Abstract
Septins are a unique family of GTPases, which were discovered 50 years ago as essential genes for the asymmetric cell shape and division of budding yeast. Septins assemble into filamentous nonpolar polymers, which associate with distinct membrane macrodomains and subpopulations of actin filaments and microtubules. While structurally a cytoskeleton-like element, septins function predominantly as spatial regulators of protein localization and interactions. Septin scaffolds and barriers have provided a long-standing paradigm for the generation and maintenance of asymmetry in cell membranes. Septins also promote asymmetry by regulating the spatial organization of the actin and microtubule cytoskeleton, and biasing the directionality of membrane traffic. In this 50th anniversary perspective, we highlight how septins have conserved and adapted their roles as effectors of membrane and cytoplasmic asymmetry across fungi and animals. We conclude by outlining principles of septin function as a module of symmetry breaking, which alongside the monomeric small GTPases provides a core mechanism for the biogenesis of molecular asymmetry and cell polarity.
Fifty years ago, Nobel laureate Lee Hartwell published the first three genes from his pioneering screen for mutants that alter the cell division cycle (cdc) of the budding yeast Saccharomyces cerevisiae (Hartwell et al., 1970). Among them, cdc3 and subsequently cdc10, cdc11, and cdc12 were all reported to develop multiple elongated buds, failing to undergo cytokinesis (Hartwell et al., 1970; Hartwell, 1971). Electron microscopy observations indicated that the products of these genes formed a filamentous network at the mother–bud cortex (Byers and Goetsch, 1976). Further characterization and cloning of these genes by John Pringle, who named them septins, marked the birth of a new class of GTP-binding proteins with important functions in the spatial organization of eukaryotic cells (Pringle, 2008).
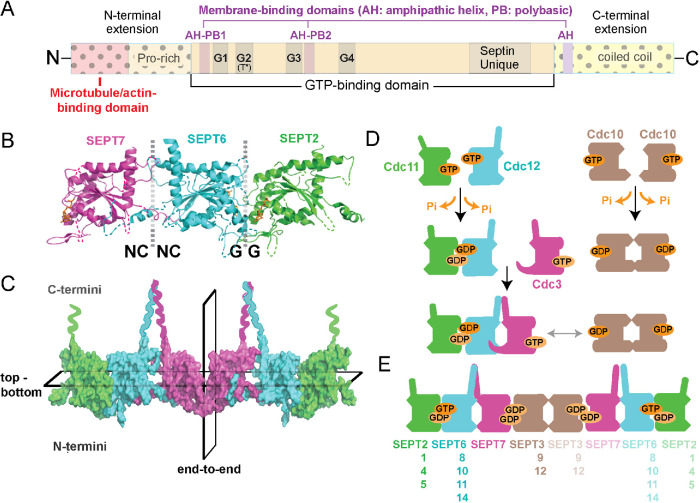
Septins comprise a family of paralogous genes, which arose early in eukaryotic evolution and expanded in fungi and animals, while largely absent from plants (Pan et al., 2007). In mice and humans, 13 septin genes express a diversity of paralogues and isoforms, which are classified under four groups (SEPT2, SEPT6, SEPT7, and SEPT3) and consist of a conserved core GTP-binding domain with variable N- and C-terminal extensions (Figure 1A; Kinoshita, 2003; Mostowy and Cossart, 2012). Opening a new era for the septin field, the x-ray crystal structure of the mammalian SEPT2/6/7 complex revealed that septins dimerize in tandem via their GTP-binding domains by utilizing two different binding interfaces, which alternate every other monomer (Sirajuddin et al., 2007). Through a serial head-to-head and tail-to-tail binding mode, septins assemble linearly into nonpolar oligomers and polymers (Figure 1B). The SEPT2/6/7 structure suggested that the minimal septin unit is a palindromic hexamer, in which SEPT2/6/7 trimers are arranged symmetrically from a central homodimeric SEPT2-SEPT2 interface (Sirajuddin et al., 2007). New evidence, however, shows that SEPT2/6/7 assembles into a hexamer with SEPT7, or SEPT9 in the case of the hetero-octameric SEPT2/6/7/9 complex, forming the central homodimeric contact (McMurray and Thorner, 2019; Mendonca et al., 2019; Soroor et al., 2019). This order is consistent with the arrangement of the budding yeast hetero-octamer (Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11) with Cdc10, the yeast septin most homologous to SEPT9, being the central homodimer (Bertin et al., 2008; McMurray and Thorner, 2019). In contrast to the symmetric end-to-end arrangement of their subunits, septin multimers are characterized by a top–bottom asymmetry with their C- and N-terminal extensions extending orthogonally toward opposite sides of the linear axis of multimerization (Figure 1C; John et al., 2007; Sirajuddin et al., 2007; Bertin et al., 2008). Although it is not functionally understood, this asymmetry may underlie an anisotropic mode of interaction with cellular components and protein complexes, which could be further modulated by the identity and combination of septin subunits.
FIGURE 1:
Structure and assembly of septin GTPases. (A) Schematic shows the main domains of septin GTPases, which consist of a highly conserved GTP-binding domain with G1 (GxxxxGK[ST]), G3 (DxxG), and G4 (AKAD) motifs, and a septin unique element. Septins contain a catalytic threonine residue (T*), which corresponds to the Thr of the G2 motif of Ras GTPases and is absent from septin paralogues that lack GTPase activity. The N- and C-terminal extensions of the septin GTP-binding domain vary in sequence and length, and contain proline-rich and coiled-coil domains, respectively. Domains of cytoskeleton- and membrane-binding (amphipathic helices and polybasic sequences) are denoted and differ among septin paralogues. (B, C) The x-ray crystal structure of SEPT2/6/7 (PDB: 2qag; B) shows the NC-NC and G-G interfaces of dimerization, which alternate between the GTP-binding domains of consecutive septin subunits. The C- and N-terminal ends of the G-domains are located at the top and bottom of the dotted vertical lines, respectively, and the guanine nucleotide is depicted in orange. A surface representation of the SEPT2/6/7 heterohexamer (C) shows the symmetric (nonpolar) end-to-end arrangement of septin paralogues from the central homodimeric SEPT7 interface and the asymmetric positioning of the C- and N-terminal ends across the horizontal plane of multimerization. (D) Schematic of the assembly of yeast septin complexes, which is driven by successive events of homo- and heterodimerization that are determined by GTP binding and hydrolysis. (E) Schematic shows the subunit order and identity of mammalian septin hetero-octamers, and the interchangeability of paralogue subunits of the same septin group (Kinoshita rule).
