To the Editor:
The capacity of a living cell to respond to external mechanical stresses is critical for many biological processes. Biomechanical and biological studies conducted over the past 30 years clearly indicate that external mechanical forces trigger essential signaling and structural transformations in cellular and nuclear compartments and are involved in changes in the composition of the nuclear envelope, chromatin organization, and gene expression (Cooke, 2003; Ando and Yamamoto, 2011; Chiu and Chien, 2011). Despite all these significant advances, further studies are still needed to elucidate the origin of these biological processes.
In an effort to help address this need, an original and elegant work published in Molecular Biology of the Cell in July 2020 (Hobson et al., 2020) has extended our understanding of the cellular effects of mechanical forces. This study deserves to be highlighted because it represents an important step for biologists, physicists, and biomechanical researchers characterizing the determinants and distribution of intracellular spatial stress.
In this paper, using an atomic force microscope (AFM), the authors investigated the relationship between the compressive force exerted on the nucleus, the mechanical properties of the nuclear constituents, and the morphological changes in the nuclear envelope and volume.
Three cell populations were used for this study: SKOV3 (WT), SKOV3 treated with trichostatin-A (TSA), and SKOV3 transfected with a small interfering RNA (siRNA) plasmid to lower lamin A/C (LA/C KD) levels. These biological interventions were performed to determine whether the roles of chromatin and lamin A/C in the cellular response to compression could be decoupled. The authors used their own original system combining AFM and side-view microscopy (AFM–LS), which allowed them to visualize the dynamics of cellular deformations in the imaging plane of the light sheet while simultaneously recording the force response of the AFM during indentation (Nelsen et al., 2020). They accurately quantified the variations of two nuclear morphological characteristics during cell indentation and deformation, namely the nuclear perimeter (NP) and nuclear cross-sectional area (NCSA). On the basis of their experimental measurements, they suggested the existence of two regimes of force: the first resulting from the cellular mechanical response generated by the nuclear volume only, and the second from combined volume and nuclear envelope responses.
A two-parameter empirical model is proposed to describe this two-phase force response. Their unidimensional model is based on correlation analysis between the amplitude of the force measured by AFM (F) and the two nuclear morphological characteristics, ΔNCSA and ΔNP2, where ΔNCSA and ΔNP are the absolute variations of NCSA and NP, respectively. The two coefficients of their two-force regime law EV and ESA were defined as effective mechanical resistances to variations in nuclear volume and envelope surface, respectively. A finite element model was used to validate the proposed empirical force law.
Thanks to their two-regime force model, they successfully:
Differentiate the magnitude of the resistive force of each of the nuclear constituents during AFM cell indentation.
Highlight the effects of the TSA and siRNA treatments on the changes of mechanical properties of the nuclear constituents, through the quantifications of their two criteria, EV and ESA.
Although this AFM-LS experiment associated with the two-regime force model appears to be a powerful and promising tool for highlighting changes in nuclear mechanical properties, and also sheds light on the role of chromatin and lamina A/C in nuclear mechanics, there are several elements in the analysis that should be considered for possible modifications and/or corrected interpretations:
The level of deformation that occurs after applying stress (defined as force per unit area; unit, Pa) to a material depends on its intrinsic mechanical properties. In a uniaxial extension test, the relationship between an applied stress σ and strain ε (defined as the relative change in sample length with respect to the undeformed geometric configuration; a dimensionless parameter) in a biological sample is a central characterization of its mechanical behavior in the direction of the imposed traction force. The modulus of elasticity (or Young’s modulus) represents this relationship: E = stress/strain (σ/ε). Rigid materials (such as carbon fiber, bone tissue, or a collagen fiber) respond to a large increase in stress with very slight deformations, while soft materials, comparatively, deform extensively with low magnitudes of applied stress. Hobson et al. (2020) define two new coefficients, EV and ESA, which they call the effective mechanical resistances to changes in nuclear volume and nuclear membrane area, respectively. Although they use a parametric finite element model to study the correlations between the two resistances and the mechanical properties of the membrane and the nucleoplasm, the empirical model and the interpretation of the significance of these variables still have certain limitations in the absence of direct property measures. The first note on these criteria is that they result from fitting a correlative analysis on two surrogate measurements against the applied force. This has some key differences from the physical definition of an elastic (Young’s) modulus E as defined previously. While their model parameters share units with Young’s modulus (Pa), they are an empirical relation between an applied force and surrogate measurements (ΔNCSA and ΔNP2). The empirical nature of these values limits their application beyond comparative studies, as they are not the true mechanical properties of the material and further biomechanical structural analysis is not possible using these fit parameters. It is also noted that these properties were not fully demonstrated to be independent of nuclear shape and geometry, a fundamental property with which they are entangled. Thus, this implies that these two quantities are not yet established as the minimum basis for describing nuclear stiffness. As a result, these variables will not allow us to say with certainty which of the two nuclear constituents is, mechanically speaking, stiffer. Note that these are two new indices defined by the authors and are used to highlight mechanical changes in order to compare distinct cell populations.
