Abstract
Macromolecule condensates, phase separation, and membraneless compartments have become an important area of cell biology research where new biophysical concepts are emerging. This article discusses the possibility that condensates assemble on multivalent surfaces such as DNA, microtubules, or lipid bilayers by multilayer adsorption. Langmuir isotherm theory conceptualized saturable surface binding and deeply influenced physical biochemistry. Brunauer-Emmett-Teller (BET) theory extended Langmuir’s ideas to multilayer adsorption. A BET-inspired biochemical model predicts that surface-binding proteins with a tendency to self-associate will form multilayered condensates on binding surfaces. These “bound condensates” are expected to assemble well below the saturation concentration for liquid–liquid phase separation, so they can compete subunits away from phase-separated droplets and are thermodynamically pinned to the binding surface. Tau binding to microtubules is an interesting test case. The nonsaturable binding isotherm is reminiscent of BET predictions, but assembly of Tau-rich domains at low concentrations requires a different model. Surface-bound condensates may find multiple biological uses, particularly in situations where it is important that condensate assembly is spatially constrained, such as gene regulation.
INTRODUCTION
An exciting recent development in cell biology is the realization that macromolecule condensates play many important roles in subcellular organization, signaling, and disease (Hyman et al., 2014; Peng and Weber, 2019; Dignon et al., 2020; Sehgal et al., 2020). Condensates have also been termed phase-separated compartments and membraneless organelles. Here, the term “condensate” will be used to describe any local aggregate of macromolecules, while the term “phase-separated” will be restricted to its precise physical definition. Condensates form in all compartments of the cell, and may be particularly important for organizing the nucleus, which lacks membrane-bounded subcompartments. The focus will be on reversible binding reactions at thermodynamic equilibrium. Irreversible reactions, such as dissipative biochemistry and condensate hardening, are very important in condensate biology. These topics are beyond the scope of this article and are discussed elsewhere (Wang et al., 2018; Weber et al., 2019).
The purpose of this article is to discuss the possibility that condensates commonly assemble on multivalent surfaces, also called scaffolds, as an extension of saturable binding reactions. Relevant surfaces include DNA, RNA, microtubules, polysaccharides, and lipid bilayers. If surface-bound condensates do form, their physical properties are expected to lie somewhere between those of conventionally bound monolayers and phase-separated droplets. They will not meet all the physical criteria that define a separate phase, yet they are an unfamiliar form of biological matter with some liquid-like properties for which the descriptor “condensate” is justified. One possible example we will explore below is Tau protein binding to the surface of microtubules.
A natural starting point for discussing surface binding is the Langmuir isotherm, which conceptualized saturable adsorption of gases to solid surfaces (Langmuir, 1918). When combined with Hill’s analysis of the concentration dependence of drug action (Hill, 1909), it gave rise to the concept of binding affinity and became a founding principle of pharmacology and physical biochemistry (Colquhoun, 2006). Brunauer-Emmett-Teller (BET) theory was developed as an extension of Langmuir’s ideas to conceptualize nonsaturable, multilayer gas adsorption, which is commonly observed in experiments conducted near the critical pressure for liquefaction (Brunauer et al., 1938). The critical pressure of a gas is the pressure at which the gas and liquid forms are at equilibrium with each other and there is no energy change when a molecule moves from one phase to the other. Multilayer adsorption is most likely as the pressure approaches this value because the favorable free energy change associated with evaporation becomes low. The BET model was later extended to a rigorous thermodynamic formalism (Hill, 1949). It is much less familiar to biochemists than the Langmuir isotherm and provided the inspiration for this article.
Condensate assembly by phase separation
Figure 1A illustrates a phase-separated macromolecule condensate droplet in equilibrium with soluble subunits at the saturation concentration, which is also termed the critical concentration. This model has dominated recent literature. The formation of such condensates has often been compared with oil–water phase separation, hence the descriptor “liquid–liquid phase separation” (Hyman et al., 2014). However, the oil–water analogy can be misleading if applied too literally. The biochemical environment inside versus outside the separated phase is much more similar in macromolecule condensates than oil–water systems. The concentration of water and ions is quite similar, and even small protein-sized probes equipartition if they do not interact with the condensing macromolecule (Wei et al., 2017; Sanders et al., 2020). The thermodynamics of phase separation in polymer solutions was conceptualized by Flory and Huggins (Flory, 1942) and new formalisms suited to complex biological systems have been developed (Riback et al., 2020). These theories can be challenging for biochemists. An alternative perspective is to draw an analogy to gas–liquid phase transitions. Outside the condensed phase, the macromolecules in dilute solution diffuse freely and interact rarely, as in a gas. Inside the condensed phase they are close together and interact frequently, as in a liquid. Macromolecule phase separation is thus analogous to condensation of a gas. This perspective was used to describe P-granule assembly in the paper that started the field (Brangwynne et al., 2009), and it suggests that the physical chemistry of gas condensation may hold useful lessons for biochemists.