Septins are structurally and phylogenetically related to the small GTPases of the Ras superfamily (e.g., Ras, Rho, Rab; Leipe et al., 2002), but most septins hydrolyze and turn over GTP slowly and there are septin paralogues (e.g., SEPT6 group) that lack GTPase activity (Vrabioiu et al., 2004; Sirajuddin et al., 2009; Zent and Wittinghofer, 2014; Abbey et al., 2019). Growing evidence indicates that GTP-binding and hydrolysis stabilize the dimeric interface, determine the order of assembly by selecting for specific dimerization partners, and induce allosteric conformational changes, which could affect the assembly and localization of septin complexes (Figure 1D; Sirajuddin et al., 2009; Zent and Wittinghofer, 2014; Weems and McMurray, 2017; Castro et al., 2020). Based on the activity and structure of their GTPase domains, paralogues of the same group occupy and exchange within the same position of the septin complex—a posit known as the Kinoshita rule (Figure 1E; Kinoshita, 2003). However, high-order septin complexes of homomeric and alternative compositions have also been reported (Mendoza et al., 2002; Mizutani et al., 2013; Sellin et al., 2014; Karasmanis et al., 2018). Higher-order septin structures exhibit slow subunit turnover and thereby persist in place longer than dynamic cytoskeletal polymers (Hu et al., 2008; Hagiwara et al., 2011; Bridges et al., 2014). Hitherto, in the absence of any known septin guanine nucleotide activating and exchange factors (GTPase-activating proteins [GAPs]/ guanine nucleotide exchange factor [GEFs]), septin dynamics and turnover are modulated by posttranslational modifications (Hernandez-Rodriguez and Momany, 2012; Ribet et al., 2017).
Septins function broadly as scaffolds and barriers that recruit and exclude proteins, respectively, controlling the localization and interactions of membrane and cytoskeletal proteins (Caudron and Barral, 2009; Mostowy and Cossart, 2012; Spiliotis, 2018). This fundamental property of septins has been adopted by a diversity of molecular mechanisms and cellular processes. In the face of an ever-growing number of biological roles, it is often overlooked that septins function primarily as a module of spatial organization. After 50 years of research, we recount here how septins promote cellular asymmetries in fungi and animals, highlighting the evolutionary adaptation of septins as effectors of asymmetry from fungal cell membranes to the mammalian cytoskeleton.
Septin roles in fungal cell asymmetry
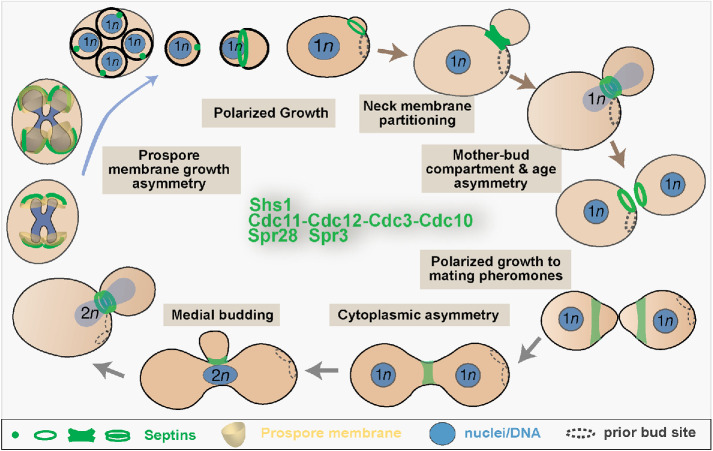
Septins perform essential functions in nearly all aspects of the asymmetric growth and division of budding yeast (Figure 2). Prior to budding, a regulatory interplay between septins and the Rho family small GTPase Cdc42 enables yeast cells to polarize growth at a single site. GTP-bound Cdc42 initially recruits septins to a cloudy patch (Gladfelter et al., 2002), where a transient direct interaction between the septin Cdc11 and Cdc24, a GEF for Cdc42, creates a short-term positive feedback loop to reinforce septin recruitment and Cdc42 activation (Chollet et al., 2020). Targeted delivery of Cdc42-containing but septin-free vesicles to the center of the septin patch transforms it into a ring (Gladfelter et al., 2002; Okada et al., 2013). Septin polymerization into circular filaments is thought to render Cdc11 inaccessible to Cdc24 (Chollet et al., 2020). Concomitantly, septins begin to recruit Cdc42 GAPs, which inhibit Cdc42, and they promote the activation of formins, which drive actin cable polymerization (Buttery et al., 2012; Okada et al., 2013). As actin cables direct more vesicles to the center of the septin ring, the nascent bud grows and concomitantly Cdc42 pushes away from septins and localizes to the bud tip, where it promotes further growth (Okada et al., 2013). Hence, septins reinforce the formation of a Cdc42 polar cap during bud emergence. During bud growth, septins pattern the localization of the actin- and formin-binding protein Hof1 along the bud cortex in a manner that aligns actin cables along the mother–bud axis, which is critical for the asymmetric growth of the bud (Garabedian et al., 2020). Septin-mutant cells lose the mother- or bud-specific asymmetric localization of a number of cortical proteins as well as mother–bud asymmetry of actin patch stability and overall cell growth (Barral et al., 2000), though the mechanistic details of how septins control these asymmetries in wild-type cells are unclear.
FIGURE 2:
Septin localization and function in the asymmetric growth and division of the budding yeast S. cerevisiae. The schematic shows in a clockwise order the different stages of the lifecycle of vegetative haploid and diploid, and sporulating S. cerevisiae cells. The localization and organization of septin complexes in vegetative (Cdc11/Shs1-Cdc12-Cdc3-Cdc10) and sporulating (Spr28-Spr3-Cdc3-Cdc10) cells are depicted as single or double rings, an hourglass-shaped collar, and dots. Shaded boxes highlight septin functions in polarized membrane growth and the asymmetric partitioning and inheritance of membrane and cytoplasmic components in different stages of the budding yeast lifecycle.
At the mother–bud neck, the septin ring expands into an hourglass collar and splits into two rings before cytokinesis (Figure 2). The hourglass structure provides a scaffold for the assembly of the actomyosin ring (Bi et al., 1998; Schneider et al., 2013) and the asymmetric distribution of many septin interactors, which localize to the mother or bud side of the neck and in between (Gladfelter et al., 2001; McMurray and Thorner, 2009). On the mother side, septins recruit chitin synthase for the generation of the bud scar, whose underlying membrane provides a landmark and spatial memory for the budding site of the next cell cycle (DeMarini et al., 1997; Kozubowski et al., 2005). On the bud side, septins scaffold kinases of the morphogenesis checkpoint, which monitors proper bud growth for entry into mitosis (Barral et al., 1999; Shulewitz et al., 1999; Theesfeld et al., 2003). At the interface of the mother–bud membranes, the double septin ring acts as a barrier to prevent the exocyst complex and membrane remodeling factors from diffusing out of the gap between them, delimiting the site of membrane growth that occurs in late cytokinesis (Dobbelaere and Barral, 2004).
