Abstract
Background
Fish is a primary source of protein and n-3 PUFA but also contains methylmercury (MeHg), a naturally occurring neurotoxicant to which, at sufficient exposure levels, the developing fetal brain is particularly sensitive.
Objectives
To examine the association between prenatal MeHg and maternal status of n-3 and n-6 PUFA with neurodevelopment, and to determine whether PUFA might modify prenatal MeHg associations with neurodevelopment.
Methods
We examined the Seychelles Child Development Study Nutrition Cohort 2 (NC2) at age 7 y. We used a sophisticated and extensive neurodevelopmental test battery that addressed 17 specific outcomes in multiple neurodevelopmental domains: cognition, executive and psychomotor function, language development, behavior, scholastic achievement, and social communication. Analyses were undertaken on 1237 mother-child pairs with complete covariate data (after exclusions) and a measure of at least 1 outcome. We examined the main and interactive associations of prenatal MeHg exposure (measured as maternal hair mercury) and prenatal PUFA status (measured in maternal serum at 28 weeks’ gestation) on child neurodevelopmental outcomes using linear regression models. We applied the Bonferroni correction to account for multiple comparisons and considered P values <0.0029 to be statistically significant.
Results
Prenatal MeHg exposure and maternal DHA and arachidonic acid (20:4n-6) (AA) status were not significantly associated with any neurodevelopmental outcomes. Findings for 4 outcomes encompassing executive function, cognition, and linguistic skills suggested better performance with an increasing maternal n-6:n-3 PUFA ratio (P values ranging from 0.004 to 0.05), but none of these associations were significant after adjusting for multiple comparisons. No significant interaction between MeHg exposure and PUFA status was present.
Conclusions
Our findings do not support an association between prenatal MeHg exposure or maternal DHA and AA status with neurodevelopmental outcomes at age 7 y. The roles of n-6 and n-3 PUFA in child neurodevelopment need further research. Am J Clin Nutr 2021;113:304–313.
Keywords: child neurodevelopment, maternal fish consumption, prenatal methylmercury, polyunsaturated fatty acids, n-6:n-3 ratio
Introduction
Globally, more than 3 billion people depend daily on fish as their primary source of protein (1). Fish is also the major source of DHA, a n-3 PUFA and a major component of the developing brain (2). The fetus acquires DHA and the n-6 PUFA arachidonic acid (20:4n-6) (AA) from maternal blood, and both are necessary for normal brain development.
Fish also contain small amounts of methylmercury (MeHg), which is produced naturally in the environment by microbial methylation of inorganic mercury. MeHg was probably present in primordial sea and freshwater food chains (3) long before anthropogenic release of Hg into the environment. While MeHg is a known neurotoxicant at high exposures, associated with poisoning episodes, and the fetal brain is especially sensitive (4, 5), there is substantial uncertainty over whether there are any neurodevelopmental consequences of MeHg exposure from consuming fish with only background concentrations of MeHg (6, 7, 8, 9). A recent review of epidemiologic studies of fish consumption and children’s neurodevelopmental outcomes (studies including sea mammals were excluded) found no studies reporting an inverse association (10).
The Seychelles Child Development Study (SCDS) is a large, prospective, longitudinal study. The Republic of Seychelles was selected as the study location because fish consumption there is very high, consumption does not include sea mammals, and the average MeHg exposure is about 10 times that of the US (11) and UK (12) populations. Our earlier studies revealed surprisingly better scores on neurodevelopmental tests in relation to increasing prenatal MeHg exposure, and we hypothesized these associations were related to the unmeasured benefits of nutrition from fish consumption (13). We reported better neurodevelopmental tests scores associated with higher n-3 PUFA status in an earlier nutrition study [Nutrition Cohort 1 (NC1)] and subsequently hypothesized that they could be sufficiently large to mask any association with MeHg toxicity if such an association is present (14, 15). To confirm these earlier findings and identify factors potentially modifying any MeHg toxicity, we enrolled a cohort of 1535 mother-child pairs [Nutrition Cohort 2 (NC2)]. We characterized the cohort for prenatal MeHg exposure, maternal nutritional status, and developmental outcomes at 20 months of age. We again found no overall association between prenatal MeHg and neurodevelopment, but found better test scores with increasing n-3 PUFA (15). We also reported that there may be an optimal balance between n-6 and n-3 PUFA (indicated by the physiological measure of the n-6:n-3 ratio in maternal serum) that may influence the maternal inflammatory milieu (16) and, in turn, may influence MeHg neurotoxicity (15). It might be expected that the relative proportions of n-6 to n-3 PUFA (as indicated by the maternal n-6:n-3 PUFA ratio) available to the fetus might affect subsequent neurodevelopment of offspring, as both n-6 and n-3 PUFA compete for the same enzymes in biosynthetic pathways, transfer across the placenta, and incorporation into neural membranes (17). In this paper, we reexamined this cohort for neurodevelopment at 7 years of age to determine whether these associations persisted using a more sophisticated and extensive neurodevelopmental test battery.
Methods
Study population
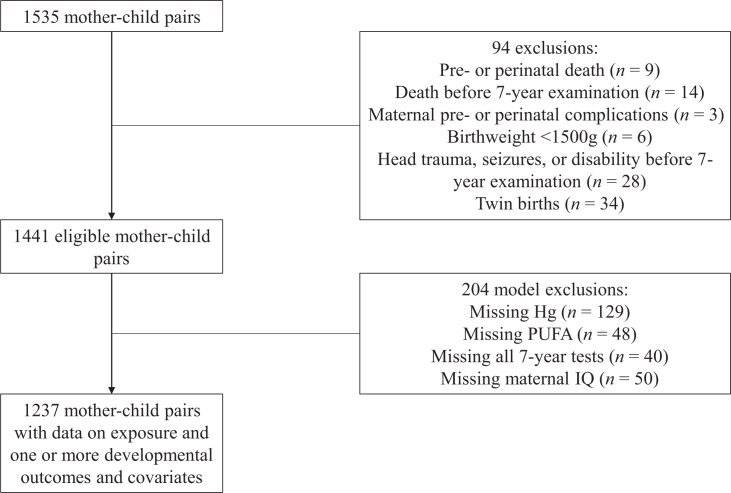
From 2008–2011, 1522 pregnant women were enrolled during their first prenatal visit (>14 weeks of gestation). There were 1441 mother-child pairs eligible for follow-up at 7 years of age after applying the following prespecified exclusion criteria: pre- or perinatal or childhood death, maternal prenatal complications, birth weight <1500 g, twin births, and illnesses or injuries affecting neurodevelopment, such as closed head trauma or meningitis (Figure 1). The study was reviewed and approved by the Seychelles Ethics Board and the Research Subjects Review Board at the University of Rochester. Original power calculations based on the magnitudes of associations observed for the Bayley Scales of Infant Development II (BSID-II) Psychomotor Developmental Index (PDI) at 9 and 30 months of age in our earlier NC1 cohort determined that the sample size needed to detect an interaction between MeHg and PUFA at 20 months of age in the NC2 cohort would be 1200 (15). Quality control procedures at all collaborating study sites are described elsewhere (15).
FIGURE 1.
Description of exclusions and missing data for the current analysis.