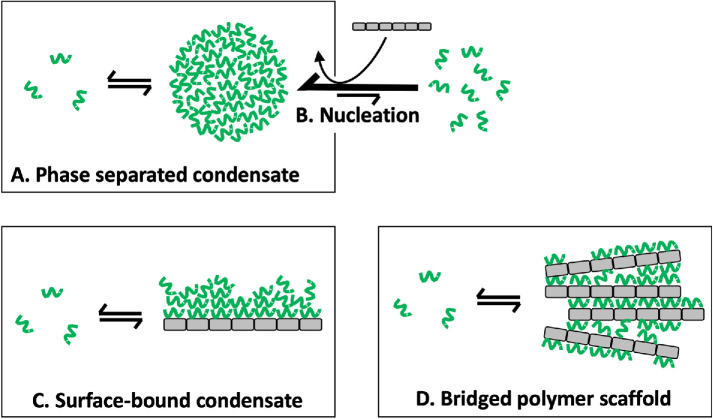
FIGURE 1:
Phase-separated vs. surface-bound condensates. (A) Phase-separated macromolecule droplet in equilibrium with soluble subunits at the saturation concentration. (B) Nucleation of phase separation by a multivalent surface (gray rectangles). (C) Proposed surface-bound condensate assembled by multilayer adsorption. This model is inspired by BET theory and is discussed in more detail below. (D) Bridged polymer scaffold model proposed for nuclear condensates by Peng and Weber (2019). Scaffolds (or scaffold loops) are brought together by the scaffold interacting with a single binding protein at two sites, or/and two binding proteins interacting with each other.
Condensate nucleation by surfaces and scaffolds
Figure 1B illustrates nucleation of phase separation by a polyvalent surface, which is often called a scaffold in biochemistry. Nucleation is well documented in physical and macromolecular systems. It can only occur if the concentration of the soluble macromolecule is initially above the saturation concentration for phase separation. Under these conditions, phase separation is thermodynamically favored, but can be kinetically slow, and subject to catalysis by heterologous particles. After nucleation, condensate droplets grow rapidly in size until the concentration of soluble macromolecule is depleted down to the saturation concentration, whereupon equilibrium is reestablished. Nucleation is distinct from the other known and proposed processes illustrated in Figure 1 in that it is a kinetic process while the other panels are descriptions of the state of a system at thermodynamic equilibrium.
Surface-bound condensates
Figure 1C illustrates a proposal for condensate assembly by multilayer adsorption that is new for macromolecules but far from new in the physical sciences. The purpose of this article is to explain why this type of condensate is likely to exist and to discuss their expected properties. Surface-bound condensates assemble by a combination of conventional surface binding and the same homointeractions that drive phase separation, but they are not expected to meet the physical criteria that define a bulk liquid phase. This concept is quite different from nucleation. Surface-bound condensates assemble below the saturation concentration, as discussed below, and are at thermodynamic equilibrium.
Bridged polymer scaffolds
Figure 1D illustrates another alternative to phase separation that was proposed for nuclear condensates by Peng and Weber (2019) who listed experimental criteria for defining whether condensates are phase separated and applied them to several nuclear condensates that assemble proximal to DNA or RNA scaffolds. They concluded that nucleoli meet the criteria to be termed a separated phase, paraspeckles do not, and other nuclear condensates are still ambiguous due to lack of experimental data. They proposed a “bridged polymer scaffold” model in which scaffolds (or scaffold loops) are brought together by the scaffold interacting with the binding protein at multiple sites, or by binding proteins interacting with each other via sites that are distinct from the scaffold binding site. All binding proteins contact the scaffold directly in this model, so it can be viewed as a condensate of scaffolds but not a condensate of binding proteins.
