Abstract
Background
New-onset diabetes after transplantation (NODAT) is a serious complication after a solid organ transplant. NODAT occurs in 2% to 53% of all solid organ transplant recipients. The identification of high-risk patients and the implementation of measures to limit the development of NODAT can improve the long-term patient prognosis.
Material/Methods
Our study group consisted of 336 patients undergoing heart transplant. Patients with prior diabetes (60 patients) were excluded from analysis. The remaining 276 patients were divided in 2 groups: with NODAT (n=109) and without NODAT (n=167). Logistic regression analysis was used for NODAT risk factor assessment.
Results
NODAT occurred in 109 (32%) out of 336 patients without diagnosed diabetes before heart transplantation. Risk factors for post-transplant diabetes mellitus, which was shown by the analysis of the collected data, were BMI at discharge (OR=1.082, CI 1.011–1.158, p=0.0233), history of diagnosed CMV infection (OR=1.464, CI 1.068–2.007, p=0.0179), and age over 51 years (OR=1.634, CI 1.274–2.095, p=0.0001).
Conclusions
1. New-onset diabetes after transplantation (NODAT) or long-lasting hypoglycemia (over 2 years after transplantation) was diagnosed in 32% patients after heart transplantation developed. 2. The risk factors of NODAT were BMI at discharge and history of diagnosed CMV infection, and age over 51 years was an independent risk factor.
MeSH Keywords: Diabetes Complications, Heart Transplantation, Risk Factors
Background
New-onset diabetes after transplantation (NODAT) is a serious complication occurring in patients receiving heart and other solid organ transplantations, which significantly increases morbidity and mortality and decreases quality of life. In the literature, the incidence of NODAT ranges from 2% to 53% [1].
The diagnosis of NODAT is currently performed using unmodified criteria for diagnosing diabetes in the general population, based on the WHO criteria proposed by the American Diabetes Association (ADA) [2].
The primary risk factors influencing the incidence of diabetes after transplantation are the commonly recognized factors for developing diabetes: overweight, obesity, positive family history, low physical activity, age >45 years, peer or ethnic groups at higher risk of diabetes (e.g., African-, Latino-, and Asian-Americans, Native Americans, and native inhabitants of the Pacific Islands), history of gestational diabetes or birth of a child weighing >4 kg, arterial hypertension, dyslipidemias, polycystic ovary syndrome, and secondary hyperparathyroidism [3,4].
The increased risk of developing NODAT is also influenced by specific drugs administered following organ transplantation: immunosuppressants, especially glucocorticosteroids and calcineurin inhibitors such as cyclosporine and tacrolimus, as well as mTOR kinase inhibitors such as sirolimus and everolimus. Other risk factors include CMV infection, hepatitis C, male sex of the recipient and donor, and immunological reactions of acute rejection [1,5,6].
An increased risk of cardiovascular incidents resulting in death and other diseases was observed in patients who developed diabetes after transplantation. Other adverse effects included infection, decreased patient survival, rejection, and early graft loss [7,8].
Identifying high-risk patients and implementing measures to limit the development of NODAT may improve a patient’s long-term prognosis.
Material and Methods
Our study group consisted of 336 patients undergoing a heart transplant at the Silesian Center for Heart Diseases in Zabrze between June 2001 and January 2018. There were 60 patients who were diagnosed with diabetes prior to surgery and these were excluded from analysis. The remaining 276 were divided in 2 groups: 109 with NODAT and 167 without NODAT. After institutional review board approval, we identified the study cohort by systematic chart review. The database was prepared and retrospective analysis was conducted. NODAT was defined as any need for chronic hypoglycemic treatment 6 months after heart transplantation.
The database consisted of clinical information, including place of residence, body weight, height, comorbidities, medications, and the occurrence of diabetes and hypertension. These data were collected from discharge documents immediately after transplantation. Laboratory results were also collected, including blood type, surface antigen of the hepatitis B virus (HBsAg), HCV antibodies, HIV antibodies, venereal disease research laboratory (VRDL), cytomegalovirus, glycemia, creatinine, uric acid, aspartate transaminase (AST), alanine transaminase (ALAT), cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), glycated hemoglobin (HbA1c), and myocardial biopsy results according to the International Society for Heart & Lung Transplantation (ISHLT) scale. One-year and longest available follow-up information (all patients had at least a 2-year follow-up post-transplant) consisted of body weight and height, blood pressure, diabetes, glycemia, cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), glycated hemoglobin (HbA1c), and myocardial biopsy results according to the International Society for Heart & Lung Transplantation (ISHLT) scale. Additional information regarding NODAT (e.g., date of hospitalization when NODAT was diagnosed and NODAT treatment) and incidence of transplant rejection (date and result of myocardial biopsy in ISHLT scale and medical therapy used) was collected. According to the “Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults”, metabolic syndrome was defined as the presence of 3 or more of the following 5 components: (1) obesity with body mass index (BMI) ≥30 kg/m2; (2) triglycerides ≥150 mg/dL or on treatment; (3) HDL <40 mg/dL in men and <50 mg/dL in women; (4) systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or antihypertensive therapy; and (5) fasting glucose ≥100 mg/dL [9].
