Abstract
Background
The bluetongue virus (BTV) is the prototype virus in the genus Orbivirus within the family Reoviridae. Recent studies indicate that BTVs are capable of infecting and selectively lysing human hepatic carcinoma cells (Hep-3B) and prostate carcinoma cells (pc-3). This study was designed to evaluate the oncolytic potential of BTV in experimental models of human renal cancer in vitro and in vivo.
Material/Methods
Five human renal cancer cell lines, ACHN, CAKI-1, OS-RC-2, 786-O, and A498, were used in this study to analyze BTV replication. These cells were lysed by oncolysis compared to normal control. Xenograft models were used to assess the efficacy and toxicity of BTVs in vivo. Data were analyzed by one-way ANOVA or two-sided unpaired t tests.
Results
The results showed HPTEC cells to be relatively resistant to cytotoxic effects of BTVs and exhibited normal growth rate even at high dose of BTVs. Nonetheless, the renal cancer cells showed a remarkably higher sensitivity to BTVs. Moreover, the ultramicroscopic subcellular changes were also detected in the renal cells. The viral particles were observed in all the RCC cell lines, but not in HPTEC cells. Intratumoral injections of BTVs significantly decreased the tumor volume as compared to animals that received no virus treatment. Infection with BTVs significantly increased the percentage of apoptotic renal cancer cells but not the HPTEC cells. Moreover, BTV triggered apoptosis in renal cancer cells via a mitochondria-mediated pathway.
Conclusions
This study for the first time demonstrated the oncolytic potential of BTV in experimental models of human renal cancer. BTV exhibits the potential to inhibit human renal cancer cell growth in vitro and in vivo.
MeSH Keywords: Apoptosis, Bluetongue Virus, Kidney Neoplasms, Oncolytic Viruses
Background
Renal cell carcinoma (RCC) is the most prevalent type of human kidney cancer, with 65 340 new cases and 14 970 related deaths reported in 2018 [1]. Currently, tyrosine kinase inhibitors (TKIs) are a valuable treatment approach for patients with RCC. However, resistance to these targeted therapies is inevitable. Thus, novel and more effective therapies to prevent relapse and to inhibit metastases are urgently needed. Oncolytic virotherapy is emerging as a new cancer therapeutic approach with significant potential [2,3]. Tremendous advancements have been made over the past decade in the development OVs. Several of these viruses have been tested experimentally and clinically [4–6]. An oncolytic virus (OVs) should have at least 2 obvious properties – effective and non-pathogenic – which means that they should exhibit the capacity to trigger oncolysis and preferential infection of tumor cells [7]. Further studies have increasingly found that OVs have the potential to function as multiplexed immune-modulating platforms [6,8].
OVs used in cancer therapy are typically characterized by gene modifications. Some non-genetically modified viruses, defined as natural OVs, can usurp the signaling pathways of tumor cells to facilitate lytic infection [9], such as interferon (IFN) signaling pathways. In contrast, some pathogenic viruses can be used as OVs after gene modification [10,11]. The bluetongue virus (BTV), a double-stranded RNA (dsRNA) virus, is the prototype virus belonging to the genus Orbivirus of the family Reoviridae. It has been reported to primarily affect sheep and cattle but not humans [12]. Recent studies indicated that BTVs are capable of infecting and selectively lysing human hepatic carcinoma cells (Hep-3B) and prostate carcinoma cells (pc-3) [13,14]. Given this background, we evaluated BTVs as a novel oncolytic agent against human RCC in vitro and in vivo.
Material and Methods
Ethics statement
This study was approved by the Ethics Committee of the Chao-Yang Hospital of Capital Medical University. Animal breeding, care, and all experiments were performed in adherence to the guidelines of the Center for Animal Experiments of Capital Medical University and approved by the Animal Ethics Committee (IRB Number: 2020-KE-12).
Cells and viruses
Approximately 85% of renal cell cancers are adenocarcinomas, and most of these are of proximal tubular origin. Therefore, the human primary proximal tubular epithelial cell (HPTEC) was used as the control for the renal cancer cell lines.
The 5 human renal cancer cell lines (ACHN, CAKI-1, OS-RC-2, 786-O, and A498) were used in the present study. The ACHN cell line was obtained from the China Centre for Type Culture (CCTCC), Wuhan, China. The CAKI-1, OS-RC-2, 786-O A498, and HPTEC cell lines were obtained from the Cell Resources Centre at the Institute of Basic Medical Sciences (IBMS), Peking, China. The characterization and propagation of HPTEC was performed as described previously [15,16].