Prenatal MeHg exposure
Maternal hair samples were collected at delivery to determine prenatal MeHg exposure. Total mercury was measured by atomic absorption spectroscopy at the University of Rochester in the longest hair segment available that reflected exposure throughout pregnancy. Hair was assumed to grow at a rate of 1.1 cm/mo (18). Mercury deposited in hair is >80% MeHg and is known to correlate with the amount of mercury deposited in the infant brain (18).
Blood sampling and PUFA analysis
As described previously (15), maternal blood was drawn at the 28-week visit, then aliquoted and stored at −80°C. In total serum, the extraction of individual PUFAs, including AA, linoleic acid (18:2n-6) (LA), α-linolenic acid (18:3n-3) (ALA), EPA, and DHA, was completed and their FAME were prepared using boron trifluoride methanol according to an adaptation of the Folch et al. method (19). Individual FAME were detected and quantified by the gold-standard technique of gas chromatography–mass spectrometry (7890A-5975C; Agilent Technologies UK Ltd.), as previously described (8). The PUFA were identified both by their retention time and corresponding qualifier ions, with reference to those commercially available fatty acid standards, and quantified by use of an internal standard (C17:0). The internal standard was added to the samples to monitor the recovery rate. All analytic standards were of >99% purity and purchased from Sigma-Aldrich. An external calibration curve was established to a range of 0.01–1.5mg/ml and was run daily alongside the quality control (QC) material to check analytic precision. The average inter-assay and intra-assay CVs of fatty acids in the QC material were 8.34% and 5.83%, respectively. Results are presented as milligrams per milliliter to indicate physiologic quantities.
Dietary assessment
Descriptive data on dietary intake of fish were collected for 1424 NC2 mothers at 28 weeks of gestation using a Fish Use Questionnaire, which recorded the frequency of fish consumption (meals/week) by women during pregnancy.
Neurodevelopmental assessment
All assessments were conducted at the dedicated Seychelles Child Development Centre by a team of trained nurses. When children were about 7 years of age (range, 7.0–7.9 years), they completed a comprehensive test battery that addressed a range of neurodevelopmental domains. Children were evaluated on 14 primary neurodevelopmental endpoints using the following tests: Clinical Evaluation of Language Fundamentals–5 (CELF-5; 6 endpoints), Kaufman Brief Intelligence Test 2 (KBIT-2; 2 endpoints), Boston Naming Test (BNT), Trailmaking A, finger tapping (FT; 2 endpoints), and Woodcock-Johnson Test of Achievement–III (WJ-III; 2 endpoints). Parents were asked to complete the following instruments for an additional 3 primary endpoints: Child Behavior Checklist (CBCL), Social Responsiveness Scale 2 (SRS-2), and the Social Communication Questionnaire (SCQ). The tests are further described below.
Language development.
The CELF-5 is a 5-part test of linguistic skills to assess functions such as comprehension, repetition, naming, and other receptive and expressive skills. These skills include an assessment of children’s understanding of linguistic concepts; the ability to interpret, recall, and execute oral directions of increasing length and complexity and to remember the names, characteristics, and order of objects (following directions); the ability to recall and reproduce sentences (recalling sentences); comprehension of grammatical rules at the sentence level (sentence comprehension); and the ability to interpret factual and inferential information (understanding spoken paragraphs) (20).
Cognition.
The KBIT-2 is a cognitive test that assesses both verbal and nonverbal intelligence. The verbal scale assesses knowledge of words and their meanings, whereas the nonverbal scale assesses the ability to solve new problems by perceiving relationships and completing analogies (21).
Executive function.
The BNT total score assesses executive function and is a 60-item measure of object naming from line drawings. It evaluates the extent to which subjects have difficulty retrieving the correct words, names, or numbers from memory. Items are rank ordered in terms of their ability to be named, reflecting the frequency or common occurrence of the items (e.g., low-frequency objects may be more difficult to name than high-frequency objects) (22). Trailmaking is a measure of psychomotor development and examines visual attention and task switching. This measure requires a subject to connect 25 consecutive targets on a sheet of paper as quickly as possible and is measured by the time taken to complete the test. Trailmaking A includes only numbers (23).
Psychomotor function.
The FT test is a neuropsychological test that examines motor functioning: specifically, motor speed and lateralized coordination. This test determines the average number of taps in 5 consecutive 10-second periods. Test scores were obtained for the dominant and nondominant hands (23).
Scholastic achievement.
The WJ-III is a measure of scholastic achievement and provides scores for academic achievement. We administered 2 subtests: letter-word identification and applied mathematical problem solving (24).
Behavior.
The CBCL is a 118-item test designed to assess behavioral problems and social competencies of children, as reported by parents. Items are scored on a 3-point scale ranging from not true to often true of the child (25).
Social communication.
The SRS-2 is a 65-item rating scale that ascertains autistic symptoms across the entire range of severity occurring in ordinary social settings. Symptoms are sampled across the domains of behaviors critical for the diagnosis of autism spectrum disorders (ASD) by DSM-IV criteria (26). SCQ is a 40-item parent questionnaire designed to screen for symptoms of ASD in children. It is based on the Autism Diagnostic Interview, the most widely accepted interview for making a research diagnosis of ASD, and has been used internationally to study ASD (27).
Covariates
As in previous analyses of this cohort (15), models were adjusted for covariates known to be associated with child neurodevelopment, including maternal age at delivery, child age at testing, child sex, Hollingshead socioeconomic status, whether or not both parents were living with the child (family status), and mother’s cognitive ability (KBIT-2 matrices). Analysis of CELF-5 outcomes also accounted for interviewer differences, because preliminary linear models that included the interviewer as a covariate showed that it was a significant predictor of CELF-5 outcomes only.
Statistical analysis
We carried out linear regression analyses to evaluate the main and interactive associations of MeHg and PUFAs on outcomes. All analyses were specified in an a priori analysis plan developed before model fitting. In main association models, we used DHA and AA because these PUFAs are considered to have direct influences on brain development (28). In the MeHg by PUFA interaction models, we used total n-3 (comprising the major quantitative PUFA, ALA, EPA, and DHA), total n-6 (comprising the major quantitative PUFA, LA, and AA), and the n-6:n-3 ratio because the balance of these PUFAs can influence the inflammatory response to MeHg toxicity in the developing brain (29) and, in turn, might modify MeHg toxicity.
We examined the main associations of MeHg and PUFAs on developmental outcomes with and without adjustment for each other; only the models with both variables are reported. We repeated these models while including an interaction between MeHg and child sex, but only 1 sex interaction was statistically significant at the conventional level (P < 0.05) and none were significant after accounting for multiple comparisons. Therefore, sex-specific associations were not considered further. We next examined interactions between MeHg and tertiles of total n-3 and total n-6 PUFAs and the n-6:n-3 ratio, in separate models. The main association models used PUFA as continuous variables, whereas we used PUFA tertiles in the interaction models for interpretability, specifically to compare MeHg slopes among subjects with low, medium, or high PUFA concentrations.
All models were fit using the statistical package R, version 3.6.2 (30). Model assumptions were checked using standard methods (31), which included graphically checking for linearity and constant variance and normality of the residuals. No substantial departures from linearity or constant variance were observed. To improve the model fit, some outcomes were transformed by either log10, square, or square root, as chosen by Box-Cox criteria and reported in the tables. After transformations, when required, there was no evidence of extreme outliers or highly influential observations.