Logistic regression analysis was used to determine the risk of NODAT associated with pre- and peri-transplant patient characteristics. We first constructed univariate models to determine the association of individual pretransplant variables with development of NODAT. Variables significant at p<0.25 in univariate models were then included in multivariate analyses. We conducted 3 multivariate analyses: 1) a standard model, in which continuous variables and discrete variables were included without categorization where age was statistically significant; 2) where age was categorized in interquartile range to determine the age risk groups where 3rd and 4th quartile range where statistically significant; and 3) where age was presented as binary values, over and under its median (we have shown the last and most informative results). The t test and Mann-Whitney U test were used for assessing the statistical significance of the difference between mean of continuous variables and Pearson’s chi-squared test was used for categorical data. All analyses were performed using STATISTICA ver. 13 (StatSoft).
Results
Clinical characteristics of the 2 study groups are shown in Table 1. In all follow-up periods – 1 year after transplantation (p=0.2453) and at the latest follow-up visit (p=0.9105) – patients who had NODAT did not have significantly higher BMI than those without.
Table 1.
Clinical characteristics of the study group, divided according to NODAT. Data is presented as means±SD or numbers (%).
| Patients without NODAT n=167 | Patients with NODAT n=109 | |
|---|---|---|
| BMI at discharge [kg/m2 ±SD] | 23.24±3.56 | 24.01±4.03 |
| BMI at 1 year follow-up [kg/m2 ±SD] | 25.19±4.00 | 25.66±4.38 |
| BMI at longest available follow-up [kg/m2 ±SD] | 26.28±4.67 | 26.04±4.75 |
| Body mass gain >5 kg at 1 year after transplantation [n%] | 88 (53.01%) | 53 (48.62%) |
| Body mass gain >5 kg at longest available follow up [n%] | 102 (61.45%) | 55 (50.46%) |
Additionally, body mass gain in the first year after transplantation (p=0.4764) and in the longest available follow-up (p=0.08169) was not significantly higher among NODAT patients. The clinical information on the 2 groups is presented in Table 2. Non-ischemic heart disease as a reason for transplantation vs. ischemic cardiomyopathy was more frequent in the NODAT group (p=0.001).
Table 2.
Selected clinical parameters in both study groups. Data is presented as numbers (%).
| Patients without NODAT n=167 | Patients with NODAT n=109 | |
|---|---|---|
| Dyslipidemia | 48 (28.92%) | 39 (35.78%) |
| Non-ischemic cardiomyopathy vs. ischemic cardiomyopathy | 113 (67.66%) | 48 (44.04%)** |
| Cigarette smoking | 5 (2.99%) | 4 (3.67%) |
| >3a ISHLT at discharge | 82 (49.10%) | 69 (63.30%)* |
| Hyperthyroidism | 7 (4.19%) | 8 (7.34%) |
| Gout or hyperuricemia | 19 (11.38%) | 11 (10.09%) |
| Thiamazole usage at discharge | 1 (0.60%) | 0 (0.00%) |
| Levothyroxine usage at discharge | 24 (14.37%) | 11 (10.09%) |
| Tacrolimus usage at discharge | 117 (70.05%) | 71 (65.14%) |
| Cyclosporin usage at discharge | 50 (29.95%) | 38 (34.86%) |
| Glucocorticoids usage at 6th month | 146 (87.43%) | 101 (87.43%) |
| Glucocorticoids usage at 12th month | 53 (31.74%) | 35 (32.11%) |
| Glucocorticoids usage at 24th month | 9 (5.39%) | 14 (12.84%)* |
p<0.05;
p<0.001.
The incidence of cardiac allograft rejection (defined as myocardial biopsy result ≥3a on ISHLT scale) was higher in NODAT patients (p=0.0442). Figure 1 shows that patients who developed NODAT were more likely to have been treated with glucosteroids (p=0.0285) at discharge in the first 2 years after transplantation compared to patients without NODAT.
Figure 1.
