Abstract
Milk oligosaccharides (MO) are bioactive compounds in mammalian milk that provide health benefits to neonates beyond essential nutrients. GNU100, a novel animal MO biosimilar, was recently tested in vitro, with results showing beneficial shifts in microbiota and increased short-chain fatty acid (SCFA) production, but other effects of GNU100 were unknown. Three studies were conducted to evaluate the safety, palatability, and gastrointestinal (GI) tolerance of GNU100. In study 1, the mutagenic potential of GNU100 was tested using a bacterial reverse mutation assay and a mammalian cell micronucleus test. In study 2, palatability was assessed by comparing diets containing 0% vs. 1% GNU100 in 20 adult dogs. In study 3, 32 adult dogs were used in a completely randomized design to assess the safety and GI tolerance of GNU100 and explore utility. Following a 2-wk baseline, dogs were assigned to one of four treatments and fed for 26 wk: 0%, 0.5%, 1%, and 1.5% GNU100. On weeks 2, 4, and 26, fresh fecal samples were collected to measure stool quality, immunoglobulin A, and calprotectin, and blood samples were collected to measure serum chemistry, inflammatory markers, and hematology. On weeks 2 and 4, fresh fecal samples were collected to measure metabolites and microbiota. On week 4, total feces were collected to assess apparent total tract macronutrient digestibility. Although revertant numbers were greater compared with the solvent control in tester strain WP2uvrA(pKM101) in the presence of metabolic activation (S9) in the initial experiment, they remained below the threshold for a positive mutagenic response in follow-up confirmatory tests, supporting that GNU100 is not mutagenic. Similarly, no cytotoxicity or chromosome damage was observed in the cell micronucleus test. The palatability test showed that 1% GNU100 was strongly preferred (P < 0.05; 3.6:1 consumption ratio) over the control. In study 3, all dogs were healthy and had no signs of GI intolerance or illness. All diets were well accepted, and food intake, fecal characteristics, metabolite concentrations, and macronutrient digestibilities were not altered. GNU100 modulated fecal microbiota, increasing evenness and Catenibacterium, Megamonas, and Prevotella (SCFA producers) and reducing Collinsella. Overall, the results suggest that GNU100 is palatable and well-tolerated, causes no genotoxicity or adverse effects on health, and beneficially shifts the fecal microbiota, supporting the safety of GNU100 for the inclusion in canine diets.
Keywords: canine nutrition, gastrointestinal functionality, microbiome, milk oligosaccharides, nutrient digestibility
Introduction
Mammalian milk contains high concentrations of structurally diverse, complex carbohydrates in a free or conjugated form known as milk oligosaccharides (MO) that act as bioactive compounds. In addition to providing essential nutrients to neonates, there is evidence that MO confer health benefits by serving as an anti-adhesive and an anti-microbial and helping to modulate immunity and epithelial cell responses and enhance brain and cognitive function of neonates (Kunz et al., 2000; Ruiz-Palacios et al., 2003; Eiwegger et al., 2004; Newburg et al., 2005; Newburg, 2009; Bode, 2012; Jantscher-Krenn and Bode, 2012; Hester et al., 2013; Kavanaugh et al., 2013; Manthey et al., 2014; Comstock et al., 2017; Plaza-Díaz, 2018; Bode, 2020; Kong et al., 2019). In addition, MO are known for their prebiotic effect, with in vitro fermentation studies showing fermentation of MO by beneficial microbes that produce short-chain fatty acids (SCFA) (Ward et al., 2006; LoCascio et al., 2009; Asakuma et al., 2011).
There are hundreds of MO that have been identified in mammals, being composed of various monomers, including d-glucose, d-galactose, N-acetylglucosamine, l-fucose, and sialic acid (N-acetylneuraminic acid) (Kunz et al., 2000; Kobata, 2010; Bode, 2020). Over 20 MO structures have been identified from dogs through liquid chromatography coupled with mass spectrometry (Macias Rostami et al., 2014; Hughes et al., 2020; Wrigglesworth et al., 2020). Though it has been of great interest to find substitutes of MO that can provide the same health benefits, success has been limited due to the lack of structural diversity and complexity of traditional oligosaccharides (fructooligosaccharides [FOS] and galactooligosaccharides [GOS]) as well as a difficulty in large-scale supply (Bode et al., 2016; Thomson et al., 2018). GNU100 is a source of an animal milk oligosaccharide (AMO) biosimilar that is isolated from hydrolyzed porcine intestinal mucosa. It contains about 30 complex conjugated oligosaccharides composed of O-linked glycans bound to peptidic moieties. The structure of intestinal mucin glycans is similar to MO, in that both have a galactose and N-acetylglucosamine elongated core structure and fucose or sialic acid group terminal chains (Marcobal et al., 2011). Functionally, both mucin glycans and MO have been shown to enrich similar taxa of gut resident microbes, help mitigate perturbations in the gut microbiota, and provide host health benefits in mice (Pruss et al., 2021). In addition, mucin glycans have been shown to provide mucosal immune homeostasis through SCFA production in a rat model (Hino et al., 2020).
The results of a previous in vitro fermentation study showed that when GNU100 was inoculated with canine feces, there was a beneficial shift in microbial composition (increased Bifidobacterium and Lactobacillus; reduction in Escherichia/Shigella and Salmonella) and an increased production in fermentative metabolites (SCFA [acetate, propionate, and butyrate] and ammonia; Oba et al., 2020). Although AMO have been studied using in vitro fermentation assays, to our knowledge, nobody has tested them in dogs. Therefore, we performed three experiments to: 1) assess the genotoxicity of GNU100 by using standard in vitro assays recommended by regulatory authorities to qualify novel ingredient for safe use in food, 2) test GNU100 palatability in dogs, and 3) evaluate primarily the safety and gastrointestinal (GI) tolerance of GNU100 and explore its utility by conducting a 26-wk study in healthy adult dogs. We hypothesized that GNU100 would not demonstrate genotoxic effects, would increase the palatability of food fed to dogs, and would be a safe, well-tolerated ingredient by dogs without having a negative impact on serum chemistry or hematology, fecal characteristics, or nutrient digestibility and by favorably modulating gut microbiota and metabolites.
Materials and Methods
Experiment 1: genotoxicity test
To test the genotoxicity of GNU100, two different in vitro assays were conducted. First, a bacterial reverse mutation assay was conducted to evaluate the mutagenic potential of GNU100. A second assay using mammalian cells was done to test cytotoxicity and the potential of inducing micronucleated cells by GNU100. A correction factor was applied to assay dilutions in all tests conducted to account for the purity profile of the test substance.
Bacterial reverse mutation assay
This assay was conducted in accordance with Organisation for Economic Cooperation and Development (OECD) Guideline Test No. 471 (OECD, 1997) using the treat and plate method as described by Ames et al. (1975) and Maron and Ames (1983). The first assay was conducted using Salmonella typhimurium tester strains TA98, TA100, TA1535, and TA1537 and Escherichia coli tester strain WP2 uvrA(pKM101). The study consisted of two phases conducted in the presence and the absence of metabolic activation (Aroclor 1254-induced rat liver S9-mix, obtained from Trinova Biochem GmbH, Giessen, Germany). The S9 mixture was included at 5% (v/v) in the initial mutation assay and at 10% (v/v) in the confirmatory assay. In the initial assay, the concentrations of GNU100 tested were 0, 52, 164, 512, 1,600, and 5,000 µg/plate, while in the second confirmatory phase, the concentrations were 0, 492, 878, 1,568, 2,800, and 5,000 µg/plate. To verify a mutagenic response observed in the second confirmatory phase of the test in the tester strain WP2uvrA(pKM101), an additional experiment was performed using the same concentrations as in the second phase in the presence of 10% (v/v) S9-mix. The negative (vehicle) control and positive controls were evaluated concurrently with the treatment groups. The negative control was cell culture grade water. Positive controls in the absence of S9 metabolic activation included dimethylsulfoxide solutions of sodium azide, 2-nitrofluorene, and methylmethane sulfonate. A positive control in the presence of S9 activation was a dimethylsulfoxide solution of 2-aminoanthracene.
It has been reported that biological materials capable of releasing amino acids can cause increases in the number of revertant colonies that are not related to a mutagenic mode of action and thus give false-positive results in this type of assay (Thompson et al., 2005). As GNU100 is a complex of oligosaccharides and peptides that fits this criterion, a “treat and wash” variation of the bacterial reverse mutation test was also conducted with E. coli tester strain WP2uvrA with and without rat S9 mix. This method was developed specifically to test peptide- and amino acid-containing materials. The following concentrations of GNU100 were tested: 0, 160, 310, 620, 1,200, 2,500, and 5,000 µg/plate. The negative (vehicle) control was cell culture grade water and was plated in the presence and absence of S9 mix. Positive control in the absence of S9 metabolic activation was a dimethylsulfoxide solution of 4-nitroquinoline N-oxide. The positive controls in the presence of S9 metabolic activation were a dimethylsulfoxide solution of 2-aminoanthracene and an aqueous solution of cyclophosphamide monohydrate.
For the evaluation of mutagenicity, a test was considered positive (mutagenic) if the total number of revertants in the tester strains TA100 or WP2uvrA was greater than two times the concurrent vehicle control or the total number of revertants in tester strains TA1535, TA1537, or TA98 was greater than three times the concurrent vehicle control.
In vitro mammalian cell micronucleus test
The in vitro mammalian cell micronucleus test was conducted in accordance with OECD Guideline Test No. 487 (OECD, 2010). Prior to treatment, L5178Y TK+/− mouse lymphoma cells (obtained from American Type Culture Collection ATCC, Manassas, VA) were counted and suspended at approximately 3 × 105 cells/mL in the treatment medium (culture medium and 5% inactivated horse serum). Based on a preliminary study, the following concentrations of GNU100 were selected for the main test: 0, 625, 1,250, and 2,500 µg/mL (the latter being the highest analyzable dose level because higher dose levels induced an important increase in osmolality). Cells were treated with GNU100, vehicle control (sterile water), or a positive control for 3 h (“short-term exposure”) in either the presence or the absence of metabolic activation (Aroclor 1254-induced rat liver S9 mix, obtained from Molecular Toxicology, INC, Boone, NC 28607, USA) or for 24 h (“long-term exposure”) in the absence of S9. After short-term exposure in the absence or presence of S9, the test article was removed by washing the cells twice and suspending in a culture medium containing 10% inactivated horse serum followed by incubation at 37 °C in a humidified atmosphere of 5% CO2/95% air for a recovery period of 24 h. Positive controls in the absence of S9 consisted of 5 µg/mL of colchicine in both the 3-h and 24-h treatment periods and 0.2 and 0.05 µg/mL of mitomycin C in the 3-h and 24-h treatment groups, respectively. In the presence of S9, 3 µg/mL of cyclophosphamide was used. Cells were exposed in 24-well plates, at 37 °C in a humidified atmosphere of 5% CO2/95% air. Each treatment was coupled to an assessment of cytotoxicity at the same dose levels. Cytotoxicity was evaluated by determining the population doubling of cells as described by Greenwood et al. (2004).