We report the individual associations and corresponding slope (beta), SE, and P values. Associations for prenatal MeHg and maternal PUFA were scaled by the IQR so that the predicted outcome changes per unit increase in covariates are within the possible values observed in our data. Therefore, regression coefficients for MeHg and PUFAs express the expected change in each outcome when the exposure changes by the amount of the IQR. We applied the Bonferroni correction to account for multiple comparisons. We focused on 17 primary neurodevelopmental outcomes and considered a P value less than 0.05/17 (0.0029) as statistically significant after correction for multiplicity.
Results
We conducted analyses on 1237 participants with complete covariate data and a measure of at least 1 outcome (Figure 1). Summary statistics for selected maternal characteristics, prenatal MeHg and PUFA status, child outcomes, and model covariates are presented in Table 1. The mean self-reported maternal fish intake per week in this cohort was 8.6 meals (SD, 4.57).
TABLE 1.
Summary statistics for maternal biomarkers, child outcomes at 7 years of age, and model covariates
| Variable | n | Mean (SD) | Minimum | Median | Maximum |
|---|---|---|---|---|---|
| Maternal prenatal biomarkers | |||||
| Hair MeHg, ppm | 1237 | 3.91 (3.47) | 0.01 | 2.89 | 31.66 |
| Total n-3 PUFA, mg/mL | 1237 | 0.27 (0.09) | 0.12 | 0.27 | 0.63 |
| Total n-6 PUFA, mg/mL | 1237 | 1.10 (0.29) | 0.42 | 1.09 | 2.70 |
| n-6:n-3 ratio | 1237 | 4.35 (1.64) | 1.55 | 3.95 | 15.80 |
| DHA, mg/mL | 1237 | 0.18 (0.08) | 0.04 | 0.18 | 0.52 |
| AA, mg/mL | 1237 | 0.20 (0.08) | 0.04 | 0.21 | 0.38 |
| Child 7-year outcomes | |||||
| Trailmaking time A | 1132 | 92.7 (42.1) | 27 | 64 | 300 |
| BNT total | 1229 | 23.2 (5.3) | 8 | 23 | 45 |
| CELF-5 total | 1200 | 81.7 (16.6) | 6 | 82 | 135 |
| CELF-5 FD | 1224 | 11.5 (4.1) | 0 | 11 | 24 |
| CELF-5 LC | 1231 | 17.7 (3.0) | 0 | 18 | 25 |
| CELF-5 RS | 1219 | 26.9 (7.7) | 3 | 26 | 57 |
| CELF-5 SC | 1222 | 19.5 (4.3) | 0 | 21 | 26 |
| CELF-5 USP | 1215 | 5.8 (3.5) | 0 | 3 | 18 |
| CBCL total | 1218 | 42.4 (24.3) | 0 | 25 | 191 |
| KBIT-2 word knowledge | 1233 | 20.4 (7.8) | 4 | 22 | 47 |
| KBIT-2 matrices | 1229 | 17.6 (4.9) | 0 | 16 | 33 |
| WJ-III applied problems | 1225 | 23.5 (3.7) | 0 | 24 | 34 |
| WJ-III letter word | 1224 | 52.8 (22.4) | 2 | 62 | 76 |
| FT dominant hand | 1222 | 31.2 (5.1) | 5 | 31.2 | 45.8 |
| FT nondominant hand | 1223 | 27.4 (4.6) | 4.2 | 27.4 | 46.4 |
| SCQ total | 1227 | 8.4 (4.3) | 0 | 9 | 31 |
| SRS-2 total | 1235 | 48.0 (19.3) | 6 | 46 | 129 |
| Covariates | |||||
| Child age at testing 7 years | 1237 | 7.4 (0.2) | 7 | 7.3 | 7.9 |
| SES at 7 years | 1237 | 33.3 (10.8) | 8 | 32 | 63 |
| Raw maternal KBIT-2 matrices | 1237 | 29.6 (7.0) | 4 | 30 | 46 |
| Mothers’ age at delivery | 1237 | 27.1 (6.3) | 16.3 | 26.1 | 46.8 |
| Child sex, female | 1237 | 48% | |||
| Family status at 7 years | |||||
| Both parents living with child | 1237 | 52% | |||
Total n-3 PUFA is defined as the sum of a-linolenic acid + EPA + DHA; Total n-6 PUFA is defined as the sum of linoleic acid + AA. Abbreviations: AA, arachidonic acid; BNT, Boston Naming Test; CBCL, Child Behavior Checklist; CELF-5, Clinical Evaluation of Language Fundamentals-5; FD, following directions; FT, finger tapping; KBIT-2, Kaufman Brief Intelligence Test 2; LC, linguistic concepts; MeHg, methylmercury; RS, recalling sentences; SC, sentence comprehension; SES, socioeconomic status; SCQ, Social Communication Questionnaire; SRS-2, Social Responsiveness Scale 2; USP, understanding spoken paragraphs; WJ-III, Woodcock-Johnson Test of Scholastic Achievement–III.
The main associations between MeHg and child outcomes are presented in Table 2 for models with DHA and AA (Model 1) and with the n-6:n-3 ratio (Model 2). Prenatal MeHg was not significantly associated with any outcomes. While increasing prenatal MeHg exposure was associated (0.0029 < P < 0.05) with lower (i.e., worse) WJ-III letter word scores and lower (i.e., better) square root (SCQ total) scores, these associations were not statistically significant after correction for multiplicity.
TABLE 2.
Prenatal methylmercury exposure in Models 1 and 2 in relation to child neurodevelopmental outcomes at 7 years of age: Seychelles Child Development Study Nutrition 2 Cohort
| Outcome | n | MeHg: Model 11 | MeHg: Model 22 | ||
|---|---|---|---|---|---|
| Beta, SE | P | Beta, SE | P | ||
| Log, Trailmaking time A | 1132 | 0.01, 0.01 | 0.26 | 0.02, 0.01 | 0.24 |
| Square root, BNT total | 1229 | 0.02, 0.02 | 0.15 | 0.02, 0.02 | 0.16 |
| CELF-5 total | 1200 | − 0.20, 0.49 | 0.68 | − 0.25, 0.48 | 0.61 |
| CELF-5 FD | 1224 | − 0.12, 0.13 | 0.35 | − 0.12, 0.13 | 0.33 |
| CELF-5 LC^2 | 1231 | − 0.71, 2.92 | 0.81 | − 1.06, 2.90 | 0.72 |
| CELF-5 RS | 1219 | − 0.09, 0.23 | 0.68 | − 0.11, 0.23 | 0.64 |
| CELF-5 SC^2 | 1222 | 0.52, 4.33 | 0.90 | 0.32, 4.30 | 0.94 |
| Square root, CELF-5 USP | 1215 | − 0.00, 0.02 | 0.90 | − 0.00, 0.02 | 0.84 |
| Square root, CBCL total | 1218 | − 0.01, 0.05 | 0.81 | − 0.01, 0.05 | 0.80 |
| KBIT-2 word knowledge | 1233 | − 0.28, 0.24 | 0.25 | − 0.30, 0.24 | 0.22 |
| KBIT-2 matrices | 1229 | 0.07, 0.15 | 0.63 | 0.06, 0.15 | 0.69 |
| WJ-III applied problems^2 | 1225 | 0.52, 5.00 | 0.92 | 0.32, 4.98 | 0.95 |
| WJ-III letter word | 1224 | − 1.36, 0.68 | 0.05 | − 1.39, 0.68 | 0.04 |
| FT dominant hand | 1222 | 0.00, 0.15 | 0.97 | 0.01, 0.15 | 0.96 |
| FT nondominant hand | 1223 | − 0.14, 0.14 | 0.33 | − 0.13, 0.14 | 0.33 |
| Square root, SCQ total | 1227 | − 0.05, 0.02 | 0.04 | − 0.05, 0.02 | 0.04 |
| Square root, SRS-2 total | 1235 | − 0.01, 0.04 | 0.86 | − 0.01, 0.04 | 0.83 |
A regression analysis was performed for each outcome separately. Beta indicates the estimated regression coefficients. B and SE values are expressed as changes in outcomes per IQR increase in exposure (3.75 ppm). The P values have not been adjusted for multiple comparisons. P values are considered statistically significant when P < 0.0029. Abbreviations: AA, arachidonic acid; BNT, Boston Naming Test; CBCL, Child Behavior Checklist; CELF-5, Clinical Evaluation of Language Fundamentals–5; FD, following directions; FT, finger tapping; KBIT-2, Kaufman Brief Intelligence Test 2; LC, linguistic concepts; MeHg, methylmercury; RS, recalling sentences; SC, sentence comprehension; SCQ, Social Communication Questionnaire; Sqrt, SCQ total score; SRS-2, Social Responsiveness Scale 2; USP, understanding spoken paragraphs; WJ-III, Woodcock-Johnson Test of Scholastic Achievement–III.
