IHL content (P = 0.72).
Effect of fructose restriction on glucose tolerance and serum lipid concentrations
Plasma glucose excursions during a 2-h 75-g OGTT were not significantly different between the 2 groups (difference between change from baseline: 32.3; 95% CI: −62.3, 119.0) (Figure 3B). HOMA2-IR was affected neither in the intervention nor in the control group (change from baseline: 0.12; 95% CI: −0.17, 0.51; and 0.06; 95% CI: −0.13, 0.19, respectively), nor was there a difference between the groups (difference between change from baseline: 0.10; 95% CI: −0.21, 0.42). In addition, no effects were observed on serum lipids (Figure 3C–F).
Discussion
In this double-blind randomized controlled trial, we have shown that a 6-wk fructose-restricted diet resulted in a small decrease in IHL content in adult overweight individuals with a high FLI in comparison with an isocaloric comparator. No effects were observed on glucose tolerance or serum lipid concentrations.
Although glucose and fructose are both simple sugars with identical molecular formulas, their metabolic fate differs greatly. In contrast to glucose, which is widely metabolized throughout the body, a proportion of ingested fructose is already cleared in the small intestine, as shown by animal studies (29). After absorption, fructose reaches the portal blood where it is almost entirely taken up by the liver to be converted to glucose, lactate, glycogen, or triglycerides (30). Furthermore, at a low dose, fructose facilitates hepatic glucose disposal by dissociation of the glucokinase (GCK)-glucokinase regulatory protein (GKRP) complex (GCK-GKRP) (16, 31). Dissociation of this complex appears clinically relevant, because carriers of a missense variant in GCKR (glucokinase regulatory protein gene) — the gene that encodes a GKRP protein that binds GCK less effectively — are prone to develop NAFLD (32). Thus, although clear metabolic differences between fructose and glucose metabolism exist, such differences cannot be easily extrapolated to net effects on metabolic health, including IHL content, and experiments such as the current trial may provide important insights.
Dose-dependent metabolic pathways such as those aforementioned may also explain the conflicting results of previous clinical trials (5, 13). To date, fructose intervention studies have mainly focused on the role of fructose added to the diet and shown contrasting effects on IHL content (5, 33–36). Recently, Schwarz et al. (13) showed that restriction of fructose from the diet reduced IHL content in obese children and adolescents. The lack of a control group, however, makes it difficult to conclude whether this change in IHL content can be attributed to fructose restriction per se or, alternatively, to the change in the diet in general or other behaviors that are associated with participation in a clinical trial [i.e., the Hawthorne effect (37)].
The current study is the first that we know of to have used a randomized controlled study design in which both the intervention and control groups followed the same fructose-restricted diet. Together with a similar caloric intake in both treatment arms (as a consequence of the isocaloric glucose supplementation in the intervention group), we were able to study the effect of fructose restriction per se. Indeed, the results in the control group show that the diet by itself was already associated with a decrease in IHL content, independently of a change in fructose intake (which was supplemented). More importantly, by comparing both treatment arms we are able to show that fructose restriction per se reduces IHL content, albeit with a small effect size (0.7% point).
In the present study, we did not observe any effect of fructose restriction on serum lipid concentrations, glucose tolerance, or HOMA2-IR, which was somewhat surprising because these variables have been associated with IHL content (38, 39). A possible explanation might be that the effect of fructose restriction on IHL content was too small to result in a detectable change in serum lipid concentrations and/or glucose tolerance. In addition, we did not assess insulin sensitivity with the gold-standard method, i.e., a hyperinsulinemic-euglycemic clamp. Another possible explanation might be that the duration of the intervention was too short. Yet, this period is comparable with most other fructose feeding trials, which varied between 3 and 10 wk in duration (40). Moreover, it has been shown that IHL accumulation can resolve within days (13, 41). We, therefore, believe that a 6-wk intervention period — in which the cumulative difference in fructose consumption was almost 2 kg — is sufficient to reach a steady state in these metabolic outcomes.