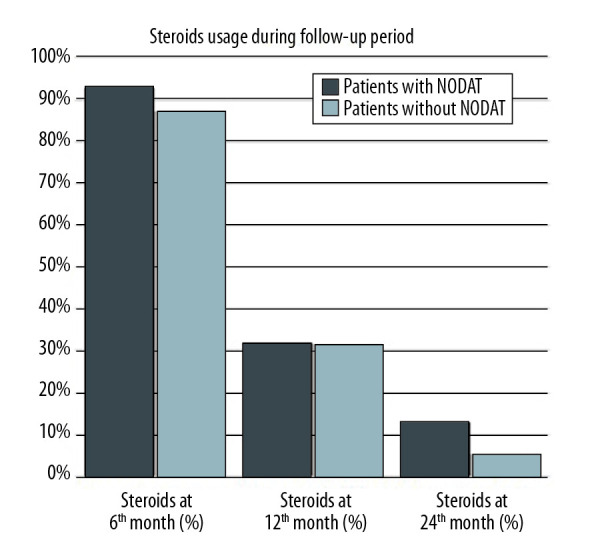
Steroids usage during follow-up period.
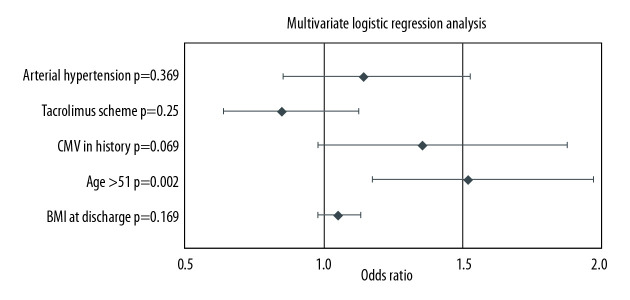
Table 3 presents a one-way logistic regression analysis of the 2 study groups. BMI at discharge (OR 1.082; CI 1.011–1.158; p=00233), CMV in history (OR 1.464; CI 1.068–2.007; p=0.0179), and age over 51 years (OR 1.634; CI 1.274–2.095; p=0.0001) were risk factors of NODAT development. Results of multivariate logistic regression analysis are presented in Figure 2. Age over 51 years (OR 1.520; CI 1.173–1.971; p=0.002) was an independent risk factor of NODAT onset in our model.
Table 3.
Univariate logistic regression analysis of the study groups.
| OR | Cl | p | |
|---|---|---|---|
| BMI at discharge [kg/m2 ±SD] | 1.082 | 1.011–1.158 | 0.0233 |
| Clinically relevant rejection (>3a) per patient per year | 1.018 | 0.577–1.796 | 0.9510 |
| Number of biopsies >3a | 1.090 | 0.907–1.311 | 0.3574 |
| Sex [M] | 0.978 | 0.726–1.316 | 0.8817 |
| Arterial hypertension | 1.312 | 0.999–1.723 | 0.0511 |
| HDL <40 mg/dl or triglycerides >150 mg/dl | 0.975 | 0.765–1.241 | 0.8348 |
| Tacrolimus scheme | 0.829 | 0.635–1.084 | 0.1704 |
| CMV in history | 1.464 | 1.068–2.007 | 0.0179 |
| Age >51 | 1.634 | 1.274–2.095 | 0.0001 |
Figure 2.
Multivariate analysis of NODAT risk factors.
Discussion
We found a significant number of cases of NODAT in patients after HT who did not exhibit impaired glucose tolerance before the transplant. NODAT risk factors included CMV in the history and increase in BMI at discharge, and age over 51 years was an independent risk factor.
The incidence of NODAT varies widely (2–53%) depending on the criteria used to diagnose diabetes after transplantation [1]. According to the latest report of the International Society for Heart and Lungs Transplantation (ISHLT), the incidence of diabetes after the first year following a heart transplant was 23% and after 5 years it increased to 37%, which constitutes a significant increase compared to an earlier report (2002) of a 32% incidence rate after a control period of 5 years [10]. The higher level of NODAT diagnosis in the sample group (46%) is most likely related to the longer period of observation following heart transplantation in the analyzed group, as the incidence of NODAT increases with time after a transplant, although it is usually diagnosed within the first 6 months [1].