Septin barriers appear to be dispensable for cytokinesis (Wloka et al., 2011), but they are essential for mother–daughter asymmetry in organelle content and age. Septins compartmentalize the endoplasmic reticulum (ER) associated with the plasma membrane of the bud neck (Luedeke et al., 2005). The septin ring excludes ribosomes, creating smooth ER at the bud neck (Luedeke et al., 2005). Moreover, as the cortical ER moves into the growing bud, the septin ring protects the bud from inheriting ER membranes with aggregates of misfolded proteins (Clay et al., 2014). Septin-ring–dependent enrichment of sphingolipids in bud neck membranes is required for the diffusion barrier, but it is unknown how septins may control sphingolipid localization (Clay et al., 2014). Septins also act as a sphingolipid-dependent barrier for the diffusion of nuclear pore complexes (NPCs) from the mother to bud ER membranes, which are continuous with the nuclear envelope (NE; Shcheprova et al., 2008). Lack of NPC diffusion correlated with the asymmetric retention of nonchromosomal DNA circles within the mother compartment, which reduces replicative span (Shcheprova et al., 2008). However, the shape of the dividing nucleus and length of mitosis were also proposed to restrict the diffusion of DNA circles, which entered the bud upon artificial tethering to nuclear pores (Gehlen et al., 2011; Khmelinskii et al., 2011). Reconciling these findings, subsequent work showed that in aged cells DNA circles are coupled to only a subset of NPCs by the Spt-Ada-Gcn5 acetyltransferase (SAGA) complex, hindering diffusion into the bud beyond the constraints imposed by the nuclear shape (Denoth-Lippuner et al., 2014). Thus, the septin-dependent ER/NE diffusion barrier plays a fundamental role in yeast aging.
Polarized budding yeast growth occurs not only intrinsically, taking place adjacent to or opposite the site of the preceding cell division, but also in response to mating pheromones, which override intrinsic signals. Septins encircle polarity factors at the polar cap of the shmoo-shaped tips that grow toward the pheromone gradient, and septin mutants are defective in pheromone tracking and polarized morphogenesis (Kelley et al., 2015). Upon mating, a distinct septin structure at the site of cell fusion limits diffusion of organelles and large cytoplasmic complexes between the mating partners, which promotes the asymmetrical inheritance of mitochondrial DNA by diploid buds (Tartakoff et al., 2013).
Septins are similarly required for the polarized membrane growth that occurs during sporulation (Onishi et al., 2010; Heasley and McMurray, 2016). Spore membrane biogenesis is like inside-out budding as new membranes and cell walls form around the four haploid nuclei, which result from the meiosis of a diploid cell. Each membrane emerges from a single point, the pole of a postmeiosis II spindle, and grows outward as a cup before closing at another single point to form a sphere (Figure 2). Septins localize to bar- and horseshoe-shaped structures along the growing membranes. In septin mutants the membranes grow in the wrong directions and often fail to close (Onishi et al., 2010; Heasley and McMurray, 2016). After their formation, spores bud in a prepolarized manner (Joseph-Strauss et al., 2007). Strikingly, a septin spot is the only known polarity marker that demarcates the site of new membrane and wall synthesis. A septin ring of unknown function encircles the site of membrane outgrowth before a new septin ring forms where the spore first buds, completing the S. cerevisiae life cycle (Joseph-Strauss et al., 2007).
While budding yeasts have taught us much about septins and asymmetry, we have learned many fundamental lessons from other fungi. In the fission yeast Schizosaccharomyces pombe, septins act as scaffolds and/or diffusion barriers to guide the orderly recruitment of the cytokinetic machinery to the division site, which occurs at the equatorial plane of the cell. Septin mutants undergo cytokinesis, but fail in cell separation due to defects in recruiting the cell wall degrading enzymes (Zheng et al., 2018). In filamentous fungi, multiple distinct septin-based structures are found in a single cell, and function in limiting the polarity sites and branching of hyphae (Khan et al., 2015; Momany and Talbot, 2017). In Ashbya gossypii, septins form rings and lateral bundles, and localize to the membrane curvature of hyphal branch points, which depends on a C-terminal amphipathic helix (Cannon et al., 2019). Notably, septin recognition of micron-scale membrane curvature is a conserved property of septins from fungi to animals (Bridges et al., 2016). The pathogenic rice blast fungus Magnaporthe oryzae undergoes polarized growth to develop a specialized structure called the appressorium, which contacts the plant surface and uses extreme pressure to penetrate the host (Momany and Talbot, 2017). Here, a septin ring acts as a diffusion barrier to retain actin-polymerizing proteins, and scaffolds actin assembly and the localization of the exocyst complex (Dagdas et al., 2012; Gupta et al., 2015). Overall, fungi have provided compelling systems to explore septin assembly and functions in cell asymmetry.
Septins promote asymmetry in the plasma membrane of animal cells
Studies of septins in animal cells have revealed a remarkable conservation with their fungal counterparts in promoting cell asymmetry. Septins are required for plasma membrane protrusions and compartments (e.g., uropodia, filopodia, cilia) that underlie the morphogenesis of distinct cell types and tissues and arise from local membrane asymmetries, microdomains of distinct protein enrichment (Caudron and Barral, 2009; Tooley et al., 2009; Hu et al., 2010, 2012; Dolat et al., 2014a). Homozygous deletions of several septin genes in mice cause early embryonic lethality and male sterility, demonstrating how essential septins are for development and physiology (Dolat et al., 2014a).
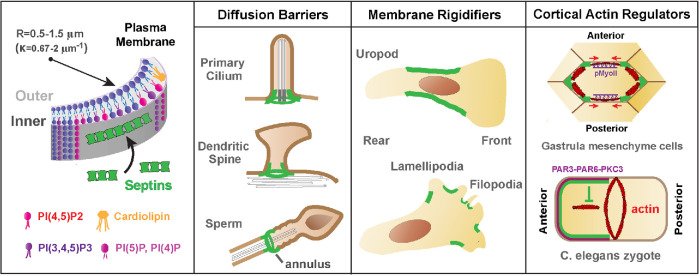
On the plasma membrane of animal cells, septin assembly and localization involves signaling cues and selective binding to microdomains with distinct phospholipid content and curvature (Figure 3). The signaling pathways that instruct the membrane sites of septin assembly in multicellular organisms are not as well studied as in fungi. The WD repeat-containing planar polarity effector and the PAR complex have been implicated in the localization of membrane septins (Cui et al., 2013; Park et al., 2015; Jordan et al., 2016). Septins have intrinsic preferences for select phospholipids including phosphoinositides (e.g, phosphatidylinositol 4,5-bisphosphate) and cardiolipin, a membrane curvature–specific lipid (Zhang et al., 1999; Krokowski et al., 2018). Taken together with a strong affinity for membrane micron-scale curvatures that is mediated by amphipathic helices (Bridges et al., 2016; Cannon et al., 2019), septins become enriched on the inner saddle-like areas of the plasma membrane, outlining the base-neck border of protrusions such as filopodia, lamellipodia, and dendritic spines (Figure 3).