Our study has strengths and limitations. First, the sample size was relatively small, but should be viewed in perspective of the major dietary restrictions that were imposed. More importantly, our study was adequately powered with an expected dropout rate. Second, we used the FLI as a screening tool for fatty liver (42). Despite a high positive likelihood ratio to rule in fatty liver with an FLI ≥ 60 (43–45), the median IHL content of the study participants was only 3.4%. This was not attributable to regression to the mean, because FLI was high at both the screening and baseline visits (91 and 87, respectively). Nevertheless, sensitivity analysis with stratification for high and low liver fat showed that similar effect sizes were observed in individuals with a high IHL content at baseline. Furthermore, we did not observe a dose–response relation between the change in fructose intake and the change in IHL content. It should, however, be noted that this study was not designed and, hence, not powered to assess a dose–response relation. Third, owing to a slow inclusion rate we decided to abandon the inclusion criterion of fructose intake ≥ 45 g/d. To safeguard a detectable effect on IHL content, the supplementation of either glucose or fructose was increased to 45 g/d in participants with a low habitual fructose intake. Again, a sensitivity analysis did not show any significant difference when we stratified for fructose intake at baseline. Fourth, supplemented fructose may not have the same metabolic effects as fructose-containing food products. The food matrix may play a role in the timing of fructose absorption — in the current study the supplemented fructose was dissolved in water or food — and may cause an interaction between different nutrients. For instance, it has been postulated that that the presence of vitamin C in fruit and vegetables abolishes the toxic effects of fructose (46–48). Fifth, despite major efforts to achieve an isocaloric diet in comparison with the habitual baseline diet of participants, BMI decreased similarly in both the intervention and control groups, which could be explained by the Hawthorne effect.
A major strength of this study is the double-blind randomized controlled design, using an isocaloric comparator. As previously mentioned, both groups followed the same fructose-restricted diet, which is of importance given the differential impact of macronutrients on IHL content (49, 50). Despite this design, we did find some small, probably accidental, differences in carbohydrate intake expressed as percentage of total energy intake (i.e., relatively higher in the intervention group), which tended to be in exchange for protein intake (i.e., relatively lower in the intervention group). Given the previously described effects of carbohydrates and proteins on IHL content (i.e., predisposing and protective, respectively) (49, 50), the effects of these subtle differences in macronutrient composition on IHL content, if any, have probably mitigated the observed difference in the current study. Finally, not only did we use a 3-d food journal combined with personal interviews to assess fructose intake throughout the study, we were also able to objectively confirm adherence to the study protocol by measuring urinary fructose concentrations, a biomarker of fructose intake (28).
In conclusion, in this double-blind randomized controlled trial, we showed that a 6-wk fructose restriction per se results in a small but statistically significant decrease in IHL content in overweight adult individuals with a high FLI in comparison with an isocaloric control group. No effects were observed on glucose tolerance or serum lipid concentrations.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jean LJM Scheijen, Amée M Buziau, Petra M Niessen, Jos Op ‘t Roodt, Yvo HAM Kusters, and Armand MA Linkens (Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands) for their assistance during the execution of the study.
The authors’ responsibilities were as follows — NS: performed the measurements, conducted the analyses, and wrote the manuscript; NS, MCGJB, CDAS, and NCS: researched the data; MCGJB, CDAS, and NCS: conceived the study, supervised the analyses, and provided substantial revisions to the manuscript; MCGJB, CDAS, NCS, MEK, PV, VBS-H, PIHGS, MDGVdE, EMCvdP, EJMF, and CGS: reviewed the manuscript; MEK: conducted the scanning protocol; MEK, PV, VBS-H, PIHGS, MDGVdE, EMCvdP, EJMF, and CGS: provided revisions to the manuscript; PV and VBS-H: performed the magnetic resonance spectroscopy analyses; PIHGS: assisted NS during the measurements; MDGVdE: performed the randomization procedure and preparation of the glucose/fructose supplementation; EMCvdP: supervised the dietary counseling; EJMF and CGS: facilitated the measurements; MCGJB: is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Nutricia Research Foundation grant 2016-33 (to MCGJB) and Netherlands Heart Foundation grant 2015T042 (to MCGJB). The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: FLI, fatty liver index; GCK, glucokinase; GKRP, glucokinase regulatory protein; HOMA2-IR, homeostasis model assessment of insulin resistance; IHL, intrahepatic lipid; MR, magnetic resonance; NAFLD, nonalcoholic fatty liver disease; OGTT, oral-glucose-tolerance test; TR, repetition time; 1H-MRS, proton magnetic resonance spectroscopy.
Contributor Information
Nynke Simons, Division of Endocrinology and Metabolic Diseases, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; Laboratory for Metabolism and Vascular Medicine, Division of General Internal Medicine, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands.
Pandichelvam Veeraiah, Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht, The Netherlands.
Pomme I H G Simons, Division of Endocrinology and Metabolic Diseases, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; Laboratory for Metabolism and Vascular Medicine, Division of General Internal Medicine, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands.
Nicolaas C Schaper, Division of Endocrinology and Metabolic Diseases, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands; CAPHRI School for Public Health and Primary Care, Maastricht, The Netherlands.