At the completion of the cytotoxicity assessments, cells were washed with culture medium (containing 10% inactivated horse serum) and 1% pluronic acid. Cells were suspended in 49.5% culture medium (containing 10% inactivated horse serum), 50% phosphate-buffered saline (PBS), and 0.5% pluronic acid, before being fixed. Following fixation, the cells were kept at 5 °C. Cells were prepared on glass slides and air-dried before being stained for approximately 15 min in 5% Giemsa. All slides were encoded for “blind” scoring. The analysis was performed at 1,000× magnification. The presence of micronuclei was assessed according to criteria described by Miller et al. (1995). Micronuclei frequencies were analyzed in 1,000 mononucleated cells per culture (a total of 2,000 mononucleated cells per dose). The number of cells with micronuclei and the number of micronuclei per cell were recorded separately for each treated and control culture.
Experiment 2: palatability test
Twenty beagle dogs were used for a standard 2-d palatability test conducted at Kennelwood Inc. (Champaign, IL). A control diet coated with 5% of a mixed fat source was compared with the same diet coated with 5% mixed fat source + 1% GNU100. Dietary ingredients and guaranteed analysis are presented in Supplementary Table S1. Dogs were offered two bowls per day, each containing 400 g of test diets. Food bowls were presented for 30 min each day, and to prevent left–right bias, the bowl position was reversed on the second day of the test. Total daily consumption and first choice preference were reported for each dog.
Experiment 3: safety and tolerability study
Animals, experimental design, and diets
All animal procedures were approved and conducted at Summit Ridge Farms (Susquehanna, PA). The staff conducting the study were blinded to treatments to avoid any potential bias in the evaluation of general health observations and fecal scores. Prior to the study, a physical examination by a licensed veterinarian was conducted, and blood samples for serum chemistry and hematology measurements were collected to confirm health.
Thirty-two beagle dogs (age = 1 to 8 yr old; 5 male and 27 female beagle dogs; body weight [BW] = 7.6 ± 2.31 kg) were used in a completely randomized design study. Initially, 36 dogs were acclimated to the control (0%) diet for 14 d prior to study initiation. After the acclimation period, 32 dogs were selected and randomly assigned into one of four dietary treatments (n = 8/group) and fed for 26 wk. Dogs were housed in individual cages, with ad libitum access to fresh water. Even though dogs were housed individually, they received regular exercise and socialization with humans and other dogs according to kennel standard operating procedures and in line with the USDA Animal Welfare Act.
Dogs were fed dry, extruded diets formulated to meet all Association of American Feed Control Officials (AAFCO, 2019) nutrient recommendations for adult dogs at maintenance. All diets contained poultry byproduct meal (low ash), brewer’s rice, poultry fat, corn, vitamin/mineral premixes, a palatant, and the test ingredient (GNU100) and formulated to contain approximately 35% protein, 15% fat, 7% ash, and 5% fiber. Diets were manufactured at Wenger Manufacturing, Inc. (Sabetha, KS). For these diets, Wenger used their large paddle mixer (capacity = ~550 to 700 kg). For each batch, the appropriate amount of GNU100 was pre-blended into about 50 kg of dry diet mix. The pre-blend was then be added to the entire batch. Each batch was mixed for at least 10 to 15 min to ensure complete mixing. Dietary treatments consisted of: 1) control diet (CT; 0% GNU100), 2) low dose (LD; 0.5% GNU100), 3) medium dose (MD; 1.0% GNU100), and 4) high dose (HD; 1.5% GNU100). The ingredient and analyzed chemical composition of the diets are listed in Table 1.
Table 1.
Ingredient and chemical composition of experimental diets fed to dogs
| CT | LD | MD | HD | |
|---|---|---|---|---|
| Ingredient | ---------- %, as-is ---------- | |||
| Poultry byproduct meal (low ash) | 43.27 | 43.27 | 43.27 | 43.27 |
| Brewer’s rice (US #2) | 39.77 | 39.27 | 38.77 | 38.27 |
| Poultry fat | 8.65 | 8.65 | 8.65 | 8.65 |
| Corn, whole (US #2) | 4.81 | 4.81 | 4.81 | 4.81 |
| Palatant | 1.92 | 1.92 | 1.92 | 1.92 |
| Test ingredient (GNU100) | 0.00 | 0.50 | 1.00 | 1.50 |
| Salt (sodium chloride) | 0.48 | 0.48 | 0.48 | 0.48 |
| Potassium chloride, 50% K | 0.43 | 0.43 | 0.43 | 0.43 |
| Taurine | 0.19 | 0.19 | 0.19 | 0.19 |
| Mineral premix1 | 0.18 | 0.18 | 0.18 | 0.18 |
| Vitamin premix2 | 0.18 | 0.18 | 0.18 | 0.18 |
| Choline chloride | 0.13 | 0.13 | 0.13 | 0.13 |
| Chemical composition3 | ||||
| Dry matter, % | 93.7 | 92.6 | 93.1 | 93.4 |
| ---------- % dry matter ---------- | ||||
| Organic matter | 93.3 | 93.2 | 93.3 | 93.2 |
| Crude protein | 37.2 | 37.0 | 35.9 | 36.2 |
| Acid-hydrolyzed fat | 17.6 | 17.3 | 15.7 | 16.8 |
| Total dietary fiber | 9.3 | 8.5 | 8.1 | 8.6 |
| Gross energy, kcal/g | 5.25 | 5.22 | 5.1 | 5.19 |
1Provided per kg diet: Mn (as MnSO4), 66.00 mg; Fe (as FeSO4), 120 mg; Cu (as CuSO4), 18.00 mg; Co (as CoSO4), 1.20 mg; Zn (as ZnSO4), 240 mg; I (as KI), 1.80 mg; and Se (as Na2SeO3), 0.24 mg.
2Provided per kg diet: vitamin A, 5.28 mg; vitamin D3, 0.04 mg; vitamin E, 120.00 mg; vitamin K, 0.88 mg; thiamin, 4.40 mg; riboflavin, 5.72 mg; pantothenic acid, 22.00 mg; niacin, 39.60 mg; pyridoxine, 3.52 mg; biotin, 0.13 mg; folic acid, 0.44 mg; and vitamin B12, 0.11 mg.
3Chemical composition analysis was performed from diet subsample at the University of Illinois.
Gnubiotics Sciences SA (Epalinges, Switzerland) provided the test ingredient. GNU100 is a complex of oligosaccharides and peptides isolated from the hydrolyzed porcine intestinal mucosa with the typical physicochemical characteristics described in Table 2 and Supplementary Table S2.
Table 2.
Physicochemical characteristics of GNU100 used for the study
| Parameter | Value |
|---|---|
| Sensorial | White to yellow powder |
| Oligosaccharide and peptide complex | 42.7% dry matter |
| Free amino acids | 41.7% dry matter |
| Ash | 15.6% dry matter |
| Moisture | 4.0% |
| pH (2 w/v%, in deionized water at 20 °C) | 5.6 |
| Molecular weight distribution | |
| >10,000 Da | 0.0% |
| 5,000 to 10,000 Da | 0.2% |
| 2,000 to 5,000 Da | 5.7% |
| 1,000 to 2,000 Da | 38.9% |
| <1,000 Da | 55.3% |
Daily food intake, weekly BW, and weekly body condition scores
Dogs were fed once a day to maintain BW throughout the study. Daily food consumption was recorded to monitor intake, with adjustments made weekly if needed. BW was measured by personnel on a weekly basis during the study. Body condition scores (BCS; 9-point scale) according to Laflamme (1997) were measured at the beginning and end of the study and weekly during the study.
Fecal sample collection and analyses
Fresh fecal samples were collected on weeks 2 and 4 for fecal microbiota composition and fermentative metabolites (SCFA, branched-chain fatty acids [BCFA], phenols and indoles, ammonia) and on weeks 2, 4, and 26 for the measurement of fecal scores, pH, immunoglobulin A (IgA), and calprotectin. On week 4, in addition to the fresh fecal samples collected, total fecal samples were collected from each dog for five consecutive days to determine the apparent total tract macronutrient digestibility. Total feces were weighed and stored at −20 °C until later analysis.
Fecal scores were assigned as follows: 1 = watery diarrhea; 1.5 = diarrhea; 2 = moist, no form; 2.5 = moist, with some form; 3 = moist, formed; 3.5 = well formed, sticky; 4 = well formed; 4.5 = hard, dry; and 5 = hard, dry, crumbly feces. Fecal pH measurement was taken from the fresh fecal samples immediately, and then aliquots of the samples collected were distributed as follows: approximately, 4 g of aliquots for the analysis of phenol and indole was stored in 15 mL conical tubes in duplicates and frozen at −20 °C. Fresh fecal aliquots were transferred to sterile 2 mL cryogenic vials (Nalgene, Rochester, NY), frozen immediately on dry ice, and stored at −70 °C until microbiota, IgA, and calprotectin analyses. Another aliquot of approximately 5 g of feces was placed in 60 mL Nalgene bottles containing 5 mL of 2N HCl and stored at −20 °C until SCFA, BCFA, and ammonia analyses. Finally, the remaining samples were placed in a whirl-pak bag and stored at −20 °C for dry matter determination.
Fecal SCFA and BCFA concentrations were determined according to Erwin et al. (1961) using a gas chromatograph (Hewlett-Packard 5890A series II, Palo Alto, CA) and a glass column (180 cm × 4 mm i.d.) packed with 10% SP-1200/1% H3PO4 on 80/100+ mesh Chromosorb WAW (Supelco Inc., Bellefonte, PA). Nitrogen was the carrier with a flow rate of 75 mL/min. Oven, detector, and injector temperatures were 125, 175, and 180 °C, respectively. Fecal concentrations of phenol and indole were evaluated using gas chromatography according to Flickinger et al. (2003), and fecal ammonia concentration was measured according to the method of Chaney and Marbach (1962).
