SUMMARY
Neutrophil extracellular traps (NETs) can promote tumor growth and metastases, but whether NETs impact the tumor immune microenvironment remains under-explored. In this issue of Immunity, Teijeira et al. (2020) discover that NETs shield tumor cells from cytotoxic immune cells resulting in impaired tumor clearance.
Neutrophils execute many processes including reactive oxygen species (ROS) production, phagocytosis, degranulation, and extrusion of neutrophil extracellular traps (NETs). NET formation was first described in 2004 as a mechanism to kill bacteria (Brinkmann et al., 2004), but was subsequently discovered in cancer (Demers et al., 2012), where its function is still relatively unknown.
NETosis refers to the unique cell-death process whereby the nuclear envelope of neutrophils is disassembled and decondensed chromatin is released into the cytoplasm. The sticky chromatin scaffold accumulates anti-microbial peptides and proteases from the cytoplasmic compartment, and extrudes into the extracellular space where its web-like structure can trap and kill pathogens (Figure 1). The enzyme Peptidylarginine deiminase 4 (PAD4) citrullinates histones to drive the initial chromatin decondensation that allows NET extrusion. Consistently, neutrophils from Pad4−/− mice are unable to form NETs. NETs have been observed in numerous inflammatory conditions including thrombosis, and cardiovascular and autoimmune diseases (Papayannopoulos, 2018). While NETs can effectively trap and neutralize pathogens, in some conditions, NETs can exacerbate inflammation and cause cell damage.
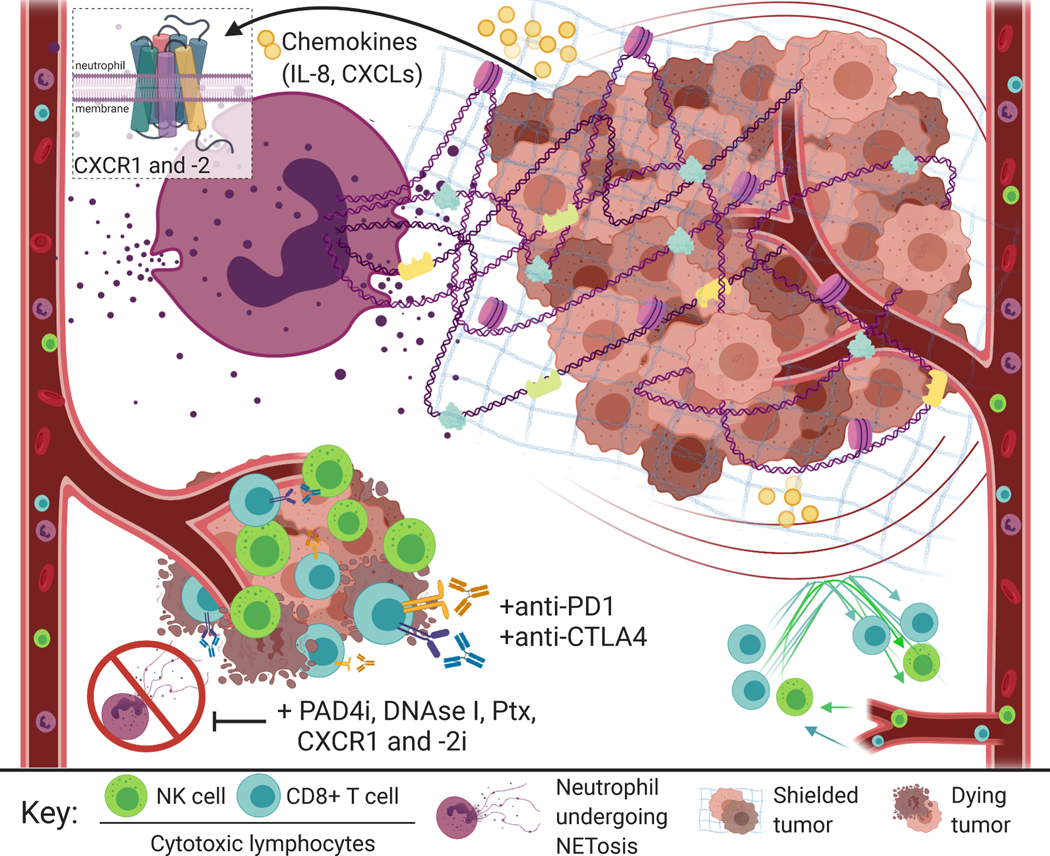
Figure 1. Neutrophil Extracellular Traps Shield Tumor Cells from the Effects of Cytotoxic Immune Cells and Immunotherapies.