M Eline Kooi, Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht, The Netherlands.
Vera B Schrauwen-Hinderling, Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht, The Netherlands; Department of Nutrition and Movement Sciences, Maastricht University Medical Center, Maastricht, The Netherlands.
Edith J M Feskens, Division of Human Nutrition and Health, Wageningen University, Wageningen, The Netherlands.
E M C (Liesbeth) van der Ploeg, Department of Dietetics, Maastricht University Medical Center, Maastricht, The Netherlands.
Mathias D G Van den Eynde, Laboratory for Metabolism and Vascular Medicine, Division of General Internal Medicine, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands.
Casper G Schalkwijk, Laboratory for Metabolism and Vascular Medicine, Division of General Internal Medicine, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands.
Coen D A Stehouwer, Laboratory for Metabolism and Vascular Medicine, Division of General Internal Medicine, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands; Division of General Internal Medicine, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands.
Martijn C G J Brouwers, Division of Endocrinology and Metabolic Diseases, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; Laboratory for Metabolism and Vascular Medicine, Division of General Internal Medicine, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands.
Data availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on request.
References
- 1. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–43. [DOI] [PubMed] [Google Scholar]
- 2. Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–34. [DOI] [PubMed] [Google Scholar]
- 3. Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DHet al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174(4):516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, Ha V, Di Buono M, Jenkins AL, Leiter LAet al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68(4):416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. 2018;128(2):545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sievenpiper JL, de Souza RJ, Jenkins DJ. Sugar: fruit fructose is still healthy. Nature. 2012;482(7386):470. [DOI] [PubMed] [Google Scholar]
- 8. Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela B-A. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45(5):1012–18. [DOI] [PubMed] [Google Scholar]
- 9. Armutcu F, Coskun O, Gürel A, Kanter M, Can M, Ucar F, Unalacak M. Thymosin alpha 1 attenuates lipid peroxidation and improves fructose-induced steatohepatitis in rats. Clin Biochem. 2005;38(6):540–7. [DOI] [PubMed] [Google Scholar]
- 10. Koo H-Y, Miyashita M, Cho BHS, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun. 2009;390(2):285–9. [DOI] [PubMed] [Google Scholar]
- 11. Roglans N, Vilà L, Farré M, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC. Impairment of hepatic Stat-3 activation and reduction of PPARα activity in fructose-fed rats. Hepatology. 2007;45(3):778–88. [DOI] [PubMed] [Google Scholar]
- 12. Samuel VT, Liu Z-X, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang X-m, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwarz J-M, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, Jones GM, Palii SP, Velasco-Alin M, Pan Ket al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017;153(3):743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gluchowski NL, Becuwe M, Walther TC, Farese RV Jr. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14(6):343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiStefano JK Fructose-mediated effects on gene expression and epigenetic mechanisms associated with NAFLD pathogenesis. Cell Mol Life Sci. 2020;77(11):2079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen KF, Laurent D, Yu C, Cline GW, Shulman GI. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50(6):1263–8. [DOI] [PubMed] [Google Scholar]
- 17. Buziau AM, Schalkwijk CG, Stehouwer CDA, Tolan DR, Brouwers M. Recent advances in the pathogenesis of hereditary fructose intolerance: implications for its treatment and the understanding of fructose-induced non-alcoholic fatty liver disease. Cell Mol Life Sci. 2020;77(9):1709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L, Liu D-W, Yan H-Y, Wang Z-Y, Zhao S-H, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17(6):510–19. [DOI] [PubMed] [Google Scholar]
- 19. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sluik D, Engelen AI, Feskens EJ. Fructose consumption in the Netherlands: the Dutch national food consumption survey 2007–2010. Eur J Clin Nutr. 2015;69(4):475–81. [DOI] [PubMed] [Google Scholar]
- 21. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. [DOI] [PubMed] [Google Scholar]
- 22. Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment] NEVO-tabel [Dutch Food Composition Table]. In: Nederlands Voedingsstoffenbestand 2010 [Dutch Food Composition Database 2010]. Den Haag: RIVM; 2010. [Google Scholar]
- 23. Lindeboom L, Nabuurs CI, Hesselink MK, Wildberger JE, Schrauwen P, Schrauwen-Hinderling VB. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am J Clin Nutr. 2015;101(1):65–71. [DOI] [PubMed] [Google Scholar]