Model 1 was adjusted for maternal DHA and AA status, maternal age, maternal IQ, child age, child sex, Hollingshead socioeconomic status, and family status. CELF-5 outcomes are also adjusted for interviewer. Effect estimates for covariates are shown in Supplemental Table 1.
Model 2 was adjusted for maternal n-6:n-3 ratio, maternal age, maternal IQ, child age, child sex, Hollingshead socioeconomic status, and family status. CELF-5 outcomes are also adjusted for interviewer.
Table 3 shows associations between maternal PUFA status and neurodevelopmental outcomes. Neither DHA nor AA were significantly associated with any of the outcomes at age 7 y. Our results suggest that a higher ratio of total n-6 to total n-3 was associated with higher (i.e., better) BNT scores, total CELF-5 scores, CELF-5 following directions scores, and KBIT-2 matrices scores (0.0029 < P < 0.05), but none of these associations were statistically significant after accounting for multiple comparisons.
TABLE 3.
Maternal PUFA status in Models 1 and 2 in relation to child neurodevelopmental outcomes at 7 years of age: Seychelles Child Development Study Nutrition 2 Cohort
| Outcome | n | Model 11 | Model 21 | ||||
|---|---|---|---|---|---|---|---|
| DHA | AA | n-6:n-3 ratio | |||||
| Beta, SE | P | Beta, SE | P | Beta, SE | P | ||
| Log, trail-making A | 1132 | 0.01, 0.02 | 0.52 | − 0.01, 0.02 | 0.77 | − 0.01, 0.01 | 0.52 |
| Square root, BNT Total | 1229 | − 0.04, 0.03 | 0.13 | 0.03, 0.03 | 0.31 | 0.03, 0.01 | 0.05 |
| CELF-5 total | 1200 | − 1.30, 0.84 | 0.12 | − 0.13, 0.79 | 0.87 | 0.99, 0.46 | 0.03 |
| CELF-5 FD | 1224 | − 0.13, 0.22 | 0.56 | − 0.29, 0.20 | 0.16 | 0.24, 0.12 | 0.05 |
| CELF-5 LC^2 | 1231 | − 8.99, 5.01 | 0.07 | 2.43, 4.67 | 0.60 | 4.42, 2.70 | 0.10 |
| CELF-5 RS | 1219 | − 0.24, 0.40 | 0.55 | − 0.10, 0.38 | 0.79 | 0.15, 0.22 | 0.48 |
| CELF-5 SC | 1222 | − 5.12, 7.43 | 0.49 | − 2.93, 6.95 | 0.67 | 4.84, 3.99 | 0.23 |
| Square root, CELF-5 USP | 1215 | − 0.05, 0.04 | 0.19 | 0.03, 0.03 | 0.32 | 0.01, 0.02 | 0.45 |
| Square root, CBCL total | 1218 | 0.06, 0.09 | 0.54 | − 0.09, 0.09 | 0.33 | − 0.04, 0.05 | 0.48 |
| KBIT-2 word knowledge | 1233 | − 0.54, 0.42 | 0.19 | 0.25, 0.39 | 0.52 | 0.29, 0.23 | 0.20 |
| KBIT-2 matrices | 1229 | − 0.48, 0.26 | 0.06 | − 0.03, 0.24 | 0.90 | 0.40, 0.14 | 0.004 |
| WJ-III applied problems^2 | 1225 | − 0.76, 8.63 | 0.93 | − 0.63, 8.08 | 0.94 | − 1.88, 4.63 | 0.69 |
| WJ-III letter word | 1224 | − 1.19, 1.18 | 0.32 | − 0.24, 1.11 | 0.83 | 1.19, 0.64 | 0.06 |
| FT dominant hand | 1222 | − 0.08, 0.27 | 0.75 | − 0.08, 0.25 | 0.76 | 0.20, 0.14 | 0.16 |
| FT nondominant hand | 1223 | − 0.15, 0.24 | 0.54 | 0.04, 0.23 | 0.86 | 0.20, 0.13 | 0.12 |
| Square root, SCQ total1 | 1227 | 0.02, 0.04 | 0.69 | − 0.02, 0.03 | 0.50 | − 0.00, 0.02 | 0.99 |
| Square root, SRS-2 total | 1235 | 0.03, 0.07 | 0.69 | − 0.09, 0.06 | 0.18 | − 0.01, 0.04 | 0.85 |
Regression analysis was performed for each outcome separately. Beta values are estimated regression coefficients. B and SE values are expressed as changes in outcomes per IQR increase in exposure (DHA: 0.128 mg/mL; AA: 0.11 mg/mL; n-6:n-3 ratio: 1.675). The P values have not been adjusted for multiple comparisons. P values are considered statistically significant when P < 0.0029. Abbreviations: AA, arachidonic acid; BNT, Boston Naming Test; CBCL, Child Behavior Checklist; CELF-5, Clinical Evaluation of Language Fundamentals–5; FD, following directions; FT, finger tapping; KBIT-2, Kaufman Brief Intelligence Test 2; LC, linguistic concepts; MeHg, methylmercury; RS, recalling sentences; SC, sentence comprehension; SCQ, Social Communication Questionnaire; SRS-2, Social Responsiveness Scale 2; USP, understanding spoken paragraphs; WJ-III, Woodcock-Johnson Test of Scholastic Achievement–III.
Models adjusted for prenatal MeHg exposure, maternal age, maternal IQ, child age, child sex, Hollingshead socioeconomic status, and family status. CELF-5 outcomes are also adjusted for interviewer. Model 1 was adjusted for maternal DHA and AA status; Model 2 was adjusted for maternal n-6:n-3 ratio.
Results from MeHg-PUFA interaction models are presented in Table 4. Interactions between MeHg and n-3 PUFA tertiles were suggestive [P = 0.030; 2 degrees of freedom (df) test] only for the FT test on the nondominant hand. Similarly, interactions between MeHg and n-6 PUFA tertiles were suggestive (P = 0.032; 2 df test) only for the log10 Trailmaking A time. However, these interactions were not statistically significant after accounting for multiplicity. There were also no significant interactions between MeHg and n-6:n-3 PUFA ratios.