Human neutrophils form neutrophil extracellular traps (NETs) following activation of CXCR1 and −2 receptors by chemokines secreted by tumor cells, including IL-8 and CXCL-1, −2, and −8 (top left). NETs shield tumor cells from the cytotoxic effects of anti-tumor immune cells—specifically NK cells and CD8+ T cells—which can result in increased tumor growth (top right). Blocking NET formation via pharmacological PAD4 inhibitors, DNAse-I, Pertussis-toxin (Ptx), or CXCR1 and −2 inhibitors allows tumor cell contact with cytotoxic immune cells. Teijeira et al. demonstrate NETosis blockade by PAD4 inhibition can increase the efficacy of anti-PD1 plus anti-CTLA4 immune checkpoint inhibitors—highlighting exciting potential for a new therapeutic strategy where NET blockade maximizes the effect of immunotherapy.
The discovery of NETs in cancer provoked a major question: Do NETs have anti- or pro-tumor functions? NETs have been associated with tumor cell proliferation and metastasis in multiple cancer types (Cedervall et al., 2016). Recent studies have shed light on mechanisms by which NETs promote tumor growth. These include NET-protease-induced remodeling of laminin to trigger integrin signaling and awaken dormant tumor cells (Albrengues et al., 2018), trapping circulating tumor cells to enable their proliferation and survival (Cools-Lartigue et al., 2013), or altering tumor cell bioenergetics (Yazdani et al., 2019). Given that NETs can impact the function of immune cells in non-cancer contexts (Papayannopoulos, 2018), it is important to determine how NETs influence the tumor immune microenvironment.
In this issue of Immunity, Teijeira et al. (2020) present findings that illuminate how NETosis can influence the tumor immune landscape and tumor response to immunotherapy. Teijeira et al. first set out to determine which chemokines have the capacity to trigger NETosis. The authors focus on CXCL chemokines as central mediators of tumor-induced NETosis because they are abundantly expressed in solid tumors and can recruit pro-tumor neutrophils (Kim et al., 2014). These chemokines, namely proinflammatory Interleukin-8 (IL-8), along with CXCL1, −2, and −8, recruit neutrophils by interacting with CXCR1 and 2 receptors on the neutrophil membrane. Teijeira et al. utilized a panel of neutrophil chemoattractants to show that these stimuli were capable of inducing NETosis in both human neutrophils as well as granulocytic myeloid-derived-suppressor-cells (GR-MDSCs). Using Gi subunit inhibitor, Pertussis toxin (Ptx) and the CXCR1 and −2 inhibitor Reparixin, the authors demonstrated NET induction to be dependent on G-protein coupled receptor (GPCR) activity during CXCR1, −2 receptor activation. Conditioned supernatant of human cancer cell lines, rich in CXCR1 and −2 agonists, induced NETosis in human neutrophils and GR-MDSCs, and this induction was fully prevented by CXCR1, −2 blockade. Reparixin and Ptx also blocked NET formation in human tumor organoids and subcutaneous mouse models of mammary and human colorectal adenocarcinomas. Together, the authors demonstrate that chemokines released from human tumor cells activate CXCR1 and −2 receptors to induce NETosis.
To shed light on the unexplored question of how NETs impact the tumor immune microenvironment, Teijeira et al. utilized a variety of methods to study NET interaction with immune cell populations—specifically, cytotoxic CD8+ T cells and IL-15 activated natural killer (NK) cells. They hypothesized that tumor cell encapsulation by NETs may shield tumor cells from interactions with neighboring anti-tumor immune cells. In support of this notion, the authors generated a co-culture system of “NET-ed” tumor cells and cytotoxic lymphocytes, and found that NETs effectively impeded the activity of NK and CD8+ T cells against tumor cells. Consistent with previous studies implicating NETs in metastases, the authors revealed that metastatic spread to the lungs in subcutaneous models of 4T1 breast cancer was prevented by NET inhibition using DNAse I injection or pharmacological PAD4 inhibition. In a T-cell deficient version of this model that retained NK cells, metastatic events were limited by NETosis blockade. However, in a model lacking both T and NK cells, NETosis blockade did not prevent metastasis. Together, these data suggest that NK cells, more than CD8+ T cells, have the dominant ability to limit metastatic spread in this model, and that NK cell cytotoxic activity is blocked by NETs.
Following the discovery that NETs can impact tumor cell interaction with surrounding immune cell populations, the authors sought to test whether NETs may influence response to immunotherapy. In the context of primary tumor growth, NETosis blockade by PAD4 inhibition was insufficient to delay tumor growth of subcutaneous 4T1 breast cancer in mice. However, when PAD4 inhibition was combined with anti-PD1 plus anti-CTLA4 immune checkpoint blockade, tumor growth was dramatically reduced in the 4T1 breast cancer model. Though NK cells were observed to primarily limit metastases in vivo, tumor growth inhibition in the absence of NETs was shown to be dependent on CD8+ T-cell activity. Together, these results lead to the exciting conclusion that destroying tumor-cell-shielding NETs may enhance the response to immunotherapy.