FIGURE 3:
Septins associate with cell membranes and function in plasma membrane asymmetry. Septins associate preferentially with membrane domains of micron-scale curvature with a radius (R) of ∼0.5–1.5 μm or curvature parameter (k) of ∼0.67–2 μm−1. In addition, septins bind preferentially phosphoinositides and cardiolipin, a cone-shaped lipid that localizes in curved membrane domains. Septins promote asymmetry by acting as barriers of lateral diffusion, membrane rigidifiers, and regulators of the organization and dynamics of cortical actin. Septins are essential components of diffusion barriers at the base of primary cilia, dendritic spines, and the midpiece of spermatozoa, where the annulus structure is located. By rigidifying specific subdomains of the plasma membrane, septins delimit areas of membrane activity, which is critical for the position of membrane protrusions in the rear (uropodia) and front (lamellipodia, filopodia) of migrating cells. In gastrulating frog embryos and C. elegans zygotes, septins locally regulate the organization and dynamics of cortical actin, which is critical for the asymmetric contraction of actomyosin that underlies convergent extension and cytokinesis.
Septins promote asymmetry by restricting lateral diffusion, enhancing membrane rigidity and spatially regulating membrane–cytoskeleton interactions. Septins are required for the maintenance of diffusion barriers at the base of primary cilia, dendritic spines, and the midpiece of spermatozoa (Hu et al., 2010; Kwitny et al., 2010; Ewers et al., 2014). Diffusion of transmembrane and inner leaflet-anchored proteins across the midbody of dividing cells may also be impeded by septins (Schmidt and Nichols, 2004). It is unclear whether septin filaments directly impede lateral diffusion or whether they are an essential component of a larger actin–spectrin skeleton with barrier properties. Immuno-EM studies indicate that septins are indeed part of the membrane actin skeleton (Hagiwara et al., 2011). Moreover, in the axon initial segment, which functions as a diffusion barrier, septins interact with ankyrin G (Hamdan et al., 2020). Septins have also been proposed to rigidify the plasma membrane, suppressing cortical protrusions and blebs, which are asymmetrically confined to septin-free membrane areas (Gilden and Krummel, 2010). For example, T-cell uropods are delimited by a septin corset-like arrangement, which braces the perinuclear cortex and enables the front–back polarity of migrating T-cells (Gilden et al., 2012).
Membrane septins bend actin filaments into circular arrays and scaffold the localization and activation of myosin-II, and thus they can promote asymmetry in membranes by locally regulating the cortical actomyosin (Joo et al., 2007; Mavrakis et al., 2014). In gastrulating frog embryos, septins localize to the vertices of mediolateral cell–cell contacts, where they stabilize actin and maintain the planar polarity of phosphorylated myosin along the anteroposterior contacts (Shindo and Wallingford, 2014). Thereby, actomyosin contraction is spatially restricted along the anteroposterior junctions, which promotes convergent extension by pulling mediolateral contacts closer to one another (Figure 3). In Caenorhabditis elegans embryos, septins polarize to the anterior pole away from the contractile actomyosin ring, inhibiting actin assembly outside the plane of cytokinesis (Jordan et al., 2016). Moreover, C. elegans septins are required for the asymmetric ingression of the cleavage furrow, which progresses directionally with a dorsal-to-ventral orientation (Maddox et al., 2007). Independently of their roles in cytokinesis, cortical septins provide spatial cues for the orientation of the axis of division in Drosophila larval neuroblasts, which is determined by the position of the last-born daughter cell (Loyer and Januschke, 2018). Notably, in neuronal crest cells, which generate dorsal root ganglia neurons after cell division, cortical septins provide a spatial memory for the outgrowth of axons from premitotic sites of membrane protrusions (Boubakar et al., 2017).
In sum, septins associate preferentially with micrometer-scale membrane domains of negative Gaussian curvature and distinct phospholipid content. While it is unclear how they impede the lateral diffusion of proteins and lipids, septins promote macroscale asymmetries by enabling molecular partitioning across adjacent membrane compartments. At the nexus, septins also facilitate asymmetries of submicrometer scale by clustering membrane proteins or lipids. For example, septins are required for the organization of PI(4,5)P2 into clusters that surround the calcium release-activated calcium channel ORAI1 at plasma membrane–ER junctions (Sharma et al., 2013). How septins interact with and organize on membrane bilayers, and how they modulate the mobility and distribution of plasma membrane proteins and lipids are key questions. As septins associate with the membranes of various intracellular organelles, it is also unclear how septins function in the generation and/or maintenance of organelle membrane domains and membrane–membrane contacts (Akil et al., 2016; Dolat and Spiliotis, 2016; Pagliuso et al., 2016; Sirianni et al., 2016; Song et al., 2019).
Septin roles in cytoskeleton-based mechanisms of cell asymmetry
Cell asymmetry requires the assembly of local microtubule and actin networks with unique organization and dynamics. Due to their structural polarity and dynamic growth, microtubules and actin filaments can spatially bias membrane traffic and determine the location, shape, and/or directionality of membrane protrusions and adhesions. Hence, microtubules and actin filaments are keys for morphing cells into asymmetric shapes.
Septins localize to subsets of microtubules and actin filaments, and promote cell asymmetry by regulating the spatial organization of the cytoskeleton and membrane traffic (Figure 4). In a diversity of mammalian cell types, septins colocalize with microtubules and actin filaments of the perinuclear and peripheral cytoplasm (Spiliotis, 2018). It is not well understood how this region-specific association is established, but it may involve effectors of Rho signaling and depend on the composition (subunit isotypes) and posttranslational modifications of microtubules and actin filaments. Alternatively, cytoskeletal sites of binding might be indirectly determined by septin binding and turnover on proximal membranes. Thus, septin roles in cytoskeleton-based mechanisms of cell asymmetry are not mutually exclusive of their functions in membrane asymmetry, which feeds back locally to the cytoskeleton.