The Langmuir binding isotherm and its influence on biochemistry
The processes illustrated in panels B–D in Figure 1 all center around a macromolecule in solution binding to a polyvalent surface or scaffold. This kind of binding reaction is very familiar in biochemistry. Its intellectual roots go back to the physical chemistry of gas adsorption to solid surfaces, which was characterized in the early 20th century, in particular by Irving Langmuir, who was awarded a Nobel prize for his work on surface chemistry in 1932. Langmuir conceptualized gas molecules binding to solid surfaces in a model comprising a lattice of binding sites that are identical in binding energy, saturable, and noninteracting. This predicts a hyperbolic binding isotherm (Figure 2A), which was observed in experiments. Earlier, Hill observed a hyperbolic relationship for the concentration dependence of drug-induced muscle contraction (Hill, 1909). He proposed this was due to the drug activating some sort of saturable chemical switch that we now know to be cell surface receptors. These ideas were gradually unified into a general theory of saturable binding, described by a hyperbolic binding curve, which is important throughout modern biochemistry and pharmacology (Colquhoun, 2006). Examples include drugs binding to membrane receptors, transcription factors binding to DNA, and microtubule-associated proteins (MAPs) binding to microtubules. If adjacent molecules on a binding lattice interact, the binding curve becomes cooperative, and can often be fit to a Hill equation with an exponent different from 1. However, binding is still saturable. In classic biochemistry, the nonsaturable component of a binding reaction is considered nonspecific and is routinely subtracted away from total binding to measure binding affinity and stoichiometry (Pollard, 2010). The possibility that surface binding can lead to condensate assembly by multilayer adsorption forces us to reconsider these fundamental concepts. If this occurs, binding will be nonsaturable, but still specific.
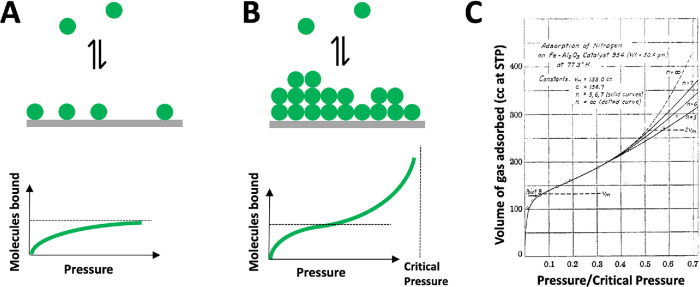
FIGURE 2.
Gas-binding isotherms. (A) Langmuir isotherm theory conceptualized saturable adsorption of gases to surfaces (Langmuir, 1918). Adsorption increases hyperbolically with pressure and saturates at one molecule per site (dotted line). (B) BET isotherm theory conceptualized multilayer gas adsorption using a simple model with no cooperativity or surface tension (Brunauer et al., 1938). At low pressures, the BET isotherm approximates the Langmuir isotherm and asymptotes toward one molecule per site (dotted line). As pressure increases, multilayer adsorption becomes significant. Binding increases over the saturation value and asymptotes toward the y-axis. The horizontal dotted line denotes one molecule bound per site; the vertical dotted line denotes the critical pressure for formation of bulk liquid nitrogen. (C) Example of experimental data interpreted using the BET model, in this case binding of nitrogen to an iron–alumina catalyst at 77°K (ibid.). The x-axis is expressed as pressure/critical pressure. Circles are measurements; lines are a family of fits to a mathematical formulation of the BET model. Note the characteristic S-shaped binding isotherm, which is a signature of multilayer adsorption.
Beyond Langmuir: the BET isotherm
Adsorption of gases to surfaces is often more complicated than the Langmuir model predicts. For example, multilayer adsorption often occurs near, but below, the critical pressure for gas–liquid phase transition. BET theory was developed as an extension of Langmuir theory to conceptualize this phenomenon (Figure 2, B and C). BET theory makes many simplifying assumptions and is only predictive over a narrow range of pressures (Hill, 1949), but it still provides a useful conceptual tool. Multilayer adsorbates form over a range of pressures well below the critical pressure for liquefaction (Figure 2, B and C), so they do not have a defined critical pressure. They are energetically favored over phase-separated droplets because the favorable energy of the direct-binding layer overcomes the cost of surface exposure that is incurred by liquid droplets. In the words of the BET authors, “In the gas phase the large free surface energy of small clusters makes their formation improbable. On the other hand, in adsorption the ‘liquid surface’ is practically complete after the first layer has been adsorbed, so that during the formation of successive layers hardly any surface tension has to be overcome.”
The strength of the BET model comes from its simplicity, which allowed a simple mathematical formulation (ibid.). This simplicity makes its central argument, that multilayered adsorbates are readily formed, broadly applicable, but also limit its explanatory power as we try to extrapolate from gas adsorption to more complex systems. BET theory does not consider cooperativity or possible surface tension of the free surface of the multilayered adsorbate. When bulk liquids coat fibers or flat surfaces, they can either form a stable layer of uniform thickness, or bead up, driven by surface tension at the free surface. In the physics literature, uniform coating is called “wetting” and beading up is called “dewetting.” The tendency of a liquid film to dewet depends on whether its free energy per unit area as a function of film thickness is monotonic (stable) or exhibits a peak (unstable; Seemann et al., 2001). Unstable films adsorbed on a fiber undergo a Plateau–Rayleigh instability and bead up with a characteristic periodicity (Haefner et al., 2015). A review of these topics for physical systems would require a separate article. The relevance of cooperative binding and dewetting instabilities to surface-bound macromolecule condensates are open research questions, as discussed below in the context of Tau binding to microtubules.