TABLE 4.
Interaction models for prenatal methylmercury exposure against child outcomes at 7 years of age with PUFA status
| Outcome | n | Interaction with n-3, MeHg slope1 | Interaction with n-6, MeHg slope1 | Interaction with n-6:n3 ratio, MeHg slope1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n-3 PUFA lowest fertile Beta, SE, P | n-3 PUFA middle tertile Beta, SE, P | n-3 PUFA highest tertile Beta, SE, P | Hg by n-3 interaction P value P | n-6 PUFA lowest tertile Beta, SE, P | n-6 PUFA middle tertile Beta, SE, P | n-6 PUFA highest tertile Beta, SE, P | Hg by n-6 interaction P value P | n-6/n-3 PUFA lowest tertile Beta, SE, P | n-6/n-3 PUFA middle tertile Beta, SE, P | n-6/n-3 PUFA highest tertile Beta, SE, P | Hg by n-6/n-3 ratio interaction P value P | ||
| Log, Trailmaking time A | 1132 | 0.00, 0.01, P = 0.88 | 0.00, 0.01, P = 0.96 | 0.01, 0.01, P = 0.11 | 0.49 | −0.01, 0.01, P = 0.27 | 0.02, 0.01, P = 0.02 | 0.01, 0.01, P = 0.25 | 0.03 | −0.00, 0.01, P = 0.80 | 0.00, 0.01, P = 0.48 | 0.02, 0.01, P = 0.04 | 0.18 |
| Square root, BNT | 1229 | 0.00, 0.01, P = 0.82 | 0.01, 0.01, P = 0.19 | 0.00, 0.01, P = 0.48 | 0.78 | 0.00, 0.01, P = 0.55 | 0.00, 0.01, P = 0.64 | 0.01, 0.01, P = 0.12 | 0.70 | 0.01, 0.01, P = 0.31 | 0.00, 0.01, P = 0.52 | 0.01, 0.01, P = 0.37 | 0.97 |
| CELF-5 total | 1200 | −0.22, 0.25, P = 0.37 | 0.29, 0.21, P = 0.18 | −0.28, 0.21, P = 0.18 | 0.12 | −0.04, 0.21, P = 0.86 | 0.03, 0.23, P = 0.90 | −0.15, 0.23, P = 0.51 | 0.85 | 0.01, 0.20, P = 0.95 | −0.20, 0.23, P = 0.37 | −0.05, 0.25, P = 0.85 | 0.76 |
| CELF-5 FD | 1224 | −0.01, 0.06, P = 0.86 | 0.01, 0.06, P = 0.81 | −0.09, 0.05, P = 0.10 | 0.38 | −0.06, 0.05, P = 0.25 | −0.01, 0.06, P = 0.89 | −0.02, 0.06, P = 0.79 | 0.77 | −0.06, 0.05, P = 0.21 | −0.07, 0.06, P = 0.26 | 0.06, 0.07, P = 0.36 | 0.26 |
| CELF-5 LC^2 | 1231 | −0.98, 1.46, P = 0.50 | 0.51, 1.28, P = 0.69 | −0.39, 1.26, P = 0.76 | 0.73 | −0.68, 1.23, P = 0.58 | −0.41, 1.40, P = 0.77 | 0.65, 1.35, P = 0.63 | 0.75 | 1.36, 1.17, P = 0.25 | −2.39, 1.34, P = 0.08 | −0.08, 1.52, P = 0.96 | 0.11 |
| CELF-5 RS | 1219 | −0.13, 0.12, P = 0.26 | 0.14, 0.10, P = 0.16 | −0.11, 0.10, P = 0.27 | 0.11 | −0.01, 0.10, P = 0.90 | −0.03, 0.11, P = 0.78 | −0.03, 0.11, P = 0.77 | 0.99 | −0.01, 0.09, P = 0.96 | −0.06, 0.11, P = 0.55 | −0.04, 0.12, P = 0.74 | 0.92 |
| CELF-5 SC | 1222 | −1.16, 2.17, P = 0.59 | 3.31, 1.89, P = 0.08 | −1.86, 1.85, P = 0.32 | 0.11 | 1.60, 1.83, P = 0.38 | 0.61, 2.07, P = 0.77 | −2.08, 2.00, P = 0.30 | 0.38 | 1.12, 1.74, P = 0.52 | −0.77, 1.99, P = 0.70 | −0.77, 2.26, P = 0.73 | 0.71 |
| Square root, CELF-5 USP | 1215 | −0.00, 0.01, P = 0.78 | −0.00, 0.01, P = 0.94 | 0.00, 0.01, P = 0.97 | 0.97 | −0.00, 0.01, P = 0.72 | 0.01, 0.01, P = 0.33 | −0.01, 0.01, P = 0.39 | 0.40 | 0.00, 0.01, P = 0.68 | −0.01, 0.01, P = 0.52 | −0.00, 0.01, P = 0.86 | 0.75 |
| Square root, CBCL total | 1218 | −0.02, 0.03, P = 0.58 | −0.00, 0.02, P = 0.99 | 0.00, 0.02, P = 0.88 | 0.86 | −0.02, 0.02, P = 0.33 | 0.04, 0.03, F= 0.18 | −0.02, 0.03, P = 0.55 | 0.21 | −0.02, 0.02, P = 0.36 | 0.00, 0.03, P = 0.92 | 0.02, 0.03, P = 0.58 | 0.58 |
| KBIT word knowledge | 1233 | −0.20, 0.12, P = 0.10 | −0.07, 0.11, P = 0.49 | 0.01, 0.11, P = 0.93 | 0.42 | −0.19, 0.10, P = 0.06 | −0.04, 0.12, P = 0.72 | 0.04, 0.11, P = 0.71 | 0.28 | −0.07, 0.10, P = 0.49 | −0.07, 0.11, P = 0.53 | −0.12, 0.13, P = 0.36 | 0.95 |
| KBIT matrices | 1229 | −0.01, 0.07, P = 0.91 | 0.05, 0.07, P = 0.47 | 0.01, 0.06, P = 0.94 | 0.84 | 0.03, 0.06, P = 0.69 | −0.01, 0.07, P = 0.90 | 0.05, 0.07, P = 0.51 | 0.85 | 0.02, 0.06, P = 0.79 | 0.03, 0.07, P = 0.69 | 0.01, 0.08, P = 0.92 | 0.98 |
| WJ-III applied problems ^2 | 1225 | −1.44, 2.51, P = 0.57 | 1.91, 2.19, P = 0.38 | −0.04, 2.16, P = 0.99 | 0.59 | −0.19, 2.11, P = 0.93 | −2.30, 2.42, P = 0.34 | 2.70, 2.32, P = 0.24 | 0.31 | 0.09, 2.01, P = 0.97 | −0.60, 2.31, P = 0.80 | 0.76, 2.62, P = 0.77 | 0.93 |
| WJ-III letter word | 1224 | −0.17, 0.34, P = 0.62 | −0.18, 0.30, P = 0.55 | −0.70, 0.30, P = 0.02. | 0.36 | −0.33, 0.29, P = 0.26 | −0.31, 0.33, P = 0.34 | −0.46, 0.32, P = 0.15 | 0.94 | −0.31, 0.28, P = 0.26 | −0.82, 0.32, P = 0.01 | 0.05, 0.36, P = 0.88 | 0.17 |
| FT dominant hand | 1222 | 0.06, 0.08, P = 0.43 | 0.04, 0.07, P = 0.57 | −0.08, 0.07, P = 0.24 | 0.31 | 0.02, 0.07, P = 0.81 | 0.03, 0.07, P = 0.71 | −0.03, 0.07, P = 0.64 | 0.82 | 0.03, 0.06, P = 0.62 | −0.10, 0.07, F= 0.16 | 0.09, 0.08, P = 0.26 | 0.17 |
| FT nondominant hand | 1223 | −0.06, 0.07, P = 0.39 | 0.08, 0.06, P = 0.17 | −0.14, 0.06, P = 0.02 | 0.03 | 0.02, 0.06, P = 0.75 | −0.01, 0.07, P = 0.85 | −0.12, 0.06, P = 0.07 | 0.27 | −0.04, 0.06, P = 0.51 | −0.04, 0.06, P = 0.51 | −0.02, 0.07, P = 0.82 | 0.96 |
| Square root, SCQ total | 1227 | −0.01, 0.01, P = 0.29 | −0.01, 0.01, P = 0.14 | −0.01, 0.01, P = 0.27 | 0.97 | −0.02, 0.01, P = 0.03 | −0.00, 0.01, P = 0.67 | −0.01, 0.01, P = 0.34 | 0.50 | −0.03, 0.01, P < 0.005 | −0.00, 0.01, P = 0.99 | −0.01, 0.01, P = 0.65 | 0.13 |
| Square root, SRS-2 total | 1235 | −0.00, 0.02, P = 0.91 | −0.00, 0.02, P = 0.82 | 0.00, 0.02, P = 0.88 | 0.96 | −0.01, 0.02, P = 0.53 | 0.01, 0.02, P = 0.75 | 0.00, 0.02, P = 0.91 | 0.78 | −0.01, 0.02, P = 0.36 | 0.01, 0.02, P = 0.72 | 0.00, 0.02, P = 0.82 | 0.62 |