FIGURE 4:
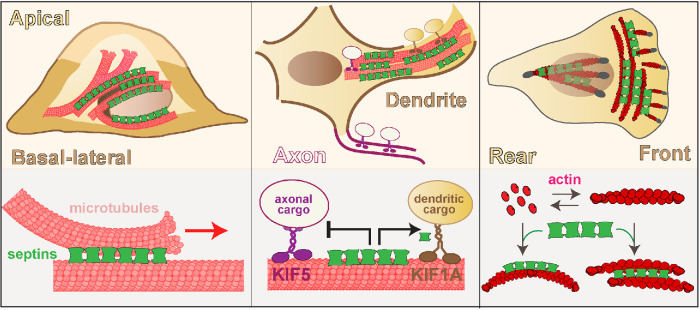
Septins roles in cytoskeleton-based mechanisms of apicobasal, axonodendritic, and front–rear polarity. Microtubule-associated septins guide microtubule organization and membrane traffic along the apicobasal axis of polarizing epithelia. In neuronal dendrites, septin 9 reinforces the polarity of neuronal membrane traffic by impeding the transport of axonal cargo of kinesin-1/KIF5 and enhancing the anterograde movement of dendritic cargo of kinesin-3/KIF1A. In cells undergoing epithelial-to-mesenchymal transition, septins associate with the actin stress fibers of the leading lamellae and promote the asymmetric organization of the actin network and focal adhesion turnover, which is critical for the front–rear polarity of cell migration.
In epithelia and neurons, microtubule-associated septins are functionally important for apicobasal and axon–dendrite polarity (Spiliotis et al., 2008; Bowen et al., 2011; Karasmanis et al., 2018). In polarizing epithelia, septins associate with subsets of microtubules, steering microtubule plus end growth and microtubule–microtubule interactions (Bowen et al., 2011). Hence, septins provide a navigation mechanism for microtubule organization along the developing apicobasal axis of polarity. In addition, microtubule-associated septins are required for Golgi-to-plasma membrane transport of vesicles with apical and basolateral membrane proteins (Spiliotis et al., 2008). Septin depletion abrogates exocytic membrane traffic, disrupting the growth and differentiation of the epithelial cell membrane into apical and basolateral domains (Spiliotis et al., 2008). In fully polarized epithelia, it is unknown whether specific septin paralogues and complexes function exclusively in the apical or basolateral routes of membrane traffic. In hippocampal neurons, however, septin 9 localizes to dendritic microtubules, reinforcing the polarity of membrane traffic by hindering the transport of axonal cargo of kinesin-1/KIF5 and promoting the anterograde movement of dendritic cargo of kinesin-3/KIF1A (Karasmanis et al., 2018). This differential regulation of kinesin-driven transport is critical for axon–dendrite polarity, and in principle may constitute a general mechanism by which septins polarize membrane traffic.
Growing evidence indicates that septins function in the mechanisms of mechanotransduction that induce and sustain front–rear polarity in migrating cells (Lam and Calvo, 2019). Cells migrate toward stiffer extracellular matrices and chemoattractants by forming a leading protrusive front and retracting rear. Front–rear polarity is largely supported by an asymmetry in the organization of the actomyosin stress fibers and the turnover of focal adhesions. Septins colocalize with actin stress fibers in a diversity of cell types, and alterations in septin expression impair directional migration. In cells undergoing epithelial-to-mesenchymal transition, septins are enriched in the leading lamella localizing at the interphase of a contractile network of curved actin stress fibers (transverse arc stress fibers) and the radial stress fibers, which are anchored to focal adhesions (Dolat et al., 2014b). Septin depletion collapses the transverse arc actin network with concomitant loss of stability and front-to-rear maturation of focal adhesions. Taken together with loss of directional cell migration, this phenotype underscores a critical role of septins in the front–rear asymmetry of cell migration. In cancer-associated fibroblasts, septins function together with the Cdc42 effector Cdc42EP3 in actin organization in a mechanosensitive manner, responding to stiffer matrices via association with perinuclear actin stress fibers (Calvo et al., 2015). Of note, septins may function in nuclear mechanotransduction, nuclear movement, and/or the regulation of subnuclear focal adhesions (Verdier-Pinard et al., 2017; Lam and Calvo, 2019). In parallel with their functions in the actin cytoskeleton, septins also likely contribute to the front–rear asymmetry of migrating cells by spatially regulating membrane–microtubule interactions (Shindo et al., 2018).
Septin association with subsets of actin filaments and microtubules is a salient characteristic that enables local regulation of cytoskeleton-based processes, a key functionality in the generation of cellular asymmetries. Progress in understanding how septins interact with actin and microtubules, and how they mechanistically impact cytoskeletal dynamics has been slow. High-resolution cryoelectron microscopy studies of septins in complex with actin and microtubules are necessary to provide structural insights into how septins modulate cytoskeletal dynamics and interactions with actin-binding and microtubule-associated proteins. Septins have yet to be studied in the context of the basic mechanisms of actin nucleation and polymerization, which can have important implications for actin assembly and symmetry breaking at membrane sites of septin enrichment. Advances in these areas and better understanding of the signaling pathways that determine the cytoskeletal locales of septin function will provide a fuller picture of septins as cytoskeletal agents of cell asymmetry.
Principles and unknowns of septin function as a symmetry-breaking module
The spatial organization of eukaryotic cells requires microscale asymmetries, which are established and maintained in a region-specific manner, and at the macroscale level promote the polarization of cellular shapes and processes. It is little understood how these asymmetries arise amidst membrane and cytoplasmic fluidity. Part small GTPase-like regulators and part cytoskeleton-like polymers, septins are inherently tailored to break symmetry by facilitating protein localization and interactions that persist in place and time. In summary of past and recent findings, there are four major principles of septin function as a symmetry-breaking module:
Septins are spatio-specific and modular. Septins assemble on select intracellular regions (e.g., micron-scale membrane curvatures, perinuclear microtubule bundles, peripheral transverse arc stress fibers). Septin assembly is modular, consisting of alternative combinations of paralogue and isoform subunits, which in turn determine intracellular localization and interactions.
Septins form higher-order structures, which turn over slowly and thereby persist spatially and temporally. Thus, septins can serve as imprints or landmarks of previous structures and molecular events, providing a type of spatial memory.
Septins scaffold protein localization and interactions. Septin scaffolds induce molecular asymmetries by recruiting and clustering protein interactors, and facilitating the assembly of macromolecular complexes.
Septins partition proteins in distinct membrane or cytoskeletal domains. Septins exclude proteins locally by impeding lateral diffusion or binding.
While most evident in membrane septins, these principles also apply to septins that associate with actin and microtubules and the membrane–cytoskeleton interface. On microtubule lattices, where many microtubule-associated proteins (MAPs) undergo diffusion through transient electrostatic interactions, septins selectively inhibit or promote microtubule binding of specific MAPs and kinesin motors (Spiliotis et al., 2008; Karasmanis et al., 2018). Similar modulation is likely to take place on actin filaments, where septins could specify domains of unique actin-binding protein composition promoting the formation of actin networks of distinct architecture and dynamics. A region-specific control of cytoskeletal organization may also occur by membrane-associated septins sequestering actin-nucleating promoting factors or directly interacting with the dynamic ends of actin filament or microtubules (Dagdas et al., 2012; Hu et al., 2012; Nolke et al., 2016; Nakos et al., 2019). In the cytoplasm, a templated assembly of septins along subpopulations of microtubules and actin filaments may provide a form of cytoskeletal memory as previously proposed for vimentin intermediate filaments (Gan et al., 2016). By outlasting their shorter-lived templates, septins are likely to provide cytoplasmic landmarks for orienting microtubule and actin growth along previously held patterns of spatial organization. In polarized cell types, this region-specific patterning would be critical for the continuous organization of cytoskeletal networks along the axis of cell polarity.