A BET-inspired, qualitative picture for macromolecules
If a molecule as simple as nitrogen can undergo multilayer adsorption on a multivalent surface, it seems reasonable to propose that the same could occur with proteins. Figure 3 proposes a qualitative extension of BET theory to a macromolecule system. The surface, or scaffold, could be DNA, RNA, microtubules, polysaccharide, plasma membrane, etc. The binding protein could be a transcription factor, MAP, lipid-binding protein, etc. The system is at thermodynamic equilibrium throughout and ignores kinetic complexities such as dissipative biochemistry and hardening. Importantly, the x-axis corresponds to the concentration of soluble binding protein, not total protein. The figure thus depicts an open system where the soluble protein concentration is experimentally controlled, and is not depleted by the binding reactions. To consider the situation inside cells, depletion of soluble protein by binding and phase separation reactions would have to be taken into account.
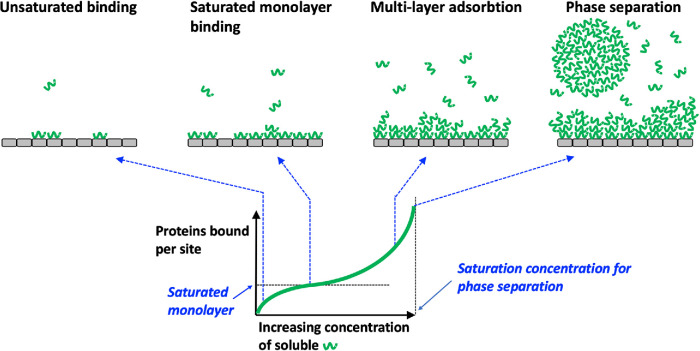
FIGURE 3:
Hypothetical, BET-inspired protein-binding isotherm. Gray rectangles represent a multivalent binding surface such as a DNA helix, microtubule, or lipid bilayer. Green squiggles represent a binding protein with self-association propensity. The figure illustrates the state of the system as the concentration of soluble binding protein increases up to the saturation concentration for phase separation. Note that multilayer binding occurs over a range of soluble concentrations.
The first two panels in Figure 3 illustrate the familiar low-concentration, Langmuir regime, where increasing concentration of the protein leads to increased binding to saturable sites. The third panel illustrates the proposed multilayer regime, where additional proteins join the condensate by interacting with each other and not directly with the surface. The fourth panel illustrates the situation when the saturation concentration is reached and condensate droplets become thermodynamically stable as a separate phase. The x-axis in Figure 3 ends at this concentration because all further binding protein added to the system will go into the liquid phase at equilibrium. Phase-separated droplets are likely to assemble in proximity to the binding surface because it catalyzes their formation. However, once the saturation concentration has been reached, phase-separated droplets are equally stable touching the binding surface or free, so they can move away. Their surface tension will pull them toward a spherical shape and promote fusion.
Figure 3 makes four important, and perhaps unfamiliar, predictions:
Surface-bound macromolecule condensates may be common in cells. This is a speculation, but the conditions needed for multilayer adsorption seem plausible for many binding proteins, particularly those with unstructured domains and a tendency to phase separate as pure proteins. The protein must have some tendency to self-associate by multivalent homophilic interactions. Binding sites on the surface must be sufficiently close together to allow self-interaction once bound. Surface- and hemophilic-binding interactions must use different interfaces so they can occur simultaneously.
Surface-bound condensates are bounded in size. The depth of the surface-bound condensate in Figure 3 depends on the concentration of soluble protein. It will exhibit local fluctuations due to random association and dissociation events, but on average it is time invariant.
Surface-bound condensates compete subunits away from phase-separated droplets. Surface-bound condensates can form over a range of soluble protein concentrations, starting well below the saturation concentration for bulk phase separation. When phase-separated droplets and surface-bound condensates compete for the same pool of soluble protein, the surface-bound condensates will win out because they are energetically favored.
Surface-bound condensates are thermodynamically “pinned” to the surface. Phase-separated droplets may be nucleated by a surface, but once formed, they are equally stable touching or far from that surface. This limits their spatial organizing capability. In contrast, surface-bound condensates are only stable on the surface and cannot move away. Combined with prediction 3), this effect will focus binding protein accumulation at binding sites, even if those sites are saturated. Spatial pinning may be one of the most important biological properties of surface-bound condensates. For example, condensate function in gene regulation likely depends on proximity of the condensed macromolecules to the regulated gene (Boija et al., 2018). Surface-bound condensates, which are spatially pinned, may be more suitable for this purpose than phase-separated condensates, which are not.