Fecal protein was extracted according to Vilson et al. (2016). Fecal samples (250 mg) were vortexed with 750 uL of extraction buffer containing 50 mM ethylenediaminetetraacetic acid (ThermoFisher, Waltham, MA) and 100 μg/L soybean trypsin inhibitor (Sigma, St. Louis, MO) in PBS/L percent bovine serum albumin (Tocris Bioscience, Bristol, UK). Phenylmethanesulphonyl fluoride (12.5 μL, 350 mg/L; Sigma, St. Louis, MO) was added into each tube and centrifuged for 10 min. Supernatants were collected for measurements of fecal IgA (E-40A; Immunology Consultants Laboratory, Portland, OR) and calprotectin (MBS030023; MyBiosource, Inc., San Diego, CA) using commercial enzyme-linked immunosorbent assay (ELISA) kits.
Fecal microbiota analysis and bioinformatics
Fecal bacterial DNA was extracted from feces collected according to the manufacturer’s instructions using the MO BIO PowerSoil Kit (MO BIO Laboratories, Carlsbad, CA). The concentration of extracted DNA was quantified using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY), and DNA quality was assessed using agarose gel electrophoresis. DNA samples were stored at −80 °C until samples were analyzed using Pet 16-sequencing technology. Genomic DNA from fecal samples at 2 wk (CT, n = 7; LD, n = 7; MD, n = 8; HD, n = 8) and 4 wk (CT, n = 6; LD, n = 7; MD, n = 8; HD, n = 8) were used for sequencing analysis.
Sequencing analysis was performed by Gnubiotics Sciences SA (Epalinges, Switzerland). In short, a targeted polymerase chain reaction-based sequencing approach was used, where unique DNA regions of the 16S rRNA gene were targeted to generate amplicons. Amplicon library was sequenced using MiSeq Illumina platform with a 2 × 150 cycle Illumina sequencing kit (MS-102-2002). Two negative control reactions without the DNA template and two negative control reactions without the amplification primers were included in the run. Fastq files generated by the sequencer were used to de-multiplex and analyze the raw reads, using a publicly available Divisive Amplicon Denoising Algorithm (DADA2) pipeline. Read counts generated by the pipeline were divided by the total sample read count to obtain relative abundances. Microbiota data ordination was done by principal component analysis (PCA), with the assessment of differences among microbial profiles of the four groups was done by one-way permutational multivariate analysis of variance (Bray–Curtis similarity distance) using paleontological Statistics (PAST; v3.12) software. Alpha-diversity analyses, including richness (number of amplicon sequence variants), Shannon diversity index (H), and evenness (e^H/S), were calculated, with significant differences being calculated using Mann–Whitney U test in PAST; v3.12. Differences were considered significant with P < 0.05 and trends at P < 0.10. Predominant bacterial phyla, class, and genus were analyzed using the Mixed Models procedure of SAS 9.3 (SAS Institute, Cary NC).
Diet and apparent total tract digestibility analyses
Test diet subsamples and dried fecal samples collected for digestibility were ground through a Wiley mill (model 4, Thomas Scientific, Swedesboro, NJ) through a 2-mm screen. Dry matter and organic matter were measured according to the Association of Official Analytical Chemists (AOAC) methods (AOAC, 2006; method 934.01 for dry matter and method 942.05 for organic matter). Crude protein content was calculated from total nitrogen values measured by LECO (TruMac N, Leco Corp., St. Joseph, MI; AOAC, 2002; method 922.15). Acid-hydrolyzed fat content was determined using methods according to the American Association of Cereal Chemists (AACC, 1983; method 30-14) and (Budde, 1952). Total dietary fiber was determined for diet samples according to (Prosky et al., 1985) and AOAC (2002; method 985.29). Gross energy was measured using a bomb calorimeter (Model 6200, Parr Instruments, Moline, IL). Apparent total tract macronutrient digestibility of nutrients and energy was calculated using the following equation: Digestibility (%) = ([nutrient intake (g/d) − fecal output (g/d)]) / (nutrient intake (g/d)) × 100%.
Blood sample collection and analyses
On weeks 2, 4, and 26, blood samples were collected for serum chemistry, hematology, inflammatory cytokines, immunoglobulin E (IgE), and C-reactive protein (CRP). Up to 8 mL of fasted blood sample was collected via jugular venipuncture. Blood samples were aliquoted so that 6 mL was used for serum separating tubes and 2 mL used for ethylenediaminetetraacetic acid tubes. Blood collected into serum separating tubes was centrifuged for 15 min at 3,000 RPM and stored into cryovials. One was sent to Antech Diagnostics for serum chemistry analysis, with two sent to the University of Illinois for the measurement of inflammatory cytokines. Blood samples stored in ethylenediaminetetraacetic acid tubes were sent to Antech Diagnostics for hematology analysis. Serum concentrations of IgE (ICL, Portland, OR), interleukin-6 (Abcam, Cambridge, MA), tumor necrosis factor-alpha (R&D Systems, Minneapolis, MN), and CRP (Abcam, Cambridge, MA) were evaluated using commercial ELISA kits.
General health observations
A complete physical examination was given to all dogs by a licensed veterinarian at study initiation and at study completion. Qualified personnel performed clinical observations twice daily. Variables associated with GI tolerance, such as abnormal stool quality, evidence of vomiting, abnormal behavior, poor coat quality, poor BCS, were recorded.
Coat and skin evaluation
Skin and coat scores were evaluated prior to study initiation, every 4 wk, and at study completion according to Rees et al. (2001): hair: 1 = dull, coarse, dry; 2 = poorly reflective, non-soft; 3 = medium reflective, medium soft; 4 = highly reflective, very soft; and 5 = greasy; skin: 1 = dry; 2 = slightly dry; 3 = normal; 4 = slightly greasy; and 5 = greasy.
Statistical analysis
All data were analyzed using the Mixed Models procedure of SAS 9.3 (SAS Institute, Cary NC). The main effect of dietary treatment was tested with dogs considered as a random effect. Data normality and homogeneity of variance were tested using SAS. Differences among treatments were determined using a Fisher-protected least significant difference with a Tukey adjustment to control for experiment-wise error. A probability of P < 0.10 was accepted as being statistically significant for safety measures and P < 0.05 for other measures. Data were reported as means ± pooled SEM.
Results
Genotoxicity tests
Bacterial reverse mutation assay
In the initial test of “treat and plate,” no increases in the number of revertants were observed in any of the tester strains treated with GNU100 at any concentration in the absence or presence of S9-mix (5%; Supplementary Table S3). Based on the results of the initial mutation assay, it was determined that the maximum concentration (5,000 µg/plate) should be included in the confirmatory assay. In the confirmatory assay of “treat and plate,” no increase in the number of revertants was observed in tester strains TA1535, TA1537, TA98, and TA100 at any concentration with or without S9 mix (10%; Supplementary Table S4). In tester strain WP2uvrA(pKM101) without S9 mix, GNU100 did not elicit an increase in revertants that reached the threshold for a positive mutagenic response. However, in the presence of S9 mix (10%), GNU100 elicited a concentration-dependent increase in revertants, reaching a 2.3-fold increase at a concentration of 5,000 µg/plate (Supplementary Table S4). In the additional confirmatory experiment in tester strain WP2uvrA(pKM101) in the presence of S9 mix (10%), GNU100 did not elicit an increase in revertants that reached the threshold for a positive mutagenic response (Supplementary Table S4). Based on the equivocal results (one positive and one negative result), the test was considered inconclusive for tester strain WP2uvrA(pKM101). In the additional “treat and wash” assay, a method developed to avoid false-positive results induced by peptides and amino acid-containing material, GNU100 did not elicit any increases in revertants in WP2uvrA above the threshold for a positive mutagenic response at any concentration in the absence or presence of S9 (10%; Supplementary Table S5). In all tests, negative and positive control substances elicited acceptable results that were within the range of historical values.
In vitro mammalian cell micronucleus test
No cytotoxicity was induced at any of the dosage levels tested, with a lack of any statistically significant or dose-related changes to population doubling. GNU100 did not induce any chromosome damage, or damage to the cell division apparatus in L5178Y TK+/− cells, either in the presence or in the absence of a rat liver S9 mixture compared with the negative control. GNU100 did not elicit any statistically significant increases in the frequency of micronucleated cells at any concentration. Frequencies of micronucleated cells remained within the historical control range (Supplementary Table S6). An incubation time of 24 h was not shown to influence genotoxicity, and the recovery period had no impact, as no significant chromosomal damage was observed. Positive controls induced the expected clastogenic or aneugenic response in L5178Y TK+/− cells. These were different (P < 0.001) than the vehicle control and consistent with historical values of positive control responses.
Palatability test
In the palatability test, a 3.6:1 total consumption ratio was observed for the MD vs. CT, showing a significant preference (P < 0.05) for the diet containing GNU100. Data collected from both days indicate that the MD was consumed first on 31/40 occasions, compared with 9/40 for CT.
General health observations
Thirty-two dogs were initially enrolled into the study. One of the dogs fed the LD treatment was removed from the study on day 140 due to chronic food regurgitation. The dog maintained its BW throughout the study and its blood work was normal. The staff veterinarian, however, noted that the dog would eat food readily and then regurgitate. Ultrasound scans of the stomach and abdomen did not show any abnormalities. The dog was ultimately diagnosed with megaesophagus, and this condition was not due to the treatment diet. All other dogs remained healthy without signs of GI discomfort or intolerance throughout the study. The condition of the skin and hair did not differ among treatments during the experiment, with all dogs having hair that remained in a state described as a medium level of reflectivity and softness, and the skin was scored as normal (data not shown).
Food intake, BW, and apparent total tract macronutrient and energy digestibility
The average daily food intake was 155 g/d and was not affected by GNU100 supplementation (Table 3). Dog BW was 7.7 kg on average and was not different among different treatment groups throughout the study. Supplementation of GNU100 did not influence the apparent total tract digestibility of dry matter (79.2%), organic matter (85.1%), crude protein (78.5%), acid-hydrolyzed fat (96.7%), or energy (89.2%) (Table 3).
Table 3.