Despite much progress over the last two decades, we have merely begun discovering septins as a core mechanism for the spatial organization of cell biology. Many unknowns remain. The signaling inputs that interface with the assembly of mammalian septins are virtually unknown. The spatio-specificity of septin assembly on subnetworks and regions of the cytoskeleton is poorly understood. Septin modularity is little explored, and more work is needed to determine how different combinations of septin paralogues and isoforms bestow alternative localizations and properties. Deciphering this septin code is critical for determining which septin complexes do what, and whether certain septin paralogues can function as homomers independently of the canonical model of heteromeric assembly. In light of GTP hydrolysis favoring septin homodimerization and the recent reordering of the SEPT2/6/7/9 hetero-octamer, it is plausible that paralogues with faster GTPase activities (e.g., SEPT9) and in excess of their cognate partners evade heteromerization into typical complexes. More studies are needed in cell types and disease states, in which certain septin paralogues or isoforms are disproportionally expressed and may take unique or pathogenic functions. Similarly, studies of septins in polarized cell types and stem cell systems, where asymmetry is key for cell fate and renewal, are lacking, and so is our understanding of septin functions in cell regeneration and repair. With many more open questions than answers, the next 50 years of septin research promises important breakthroughs in our basic understanding of cellular asymmetry and potentially the development of septin-based therapies in regenerative medicine and beyond.
Acknowledgments
We thank David Drubin (University of California, Berkeley) for his generous invitation to author this perspective and Kostas Nakos for help with figure images. We apologize to our colleagues for omitting any references owing to journal limitations. We acknowledge support by National Institutes of Health Grants no. 5R01 GM-097664-09 and no. 1R35 GM-136337-01 to E.T.S., and National Institutes of Health Grant no. 5R01 GM-124024-04 and National Science Foundation Grant no. 1928900 to M.A.M.
Abbreviations used:
- ER
endoplasmic reticulum
- MAP
microtubule-associated protein
- NPCs
nuclear pore complexes
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- SEPT
septin.
Footnotes
REFERENCES
- Abbey M, Gaestel M, Menon MB (2019). Septins: active GTPases or just GTP-binding proteins? Cytoskeleton (Hoboken) , 55–62. [DOI] [PubMed] [Google Scholar]
- Akil A, Peng J, Omrane M, Gondeau C, Desterke C, Marin M, Tronchere H, Taveneau C, Sar S, Briolotti P, et al (2016). Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubule-dependent mechanism hijacked by HCV. Nat Commun , 12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M (2000). Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell , 841–851. [DOI] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S, Snyder M (1999). Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev , 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E (2008). Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci USA , 8274–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR (1998). Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol , 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubakar L, Falk J, Ducuing H, Thoinet K, Reynaud F, Derrington E, Castellani V (2017). Molecular memory of morphologies by septins during neuron generation allows early polarity inheritance. Neuron , 834–851. e835. [DOI] [PubMed] [Google Scholar]
- Bowen JR, Hwang D, Bai X, Roy D, Spiliotis ET (2011). Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. J Cell Biol , 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges AA, Jentzsch MS, Oakes PW, Occhipinti P, Gladfelter AS (2016). Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol , 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges AA, Zhang H, Mehta SB, Occhipinti P, Tani T, Gladfelter AS (2014). Septin assemblies form by diffusion-driven annealing on membranes. Proc Natl Acad Sci USA , 2146–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery SM, Kono K, Stokasimov E, Pellman D (2012). Regulation of the formin Bnr1 by septins anda MARK/Par1-family septin-associated kinase. Mol Biol Cell , 4041–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B, Goetsch L (1976). A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol , 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F, Ranftl R, Hooper S, Farrugia AJ, Moeendarbary E, Bruckbauer A, Batista F, Charras G, Sahai E (2015). Cdc42EP3/BORG2 and septin network enables mechano-transduction and the emergence of cancer-associated fibroblasts. Cell Rep , 2699–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon KS, Woods BL, Crutchley JM, Gladfelter AS (2019). An amphipathic helix enables septins to sense micrometer-scale membrane curvature. J Cell Biol , 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro D, da Silva SMO, Pereira HD, Macedo JNA, Leonardo DA, Valadares NF, Kumagai PS, Brandao-Neto J, Araujo APU, Garratt RC (2020). A complete compendium of crystal structures for the human SEPT3 subgroup reveals functional plasticity at a specific septin interface. IUCrJ , 462–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron F, Barral Y (2009). Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell , 493–506. [DOI] [PubMed] [Google Scholar]
- Chollet J, Dunkler A, Bauerle A, Vivero-Pol L, Mulaw MA, Gronemeyer T, Johnsson N (2020). Cdc24 interacts with septins to create a positive feedback loop during bud site assembly in yeast. J Cell Sci , 240283. [DOI] [PubMed] [Google Scholar]
- Clay L, Caudron F, Denoth-Lippuner A, Boettcher B, Buvelot Frei S, Snapp EL, Barral Y (2014). A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. Elife , e01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Chatterjee B, Lozito TP, Zhang Z, Francis RJ, Yagi H, Swanhart LM, Sanker S, Francis D, Yu Q, et al (2013). Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol , e1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagdas YF, Yoshino K, Dagdas G, Ryder LS, Bielska E, Steinberg G, Talbot NJ (2012). Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science , 1590–1595. [DOI] [PubMed] [Google Scholar]
- DeMarini DJ, Adams AE, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR (1997). A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol , 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoth-Lippuner A, Krzyzanowski MK, Stober C, Barral Y (2014). Role of SAGA in the asymmetric segregation of DNA circles during yeast ageing. Elife , 03790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y (2004). Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science , 393–396. [DOI] [PubMed] [Google Scholar]