Experimental criteria for discriminating different kinds of condensate
Peng and Weber (2019) proposed five criteria for determining whether a macromolecule aggregate in a cell is a phase-separated droplet: molecular mobility of components, spherical shape, fusion, buffering the concentration of the soluble components, and slower diffusion of components at the condensate boundary compared with inside and outside. Ostwald ripening might be added to this list, because it is expected of any condensate with a surface tension (Hyman et al., 2014). Below we list criteria for identifying surface-bound condensates and distinguish them from phase-separated droplets and bridged polymer scaffolds. Interesting condensates to test using these criteria, where the termed “phase-separated” has been applied in the literature, include transcription factors that aggregate on DNA (Boija et al., 2018), nuclear paraspeckles (Peng and Weber, 2019), and MAPs that aggregate on microtubules. The latter include ZNF207 (a.k.a. BuGZ) and TPX2 in mitotic spindles (Jiang et al., 2015; King and Petry, 2020), and Tau in axons (discussed below).
Internal dynamics: Liquid-like molecular mobility is expected for both phase-separated droplets and surface-bound condensates. Bridged polymer scaffolds, in contrast, are expected to exhibit binding–unbinding dynamics more similar to saturable monolayer binding.
Shape: Only phase-separated droplets exhibit a well-defined surface tension. They are expected to be spherical, unless deformed by hardening reactions. For both surface-bound condensates and bridged polymer scaffolds, shape is determined by the scaffold and will typically be nonspherical.
Nucleation: Phase-separated droplets will often exhibit nucleated condensation, leading to sudden appearance and rapid growth. Surface-bound condensates typically will not, because their assembly Is dominated by reversible binding reactions. However, macromolecules, unlike gases, can undergo slow conformational transitions that are catalyzed by polymeric conformations of the same molecule, leading to nucleation by catalysis of conformational switching (Caspar and Namba, 1990). Thus, surface-bound condensates could, in principle, exhibit nucleated assembly. Nucleation of bridged polymer scaffolds is hard to predict and will depend on the kinetics of bridging reactions more than binding reactions.
Size change: Phase-separated droplets tend to undergo fusion and Ostwald ripening and are expected to coarsen over time (Hyman et al., 2014). Bridged polymer scaffolds will not exhibit these reactions. Whether surface-bound condensates dewet and bead up is an open research question, but is unlikely for thin layers. Constant size over time (or slow growth due to protein synthesis) is a signature of a surface-bound condensate or bridged polymer scaffold.
Location and movement: Phase-separated droplets are expected to be equally stable at all locations where their soluble subunit is present at the saturation concentration. They might be nucleated at specific sites, but they can move freely without loss of stability. Surface-bound condensates and bridged polymer scaffolds are thermodynamically pinned to their binding sites and will be immobile unless the scaffold moves. This is another important signature.
Molecular stoichiometry: A central distinction between surface-bound condensates and bridged polymer scaffolds is the number of bound molecules per scaffold site. This value is below or close to 1 in the bridged polymer scaffold model and higher than 1 in the surface-bound condensate model.
Proximity of bound molecules to the scaffold: Another way to discriminate bridged polymer scaffolds from the other models is to measure interactions between the scaffold and binding molecules. This interaction is always direct in bridged polymer scaffolds, while the other models are dominated by binding reactions between the binding proteins away from the scaffold.
Tau binding to microtubules
Tau is a microtubule-binding protein in axons that is famous for its tendency to aggregate in neurodegenerative diseases (Kosik, 1990; Goedert and Spillantini, 2019). A history of careful physical measurement makes the Tau–microtubule system an interesting test case for surface-bound condensates. The normal function of Tau was originally proposed to be stabilization of axonal microtubules (Drubin and Kirschner, 1986). Recently, a more nuanced picture has emerged where Tau competes with MAPs that more potently stabilize microtubules and may promote dynamics, as well as regulate motors and severing proteins (Baas and Qiang, 2019). Pure Tau undergoes liquid–liquid phase separation in the presence or absence of crowding agents (Wegmann et al., 2018; Kosik and Han, 2019), so it might form bounded condensates on microtubules.