The HPTEC cells were subcultured in Renal Epithelial Cell Growth Medium (PromoCell). Bluetongue virus serotype 10 (BTV-10) was used throughout this study as described previously [14]. The origin, passage, and propagation of the virus was determined as described previously. The virus progeny was titrated by a standard plaque assay, stocked at a 50% tissue culture infectious dose (TCID50) of 107/mL at −70°C. Dead BTVs (D-BTVs) were prepared by irradiating with UV light for 1 h.
Antibodies and reagents
The following antibodies were obtained from Cell Signaling Technology: cleaved caspase-3, cleaved caspase-9, cytochrome c, COX IV, β-actin. The p53, Bax, PARP, VEGF, and Ki67, and the GAPDH antibodies were purchased from Santa Cruz Biotech. The pan-caspase inhibitor Z-VAD-FMK was purchased from Promega and prepared with dimethyl sulfoxide (DMSO).
Morphological and ultramicroscopic determination
Cell monolayers were washed with phosphate-buffered saline (PBS) and incubated with viruses at the indicated multiplicity of infection (MOI). Virus adsorptions were carried out for 2 h at 37°C in 5% CO2, followed by an incubation in growth medium supplemented with 2% fetal bovine serum (FBS).
After a 72-h infection with BTVs at a MOI of 10, the morphologic changes of all the cell lines, including HPTEC, were examined with the help of a microscope. The cells were subsequently collected and prepared for ultramicroscopic analysis by electron microscopy (EM; Hitachi H-600, Japan) as described previously [17]. The intracellular multiplication of BTVs was also examined to confirm the selective oncolytic effect of BTVs on human renal cancer cells.
Cell viability assay
Cell viability assays were performed to determine the oncolytic activity of BTVs. In brief, the renal cancer cell lines and HPTEC cells were plated at the density of 10 000 cells/well in 96-well flat-bottom plates with 150 μL of media. After the cells adhered to the surface, they were infected with BTVs at MOI of 0.001, 0.01, 0.1, 1, or 10 in triplicate for 24, 48, or 72 h. After treatment, the percentage survival for each group was determined as described previously [14]. The experiments were performed in triplicate.
Flow cytometry analysis of apoptosis
Apoptosis was detected by the flow cytometric analysis of phosphatidylserine membrane redistribution using an annexin V and propidium iodide (PI) double staining kit (MultiSciences, Hangzhou, China). The experiment was performed according to the manufacturer’s instructions. A total of 100 000 cells were either mock-treated or infected with BTVs at a MOI of 10 for 24 h. The cell population in the right 2 quadrants (Annexin V-positive) depict the apoptotic cells. To further determine whether the BTV-induced apoptosis was caspase-dependent, the cells were incubated with 10 μM of Z-VAD-FMK for 24 h prior to infection with BTVs. Control cultures were treated with DMSO (used as a solvent for the peptide inhibitors). The percentage of apoptotic cells was determined by performing 3 independent experiments.
DNA fragmentation analysis
The CAKI-1 cells were infected with BTVs at a MOI of 10 for 48, 72, or 96 h. After infection, adherent and floating cells were harvested for the analysis of DNA fragmentation. The DNA was extracted using a DNA Ladder Extraction Kit (C0008, Biotime, China) and subsequently run on a 2% agarose gel for the analysis of DNA fragmentation.
Western blot
Cells were infected with BTVs at a MOI of 10 as described above. After BTVs treatment, western blot analysis was performed as described previously [18]. All western blot experiments were performed in triplicate.
Xenograft tumor model in nude mice animals
Male BALB/c nu mice (4 to 6 weeks old) were purchased from the Beijing HFK Bioscience Co., Ltd. The mice were maintained in a specific pathogen-free (SPF) environment under standard laboratory conditions. The xenografts tumor model was established by subcutaneous injection of OS-RC-2 cells (1×106) mixed with Matrigel (BD Biosciences) (50 μL of cell suspension in 50 μL of Matrigel) into the left flank of mice. As the tumors reached 120 to 150 mm3, the mice were randomly divided into 3 groups (6 mice/group) and treated by intratumoral injections of 1×106 plaque-forming units (PFU) per dose every 3 days for 6 consecutive doses. The D-BTVs and PBS were used as the control agents. As a measure of safety, the body weight was monitored continuously. At the beginning of each BTV injection, the tumor size and body weight were determined. The tumor volume was calculated by the following formula: V (mm3)=Length×Width2/2. The treated mice showing 20% to 30% weight loss were euthanized to relieve their suffering. At the end of the experiment, all mice were sacrificed under anesthesia. Tumors were harvested for further experimentation. The brain, liver, spleen, lung, and kidneys were harvested for pathological analysis.