Regression analysis was performed for each outcome separately. Beta values are estimated regression coefficients. B and SE values are expressed as change in outcome per interquartile range increase in exposure (3.75 ppm). The P values have not been adjusted for multiple comparisons. P values are considered statistically significant when P < 0.0029. Abbreviations: BNT, Boston Naming Test; CBCL, Child Behavior Checklist; CELF-5, Clinical Evaluation of Language Fundamentals–5; FD, following directions; FT, finger tapping; KBIT-2, Kaufman Brief Intelligence Test 2; LC, linguistic concepts; MeHg, methylmercury; RS, recalling sentences; SC, sentence comprehension; SCQ, Social Communication Questionnaire; SRS-2, Social Responsiveness Scale 2; USP, understanding spoken paragraphs; WJ-III, Woodcock-Johnson Test of Scholastic Achievement–III.
Models shown were adjusted for maternal age, maternal IQ, child age, child sex, Hollingshead socioeconomic status, and family status. CELF-5 outcomes are also adjusted for interviewer. Models including the n-3 by MeHg interaction were additionally adjusted for n-6 PUFA, and models including the n-6 by MeHg interaction were additionally adjusted for n-3 PUFA. Serum concentrations for the tertiles were as follows: high n-3 PUFAs, >0.308 mg/mL; medium n-3 PUFAs, 0.228–0.308 mg/mL; low n-3 PUFAs, <0.228 mg/mL; high n-6 PUFAs, >1.208 mg/mL; medium n-6 PUFAs, 0.962–1.208 mg/mL; low n-6 PUFAs, <0.962 mg/mL; high n-6/n-3, >4.496; medium n-6/n-3, 3.523–4.496; low n-6/n-3, <3.523.
Covariate associations in Supplemental Table 1 show better test scores for girls and for participants with a higher socioeconomic status, greater maternal intelligence scores, and older age at testing (P < 0.01 for most of these associations).
Discussion
We studied whether child neurodevelopmental outcomes are associated with prenatal MeHg exposure or maternal PUFA using a sophisticated and extensive test battery in the large SCDS NC2 cohort. Maternal MeHg exposure was not associated with 17 child neurodevelopmental outcomes at 7 years of age in this cohort. There were also no significant associations with maternal DHA or AA. Four outcomes encompassing executive function, cognition, and linguistic skills showed trends of better performance with an increasing maternal n-6:n-3 PUFA ratio (0.0029 < P < 0.05). Although these associations were not significant after adjusting for multiple comparisons, we believe these findings deserve more careful consideration because they appear consistently across a broad range of neurodevelopmental domains. Our analyses did not identify any statistically significant interaction between maternal MeHg exposure and PUFA status.
In the current assessment, we focused on the n-3 and n-6 PUFA to determine whether the outcome associations with PUFA observed at 20 months of age in this cohort (15) persisted at 7 years of age. The associations of neurodevelopment with the n-3 PUFA, DHA, and the PUFA interaction with MeHg that we found at 20 months in this cohort were not present at 7 years of age. Rather, in the current evaluation we observed multiple suggestive outcome associations indicative of better child development as the n-6:n-3 physiological ratio increased (BNT, the total CELF-5 score, the CELF-5 following directions score, and the KBIT matrices). These findings would be contrary to our hypothesis that a greater n-3 status (i.e., lower n-6:n-3 ratio) should offer protection against early oxidative or inflammatory insults to the developing brain by MeHg. Our hypothesis had regarded the n-6:n-3 ratio as an indirect measure of systemic inflammation, reflecting the potential for greater production of n-6 PUFA– derived eicosanoids (more proinflammatory than those derived from n-3 PUFAs) (29) and thus lower protection against any proinflammatory effects of MeHg. Therefore, it was surprising that our findings were suggestive of better performance on several neurodevelopmental tests with increases in the n-6:n-3 ratio. However, studies in healthy human adults have found that increased intake of the n-6 PUFAs LA and AA does not increase the concentrations of many inflammatory markers (32).
At age 20 months we also, unexpectedly, found associations between lower test scores on the Mental Development Index (MDI) using the BSID-II and increasing maternal DHA. (15). In that paper, we suggested that there might be an optimal balance between the n-6 PUFAs, specifically AA, and the n-3 PUFAs, specifically DHA. Studies in Tanzania where tribes with differing fish consumption were studied reported that the relationship between DHA and AA among those with low DHA status was positive, but reversed among those with high DHA status in pregnancy and postpartum (33, 34, 35). These studies suggest that the status of DHA impacts the physiological availability of AA and vice versa. The placenta is an important organ for the control of PUFA uptake from the maternal circulation and transfer to the fetal circulation (36). Ex vivo work points to a general order for placental uptake of AA > DHA > ALA > LA, but the placental transfer to the fetus differs and favors DHA > ALA > LA > AA (37). The placenta is considered to have a minimum requirement for AA for eicosanoid production. If the maternal AA status is relatively low, more AA will be retained by the placenta and could further decrease the amount of AA available for fetal neurodevelopment. Recently, the importance of a balance between DHA and AA in infant formula has received much attention, and a position paper by the European Academy of Paediatrics and the Child Health Foundation concluded that AA content should at least equal the DHA content (38). In populations that eat high amounts of fish, such as that in the Seychelles, the physiologically active n-6, AA, might become the limiting PUFA for neurodevelopment, resulting in benefits at higher n-6 intakes.