In this study, due to the limitations of the experimental protocol, only NCSA and NP can be measured feasibly. Consequently, the reader could think that this force model involving the two geometric quantities, ΔNCSA and ΔNP, remains “equivalent” to a force model based on changes of nuclear volume (ΔNV) and nuclear membrane area (ΔNMA) when assuming axisymmetric nuclear shape. However, this is not true as no coefficients in the proposed force model refer to the volume of the nucleus or to the surface of its envelope. This implication could confuse the reader because much of the discussion of this paper focuses on published studies measuring or reporting the latter geometrical quantities. Note that the introduction and discussion of the model and results directly imply three-dimensional parameters from two-dimensional measurements. This can be inaccurate; the force model in its current form does not implicitly integrate this assumption as suggested by the authors. Indeed, even in the simplest case of an axisymmetric nuclear shape, the four geometric variables, ΔNP, ΔNCSA, ΔNMA, and ΔNV, remain independent, which prevents ΔNV and ΔNMA from appearing in the force model of Hobson et al. (2020).
Finally, we would like to emphasize that a decrease in the NCSA does not always mean a decrease in nuclear volume. This could simply be illustrated by considering the axial squeezing of an incompressible cylindrical geometry from its initial configuration (radius R0, length L0, cross-sectional area S0, and volume V0) to its deformed cylindrical configuration (radius r, length l, cross-sectional area s, and volume v). Assuming the incompressibility constraint (v = V0), it can easily be shown that the deformed area s becomes smaller than the nondeformed area S0 (s = kS0, with k = R0/r < 1). It is interesting to note that the more the idealized cylindrical nuclear geometry is compressed, the more the NCSA decreases, as measured on the SKOV3 cells (Figure 1). Therefore, it is important to mention that the measured decrease in NCSA cannot and should not be related to the compressibility of the nucleus (i.e., the change in its volume).
FIGURE 1:
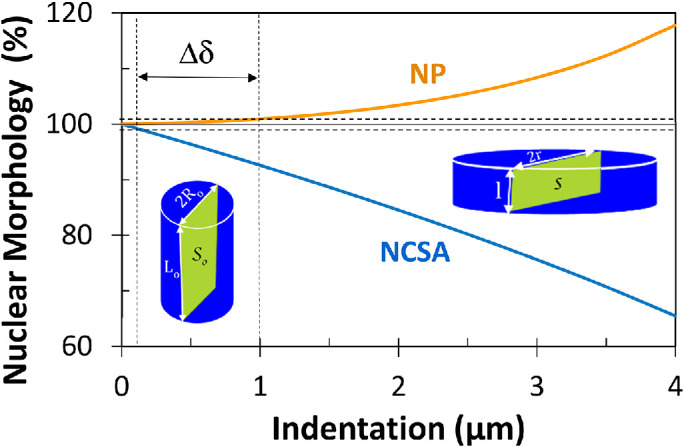
Variations of the NCSA and NP as percentage vs. indentation (δ) for the axial squeezing of an incompressible cylindrical geometry. Calculations were performed with L0 = 7 µm and R0 = 7.5 µm. The deformed length is given by l = L0 – δ. The parameter Δδ was defined as the difference in indentation at which NP and NCSA reach 1% change. Note that a nonzero value of Δδ supports the hypothesis of a two-regime force response. Notably, this simple example shows that the two-regime force response could remain valid even with the assumption of an incompressible nucleus since Δδ = 0.9 µm.
We intend our comments to be constructive and complementary to this study, as we believe studies such as this one are rare and important to our scientific community and allow for real discussions that should lead to significant advances. In this Letter, we do not question the fundamental model, which we find elegant, rigorous, correct, and useful. Instead, we seek to inform biologists and future users of the underlying assumptions and precautions to be taken in applying this model and interpreting the results.
Acknowledgments
This work was supported by National Institutes of Health R01 HL133254 and HL14833 (to J.P.C.).
Footnotes
REFERENCES
- Ando J, Yamamoto K (2011). Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal , 1389–1403. [DOI] [PubMed] [Google Scholar]
- Chiu JJ, Chien S (2011). Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev , 327–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JP (2003). Flow, NO, and atherogenesis. Proc Natl Acad Sci USA , 768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson CM, Kern M, O’Brien ET, Stephens AD, Falvo MR, Superfine R (2020). Correlating nuclear morphology and external force with combined atomic force microscopy and light sheet imaging separates roles of chromatin and lamin A/C in nuclear mechanics. Mol Biol Cell , 1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen E, Hobson CM, Kern ME, Hsiao JP, O’Brien ET, Watanabe T, Condon BM, Boyce M, Grinstein S, Hahn KM, et al (2020). Combined atomic force microscope and volumetric light sheet system for mechanobiology. Sci Rep , 8133. [DOI] [PMC free article] [PubMed] [Google Scholar]