Mean daily intake, BW, and apparent total tract macronutrient and energy digestibility of dogs supplemented with different dosages of GNU100
| GNU1001 | ||||||
|---|---|---|---|---|---|---|
| CT | LD | MD | HD | SEM | P-value | |
| Daily intake, g/d | 161.5 | 158.5 | 149.6 | 152.3 | 12.18 | 0.89 |
| BW, kg | 7.7 | 7.9 | 7.7 | 7.6 | 0.86 | 0.99 |
| Initial BW, kg | 7.6 | 7.7 | 7.7 | 7.6 | 0.87 | 0.99 |
| Final BW, kg | 7.6 | 8.2 | 7.8 | 7.7 | 0.88 | 0.97 |
| ---------- Digestibility2, % ---------- | ||||||
| Dry matter | 78.0 | 79.6 | 80.9 | 78.1 | 1.48 | 0.49 |
| Organic matter | 84.2 | 85.5 | 86.2 | 84.5 | 1.07 | 0.57 |
| Crude protein | 77.8 | 78.9 | 79.7 | 77.4 | 1.53 | 0.71 |
| Acid-hydrolyzed fat | 94.5 | 94.8 | 94.4 | 94.8 | 0.36 | 0.74 |
| Gross energy | 84.6 | 85.7 | 86.1 | 84.7 | 1.05 | 0.70 |
1CT, control diet; LD, low dose diet containing 0.5% GNU100; MD, medium dose diet containing 1.0% GNU100; HD, high dose diet containing 1.5% GNU100.
2Digestibility data measured from total feces collected over five consecutive days during week 4.
Fecal characteristics, metabolites, fecal IgA, and calprotectin
Fecal characteristics, including pH, fecal scores, and fecal dry matter percentage, were not affected by treatment (Table 4). Similarly, fecal fermentative metabolites were not altered by treatment. Finally, GNU100 did not alter fecal calprotectin or IgA, except at week 4 where fecal IgA concentration was greater (P < 0.05) in dogs fed CT than those fed MD.
Table 4.
Mean fecal characteristics, metabolites, IgA, and calprotectin concentrations of dogs fed GNU100-supplemented diets
| GNU1001 | |||||||
|---|---|---|---|---|---|---|---|
| Item | Week | CT | LD | MD | HD | SEM | P-value |
| pH | 2 | 6.9 | 7.0 | 6.9 | 6.7 | 0.16 | 0.75 |
| 4 | 6.8 | 7.1 | 6.9 | 6.8 | 0.16 | 0.55 | |
| 26 | 6.7 | 6.8 | 6.8 | 6.5 | 0.16 | 0.43 | |
| Score2 | 2 | 3.1 | 3.1 | 3.2 | 3.3 | 0.16 | 0.75 |
| 4 | 3.2 | 3.1 | 3.4 | 3.2 | 0.15 | 0.43 | |
| 26 | 3.3 | 3.2 | 3.4 | 3.4 | 0.13 | 0.58 | |
| Dry matter, % | 2 | 36.7 | 34.9 | 36.2 | 36.8 | 1.18 | 0.68 |
| 4 | 34.3 | 35.6 | 36.7 | 36.4 | 1.20 | 0.55 | |
| 26 | 34.9 | 34.0 | 35.1 | 36.5 | 0.87 | 0.23 | |
| --------------- µmol/g dry matter ----------- | |||||||
| Total SCFA3 | 2 | 270.0 | 333.0 | 309.9 | 296.1 | 26.71 | 0.40 |
| 4 | 295.0 | 283.0 | 307.4 | 281.8 | 29.60 | 0.90 | |
| Acetate | 2 | 158.1 | 200.9 | 180.5 | 162.7 | 16.22 | 0.27 |
| 4 | 164.5 | 173.4 | 179.5 | 158.5 | 16.97 | 0.81 | |
| Propionate | 2 | 72.7 | 94.4 | 86.7 | 94.9 | 7.14 | 1.00 |
| 4 | 83.5 | 79.5 | 90.1 | 85.7 | 9.37 | 0.85 | |
| Butyrate | 2 | 37.3 | 38.6 | 42.7 | 38.5 | 4.40 | 1.00 |
| 4 | 37.1 | 35.5 | 37.8 | 37.6 | 4.34 | 0.98 | |
| Total BCFA3 | 2 | 23.9 | 26.9 | 27.6 | 24.3 | 3.62 | 0.81 |
| 4 | 23.1 | 27.3 | 23.1 | 25.1 | 2.93 | 0.63 | |
| Isobutyrate | 2 | 9.5 | 10.9 | 11.0 | 9.5 | 1.41 | 0.76 |
| 4 | 9.4 | 11.0 | 9.1 | 9.9 | 1.18 | 0.62 | |
| Isovalerate | 2 | 13.9 | 15.6 | 16.1 | 14.3 | 2.19 | 0.82 |
| 4 | 13.3 | 15.9 | 13.4 | 14.5 | 1.73 | 0.62 | |
| Valerate | 2 | 0.5 | 0.5 | 0.5 | 0.5 | 0.05 | 0.86 |
| 4 | 0.4 | 0.5 | 0.6 | 0.7 | 0.08 | 0.18 | |
| Total P/I3 | 2 | 6.3 | 5.3 | 3.7 | 4.1 | 1.10 | 0.41 |
| 4 | 4.8 | 5.2 | 3.8 | 4.5 | 1.09 | 0.83 | |
| Phenol | 2 | 3.0 | 2.0 | 1.2 | 1.4 | 0.72 | 0.44 |
| 4 | 2.0 | 1.6 | 1.0 | 2.1 | 0.77 | 0.69 | |
| Indole | 2 | 3.3 | 3.4 | 2.5 | 2.7 | 0.46 | 0.40 |
| 4 | 2.7 | 3.5 | 2.8 | 2.4 | 0.50 | 0.43 | |
| Ammonia | 2 | 209.0 | 223.0 | 244.2 | 212.0 | 27.37 | 0.72 |
| 4 | 200.0 | 214.0 | 203.0 | 212.0 | 17.79 | 0.82 | |
| IgA, mg/g | 2 | 6.1 | 2.3 | 2.3 | 4.4 | 1.55 | 0.28 |
| 4 | 5.6b | 2.4ab | 0.7a | 2.4ab | 1.19 | 0.03 | |
| 26 | 0.9 | 1.5 | 2.4 | 1.8 | 0.85 | 0.90 | |
| Calprotectin, ug/g | 2 | 0.12 | 0.11 | 0.13 | 0.12 | 0.02 | 0.93 |
| 4 | 0.14 | 0.14 | 0.13 | 0.15 | 0.01 | 0.55 | |
| 26 | 0.10 | 0.10 | 0.14 | 0.13 | 0.02 | 0.26 | |
1CT, control diet; LD, low dose diet containing 0.5% GNU100; MD, medium dose diet containing 1.0% GNU100; HD, high dose diet containing 1.5% GNU100.
2Fecal score: 1, watery diarrhea; 1.5, diarrhea; 2, moist, no form; 2.5, moist, with some form; 3, moist, formed; 3.5, well formed, sticky; 4, well formed; 4.5, hard, dry; and 5, hard, dry, crumbly feces.
3SCFA = acetate + propionate + butyrate; BCFA = isobutyrate + isovalerate + valerate; and P/I = phenol + indole.
a,bWithin a row, means with different superscripts differ (P < 0.05). Bolded numbers are significant P-values (P < 0.05).
Fecal microbiota
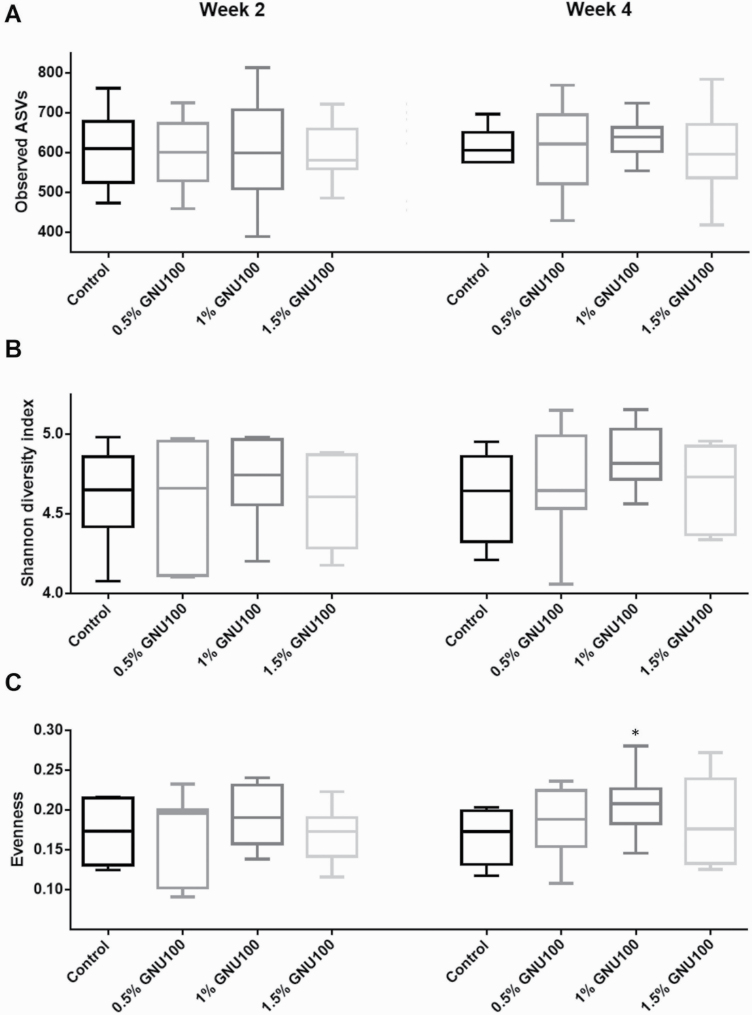
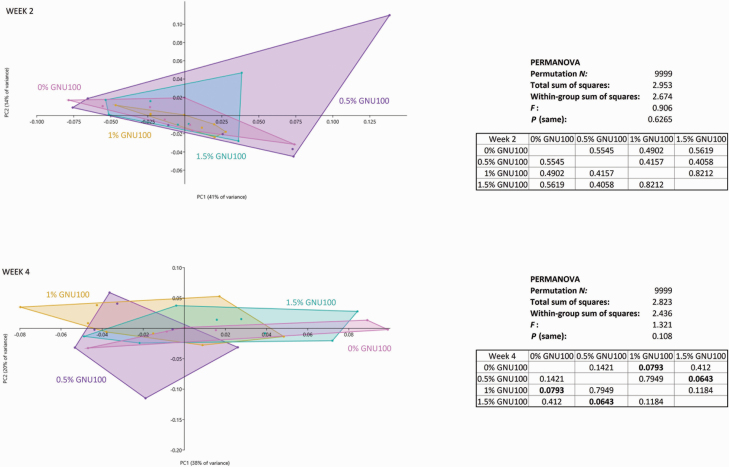
A total of 18,842,323 high-quality reads were obtained. The overall number of amplicon sequence variants detected was 2,991. The number of reads per sample ranged from 66,045 to 623,381. After the calculation of relative abundance per sample, further analyses were performed. Microbial α-diversity measures, which represent species richness and evenness within a sample, indicated that species richness and Shannon diversity index were not affected by treatment. Evenness, however, tended to be greater (P < 0.10) in dogs fed MD than those fed CT at week 4 (Figure 1). PCA demonstrated that microbial communities were not significantly affected due to treatment at week 2. At week 4, however, dogs fed MD and CT tended (P < 0.10) to differ from one another, and dogs fed LD and dogs fed HD tended (P < 0.10) to differ (Figure 2).