Tau binds to microtubules primarily through three or four repeat domains (depending on the splice isoform) that are conserved with MAP2 and MAP4 (Sündermann et al., 2016). Early biochemistry papers using cosedimentation assays reported saturable binding of Tau to microtubules with an affinity between 1 and 10 μM, a saturation stoichiometry between 1:2 and 1:5 Tau:tubulin depending on the isoform, phosphorylation state, and buffer conditions, and a Hill coefficient close to 1 (i.e., no cooperativity; Hirokawa et al., 1988; Butner and Kirschner, 1991; Gustke et al., 1992). A later study tested higher Tau concentrations and reported nonsaturable binding that resembles a BET isotherm in shape (Figure 4A). If we assume that conventional saturation binding occurs at a ratio of 1:4 Tau:tubulin, then the observed binding goes up to approximately five “layers” in the BET picture, with no sign of saturation. Pure Tau undergoes reversible liquid–liquid phase separation at around 100 μM in the absence of crowding agents (Kosik and Han, 2019), so we might expect upwards curvature in the binding isotherm according to BET theory, but none is evident. In the presence of crowding agents, Tau phase separates at lower concentrations and coats microtubules as a deep layer (Hernández-Vega et al., 2017). However, the physiological significance of the artificially crowded regime is not clear.
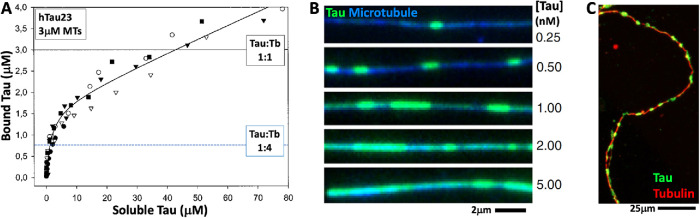
FIGURE 4:
Tau binding to microtubules. (A) Biochemical measurements using sedimentation assays revealed a nonsaturable binding isotherm (Ackmann et al., 2000). (B) Microscopy-based measurements revealed inhomogeneous binding to the surface of microtubules at very low Tau concentrations (Tan et al., 2019). The high-density regions were interpreted as condensates. (C) Microscopy-based evidence for Tau-rich domains on microtubules in an axon in cell culture (ibid.).
Recent microscopy-based measurement of Tau binding to microtubules revealed details that were missed by biochemistry, notably binding that is spatially inhomogeneous on the micron scale (Figure 4B). The bright regions were interpreted as Tau condensates on the microtubule surface whose assembly depended on Tau–Tau as well as Tau–tubulin interactions. Domain mapping revealed that multiple regions of Tau participate in these Tau–Tau interactions. Time-course observations showed that the total amount of bound Tau was time invariant, though interesting fluctuations were observed. Photobleaching and washout experiments demonstrated that binding was fully reversible. Thus, the proposed Tau condensates observed by microscopy appear to be bounded in size and dynamic. They are not spherical and do not dissociate from the microtubule surface, which argue against their being phase-separated droplets. These observations are broadly consistent with their being surface-bound condensates of the type predicted by BET theory.
Although BET theory provides an interesting starting point for discussion of Tau condensates on microtubules, strong limitations are evident. In particular, BET theory predicts spatially homogeneous binding, with only small, stochastic fluctuations, in both the unsaturated and multilayer regimes. At least two alternatives could be considered to account for the Tau patches observed by microscopy. Tau condensates on the microtubule surface might exhibit surface tension leading to a Plateau–Raleigh instability and beading up. However, it is not clear that thin Tau condensates would have a surface tension and the images do not look like nascent droplets. Alternatively, Tau might exhibit cooperative monolayer binding such that binding affinity is higher adjacent to an already bound molecule. This would lead to patches one layer deep, in which case the use of “condensate” as a descriptor may not be justified. Cooperative binding was not observed in bulk biochemical assays, so this explanation is also questionable. It is currently difficult to interpret all the data in a single model. In the future, absolute calibration of microscopy assays will be valuable to measure local Tau:tubulin ratios, and their spatial variance, which are key discriminants of alternative binding models. The spacing of bright and dim domains is also informative, because a Plateau–Raleigh instability is expected to generate a periodic pattern (Haefner et al., 2015).
In summary, the BET model provides a useful starting point for discussing Tau binding to microtubules, but it fails to predict microscopic domains at low Tau concentrations. Because cooperativity is a common feature of macromolecule interactions, the inability to account for it may be a general limitation of condensate models derived from the physical chemistry of gases. Inhomogeneity of Tau binding is not only of theoretical interest, because Tau patches were also observed in axons (Figure 4C). An intriguing possibility is that mutually exclusive MAP condensates generate functionally distinct patches on microtubules, conceptually similar to the lipid and protein microdomains that generate compositional and functional diversity on plasma membranes. Patchy binding to microtubules has been reported for other MAPs, attributed to condensate assembly and proposed to play a central role in function (Jiang et al., 2015; King and Petry, 2020). This is an exciting area of microtubule biology where new biophysical concepts are emerging, but critical appraisal of models will be important.