In addition to assessing the incidence of NODAT, we also analyzed the risk factors for the occurrence of diabetes after heart transplantation. These risk factors are similar to those observed after the transplantation of other solid organs, and can be divided into modifiable factors and non-modifiable factors that the patient has no influence over, such as recipient age. The results of many studies confirm our observations that age has a significant influence on the occurrence of NODAT and that the disease occurs more frequently in elderly patients [11,12]. Ye et al. showed that heart transplant recipients who were over 50 years old had an increased risk for development of NODAT (HR=1.20 for age ≥50 years vs. <50, p=0.01). Casio et al. studied kidney transplant recipients without previously diagnosed diabetes, finding that patients aged over 45 years were 2.9 times more likely to develop diabetes after transplantation compared to patients under age 45 (RR=2.2 comparing patients younger or older than 45 years, P<0.0001) [13]. Additionally, the incidence of NODAT during the first 6 months following transplantation is considerably higher for older adults compared to younger patients.
A modifiable risk factor that influences the development of NODAT is increase in body mass after HT, which is why patients with type 2 diabetes risk factors, such as overweight, hypertension, or dyslipidemia, should remain under strict supervision [3,14,15]. Before heart transplantation, the patients exhibited various levels of body mass, ranging from underweight to obesity. The results obtained in this study suggest that the increase in body mass following a heart transplant can be a significant factor influencing the occurrence of NODAT. Only the increase in body mass, which results in overweight or potential obesity in a number of patients, is classified as a primary risk factor for diabetes in the general population, because it is related to the insulin resistance of peripheral tissues. The increase in body mass was recognized as a risk factor in many publications [16–18], but it was not confirmed by all [19,20]. The tendency for the increase in body mass, especially during the initial period after transplantation, can be related to the unsatisfactory state of nutrition in some patients during the period of conservative treatment and to the application of massive doses of steroids, which increase hunger and insulin resistance, resulting in rapid increases in body mass, particularly at low physical activity levels. These results suggest the advisability of changing the model of nutrition therapy as well as modifying lifestyle before the heart transplant.
Another serious, potentially modifiable risk factor for developing NODAT is a history of CMV infection. Although it is typically asymptomatic, CMV infection influences the release of insulin and consequently the risk of the development of diabetes after transplantation. Hjelmesaeth et al. [21] demonstrated that CMV-infected patients who developed an active infection exhibited a significantly lower median level of insulin release compared to an uninfected control group; consequently, the risk of development of NODAT in these patients was up to 4 times higher. The explanation for this dependence given by the researchers was the possible influence of inflammatory cytokines released during CMV infection. The cytokines damage the pancreatic beta cells and result in their apoptosis, which may be a pathophysiological mechanism of reduced insulin release during CMV infection [22]. The relationship between CMV infections and the development of NODAT was confirmed in other publications [21,23], but other studies found no such relationship [18,24–26].
Conclusions
Both new-onset diabetes after transplantation (NODAT) and long-lasting hypoglycemia (over 2 years after transplantation) are serious complications after a solid organ transplant. There are few reports describing the occurrence of these complications after heart transplantation. The present study found that 32% of all heart transplant recipients develop these complications, and age over 51 years was an unmodifiable independent risk factor for the development of diabetes. Since the occurrence of diabetes is associated with an increased risk of cardiovascular complications, an increased risk of organ transplant rejection, mortality, and decreased quality of life, it is important to identify high-risk patients and to implement measures to limit the development of NODAT, which could improve the long-term patient prognosis.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Montori VM, Basu A, Erwin PJ, et al. Posttransplantation diabetes: A systematic review of the literature. Diabetes Care. 2002;25:583–92. doi: 10.2337/diacare.25.3.583. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Definition, Diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. [Google Scholar]
- 3.2019 Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clinical Diabetology. 2019;8(1):1–95. [Google Scholar]