Nevertheless, our findings at 7 years of age in NC2 are contrary to findings in another study, which reported an inverse association between a high maternal n-6:n-3 ratio (determined in maternal plasma phospholipids in 4336 women) and the child’s risk of problem behavior at age 5–6 y (39). Our findings are also contrary to other studies that have investigated the maternal dietary n-6:n-3 ratio and children’s neurodevelopmental outcomes. In 1335 mother-child pairs in the Étude des Déterminants pré- et postnatals précoces du développement et de la santé de l’ENfant (EDEN) mother-child cohort, the maternal dietary n-6:n-3 ratio during the last trimester of pregnancy, assessed post-delivery using an FFQ and a portion-size picture booklet, was inversely correlated with the child’s language development at age 2 y and with different assessments of development at age 3 y (40). Similarly, in 960 participants of the Mothers and Children’s Environmental Health (MOCEH) cohort study, the maternal dietary n-6:n-3 ratio, assessed by a 1-d 24-h recall, was inversely associated with both the MDI and the PDI of the Korean Bayley scales of infant development at age 6 months (41). A further study investigating children’s intake rather than maternal intake reported that the dietary n-6:n-3 ratio in 7–9 year old children (n = 70), assessed by three 24-h diet recalls, was inversely associated with working memory and planning problems using a subset of the Cambridge Neurophysiological Test Assessment battery (42). The conflicting results regarding maternal n-6 and n-3 PUFA and offspring neurodevelopment in mothers with high n-6:n-3 PUFA ratios (dietary or physiological) compared with the lower n-6:n-3 PUFA ratio that is more representative of mothers eating high fish intakes (mean 8.6 fish meals per week in the Seychelles cohort) require further study.
Our study strengths include a cohort enrolled early in pregnancy specifically to address the complex relationships of maternal fish consumption, MeHg exposure, and nutritional status on child neurodevelopment. The cohort size was large enough to permit prospective investigations of interactions between maternal MeHg exposure and PUFA status, as well as of the main associations of both maternal MeHg exposure and PUFA status with child neurodevelopmental outcomes. Robust biomarkers of both MeHg exposure and PUFA status were used to address the relationships, with multiple indices of child neurodevelopmental outcomes measured by a sophisticated and extensive neurodevelopmental test battery. Mothers consumed large quantities of fish, resulting in mean MeHg exposures about 10 times higher than those in US and UK women. The population does not consume sea mammals, and exposure to polychlorinated biphenyls and other toxicants was minimal. The Seychellois mothers had universal access to free systems of health care and education and live in a region with limited industrial development and no local sources of pollution, providing further natural control for study biases. Confidence in the robustness of the study design and procedures is strengthened by the finding that covariates were generally associated with neurodevelopmental outcomes in the expected direction: that is, better tests scores were observed for girls and participants with higher socioeconomic status, greater maternal intelligence scores, and older age at testing (see Supplemental Table 1). Finally, the study has been double blind from its inception and all analyses were prespecified.
Our study limitations are those inherent to all observational epidemiological studies, which can only identify associations, not causal relationships. Other limitations include the potential for measurement error in a 1-time assessment of maternal PUFA status (our maternal hair Hg measure reflects the entire pregnancy) and residual confounding owing to omission of potentially important covariates such as other fish-related nutrients. Dietary information from children during follow-up was not considered in our analyses. Furthermore, missing covariates reduced the size of the enrolled cohort available for analysis, although the demographic and exposure characteristics between those with and without missing covariates were similar (15). Finally, our study was designed to detect subtle, statistically significant associations between exposures and neurodevelopmental outcomes in otherwise healthy children. Therefore, any associations reported here are not likely to be clinically meaningful.
In conclusion, we found no significant association between prenatal MeHg exposure, maternal PUFA status, and children’s neurodevelopmental outcomes at 7 years of age. Associations showing trends of better performance on a broad range of neurodevelopmental outcomes with an increasing maternal n-6:n-3 PUFA ratio suggest that relatively higher maternal status of n-6 PUFA rather than n-3 PUFA might be more important in optimizing child neurodevelopment in the Seychelles.
Acknowledgments
We thank the Seychelles Child Development Study Nutrition Cohort 2 participants, the nursing team in Seychelles for recruitment of participants and data collection, and the laboratory staff for assistance with samples. We thank Ashley Holub and Joanne Janciuras from the University of Rochester for their assistance with database management.
The authors’ responsibilities were as follows – JJS, EvW: had full access to all data in the study, with the exception of mercury data, took responsibility for data integrity and the accuracy of data analysis, as well as the decision to submit for publication, and drafted the manuscript; JJS, EvW, EMM, CFS, GJM, PWD: were involved in the study concept, design, and funding acquisition; CFS, JH, DM, EvW: were involved in fieldwork and acquisition of the data; EvW, TML, DW, SWT: conducted the statistical analysis and interpreted the data; EvW, AJY, TML, SWT, MSM, GEW, DW: conducted the quality control assessment and were responsible for the analysis and interpretation of data; TML, AJY, DW, MSM, EMM, SWT, GEW, DM, KB, MDR, JH, CFS, GJM, PWD: contributed to critical revision of the manuscript; and all authors read and approved the final manuscript. The authors report no conflicts of interest.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request following review and approval of a proposal by the Seychelles Child Development Study (SCDS) review committee. If a proposed analysis plan involves primary mercury exposure variables, the analysis will need to be performed by the biostatistics unit at the University of Rochester. This approach is to assure the integrity of blinding of the exposure data.
Footnotes
This study was supported by the National Institutes of Health (grants R01-ES010219, P30-ES01247, and T32-ES007271) and in-kind support from the government of Seychelles.
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or any other federal agency.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Present address for DW: SUNY College of Environmental Science and Forestry, Syracuse, NY, USA.