Figure 1.
Alpha-diversity indices of fecal microbiota from dogs fed GNU100-supplemented diets. (A) richness (observed amplicon sequence variants), (B) Shannon diversity index (H), and (C) evenness (e^H/S). *Tended to be different (P < 0.10) from 0% GNU100.
Figure 2.
PCA of fecal microbiota of dogs fed GNU100-supplemented diets.
Six bacterial phyla were detected in fecal samples, with Firmicutes and Fusobacteria being the most predominant (Table 5). At week 4, the relative abundance of Actinobacteria was higher (P < 0.05) in dogs fed CT than those in the other treatment groups. The relative abundance of Actinobacteria was also higher (P < 0.05) in dogs fed HD than those fed LD. The relative abundance of Firmicutes was lower (P < 0.05) in dogs fed LD than those fed HD at week 4. The relative abundance of Proteobacteria tended to be greater (P < 0.10) in dogs fed MD at week 4.
Table 5.
Predominant bacterial phyla (mean % of total sequences) in feces of dogs fed GNU100-supplemented diets
| GNU1001 | |||||||
|---|---|---|---|---|---|---|---|
| Phylum | Week | CT | LD | MD | HD | SEM | P-value |
| Actinobacteria | 2 | 0.43 | 0.44 | 0.64 | 0.49 | 0.15 | 0.73 |
| 4 | 0.49c | 0.15a | 0.18ab | 0.43b | 0.10 | 0.04 | |
| Bacteroidetes | 2 | 5.27 | 6.77 | 5.78 | 5.47 | 1.53 | 0.91 |
| 4 | 8.54 | 13.60 | 13.89 | 7.52 | 16.65 | 0.92 | |
| Deferribacteres | 2 | 0.03 | 0.02 | 0.01 | 0.02 | 0.01 | 0.90 |
| 4 | 0.04 | 0.05 | 0.06 | 0.01 | 0.02 | 0.32 | |
| Firmicutes | 2 | 79.88 | 75.48 | 82.81 | 83.36 | 4.62 | 0.61 |
| 4 | 74.26ab | 61.83a | 63.68ab | 77.31b | 4.70 | <0.0001 | |
| Fusobacteria | 2 | 11.91 | 12.65 | 8.97 | 8.24 | 2.46 | 0.49 |
| 4 | 13.89 | 20.36 | 16.91 | 12.49 | 2.66 | 0.18 | |
| Proteobacteria | 2 | 2.13 | 4.46 | 1.63 | 2.33 | 1.31 | 0.59 |
| 4 | 2.56 | 3.75 | 5.11 | 1.97 | 0.88 | 0.07 | |
1CT, control diet; LD, low dose diet containing 0.5% GNU100; MD, medium dose diet containing 1.0% GNU100; HD, high dose diet containing 1.5% GNU100.
a–cWithin a row, means with different superscripts differ (P < 0.05). Bolded numbers are significant P-values (P < 0.05).
At the genus level, a total of 118 taxa were detected in the samples, with Clostridium_XI being the most abundant genera (Table 6). The relative abundance of Catenibacterium was higher (P < 0.05) in dogs fed HD than the other treatments at week 2, while dogs fed MD or HD had higher (P < 0.05) Catenibacterium than those fed CT or LD at week 4. At week 4, the relative abundance of Collinsella was lower (P < 0.05) in dogs fed LD compared with those fed CT and HD. Also, dogs fed MD had lower (P < 0.05) Collinsella than those fed HD at week 4. At week 2, the relative abundance of Megamonas was lower (P < 0.05) in dogs fed CT than those fed HD. The relative abundance of Turicibacter was lower (P < 0.05) in dogs fed LD than those fed MD or HD. The relative abundances of Anaerobiospirillum, Peptococcus, and Prevotella tended to be altered (P < 0.10) at week 4.
Table 6.
Predominant bacterial genera (mean % of total sequences) in feces of dogs fed GNU100-supplemented diets
| GNU1001 | |||||||
|---|---|---|---|---|---|---|---|
| Genus2 | Week | CT | LD | MD | HD | SEM | P-value |
| Acetanaerobacterium | 2 | 0.17 | 0.18 | 0.31 | 0.34 | 0.12 | 0.84 |
| 4 | 0.30 | 0.44 | 0.50 | 0.45 | 0.15 | 0.80 | |
| Allobaculum | 2 | 1.35 | 1.36 | 1.10 | 1.39 | 0.29 | 0.82 |
| 4 | 1.34 | 0.93 | 1.27 | 1.46 | 0.30 | 0.63 | |
| Alloprevotella | 2 | 2.00 | 2.39 | 1.52 | 1.50 | 0.52 | 0.59 |
| 4 | 1.92 | 3.62 | 3.21 | 2.46 | 0.66 | 0.32 | |
| Anaerobiospirillum | 2 | 0.95 | 0.75 | 0.67 | 0.45 | 0.45 | 0.93 |
| 4 | 1.31 | 1.24 | 2.25 | 0.77 | 0.39 | 0.06 | |
| Bacteroides | 2 | 2.02 | 2.86 | 2.76 | 1.82 | 0.79 | 0.72 |
| 4 | 6.09 | 6.18 | 6.72 | 2.77 | 1.37 | 0.16 | |
| Blautia | 2 | 6.69 | 6.05 | 8.29 | 6.66 | 1.12 | 0.52 |
| 4 | 6.15 | 5.37 | 5.33 | 7.10 | 1.03 | 0.56 | |
| Catenibacterium | 2 | 0.53a | 0.097a | 0.57a | 1.29b | 0.21 | 0.01 |
| 4 | 0.15a | 0.12a | 0.97b | 0.73b | 0.25 | 0.01 | |
| Clostridium_sensu_stricto | 2 | 0.55 | 0.50 | 0.81 | 0.69 | 0.19 | 0.64 |
| 4 | 0.32 | 0.20 | 0.30 | 0.31 | 0.09 | 0.76 | |
| Clostridium_XI | 2 | 33.61 | 27.70 | 30.72 | 32.60 | 3.52 | 0.66 |
| 4 | 30.10 | 21.63 | 21.99 | 29.21 | 3.28 | 0.15 | |
| Clostridium_XlVa | 2 | 0.38 | 0.26 | 0.41 | 0.36 | 0.06 | 0.35 |
| 4 | 0.46 | 0.32 | 0.40 | 0.37 | 0.07 | 0.62 | |
| Clostridium_XVIII | 2 | 0.46 | 0.30 | 0.29 | 0.28 | 0.09 | 0.46 |
| 4 | 0.39 | 0.26 | 0.19 | 0.23 | 0.07 | 0.22 | |
| Collinsella | 2 | 0.23 | 0.28 | 0.46 | 0.32 | 0.11 | 0.56 |
| 4 | 0.24bc | 0.09a | 0.12ab | 0.29c | 0.05 | 0.01 | |
| Escherichia/Shigella | 2 | 0.01 | 1.28 | 0.01 | 0.56 | 0.64 | 0.21 |
| 4 | 0.03 | 0.25 | 0.31 | 0.04 | 0.17 | 0.79 | |
| Faecalibacterium | 2 | 1.78 | 1.92 | 1.63 | 1.69 | 0.41 | 0.96 |
| 4 | 1.99 | 1.87 | 2.51 | 2.53 | 0.39 | 0.52 | |
| Fusobacterium | 2 | 11.65 | 12.26 | 8.68 | 8.00 | 2.37 | 0.44 |
| 4 | 13.53 | 19.65 | 16.37 | 12.29 | 2.58 | 0.29 | |
| Holdemanella | 2 | 0.57 | 0.41 | 0.72 | 1.08 | 0.25 | 0.30 |
| 4 | 0.55 | 0.45 | 0.93 | 0.96 | 0.26 | 0.41 | |
| Lactobacillus | 2 | 0.44 | 4.01 | 1.64 | 0.57 | 1.31 | 0.83 |
| 4 | 0.75 | 1.01 | 0.89 | 1.18 | 0.60 | 0.78 | |
| Megamonas | 2 | 0.66a | 0.83ab | 1.03ab | 2.54b | 0.50 | 0.05 |
| 4 | 0.49 | 1.37 | 1.96 | 2.47 | 0.71 | 0.11 | |
| Parasutterella | 2 | 0.41 | 0.24 | 0.19 | 0.25 | 0.15 | 0.82 |
| 4 | 0.22 | 0.42 | 0.59 | 0.32 | 0.12 | 0.19 | |
| Peptococcus | 2 | 0.09 | 0.13 | 0.34 | 0.16 | 0.08 | 0.15 |
| 4 | 0.15 | 0.31 | 0.39 | 0.80 | 0.19 | 0.10 | |
| Phascolarctobacterium | 2 | 0.37 | 0.42 | 0.56 | 0.48 | 0.09 | 0.51 |
| 4 | 0.55 | 0.86 | 0.88 | 0.60 | 0.13 | 0.19 | |
| Prevotella | 2 | 0.83 | 0.78 | 0.92 | 1.48 | 0.40 | 0.54 |
| 4 | 0.18 | 2.57 | 2.57 | 1.66 | 0.69 | 0.09 | |
| Romboutsia | 2 | 4.33 | 3.92 | 4.21 | 4.19 | 0.63 | 0.97 |
| 4 | 3.51 | 2.39 | 2.79 | 3.48 | 0.42 | 0.20 | |
| Streptococcus | 2 | 1.57 | 3.41 | 1.06 | 2.74 | 1.85 | 0.95 |
| 4 | 0.76 | 2.89 | 0.95 | 1.48 | 1.03 | 0.61 | |
| Turicibacter | 2 | 1.74ab | 0.66a | 2.14b | 1.87b | 0.49 | 0.03 |
| 4 | 0.91 | 0.56 | 1.17 | 1.22 | 0.38 | 0.57 | |
1CT, control diet; LD, low dose diet containing 0.5% GNU100; MD, medium dose diet containing 1.0% GNU100; HD, high dose diet containing 1.5% GNU100.