- Dolat L, Hu Q, Spiliotis ET, 2014a. Septin functions in organ system physiology and pathology. Biol Chem , 123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolat L, Hunyara JL, Bowen JR, Karasmanis EP, Elgawly M, Galkin VE, Spiliotis ET, 2014b. Septins promote stress fiber-mediated maturation of focal adhesions and renal epithelial motility. J Cell Biol , 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolat L, Spiliotis ET (2016). Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion. J Cell Biol , 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers H, Tada T, Petersen JD, Racz B, Sheng M, Choquet D (2014). A septin-dependent diffusion barrier at dendritic spine necks. PLoS One , e113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Z, Ding L, Burckhardt CJ, Lowery J, Zaritsky A, Sitterley K, Mota A, Costigliola N, Starker CG, Voytas DF, et al (2016). Vimentin intermediate filaments template microtubule networks to enhance persistence in cell polarity and directed migration. Cell Syst , 252–263.e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian MV, Wirshing A, Vakhrusheva A, Turegun B, Sokolova OS, Goode BL (2020). A septin-Hof1 scaffold at the yeast bud neck binds and organizes actin cables. Mol Biol Cell mbcE19120693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlen LR, Nagai S, Shimada K, Meister P, Taddei A, Gasser SM (2011). Nuclear geometry and rapid mitosis ensure asymmetric episome segregation in yeast. Curr Biol , 25–33. [DOI] [PubMed] [Google Scholar]
- Gilden J, Krummel MF (2010). Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton (Hoboken) , 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden JK, Peck S, Chen YC, Krummel MF (2012). The septin cytoskeleton facilitates membrane retraction during motility and blebbing. J Cell Biol , 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS, Bose I, Zyla TR, Bardes ES, Lew DJ (2002). Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J Cell Biol , 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ (2001). The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol , 681–689. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Dagdas YF, Martinez-Rocha AL, Kershaw MJ, Littlejohn GR, Ryder LS, Sklenar J, Menke F, Talbot NJ (2015). Septin-dependent assembly of the exocyst is essential for plant infection by magnaporthe oryzae. Plant Cell , 3277–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Tanaka Y, Hikawa R, Morone N, Kusumi A, Kimura H, Kinoshita M (2011). Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton (Hoboken) , 512–525. [DOI] [PubMed] [Google Scholar]
- Hamdan H, Lim BC, Torii T, Joshi A, Konning M, Smith C, Palmer DJ, Ng P, Leterrier C, Oses-Prieto JA, et al (2020). Mapping axon initial segment structure and function by multiplexed proximity biotinylation. Nat Commun , 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH (1971). Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res , 265–276. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Culotti J, Reid B (1970). Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci USA , 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley LR, McMurray MA (2016). Roles of septins in prospore membrane morphogenesis and spore wall assembly in Saccharomyces cerevisiae. Mol Biol Cell , 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rodriguez Y, Momany M (2012). Posttranslational modifications and assembly of septin heteropolymers and higher-order structures. Curr Opin Microbiol , 660–668. [DOI] [PubMed] [Google Scholar]
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, Spiliotis ET (2012). Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol , 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ (2010). A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science , 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Nelson WJ, Spiliotis ET (2008). Forchlorfenuron alters mammalian septin assembly, organization, and dynamics. J Biol Chem , 29563–29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CM, Hite RK, Weirich CS, Fitzgerald DJ, Jawhari H, Faty M, Schlapfer D, Kroschewski R, Winkler FK, Walz T, et al (2007). The Caenorhabditiselegans septin complex is nonpolar. EMBO J , 3296–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E, Surka MC, Trimble WS (2007). Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell , 677–690. [DOI] [PubMed] [Google Scholar]
- Jordan SN, Davies T, Zhuravlev Y, Dumont J, Shirasu-Hiza M, Canman JC (2016). Cortical PAR polarity proteins promote robust cytokinesis during asymmetric cell division. J Cell Biol , 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Strauss D, Zenvirth D, Simchen G, Barkai N (2007). Spore germination in Saccharomyces cerevisiae: global gene expression patterns and cell cycle landmarks. Genome Biol , R241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasmanis EP, Phan CT, Angelis D, Kesisova IA, Hoogenraad CC, McKenney RJ, Spiliotis ET (2018). Polarity of neuronal membrane traffic requires sorting of kinesin motor cargo during entry into dendrites by a microtubule-associated septin. Dev Cell , 204–218.e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JB, Dixit G, Sheetz JB, Venkatapurapu SP, Elston TC, Dohlman HG (2015). RGS proteins and septins cooperate to promote chemotropism by regulating polar cap mobility. Curr Biol , 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, McQuilken M, Gladfelter AS (2015). Septins and generation of asymmetries in fungal cells. Annu Rev Microbiol , 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmelinskii A, Meurer M, Knop M, Schiebel E (2011). Artificial tethering to nuclear pores promotes partitioning of extrachromosomal DNA during yeast asymmetric cell division. Curr Biol , R17–R18. [DOI] [PubMed] [Google Scholar]
- Kinoshita M (2003). Assembly of mammalian septins. J Biochem , 491–496. [DOI] [PubMed] [Google Scholar]
- Kozubowski L, Larson JR, Tatchell K (2005). Role of the septin ring in the asymmetric localization of proteins at the mother-bud neck in Saccharomyces cerevisiae. Mol Biol Cell , 3455–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokowski S, Lobato-Marquez D, Chastanet A, Pereira PM, Angelis D, Galea D, Larrouy-Maumus G, Henriques R, Spiliotis ET, Carballido-Lopez R, Mostowy S (2018). Septins recognize and entrap dividing bacterial cells for delivery to lysosomes. Cell Host Microbe , 866–874. e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwitny S, Klaus AV, Hunnicutt GR (2010). The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol Reprod , 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Calvo F (2019). Regulation of mechanotransduction: emerging roles for septins. Cytoskeleton (Hoboken) , 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe DD, Wolf YI, Koonin EV, Aravind L (2002). Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol , 41–72. [DOI] [PubMed] [Google Scholar]
- Loyer N, Januschke J (2018). The last-born daughter cell contributes to division orientation of Drosophila larval neuroblasts. Nat Commun , 3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedeke C, Frei SB, Sbalzarini I, Schwarz H, Spang A, Barral Y (2005). Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J Cell Biol , 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox AS, Lewellyn L, Desai A, Oegema K (2007). Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev Cell , 827–835. [DOI] [PubMed] [Google Scholar]
- Mavrakis M, Azou-Gros Y, Tsai FC, Alvarado J, Bertin A, Iv F, Kress A, Brasselet S, Koenderink GH, Lecuit T (2014). Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol , 322–334. [DOI] [PubMed] [Google Scholar]
- McMurray MA, Thorner J (2009). Septins: molecular partitioning and the generation of cellular asymmetry. Cell Div , 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MA, Thorner J (2019). Turning it inside out: the organization of human septin heterooligomers. Cytoskeleton (Hoboken) , 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca DC, Macedo JN, Guimaraes SL, Barroso da Silva FL, Cassago A, Garratt RC, Portugal RV, Araujo APU (2019). A revised order of subunits in mammalian septin complexes. Cytoskeleton (Hoboken) , 457–466. [DOI] [PubMed] [Google Scholar]