In a final pursuit to understand how NETs interact with the tumor immune landscape in living mice, Teijeira et al. use an elegant intravital microscopy approach to capture live cell interactions between “NET-ed” versus “free” tumor cells and cytotoxic lymphocytes. The authors visualized NET encapsulation of 4T1 tumor cells in the ears of mice and of LLC tumor cells in the liver sinusoids of mice. The “NET-ed” LLC tumor cells were shielded from contact with fluorescently-labeled, cytotoxic NK and CD8+ T cells, creating a physical barrier between tumor and immune cells. Likewise, in the ear dermis implanted with red-fluorescent B16/OVA melanocytes and green-fluorescent human NETs, ovalbumin-specific CD8+ T cells could only contact tumor cells in “NET-free” areas. Together, in a mosaic of fluorescently-labeled tumor and immune cells, the authors illuminate the capacity of NETs to shield tumor cells from interaction with cytotoxic immune cells.
Teijeira et al.’s results uncover a provocative function of NETs in impeding anti-tumor immunity and the efficacy of immunotherapy. These findings have the capacity to shape therapeutic strategies for cancer but inevitably give rise to additional questions. The authors postulate that NETs function as a physical barrier to limit contact between tumor cells and surrounding immune populations. This study does not exclude the potential that other moieties of NETs (e.g. trapped proteins, proteases, and extracellular DNA) could deter or promote T and NK cell contact and activity through other mechanisms. Likewise, it is unclear whether NETs may educate immune cells in a manner that could elicit an abscopal effect on the tumor immune landscape. Might a “NET-dense” tumor microenvironment modulate the behavior of immune cells in surrounding “NET-free” regions?
Importantly, Teijeira et al.’s findings highlight a therapeutic strategy whereby immune checkpoint blockade may be augmented by NETosis inhibition (Figure 1). There are clinical options to block chemokine receptor activation necessary for NET formation, including IL-8 neutralizing antibodies. Additionally, CXCR2 small molecule inhibitors such as AZD5069 have been tested in Phase I and II clinical trials including studies in squamous cell carcinomas, pancreatic adenocarcinoma, and others (ClinicalTrials.gov). In the context of cystic fibrosis, multiple clinical trials have been performed with NET-degrading GMP-grade DNAse-I (ClinicalTrials.gov). PAD4 inhibitors, including GSK484, and adenosine A2A receptor agonists that can block NETosis have been tested in preclinical studies (Ali et al., 2019). Thus, there are multiple avenues by which the therapeutic approach of combining NET inhibition with immunotherapy could move toward the clinic.
The current study is limited to cell culture and xenograft models of human cancer where NETs are artificially induced in tumors. Assessment of naturally-occurring NETosis in autochthonous, immune-competent tumor models would facilitate the study of how NETs interact with surrounding immune cells and immunotherapies. The use of genetically-engineered mouse models could also provide preclinical tumor burden and survival data from immune-competent hosts that would be useful for predicting how well this therapeutic strategy may be recapitulated in the clinic. Though the authors provide compelling evidence that NETs negatively influence the anti-tumor immune response, the results are often from short-term assays with artificially-induced NETs. It will be important to assess the long-term impact of NET inhibition on the tumor immune landscape and immunotherapy response to shed light on these outstanding questions.
In summary, Teijeira et al. demonstrate a requirement for CXCR1 and −2 receptor activation in NET induction and a novel role for NETs as a physical shield between human tumor cells and cytotoxic lymphocytes—specifically CD8+ T cells and NK cells (Figure 1). The results of this study highlight an exciting therapeutic approach suggesting that NET blockade may allow immune cells to “peNETrate” the tumor microenvironment and ultimately promote the efficacy of immunotherapy.
REFERENCES
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Kuttner V, et al. (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali RA, Gandhi AA, Meng H, Yalavarthi S, Vreede AP, Estes SK, Palmer OR, Bockenstedt PL, Pinsky DJ, Greve JM, et al. (2019). Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun 10, 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, and Zychlinsky A. (2004). Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- Cedervall J, Zhang Y, and Olsson AK (2016). Tumor-Induced NETosis as a Risk Factor for Metastasis and Organ Failure. Cancer Res 76, 4311–4315. [DOI] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, and Ferri L. (2013). Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. The Journal of clinical investigation 123, 3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, and Wagner DD (2012). Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 109, 13076–13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr., Papadopoulos N, Kinzler KW, Vogelstein B, and Zhou S. (2014). Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A 111, 11774–11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. (2018). Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18, 134–147. [DOI] [PubMed] [Google Scholar]
- Teijiera A, Garasa S, Gato-Cana M, Alfara C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I, et al. (2020). CXCR1 and 2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. [DOI] [PubMed] [Google Scholar]
- Yazdani HO, Roy E, Comerci AJ, van der Windt DJ, Zhang H, Huang H, Loughran P, Shiva S, Geller DA, Bartlett DL, et al. (2019). Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Research 79, 5626–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]