Multicomponent systems
All the discussion so far concerned systems of only two components: a binding molecule and a multivalent binding surface. In reality, the interior of cells is hugely complex and contains thousands of different macromolecules, most of which are present at mid-low nanomolar concentrations, as well as many potential binding surfaces. For most macromolecules tested so far, if bulk phase separation occurs in the absence of crowding agents, it does so with saturation concentrations in the high nanomolar to midmicromolar regime (Dignon et al., 2020; Riback et al., 2020). Thus, most macromolecules are not present in cells at concentrations required for assembly of large condensates by themselves, whether surface bound or phase separated. The question then arises: Do heterotypic interactions between macromolecules promote condensate assembly? This question can be broken down into two considerations: macromolecular crowding and biospecific molecular interactions.
Crowding increases the tendency of macromolecules to associate. Crowding agents such as poly(ethylene glycol) (PEG) were frequently used in the early days of the field to promote phase separation of pure macromolecules (Hyman et al., 2014). Most authors now omit them, in part because sufficient PEG can cause almost any macromolecule to phase separate. An important question remains: To what extent does macromolecular crowing inside cells promote phase separation? In a review of this topic I concluded that the cytoplasm of vertebrate cells is not very crowded at the length scale of ordinary proteins, and that crowding is not a major driver of condensate assembly at the molecular level (Mitchison, 2019). However, certain reporters pointed to significant crowding, and this interesting issue is unresolved.
The role of biospecific molecular interactions between macromolecules in promoting assembly of mixed condensates is much harder to evaluate. Can we imagine a version of Figure 3 where surface-bound condensates assemble from multiple different macromolecules interacting weakly with a surface and each other? Could it be the case that every multimeric surface in the cell that stays assembled on the timescale of protein diffusion, that is, all membranes, cytoskeletal filaments, nucleic acids, polysaccharides, etc., are coated with a dynamic multilayer of weakly adsorbed macromolecules? And if so, what are the functional consequences? These are fascinating questions for future research.
Perspective
Macromolecule condensates came as a surprise to many biochemists and the field is still grappling with how to measure and conceptualize them. BET theory reminds us that condensates have long been known in other domains of physical chemistry, and that their formation does not require special properties that are unique to macromolecules. BET theory has limited predictive power even for gas binding (Hill, 1949) and macromolecules are much more complicated than gases, particularly in their ability to engage in cooperative interactions. It is thus unlikely that BET theory will be useful for quantitative predictions in condensate biology. That said, it provides a thought-provoking conceptual starting point. It predicts that surface-binding reactions will often be nonsaturable but still specific, which requires a major change in thinking for biochemists. It further predicts that surface binding will often give rise to multilayer condensates without true phase separation and perhaps without the physical properties expected of bulk liquid phases, such as surface tension, though that is an open research question. Thermodynamic pinning of subunits to binding sites could be key to condensate function in gene expression, where it is important to localize condensate-enabled biochemistry proximal to the gene that is being regulated (Boija et al., 2018). In general, surface-bound condensates are worthy of consideration as a model of macromolecule aggregates that do not meet experimental criteria to be termed a separated phase.
Finally, this article can be read as a plea to apply the term “phase separation” more selectively. Condensate research has moved from a fringe interest 10 years ago to center stage in modern cell biology. It is increasingly influencing other research sectors, including drug discovery. This is tremendous progress, but the field needs to become more critical. Physical biochemistry matters! Not all local aggregates are condensates and not all condensates are phase-separated droplets. Different physical models will translate into different input–output relationships. Model discrimination will reveal new biology, but this will require new theory as well as more quantitative measurements.
Acknowledgments
T.J.M. is supported by National Institutes of Health Grant no. R35GM-131753. This article was inspired by conversations with Stephan Grill, Amy Gladfelter, and Tony Hyman that started at the Marine Biological Laboratory, Woods Hole Physiology Class in 2018.
Abbreviations used:
- BET
Brunauer-Emmett-Teller
- MAP
Microtubule Associated Protein
- PEG
poly(ethylene glycol).