References
- 1.FAO . Food and Agriculture Organization of the United Nations; Rome, Italy: 2018. The state of world fisheries and aquaculture 2018–Meeting the sustainable development goals. [Google Scholar]
- 2.Kuratko CN, Barrett EC, Nelson EB, Salem N., Jr. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: A review. Nutrients. 2013;5(7):2777–2810. doi: 10.3390/nu5072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hazen RM, Golden J, Downs RT, Hystad G, Grew ES, Zazzolini D, Sverjensky DA. Mercury (Hg) mineral evolution: A mineralogical record of supercontinent assembly, changing ocean geochemistry, and the emerging terrestial biosphere. Am Mineral. 2012;97:1013–1042. [Google Scholar]
- 4.Sakamoto M, Tatsuta N, Izumo K, Phan PT, Vu LD, Yamamoto M, Nakamura M, Nakai K, Murata K. Health impacts and biomarkers of prenatal exposure to methylmercury: Lessons from Minamata, Japan. Toxics. 2018;6(3):45. doi: 10.3390/toxics6030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox C, Clarkson TW, Marsh DO, Amin-Zaki L, Tikriti S, Myers GG. Dose-response analysis of infants prenatally exposed to methylmercury: An application of a single compartment model to single-strand hair analysis. Environ Res. 1989;49(2):318–332. doi: 10.1016/s0013-9351(89)80075-1. [DOI] [PubMed] [Google Scholar]
- 6.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19(6):417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Ha EH, Park H, Ha M, Kim Y, Hong YC, Lee EJ, Kim H, Chang N, Kim BN. Prenatal mercury exposure, fish intake and neurocognitive development during first three years of life: prospective cohort mothers and children’s environmental health (MOCEH) study. Sci Total Environ. 2018;615:1192–1198. doi: 10.1016/j.scitotenv.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Barbone F, Rosolen V, Mariuz M, Parpinel M, Casetta A, Sammartano F, Ronfani L, Vecchi Brumatti L, Bin M, Castriotta L, et al. Prenatal mercury exposure and child neurodevelopment outcomes at 18 months: results from the Mediterranean PHIME cohort. Int J Hyg Environ Health. 2019;222(1):9–21. doi: 10.1016/j.ijheh.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 9.van Wijngaarden E, Thurston SW, Myers GJ, Harrington D, Cory-Slechta DA, Strain JJ, Watson GE, Zareba G, Love T, Henderson J, et al. Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24 years. Neurotoxicol Teratol. 2017;59:35–42. doi: 10.1016/j.ntt.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibbeln JR, Spiller P, Brenna JT, Golding J, Holub BJ, Harris WS, Kris-Etherton P, Lands B, Connor SL, Myers G, et al. Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: Two systematic reviews. Prostaglandins Leukot Essent Fatty Acids. 2019;151:14–36. doi: 10.1016/j.plefa.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, Montes de Oca R, Schober SE, Sinks T, Jones RL, et al. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112(11):1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindow SW, Knight R, Batty J, Haswell SJ. Maternal and neonatal hair mercury concentrations: the effect of dental amalgam. BJOG. 2003;110(3):287–291. [PubMed] [Google Scholar]
- 13.Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280(8):701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- 14.Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JM, Robson PJ, Shamlaye CF, Georger LA, et al. Associations of maternal long-chain polyunsaturated fatty acids, methylmercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29(5):776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strain JJ, Yeates AJ, van Wijngaarden E, Thurston SW, Mulhern MS, McSorley EM, Watson GE, Love TM, Smith TH, Yost K, et al. Prenatal exposure to methylmercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr. 2015;101(3):530–537. doi: 10.3945/ajcn.114.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSorley EM, Yeates AJ, Mulhern MS, van Wijngaarden E, Grzesik K, Thurston SW, Spence T, Crowe W, Davidson PW, Zareba G, et al. Associations of maternal immune response with MeHg exposure at 28 weeks’ gestation in the Seychelles Child Development Study. Am J Reprod Immunol. 2018;80(5):e13046. doi: 10.1111/aji.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 18.Cernichiari E, Toribara TY, Liang L, Marsh DO, Berlin MW, Myers GJ, Cox C, Shamlaye CF, Choisy O, Davidson P, et al. The biological monitoring of mercury in the Seychelles study. Neurotoxicology. 1995;16(4):613–628. [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 20.Wiig EH, Semel E, Secord WA. 5th ed. NCS Pearson; Bloomington, MN: 2013. Clinical evaluation of language fundamentals. [Google Scholar]
- 21.Kaufman AS, Kaufman NL. 2nd ed. AGS Publishing; Circle Pines, MN: 2004. Kaufman brief intelligence test. [Google Scholar]
- 22.Kaplan E, Goodglass H, Weintraub S. Lea & Febiger; Philadelphia, PA: 1983. Boston naming test. [Google Scholar]
- 23.Reitan RM. Neuropsychology Press; Tuscon, AZ: 1993. The Halstead-Reitan neuropsychologial test battery: theory and clinical interpretation. [Google Scholar]
- 24.Woodcock RW, McGrew KS, Mather N. Riverside; Rolling Meadows, IL: 2001. Woodcock-Johnson III. [Google Scholar]
- 25.Achenbach TM, Rescorla LA. ASEBA; Burlington, VT: 2010. ASEBA child behavior checklist. [Google Scholar]
- 26.Constantino JN. 2nd ed. WPS; Torrance, CA: 2012. Social responsiveness scale. [Google Scholar]
- 27.Rutter M, Bailey A, Lord C. WPS; Torrance, CA: 2003. Social communication questionnaire. [Google Scholar]
- 28.Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36(9):1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team . R Foundation for Statistical Computing; Vienna: Austria: 2019. R: A language and environment for statistical computing. [Internet]. Available from: http://www.R-project.org/. [Google Scholar]
- 31.Weisberg S. John Wiley & Sons, Inc.; Hoboken, NJ: 2005. Applied linear regression. [Google Scholar]
- 32.Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Kuipers RS, Luxwolda MF, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA. Maternal DHA equilibrium during pregnancy and lactation is reached at an erythrocyte DHA content of 8 g/100 g fatty acids. J Nutr. 2011;141(3):418–427. doi: 10.3945/jn.110.128488. [DOI] [PubMed] [Google Scholar]
- 34.Luxwolda MF, Kuipers RS, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA. A maternal erythrocyte DHA content of approximately 6 g% is the DHA status at which intrauterine DHA biomagnifications turns into bioattenuation and postnatal infant DHA equilibrium is reached. Eur J Nutr. 2012;51(6):665–675. doi: 10.1007/s00394-011-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luxwolda MF, Kuipers RS, Smit EN, Velzing-Aarts FV, Dijck-Brouwer DA, Muskiet FA. The relation between the omega-3 index and arachidonic acid is bell shaped: synergistic at low EPA+DHA status and antagonistic at high EPA+DHA status. Prostaglandins Leukot Essent Fatty Acids. 2011;85(3-4):171–178. doi: 10.1016/j.plefa.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res. 2009;48(1):52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–255. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- 38.Koletzko B, Bergmann K, Brenna JT, Calder PC, Campoy C, Clandinin MT, Colombo J, Daly M, Decsi T, Demmelmair H, et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am J Clin Nutr. 2019;111(1):10–16. doi: 10.1093/ajcn/nqz252. [DOI] [PubMed] [Google Scholar]
- 39.Loomans EM, Van den Bergh BR, Schelling M, Vrijkotte TG, van Eijsden M. Maternal long-chain polyunsaturated fatty acid status during early pregnancy and children’s risk of problem behavior at age 5–6 years. J Pediatr. 2014;164(4):762–768. doi: 10.1016/j.jpeds.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 40.Bernard JY, De Agostini, Forhan M, de Lauzon-Guillain A, Charles B, Heude MA, Group EM-CCS B The dietary n6:n3 fatty acid ratio during pregnancy is inversely associated with child neurodevelopment in the EDEN mother-child cohort. J Nutr. 2013;143(9):1481–1488. doi: 10.3945/jn.113.178640. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Kim H, Lee E, Kim Y, Ha EH, Chang N. Association between maternal intake of n-6 to n-3 fatty acid ratio during pregnancy and infant neurodevelopment at 6 months of age: results of the MOCEH cohort study. Nutr J. 2017;16(1):23. doi: 10.1186/s12937-017-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheppard KW, Cheatham CL. Omega-6 to omega-3 fatty acid ratio and higher-order cognitive functions in 7- to 9-y-olds: a cross-sectional study. Am J Clin Nutr. 2013;98(3):659–667. doi: 10.3945/ajcn.113.058719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request following review and approval of a proposal by the Seychelles Child Development Study (SCDS) review committee. If a proposed analysis plan involves primary mercury exposure variables, the analysis will need to be performed by the biostatistics unit at the University of Rochester. This approach is to assure the integrity of blinding of the exposure data.