- Mendoza M, Hyman AA, Glotzer M (2002). GTP binding induces filament assembly of a recombinant septin. Curr Biol , 1858–1863. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Ito H, Iwamoto I, Morishita R, Kanoh H, Seishima M, Nagata K (2013). Possible role of a septin, SEPT1, in spreading in squamous cell carcinoma DJM-1 cells. Biol Chem , 281–290. [DOI] [PubMed] [Google Scholar]
- Momany M, Talbot NJ (2017). Septins focus cellular growth for host infection by pathogenic fungi. Front Cell Dev Biol , 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Cossart P (2012). Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol , 183–194. [DOI] [PubMed] [Google Scholar]
- Nakos K, Radler MR, Spiliotis ET (2019). Septin 2/6/7 complexes tune microtubule plus-end growth and EB1 binding in a concentration- and filament-dependent manner. Mol Biol Cell , 2913–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolke T, Schwan C, Lehmann F, Ostevold K, Pertz O, Aktories K (2016). Septins guide microtubule protrusions induced by actin-depolymerizing toxins like Clostridium difficile transferase (CDT). Proc Natl Acad Sci USA , 7870–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Leda M, Hanna J, Savage NS, Bi E, Goryachev AB (2013). Daughter cell identity emerges from the interplay of Cdc42, septins, and exocytosis. Dev Cell , 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Koga T, Hirata A, Nakamura T, Asakawa H, Shimoda C, Bahler J, Wu JQ, Takegawa K, Tachikawa H, et al (2010). Role of septins in the orientation of forespore membrane extension during sporulation in fission yeast. Mol Cell Biol , 2057–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuso A, Tham TN, Stevens JK, Lagache T, Persson R, Salles A, Olivo-Marin JC, Oddos S, Spang A, Cossart P, Stavru F (2016). A role for septin 2 in Drp1-mediated mitochondrial fission. EMBO Rep , 858–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Malmberg RL, Momany M (2007). Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol , 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Kim SK, Wallingford JB (2015). The planar cell polarity effector protein Wdpcp (Fritz) controls epithelial cell cortex dynamics via septins and actomyosin. Biochem Biophys Res Commun , 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JR (2008). Origins and development of the septin field. In: The Septins, ed. Hall PA, Russell HSE, Pringle JR, West Sussex, U.K.: Wiley-Blackwell, 7–34. [Google Scholar]
- Ribet D, Boscaini S, Cauvin C, Siguier M, Mostowy S, Echard A, Cossart P (2017). SUMOylation of human septins is critical for septin filament bundling and cytokinesis. J Cell Biol , 4041–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Nichols BJ (2004). A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr Biol , 1002–1006. [DOI] [PubMed] [Google Scholar]
- Schneider C, Grois J, Renz C, Gronemeyer T, Johnsson N (2013). Septin rings act as a template for myosin higher-order structures and inhibit redundant polarity establishment. J Cell Sci , 3390–3400. [DOI] [PubMed] [Google Scholar]
- Sellin ME, Stenmark S, Gullberg M (2014). Cell type-specific expression of SEPT3-homology subgroup members controls the subunit number of heteromeric septin complexes. Mol Biol Cell , 1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG (2013). An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature , 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y (2008). A mechanism for asymmetric segregation of age during yeast budding. Nature , 728–734. [DOI] [PubMed] [Google Scholar]
- Shindo A, Audrey A, Takagishi M, Takahashi M, Wallingford JB, Kinoshita M (2018). Septin-dependent remodeling of cortical microtubule drives cell reshaping during epithelial wound healing. J Cell Sci , 212647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo A, Wallingford JB (2014). PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science , 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulewitz MJ, Inouye CJ, Thorner J (1999). Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol , 7123–7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A (2007). Structural insight into filament formation by mammalian septins. Nature , 311–315. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Zent E, Wittinghofer A (2009). GTP-induced conformational changes in septins and implications for function. Proc Natl Acad Sci USA , 16592–16597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirianni A, Krokowski S, Lobato-Marquez D, Buranyi S, Pfanzelter J, Galea D, Willis A, Culley S, Henriques R, Larrouy-Maumus G, et al (2016). Mitochondria mediate septin cage assembly to promote autophagy of Shigella. EMBO Rep , 1029–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Gras C, Capin G, Gimber N, Lehmann M, Mohd S, Puchkov D, Rodiger M, Wilhelmi I, Daumke O, et al (2019). A SEPT1-based scaffold is required for Golgi integrity and function. J Cell Sci , 225557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroor F, Kim MS, Palander O, Balachandran Y, Collins R, Benlekbir S, Rubinstein J, Trimble WS (2019). Revised subunit order of mammalian septin complexes explains their in vitro polymerization properties. bioRxiv, 569871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET (2018). Spatial effects - site-specific regulation of actin and microtubule organization by septin GTPases. J Cell Sci , 207555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ (2008). Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol , 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff AM, Aylyarov I, Jaiswal P (2013). Septin-containing barriers control the differential inheritance of cytoplasmic elements. Cell Rep , 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theesfeld CL, Zyla TR, Bardes EG, Lew DJ (2003). A monitor for bud emergence in the yeast morphogenesis checkpoint. Mol Biol Cell , 3280–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley AJ, Gilden J, Jacobelli J, Beemiller P, Trimble WS, Kinoshita M, Krummel MF (2009). Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol , 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier-Pinard P, Salaun D, Bouguenina H, Shimada S, Pophillat M, Audebert S, Agavnian E, Coslet S, Charafe-Jauffret E, Tachibana T, Badache A (2017). Septin 9_i2 is downregulated in tumors, impairs cancer cell migration and alters subnuclear actin filaments. Sci Rep , 44976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu AM, Gerber SA, Gygi SP, Field CM, Mitchison TJ (2004). The majority of the Saccharomyces cerevisiae septin complexes do not exchange guanine nucleotides. J Biol Chem , 3111–3118. [DOI] [PubMed] [Google Scholar]
- Weems A, McMurray M (2017). The step-wise pathway of septin hetero-octamer assembly in budding yeast. Elife , 23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloka C, Nishihama R, Onishi M, Oh Y, Hanna J, Pringle JR, Krauss M, Bi E (2011). Evidence that a septin diffusion barrier is dispensable for cytokinesis in budding yeast. Biol Chem , 813–829. [DOI] [PubMed] [Google Scholar]
- Zent E, Wittinghofer A (2014). Human septin isoforms and the GDP-GTP cycle. Biol Chem , 169–180. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS (1999). Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol , 1458–1467. [DOI] [PubMed] [Google Scholar]
- Zheng S, Dong F, Rasul F, Yao X, Jin QW, Zheng F, Fu C (2018). Septins regulate the equatorial dynamics of the separation initiation network kinase Sid2p and glucan synthases to ensure proper cytokinesis. FEBS J , 2468–2480. [DOI] [PubMed] [Google Scholar]