Footnotes
REFERENCES
- Ackmann M, Wiech H, Mandelkow E (2000). Nonsaturable binding indicates clustering of tau on the microtubule surface in a paired helical filament-like conformation. J Biol Chem , 30335–30343. [DOI] [PubMed] [Google Scholar]
- Baas PW, Qiang L (2019). Tau: it’s not what you think. Trends Cell Biol , 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, Manteiga JC, Hannett NM, et al. (2018). Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell , 1842–1855.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science , 1729–1732. [DOI] [PubMed] [Google Scholar]
- Brunauer S, Emmett P, Teller E (1938). Adsorption of gases in multimolecular layers. J Am Chem Soc , 309–319. [Google Scholar]
- Butner KA, Kirschner MW (1991). Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol , 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar DL, Namba K (1990). Switching in the self-assembly of tobacco mosaic virus. Adv Biophys , 157–185. [DOI] [PubMed] [Google Scholar]
- Colquhoun D (2006). The quantitative analysis of drug-receptor interactions: a short history. Trends Pharmacol Sci , 149–157. [DOI] [PubMed] [Google Scholar]
- Dignon GL, Best RB, Mittal J (2020). Biomolecular phase separation: from molecular driving forces to macroscopic properties. Annu Rev Phys Chem , 53–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Kirschner MW (1986). Tau protein function in living cells. J Cell Biol , 2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory PJ (1942). Thermodynamics of high polymer solutions. J Chem Phys , 51–61. [Google Scholar]
- Goedert M, Spillantini MG (2019). Ordered assembly of tau protein and neurodegeneration. Adv Exp Med Biol , 3–21. [DOI] [PubMed] [Google Scholar]
- Gustke N, Steiner B, Mandelkow EM, Biernat J, Meyer HE, Goedert M, Mandelkow E (1992). The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett , 199–205. [DOI] [PubMed] [Google Scholar]
- Haefner S, Benzaquen M, Bäumchen O, Salez T, Peters R, McGraw JD, Jacobs K, Raphaël E, Dalnoki-Veress K (2015). Influence of slip on the Plateau–Rayleigh instability on a fibre. Nat Commun , 7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Vega A, Braun M, Scharrel L, Jahnel M, Wegmann S, Hyman BT, Alberti S, Diez S, Hyman AA (2017). Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Rep , 2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV (1909). The mode of action of nicotine and curari, determined by the form of the contraction curve and the method of temperature coefficients. J Physiol , 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TL (1949). Satistical mechnanics of adsorbtion. VII. Thermodynamic functions for the B.E.T. theory. J Chem Phys , 772–774. [Google Scholar]
- Hirokawa N, Shiomura Y, Okabe S (1988). Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol , 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, Jülicher F (2014). Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol , 39–58. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang S, Huang Y, He X, Cui H, Zhu X, Zheng Y (2015). Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell , 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MR, Petry S (2020). Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. Nat Commun , 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS (1990). Tau protein and neurodegeneration. Mol Neurobiol , 171–179. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Han S (2019). Tau condensates. Adv Exp Med Biol , 327–339. [DOI] [PubMed] [Google Scholar]
- Langmuir I (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc , 1361–1403. [Google Scholar]
- Mitchison TJ (2019). Colloid osmotic parameterization and measurement of subcellular crowding. Mol Biol Cell , 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A, Weber SC (2019). Evidence for and against liquid-liquid phase separation in the nucleus. Non-Coding RNA , 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD (2010). A guide to simple and informative binding assays. Mol Biol Cell , 4061–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback JA, Zhu L, Ferrolino MC, Tolbert M, Mitrea DM, Sanders DW, Wei M-T, Kriwacki RW, Brangwynne CP (2020). Composition-dependent thermodynamics of intracellular phase separation. Nature , 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, Riback JA, Bracha D, Eeftens JM, Iwanicki A, Wang A, et al. (2020). Competing protein-RNA interaction networks control multiphase intracellular organization. Cell , 306–324.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann R, Herminghaus S, Jacobs K (2001). Dewetting patterns and molecular forces: a reconciliation. Phys Rev Lett , 5534–5537. [DOI] [PubMed] [Google Scholar]
- Sehgal PB, Westley J, Lerea KM, DiSenso-Browne S, Etlinger JD (2020). Biomolecular condensates in cell biology and virology: phase-separated membraneless organelles (MLOs). Anal Biochem , 113691. [DOI] [PubMed] [Google Scholar]
- Sündermann F, Fernandez M-P, Morgan RO (2016). An evolutionary roadmap to the microtubule-associated protein MAP Tau. BMC Genomics , 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R, Lam AJ, Tan T, Han J, Nowakowski DW, Vershinin M, Simó S, Ori-McKenney KM, McKenney RJ (2019). Microtubules gate tau condensation to spatially regulate microtubule functions. Nat Cell Biol , 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. (2018). A molecular grammar governing the friving gorces for phase separation of prion-like RNA binding proteins. Cell , 688–699.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CA, Zwicker D, Jülicher F, Lee CF (2019). Physics of active emulsions. Rep Prog Phys Phys Soc G B , 064601. [DOI] [PubMed] [Google Scholar]
- Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C, et al. (2018). Tau protein liquid–liquid phase separation can initiate tau aggregation. EMBO J , e98049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M-T, Elbaum-Garfinkle S, Holehouse AS, Chen CC-H, Feric M, Arnold CB, Priestley RD, Pappu RV, Brangwynne CP (2017). Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat Chem , 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]