2All genera with relative abundance >0.5% of total sequences are presented.
a–cWithin a row, means with different superscripts differ (P < 0.05). Bolded numbers are significant P-values (P < 0.05).
Serum chemistry profile and blood cell counts
Serum metabolites were unaltered by treatment (Supplementary Table S7). All metabolites were within reference ranges for all dogs except for blood urea nitrogen:creatinine ratio, which was slightly higher (P < 0.05) than the range in dogs fed MD at week 2 and CT and HD at week 4. Cholesterol also tended to be lower (P < 0.1) in dogs fed HD compared with dogs fed CT at week 26. Blood cell count values for all dogs were within reference ranges, and only percent of monocytes was (P < 0.05) increased in MD at week 26 (Supplementary Table S8).
Serum IgE, cytokine, and CRP
Serum concentrations of IgE and CRP were not altered by treatment (Supplementary Table S9). Serum interleukin 6 and tumor necrosis factor alpha concentrations were measured but not reported as values were below than the detectable value (minimum detectable range: 0.9 to 4.2 pg/mL for tumor necrosis factor alpha and 0.1 ng/mL for interleukin 6) using a commercial ELISA kit.
Discussion
The main objective of the present study was to evaluate the palatability, safety, and GI tolerance of GNU100 in healthy adult dogs. Moreover, GI functionality was explored. A battery of genotoxicity in vitro studies was conducted to determine the safety of GNU100 as a nutritional ingredient in acceptance with guidelines established by the European Food Safety Authority and the US Food and Drug Administration. GNU100 was not mutagenic in the bacterial reverse mutation test with or without metabolic activation by a rat liver microsomal fraction. Also, GNU100 was not cytotoxic and did not induce any chromosome damage or damage to the cell division apparatus in mammalian somatic cells in vitro with or without metabolic activation. These results are in line with previous genotoxicity studies on MO. For example, no evidence of genetic toxicity and chromosomal aberration was noticed for 3′-fucosyllactose (Pitt et al., 2019) or a mixture of 2′-fucosyllactose and difucosyllactose (Phipps et al., 2018), thus supporting the safe use of such MO as nutritional ingredients. Together with the results obtained from molecules with similar epitopes, our genotoxicity data support a lack of genotoxicity of GNU100.
The pet food industry has grown exponentially over the past couple of decades due to the increasing number of households that own companion animals and the preference for premium products. Palatability is one of the most important factors that pet food companies consider when including a new ingredient or producing a new dog food (Li et al., 2020). Evaluating food preference is necessary to determine whether the animals will be satisfied with the new products (Tobie et al., 2015; Alegría-Morán et al., 2019). The inherent animal characteristics, together with the sensorial and nutritional properties of the food, affect the dietary habits of dogs (Hall et al., 2017). In the current study, the inclusion of MD increased palatability. The high content of amino acids, peptides, and glycopeptides of GNU100 may explain the higher palatability or preference of this ingredient in dogs. In fact, a strong preference for protein-rich foods and diets coated with other protein-rich ingredients, including Saccharomyces cerevisiae fermentation product (Lin et al., 2019), is often reported.
Testing the GI tolerance and measures of general health (serum chemistry and hematology) of animals fed novel ingredients are also of importance. In the current study, all doses of GNU100 were well tolerated, with no signs of GI distress and desirable fecal scores, pH, and dry matter percentages being present in dogs fed all treatments. Based on the data of our previous in vitro fermentation study of GNU100 using fecal inoculum that suggested moderate fermentation rates that should not drastically affect fecal characteristics in vivo (Oba et al., 2020), this outcome was expected. All serum metabolites and blood cell counts were normal throughout the study, and measures of inflammation (serum CRP and fecal calprotectin) and allergy (serum IgE) were not affected by dietary treatment. These data demonstrate that GNU100 does not cause loose stools, intestinal inflammation, or immune or allergic reactions. Lastly, because nutrient digestibility was unaffected by GNU100 inclusion, these data suggest that the use of GNU100 will not affect nutrient digestibility when used up to 1.5% of the diet.
GNU100 is composed of a diverse and complex mixture of conjugated oligosaccharides, with epitopes similar to that present in AMO. Therefore, its capacity to modulate the canine microbiome was explored in this study. MO are nondigestible glycans with frequent sialylation and fucosylation that are key components of animal milk (Boehm and Stahl, 2007; Oliveira et al., 2015; Robinson, 2019). These complex glycans act as prebiotics, anti-adhesives, and anti-microbials and play critical roles in immune cell responses (McKeen et al., 2019). They are selectively utilized as substrates by beneficial gut bacteria and are fundamental to the establishment of a healthier microbiota and promote host health (McKeen et al., 2019; Robinson, 2019). In the current study, GI microbiota were modulated over time with several microbial taxa being changed at week 2 or week 4. Also, dogs fed MD at week 4 tended to have a higher evenness than at week 2, providing potential benefits to the animals, as a more diverse microbiome is more resistant to perturbations (Lozupone et al., 2012).
Previous studies have shown that nondigestible fibers and prebiotics such as FOS can modulate microbial communities, increasing the abundance of beneficial bacterial taxa in dogs (Garcia-Mazcorro et al., 2017; Redfern et al., 2017). Changes are not always demonstrated with prebiotics or fibers; however, as dogs fed fiber-prebiotic and saccharin–eugenol blend (Rentas et al., 2020) or the fructooligosaccharide kestose (Ide et al., 2020) did not have a different microbial diversity than those in the control group. In humans, Salli et al. (2019) reported a higher microbial diversity with 2′-fucosyllactose in a simulated infant gut microbiome environment in comparison with GOS and lactose. It has been proposed that differences in concentration, diversity, and complexity of glycans may affect the microbiome composition (McKeen et al., 2019). The complexity and diversity of GNU100 could explain the present results.
In the current study, fecal SCFA and BCFA were not affected by dietary treatments even though GNU100 was shown previously to be fermented and increase the production of these metabolites in vitro (Oba et al., 2020). Although fecal metabolites are typically measured, there are often discrepancies between the amount measured in feces and the actual amount produced by microbiota in the gut due to rapid absorption and metabolism of SCFA by colonocytes (von Englehardt et al., 1989). Hence, the measurement of digesta or circulating metabolites may be of interest in future studies (Müller et al., 2019). Microbiota results indicated that some SCFA-producing taxa were altered in the feces, but more research would be necessary to confirm these effects.
The relative abundance of the Catenibacterium was greater at week 2 in dogs fed HD and at 4 wk in dogs fed MD and HD. This genus is known to be a SCFA producer (Kageyama and Benno, 2000), with increased dietary fiber being correlated with greater Catenibacterium spp. in cats (Hooda et al., 2013; Yang et al., 2013) and dogs (Jarett et al., 2019). Another taxon, Collinsella, was lower in dogs fed LD compared with CT. Similar results have been reported in dogs after the oral administration of probiotics (Xu et al., 2019). In humans, the genus Collinsella has been shown to be enriched in irritable bowel syndrome patients (Masoodi et al., 2020) and decreased in patients on a weight-loss diet (Stephens et al., 2018) so a reduction appears to be a beneficial shift. In the current study, Megamonas was greater in dogs fed HD in comparison with the CT at week 2. Similar results have been observed in dogs supplemented with inulin (Beloshapka et al., 2013) or FOS (Hidaka et al., 2008). Megamonas is another SCFA (acetate and propionate) producer that has been negatively correlated with BW in dogs (Kieler et al., 2017). The relative abundance of Peptococcus and Prevotella tended to be higher in dogs fed GNU100, while the relative abundance of Anaerobiospirillum was variable. Prevotella is a well-known fiber fermentor and SCFA producer that has been associated with GI health (Minamoto et al., 2015; Schmidt et al., 2018; Pilla and Suchodolski, 2019), but Peptococcus and Anaerobiospirillum are not well studied in dogs. The promotion of SCFA producers is in line with a previous in vitro fermentation study that demonstrated the fermentation of GNU100 with the concomitant growth of beneficial bacteria and production of SCFA (Oba et al., 2020). Furthermore, O-glycans have been shown to act as a fermentation source to produce SCFA (Yamada et al., 2019; Hino et al., 2020).
This study had many strengths, including the length of the observational period and the number of measurements made. The main aim of this study was to test the safety of GNU100, so the experimental design (completely randomized design), length of study (6 mo), and animal numbers (n = 8/treatment) suggested by AAFCO guidelines were used. Another strength was that this study tested the effects of three GNU100 dosages against a control (0%) group. The study also had a few limitations. Because safety studies require a long intervention period, a completely randomized design was used. This design is ideal for safety testing, but likely limited our ability to observe differences in fecal microbiota and metabolites, which are highly variable among animals. A crossover or Latin square design, which would allow each animal to serve as their own control, likely would have reduced variation and increased statistical power. Also, because the AAFCO protocol requires the study of a healthy animal population, the ability to observe differences is more challenging than a clinical population.
In conclusion, GNU100 was shown to be palatable and its dietary supplementation over a long period of time (6 mo) was well tolerated and did not cause detrimental effects on stool quality or nutrient digestibility. Together with the in vitro genotoxicity results, the absence of negative effects on serum chemistry, hematology, and other health biomarkers in the in vivo study conducted with healthy dogs supports the safety of GNU100 for inclusion in canine diets. Finally, GNU100 supplementation favorably impacted the fecal microbiota, notably by promoting SCFA producers and tended to increase diversity. Overall, the results suggest potential benefits on GI health of dogs.
Supplementary Material
Acknowledgment
The funding for this study was provided by Gnubiotics Sciences SA, Epalinges, Switzerland.
Glossary
Abbreviations
- AMO
animal milk oligosaccharides
- ATTD
apparent total tract digestibility
- BCFA
branched-chain fatty acids
- BCS
body condition scores
- BW
body weight
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- FOS
fructooligosaccharides
- GI
gastrointestinal
- GOS
galactooligosaccharides
- HMO
human milk oligosaccharides
- IgA
immunoglobulin A
- IgE
immunoglobulin E
- MO
milk oligosaccharides
- PBS
phosphate-buffered saline
- PCA
principal component analysis
- SCFA
short-chain fatty acids
Conflict of interest statement
S.V., R.W., Y.M., and Y.A. are employed by Gnubiotics Sciences SA. K.S.S. has served as a paid consultant for Gnubiotics Sciences SA. The remaining two authors have no conflicts of interest.