- 24. Guiu B, Petit JM, Loffroy R, Ben Salem D, Aho S, Masson D, Hillon P, Krause D, Cercueil J-P. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250(1):95–102. [DOI] [PubMed] [Google Scholar]
- 25. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–8. [DOI] [PubMed] [Google Scholar]
- 26. Kusters YH, Schalkwijk CG, Houben AJ, Kooi ME, Lindeboom L, Op ’t Roodt J, Joris PJ, Plat J, Mensink RP, Barrett EJet al. Independent tissue contributors to obesity-associated insulin resistance. JCI Insight. 2017;2(13):89695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simons N, Debray FG, Schaper NC, Kooi ME, Feskens EJM, Hollak CEM, Lindeboom L, Koek GH, Bons JAP, Lefeber DJet al. Patients with aldolase B deficiency are characterized by an increased intrahepatic triglyceride content. J Clin Endocrinol Metab. 2019;104(11):5056–64. [DOI] [PubMed] [Google Scholar]
- 28. Tasevska N, Runswick SA, McTaggart A, Bingham SA. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1287–94. [DOI] [PubMed] [Google Scholar]
- 29. Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, Liu W, Tesz GJ, Birnbaum MJ, Rabinowitz JD. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018;27(2):351–61.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun SZ, Empie MW. Fructose metabolism in humans – what isotopic tracer studies tell us. Nutr Metab (Lond). 2012;9(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shiota M, Moore MC, Galassetti P, Monohan M, Neal DW, Shulman GI, Cherrington AD. Inclusion of low amounts of fructose with an intraduodenal glucose load markedly reduces postprandial hyperglycemia and hyperinsulinemia in the conscious dog. Diabetes. 2002;51(2):469–78. [DOI] [PubMed] [Google Scholar]
- 32. Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJet al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLos Genet. 2011;7(3):e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sobrecases H, Le KA, Bortolotti M, Schneiter P, Ith M, Kreis R, Boesch C, Tappy L. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab. 2010;36(3):244–6. [DOI] [PubMed] [Google Scholar]
- 34. Le KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89(6):1760–5. [DOI] [PubMed] [Google Scholar]
- 35. Kechagias S, Ernersson Å, Dahlqvist O, Lundberg P, Lindström T, Nystrom FH. Fast-food-based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut. 2008;57(5):649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, Peltonen M, Romeo S, Lundbom J, Lundbom Net al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96(4):727–34. [DOI] [PubMed] [Google Scholar]
- 37. McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227(2):429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang B, Li M, Zhao Z, Wang S, Lu J, Chen Y, Xu M, Wang W, Ning G, Bi Yet al. Glycemic measures and development and resolution of nonalcoholic fatty liver disease in nondiabetic individuals. J Clin Endocrinol Metab. 2020;105(5):1416–26. [DOI] [PubMed] [Google Scholar]
- 40. Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, Mirrahimi A. Fructose vs. glucose and metabolism: do the metabolic differences matter?. Curr Opin Lipidol. 2014;25(1):8–19. [DOI] [PubMed] [Google Scholar]
- 41. Bortolotti M, Kreis R, Debard C, Cariou B, Faeh D, Chetiveaux M, Ith M, Vermathen P, Stefanoni N, Lê K-Aet al. High protein intake reduces intrahepatocellular lipid deposition in humans. Am J Clin Nutr. 2009;90(4):1002–10. [DOI] [PubMed] [Google Scholar]
- 42. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. [DOI] [PubMed] [Google Scholar]
- 43. Yang B-L, Wu W-C, Fang K-C, Wang Y-C, Huo T-I, Huang Y-H, Yang H-I, Su C-W, Lin H-C, Lee F-Yet al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS One. 2015;10(3):e0120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F, Umpleby M, Pfeiffer AF, Thomas EL, Bell JDet al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171(5):561–9. [DOI] [PubMed] [Google Scholar]
- 45. Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11(9):1201–4. [DOI] [PubMed] [Google Scholar]
- 46. Lanaspa MA, Sanchez-Lozada LG, Choi Y-J, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay Met al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287(48):40732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sagan KC, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005;19(12):1657–67. [DOI] [PubMed] [Google Scholar]
- 48. Johnson RJ, Stenvinkel P, Andrews P, Sánchez-Lozada LG, Nakagawa T, Gaucher E, Andres-Hernando A, Rodriguez-Iturbe B, Jimenez CR, Garcia Get al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med. 2020;287(3):252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luukkonen PK, Sadevirta S, Zhou Y, Kayser B, Ali A, Ahonen L, Lallukka S, Pelloux V, Gaggini M, Jian Cet al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41(8):1732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, Machann J, Petzke KJ, Hierholzer J, Lichtinghagen Ret al. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology. 2017;152(3):571–85.e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on request.