- 4.Ivarsson KM, Clyne N, Almquist M, Akaberi S. Hyperparathyroidism and new-onset diabetes after renal transplantation. Transplant Proc. 2014;46:145–50. doi: 10.1016/j.transproceed.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 5.Lv Ch, Chen M, Xu M, et al. Influencing factors of new-onset diabetes after renal transplant and their effects on complications and survival rate. PLoS One. 2014;9(6):e99406. doi: 10.1371/journal.pone.0099406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einollahi B, Motalebi M, Salesi M, et al. The impact of cytomegalovirus infection on new-onset diabetes mellitus after kidney transplantation: A review on current findings. J Nephropathol. 2014;3:139–48. doi: 10.12860/jnp.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedanova H, Ondrasek J, Cerny J, et al. Impact of diabetes mellitus on survival rates after heart transplantation. Biomedical Papers. 2009;153(4):283–87. doi: 10.5507/bp.2009.047. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda Y, Tenderich G, Zittermann A, et al. Heart transplantation in insulin-treated diabetic mellitus patients with diabetes-related complications. Transpl Int. 2007;20(6):528–33. doi: 10.1111/j.1432-2277.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 9.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143. [PubMed] [Google Scholar]
- 10.Lund LH, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third adult heart transplantation report – 2016; focus theme: Primary diagnostic indications for transplant. J Heart Lung Transplantat. 2016;35(10):1158–69. doi: 10.1016/j.healun.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Ye X, Kuo H, Sampaio MS, et al. Risk factors for development of new-onset diabetes mellitus in adult heart transplant recipients. Transplantation. 2010;89(12):1526–32. doi: 10.1097/TP.0b013e3181dd6bd9. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigo E, Fernandez-Fresnedo G, Valero R, et al. New-onset diabetes after kidney transplantation: Risk factors. J Am Soc Nephrol. 2006;17(12 Suppl 3):S291. doi: 10.1681/ASN.2006080929. [DOI] [PubMed] [Google Scholar]
- 13.Cosio FG, Pesavento TE, Osei K, et al. Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001;59:732–37. doi: 10.1046/j.1523-1755.2001.059002732.x. [DOI] [PubMed] [Google Scholar]
- 14.Maes BD, Kuypers D, Messiaen T, et al. Posttransplantation diabetes mellitus in FK-506-treated renal transplant recipients: Analysis of incidence and risk factors. Transplantation. 2001;72:1655–61. doi: 10.1097/00007890-200111270-00014. [DOI] [PubMed] [Google Scholar]
- 15.Shah T, Kasravi A, Huang E, et al. Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82:1673–76. doi: 10.1097/01.tp.0000250756.66348.9a. [DOI] [PubMed] [Google Scholar]
- 16.Numakura K, Satoh S, Tsuchiya N, et al. Clinical and genetic risk factors for posttransplant diabetes mellitus in adult renal transplant recipients treated with tacrolimus. Transplantation. 2005;80:1419–24. doi: 10.1097/01.tp.0000181142.82649.e3. [DOI] [PubMed] [Google Scholar]
- 17.Romagnoli J, Citterio F, Violi P, et al. Post-transplant diabetes mellitus: A case-control analysis of the risk factors. Transpl Int. 2005;18:309–12. doi: 10.1111/j.1432-2277.2004.00043.x. [DOI] [PubMed] [Google Scholar]
- 18.Cosio FG, Kudva Y, van der Velde M, et al. New-onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–21. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 19.Sulanc E, Lane JT, Puumala SE, et al. New-onset diabetes after kidney transplantation: An application of 2003 international guidelines. Transplantation. 2005;80:945–52. doi: 10.1097/01.tp.0000176482.63122.03. [DOI] [PubMed] [Google Scholar]
- 20.Gourishankar S, Jhangri GS, Tonelli M, et al. Development of diabetes mellitus following kidney transplantation: A Canadian experience. Am J Transplant. 2004;4:1876–82. doi: 10.1111/j.1600-6143.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 21.Hjelmesaeth J, Sagedal S, Hartmann A, et al. Asymptomatic cytomegalovirus infection is associated with increased risk for new-onset diabetes and impaired insulin release after renal transplantation. Diabetologia. 2004;47(9):1550–56. doi: 10.1007/s00125-004-1499-z. [DOI] [PubMed] [Google Scholar]
- 22.Hjelmesaeth J, Muller F, Jenssen T, et al. Is there a link between cytomegalovirus infection and new-onset posttransplant diabetes mellitus? Potential mechanisms of virus induced beta-cell damage. Nephrol Dial Transplant. 2005;20(11):2311–15. doi: 10.1093/ndt/gfi033. [DOI] [PubMed] [Google Scholar]
- 23.Hjelmesaeth J, Hartmann A, Kofstad J, et al. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997;64(7):979–83. doi: 10.1097/00007890-199710150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Gourishankar S, Jhangri GS, Tonelli M, Wales LH. Cock-S294. J Am Soc Nephrol. 2006;17:S291–95. [Google Scholar]
- 25.Marin M, Renoult E, Bondor C, Kessler M. Factors influencing the onset of diabetes mellitus after kidney transplantation: A single French center experience. Transplant Proc. 2005;37(4):1851–56. doi: 10.1016/j.transproceed.2005.03.140. [DOI] [PubMed] [Google Scholar]
- 26.Numakura K, Satoh S, Tsuchiya N, et al. Clinical and genetic risk factors for posttransplant diabetes mellitus in adult renal transplant recipients treated with tacrolimus. Transplantation. 2005;80:1419–24. doi: 10.1097/01.tp.0000181142.82649.e3. [DOI] [PubMed] [Google Scholar]