Literature Cited
- AACC. 1983. Approved methods. In: AACC, editor. 8th ed. St Paul (MN): American Association of Cereal Chemists. [Google Scholar]
- Alegría-Morán, R. A., S. A. Guzmán-Pino, J. I. Egaña, C. Muñoz, and J. Figueroa. . 2019. Food preferences in dogs: effect of dietary composition and intrinsic variables on diet selection. Animals (Basel). 9:219. doi: 10.3390/ani9050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames, B. N., J. Mccann, and E. Yamasaki. . 1975. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 31:347–364. doi: 10.1016/0165-1161(75)90046-1 [DOI] [PubMed] [Google Scholar]
- AOAC 2002. Official methods of analysis of AOAC International. Gaithersburg (MD): Association of Official Analysis Chemists International. [Google Scholar]
- AOAC 2006. Official methods of analysis of AOAC International. 17th ed. Arlington (VA): Association of Official Analysis Chemists International. [Google Scholar]
- Asakuma, S., E. Hatakeyama, T. Urashima, E. Yoshida, T. Katayama, K. Yamamoto, H. Kumagai, H. Ashida, J. Hirose, and M. Kitaoka. . 2011. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 286:34583–34592. doi: 10.1074/jbc.M111.248138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of American Feed Control Officials (AAFCO) 2019. Official publication 2019. Oxford (IN): AAFCO. [Google Scholar]
- Beloshapka, A. N., S. E. Dowd, J. S. Suchodolski, J. M. Steiner, L. Duclos, and K. S. Swanson. . 2013. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol. Ecol. 84:532–541. doi: 10.1111/1574-6941.12081 [DOI] [PubMed] [Google Scholar]
- Bode, L 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22:1147–1162. doi: 10.1093/glycob/cws074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, L., N. Contractor, D. Barile, N. Pohl, A. R. Prudden, G. J. Boons, Y. S. Jin, and S. Jennewein. . 2016. Overcoming the limited availability of human milk oligosaccharides: challenges and opportunities for research and application. Nutr. Rev. 74:635–644. doi: 10.1093/nutrit/nuw025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode, L 2020. Human milk oligosaccharides: Structure and functions. In: Ogra, P. L., W. A. Walker, B. Lönnerdal, editors. Milk, mucosal immunity and the microbiome: impact on the neonate. Nestlé Nutr Inst Workshop Ser. Basel, Karger, 2020, vol 94, p. 115–123. doi: 10.1159/000505339 [DOI] [PubMed] [Google Scholar]
- Boehm, G., and B. Stahl. . 2007. Oligosaccharides from milk. J. Nutr. 137(3 Suppl 2):847S–849S. doi: 10.1093/jn/137.3.847S [DOI] [PubMed] [Google Scholar]
- Budde, E. F 1952. The determination of fat in baked biscuit type of dog foods. J. Assoc. Off. Agric. Chem. 35:799–805. doi: 10.1093/jaoac/35.3.799 [DOI] [Google Scholar]
- Chaney, A. L., and E. P. Marbach. . 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. doi: 10.1093/clinchem/8.2.130 [DOI] [PubMed] [Google Scholar]
- Comstock, S. S., M. Li, M. Wang, M. H. Monaco, T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, and S. M. Donovan. . 2017. Dietary human milk oligosaccharides but not prebiotic oligosaccharides increase circulating natural killer cell and mesenteric lymph node memory T cell populations in noninfected and rotavirus-infected neonatal piglets. J. Nutr. 147:1041–1047. doi: 10.3945/jn.116.243774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiwegger, T., B. Stahl, J. Schmitt, G. Boehm, M. Gerstmayr, J. Pichler, E. Dehlink, C. Loibichler, R. Urbanek, and Z. Szépfalusi. . 2004. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr. Res. 56:536–540. doi: 10.1203/01.PDR.0000139411.35619.B4 [DOI] [PubMed] [Google Scholar]
- von Englehardt, W., K. Rönnau, G. Rechkemmer, and T. Sakata. . 1989. Absorption of short-chain fatty acids and their role in the hindgut of monogastric animals. Anim. Feed Sci. Technol. 23:43–53. doi: 10.1016/0377-8401(89)90088-6 [DOI] [Google Scholar]
- Erwin, E. S. S., G. J. J. Marco, and E. M. M. Emery. . 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6 [DOI] [Google Scholar]
- Flickinger, E. A., E. M. Schreijen, A. R. Patil, H. S. Hussein, C. M. Grieshop, N. R. Merchen, and G. C. Fahey, Jr. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x [DOI] [PubMed] [Google Scholar]
- Garcia-Mazcorro, J. F., J. R. Barcenas-Walls, J. S. Suchodolski, and J. M. Steiner. . 2017. Molecular assessment of the fecal microbiota in healthy cats and dogs before and during supplementation with fructo-oligosaccharides (FOS) and inulin using high-throughput 454-pyrosequencing. PeerJ. 05:e3184. doi: 10.7717/peerj.3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, S. K., R. B. Hill, J. T. Sun, M. J. Armstrong, T. E. Johnson, J. P. Gara, and S. M. Galloway. . 2004. Population doubling: a simple and more accurate estimation of cell growth suppression in the in vitro assay for chromosomal aberrations that reduces irrelevant positive results. Environ. Mol. Mutagen. 43:36–44. doi: 10.1002/em.10207 [DOI] [PubMed] [Google Scholar]
- Hall, N. J., F. Péron, S. Cambou, L. Callejon, and C. D. L. Wynne. . 2017. Food and food-odor preferences in dogs: a pilot study. Chem. Sens. 42:361–370. doi: 10.1093/chemse/bjx016 [DOI] [Google Scholar]
- Hester, S. N., X. Chen, M. Li, M. H. Monaco, S. S. Comstock, T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, and S. M. Donovan. . 2013. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 110:1233–1242. doi: 10.1017/S0007114513000391 [DOI] [PubMed] [Google Scholar]
- Hidaka, H., T. Adachi, and M. Hirayama. . 2008. Development and beneficial effects of fructo-oligosaccharides (Neosugar). In: McCleary, B. V., and L. Prosky, editors. Advanced dietary fibre technology. Oxford (UK): Blackwell Science Ltd; p. 471–479. [Google Scholar]
- Hino, S., T. Mizushima, K. Kaneko, E. Kawai, T. Kondo, T. Genda, T. Yamada, K. Hase, N. Nishimura, and T. Morita. . 2020. Mucin-derived O-glycans act as endogenous fiber and sustain mucosal immune homeostasis via short-chain fatty acid production in rat cecum. J. Nutr. 150:2656–2665. doi: 10.1093/jn/nxaa097 [DOI] [PubMed] [Google Scholar]
- Hooda, S., B. M. Vester Boler, K. R. Kerr, S. E. Dowd, and K. S. Swanson. . 2013. The gut microbiome of kittens is affected by dietary protein: carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br. J. Nutr. 109:1637–1646. doi: 10.1017/S0007114512003479 [DOI] [PubMed] [Google Scholar]
- Hughes, K., P. G. Jones, C. Lebrilla, and D. J. Wrigglesworth. . 2020. Pet food products comprising oligosaccharides and methods of use WO2020033708A1. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020033708
- Ide, K., M. Shinohara, S. Yamagishi, A. Endo, K. Nishifuji, and T. Tochio. . 2020. Kestose supplementation exerts bifidogenic effect within fecal microbiota and increases fecal butyrate concentration in dogs. J. Vet. Med. Sci. 82:1–8. doi: 10.1292/jvms.19-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantscher-Krenn, E., and L. Bode. . 2012. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 64:83–99. [PubMed] [Google Scholar]
- Jarett, J. K., A. Carlson, M. R. Serao, J. Strickland, L. Serfilippi, and H. H. Ganz. . 2019. Diets with and without edible cricket support a similar level of diversity in the gut microbiome of dogs. PeerJ 7:e7661. doi: 10.7717/peerj.7661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama, A., and Y. Benno. . 2000. Catenibacterium mitsuokai gen. nov., sp. nov., a gram-positive anaerobic bacterium isolated from human faeces. Int. J. Syst. Evol. Microbiol. 50(Pt 4): 1595–1599. doi: 10.1099/00207713-50-4-1595 [DOI] [PubMed] [Google Scholar]
- Kavanaugh, D. W., J. O′Callaghan, L. F. Buttó, H. Slattery, J. Lane, M. Clyne, M. Kane, L. Joshi, and R. M. Hickey. . 2013. Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS One. 8:e67224. doi: 10.1371/journal.pone.0067224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieler, I. N., S. Shamzir Kamal, A. D. Vitger, D. S. Nielsen, C. Lauridsen, and C. R. Bjornvad. . 2017. Gut microbiota composition may relate to weight loss rate in obese pet dogs. Vet. Med. Sci. 3:252–262. doi: 10.1002/vms3.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata, A 2010. Structures and application of oligosaccharides in human milk. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 86:731–747. doi: 10.2183/pjab.86.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, C., M. Elderman, L. Cheng, B. J. de Haan, A. Nauta, and P. de Vos. . 2019. Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non-digestible carbohydrates. Mol. Nutr. Food Res. 63:e1900303. doi: 10.1002/mnfr.201900303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, C., S. Rudloff, W. Baier, N. Klein, and S. Strobel. . 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr. 20:699–722. doi: 10.1146/annurev.nutr.20.1.699 [DOI] [PubMed] [Google Scholar]
- Laflamme, D 1997. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract. 25:13–18. [Google Scholar]
- Li, H., R. Wyant, G. Aldrich, and K. Koppel. . 2020. Preference ranking procedure: method validation with dogs. Animals (Basel). 10:710. doi: 10.3390/ani10040710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. Y., C. Alexander, A. J. Steelman, C. M. Warzecha, M. R. C. de Godoy, and K. S. Swanson. . 2019. Effects of a Saccharomyces cerevisiae fermentation product on fecal characteristics, nutrient digestibility, fecal fermentative end-products, fecal microbial populations, immune function, and diet palatability in adult dogs. J. Anim. Sci. 97:1586–1599. doi: 10.1093/jas/skz064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio, R. G., M. R. Niñonuevo, S. R. Kronewitter, S. L. Freeman, J. B. German, C. B. Lebrilla, and D. A. Mills. . 2009. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb. Biotechnol. 2:333–342. doi: 10.1111/j.1751-7915.2008.00072.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone, C. A., J. I. Stombaugh, J. I. Gordon, J. K. Jansson, and R. Knight. . 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230. doi: 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias Rostami, S., T. Bénet, J. Spears, A. Reynolds, E. Satyaraj, N. Sprenger, and S. Austin. . 2014. Milk oligosaccharides over time of lactation from different dog breeds. PLoS One. 9:e99824. doi: 10.1371/journal.pone.0099824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey, C. F., C. A. Autran, L. Eckmann, and L. Bode. . 2014. Human milk oligosaccharides protect against enteropathogenic Escherichia coli attachment in vitro and EPEC colonization in suckling mice. J. Pediatr. Gastroenterol. Nutr. 58:165–168. doi: 10.1097/MPG.0000000000000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal, A., M. Barboza, E. D. Sonnenburg, N. Pudlo, E. C. Martens, P. Desai, C. B. Lebrilla, B. C. Weimer, D. A. Mills, J. B. German, . et al. 2011. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 10:507–514. doi: 10.1016/j.chom.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron, D. M., and B. N. Ames. . 1983. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 113:173–215. doi: 10.1016/0165-1161(83)90010-9 [DOI] [PubMed] [Google Scholar]
- Masoodi, I., A. S. Alshanqeeti, E. J. Alyamani, A. A. AlLehibi, A. N. Alqutub, K. N. Alsayari, and A. O. Alomair. . 2020. Microbial dysbiosis in irritable bowel syndrome: a single-center metagenomic study in Saudi Arabia. JGH Open 4: 649–655. doi: 10.1002/jgh3.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeen, S., W. Young, K. Fraser, N. C. Roy, and W. C. McNabb. . 2019. Glycan utilisation and function in the microbiome of weaning infants. Microorganisms 7:190. doi: 10.3390/microorganisms7070190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, B. M., E. Pujadas, and E. Gocke. . 1995. Evaluation of the micronucleus test in vitro using Chinese hamster cells: results of four chemicals weakly positive in the in vivo micronucleus test. Environ. Mol. Mutagen. 26:240–247. doi: 10.1002/em.2850260309 [DOI] [PubMed] [Google Scholar]
- Minamoto, Y., C. C. Otoni, S. M. Steelman, O. Büyükleblebici, J. M. Steiner, A. E. Jergens, and J. S. Suchodolski. . 2015. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 6:33–47. doi: 10.1080/19490976.2014.997612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, M., M. A. G. Hernández, G. H. Goossens, D. Reijnders, J. J. Holst, J. W. Jocken, H. van Eijk, E. E. Canfora, and E. E. Blaak. . 2019. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 9:12515. doi: 10.1038/s41598-019-48775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburg, D. S 2009. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci. 87(Suppl. 13):26–34. doi: 10.2527/jas.2008-1347 [DOI] [PubMed] [Google Scholar]
- Newburg, D. S., G. M. Ruiz-Palacios, and A. L. Morrow. . 2005. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553 [DOI] [PubMed] [Google Scholar]
- Oba, P. M., S. Vidal, R. Wyss, Y. Miao, Y. Adesokan, and K. S. Swanson. . 2020. Effect of a novel animal milk oligosaccharide biosimilar on the gut microbial communities and metabolites of in vitro incubations using feline and canine fecal inocula. J. Anim. Sci. 98:1–11. doi: 10.1093/jas/skaa273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD 1997. Test No. 471: Bacterial reverse mutation test, OECD Guidelines for the Testing of Chemicals, Section 4. Paris: OECD Publishing. doi: 10.1787/20745788 [DOI] [Google Scholar]
- OECD 2010. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4. Paris: OECD Publishing. doi: 10.1787/9789264264861-en [DOI] [Google Scholar]
- Oliveira, D. L., R. A. Wilbey, A. S. Grandison, and L. B. Roseiro. . 2015. Milk oligosaccharides: a review. Int. J. Dairy Technol. 68:305–321. doi: 10.1111/1471-0307.12209 [DOI] [Google Scholar]
- Phipps, K. R., N. Baldwin, B. Lynch, J. Flaxmer, A. Šoltésová, B. Gilby, M. H. Mikš, and C. H. Röhrige. . 2018. Safety evaluation of a mixture of the human-identical milk oligosaccharides 2′-fucosyllactose and difucosyllactose. Food Chem. Toxicol. 120:552–565. doi: 10.1016/j.fct.2018.07.054 [DOI] [PubMed] [Google Scholar]
- Pilla, R., and J. S. Suchodolski. . 2019. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 6:498. doi: 10.3389/fvets.2019.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt, J., M. Chan, C. Gibson, O. Hasselwander, A. Lim, P. Mukerji, R. Mukherjea, A. Myhre, P. Sarela, P. Tenning, . et al. 2019. Safety assessment of the biotechnologically produced human-identical milk oligosaccharide 3-Fucosyllactose (3-FL). Food Chem. Toxicol. 134:110818. doi: 10.1016/j.fct.2019.110818 [DOI] [PubMed] [Google Scholar]
- Plaza-Díaz, J., L. Fontana, and A. Gil. . 2018. Human milk oligosaccharides and immune system development. Nutrients 10:1038. doi: 10.3390/nu10081038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosky, L., N. G. Asp, I. Furda, J. W. DeVries, T. F. Schweizer, and B. F. Harland. . 1985. Determination of total dietary fiber in foods and food products: collaborative study. J. AOAC Int. 68:677–679. 10.1093/jaoac/68.4.677 [DOI] [PubMed] [Google Scholar]
- Pruss, K. M., A. Marcobal, A. M. Southwick, D. Dahan, S. A. Smits, J. A. Ferreyra, S. K. Higginbottom, E. D. Sonnenburg, P. C. Kashyap, B. Choudhury, . et al. 2021. Mucin-derived O-glycans supplemented to diet mitigate diverse microbiota perturbations. ISME J. 15:577–591. doi: 10.1038/s41396-020-00798-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern, A., J. Suchodolski, and A. Jergens. . 2017. Role of the gastrointestinal microbiota in small animal health and disease. Vet. Rec. 181:370. doi: 10.1136/vr.103826 [DOI] [PubMed] [Google Scholar]
- Rees, C. A., J. E. Bauer, W. J. Burkholder, R. A. Kennis, B. L. Dunbar, and K. E. Bigley. . 2001. Effects of dietary flax seed and sunflower seed supplementation on normal canine serum polyunsaturated fatty acids and skin and hair coat condition scores. Vet. Dermatol. 12:111–117. doi: 10.1046/j.1365-3164.2001.00234.x [DOI] [PubMed] [Google Scholar]
- Rentas, M. F., R. S. Pedreira, M. P. Perini, L. W. Risolia, R. V. A. Zafalon, I. C. Alvarenga, T. H. A. Vendramini, J. C. C. Balieiro, C. F. F. Pontieri, and M. A. Brunetto. . 2020. Galactoligosaccharide and a prebiotic blend improve colonic health and immunity of adult dogs. PLoS One. 15:e0238006. doi: 10.1371/journal.pone.0238006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, R. C 2019. Structures and metabolic properties of bovine milk oligosaccharides and their potential in the development of novel therapeutics. Front. Nutr. 6:50. doi: 10.3389/fnut.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios, G. M., L. E. Cervantes, P. Ramos, B. Chavez-Munguia, and D. S. Newburg. . 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278:14112–14120. doi: 10.1074/jbc.M207744200 [DOI] [PubMed] [Google Scholar]
- Salli, K., H. Anglenius, J. Hirvonen. . 2019. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci. Rep. 9:13232. doi: 10.1038/s41598-019-49497-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M., S. Unterer, J. S. Suchodolski, J. B. Honneffer, B. C. Guard, J. A. Lidbury, J. M. Steiner, J. Fritz, and P. Kölle. . 2018. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS One. 13:e0201279. doi: 10.1371/journal.pone.0201279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, R. W., L. Arhire, and M. Covasa. . 2018. Gut microbiota: from microorganisms to metabolic organ influencing obesity. Obesity (Silver Spring). 26:801–809. doi: 10.1002/oby.22179 [DOI] [PubMed] [Google Scholar]
- Thompson, C., P. Morley, D. Kirkland, and R. Proudlock. . 2005. Modified bacterial mutation test procedures for evaluation of peptides and amino acid-containing material. Mutagenesis 20:345–350. doi: 10.1093/mutage/gei045 [DOI] [PubMed] [Google Scholar]
- Thomson, P., D. A. Medina, and D. Garrido. . 2018. Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization. Food Microbiol. 75:37–46. 10.1016/j.fm.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Tobie, C., F. Péron, and C. Larose. . 2015. Assessing food preferences in dogs and cats: a review of the current methods. Animals (Basel). 5:126–137. doi: 10.3390/ani5010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilson, Å., Å. Hedhammar, A. Reynolds, J. Spears, E. Satyaraj, R. Pelker, C. Rottman, B. Björkstén, and H. Hansson-Hamlin. . 2016. Immunoglobulins in dogs: correspondence and maturation in 15 litters of German shepherd dogs and their dams. Vet. Rec. Open 3:e000173. doi: 10.1136/vetreco-2016-000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, R. E., M. Ninonuevo, D. A. Mills, C. B. Lebrilla, and J. B. German. . 2006. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl. Environ. Microbiol. 72:4497–4499. doi: 10.1128/AEM.02515-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigglesworth, D. J., E. Goonatilleke, R. Haydock, K. R. Hughes, C. B. Lebrilla, K. S. Swanson, P. Jones, and P. Watson. . 2020. High-throughput glycomic analyses reveal unique oligosaccharide profiles of canine and feline milk samples. PLoS ONE 15:e0243323. doi: 10.1371/journal.pone.0243323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H., W. Huang, Q. Hou, L.-Y. Kwok, W. Laga, Y. Wang, H. Ma, Z. Sun, and H. Zhang. . 2019. Oral administration of compound probiotics improved canine feed intake, weight gain, immunity and intestinal microbiota. Front. Immunol. 10:666. doi: 10.3389/fimmu.2019.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, T., S. Hino, H. Iijima, T. Genda, R. Aoki, R. Nagata, K. H. Han, M. Hirota, Y. Kinashi, H. Oguchi, . et al. 2019. Mucin O-glycans facilitate symbiosynthesis to maintain gut immune homeostasis. EBiomedicine 48:513–525. doi: 10.1016/j.ebiom.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., I. Martínez, J. Walter, A. Keshavarzian, and D. J. Rose. . 2013. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 23:74–81. doi: 10.1016/j.anaerobe.2013.06.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.