Immunohistochemistry staining
The tumors were harvested and fixed in 10% formalin, embedded in paraffin, and cut into 4-μm sections. First, the tumor sections were examined by hematoxylin and eosin (H&E) staining. Subsequently, the tumor cell proliferation was evaluated by counting Ki67-positive cells. The growth ability and angiogenesis of the tumor tissue was measured by VEGF staining. The staining was assessed with a SuperPicture™ 3rd Gen IHC Detection Kit (Life Technologies). The slides were subjected to incubation with an anti-Ki67 or anti-VEGF primary antibody. The tissue sections with incubation with primary antibody severed as negative control. Finally, the slides were counterstained with hematoxylin. For the determination of the Ki67 positive cells, at least 100 cells were counted in 6 different regions and the mean number was determined. Apoptosis was measured by counting TUNEL-positive cells using the in-situ apoptosis detection kit (Roche). Under microscopy, 6 different fields were randomly selected from every sample and 100 cells were randomly selected from each field.
Statistical analysis
All data were analyzed with SPASS 13.0 and GraphPad software (Prism 5.0). Data were analyzed by one-way ANOVA or two-sided unpaired t tests. A P value at <0.05 was regarded as a statistically significant difference.
Results
Selective oncolysis of BTVs in RCC cells
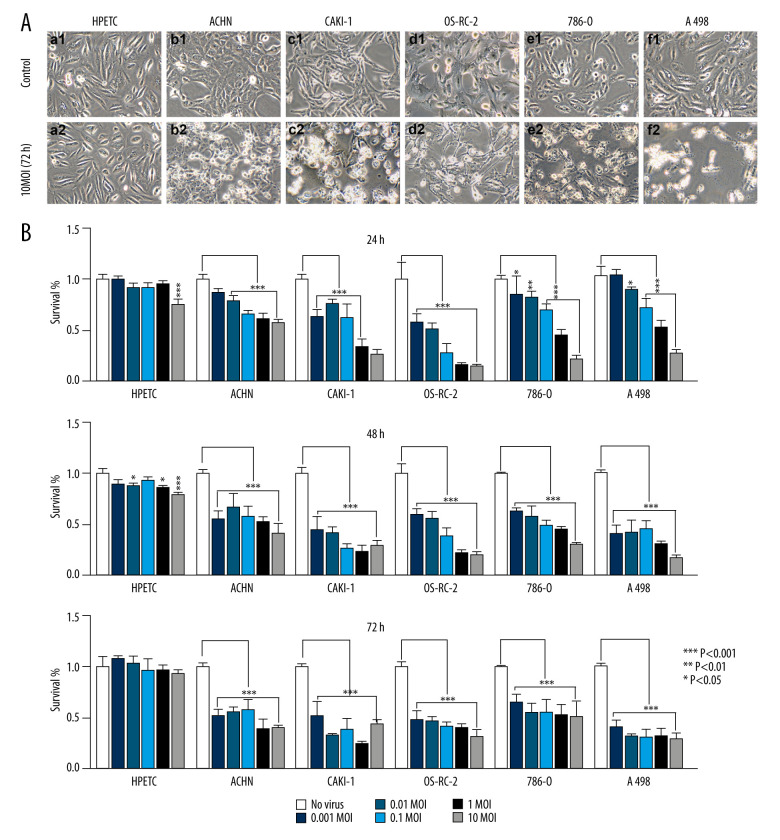
The HPTECs were characterized by immunocytochemical staining. The results revealed the HPTECs to be immunocytochemically negative for Factor VIII, but strongly positive for cytokeratin 18 and AP. The electron micrographs showed a typical epithelial cell ultrastructure, including microvilli, cell junctions, lateral interdigitation, mitochondria, and a rough endoplasmic reticulum (data not shown). All cells were infected with BTVs at MOIs between 0.01 and 10. Additionally, prominent morphological changes were observed in all cancer cell lines (Figure 1A). In contrast, the HPTEC did not exhibit any apparent cytopathic effect (CPE) after infection. Therefore, HPTEC cells were deemed not susceptible to BTVs infection. The cell survival was measured by using a CCK8 kit. The renal cancer cells showed a dramatic sensitivity to BTVs (controls versus virus-infected cells after 72-h infection, P<0.001 for all MOI), whereas HPTEC cells were found to be relatively resistant to the cytotoxicity of BTVs. BTVs initially inhibited the growth rate of HPTEC at high doses (controls versus virus-infected cells after 24- or 48-h infection, P<0.001 for MOI=10). However, they resumed growth at 72 h after infection (Figure 1B).
Figure 1.
BTVs exerted an antitumor effect against a range of renal tumor cell lines. Morphological changes in the renal cancer cell lines and human primary proximal tubular epithelial cell (HPTEC). (A) After 72 h of infection by BTVs (MOI 10 PFUs/cell), cytopathic effects (CPE) were clearly observed in all cancer cell lines, but not in HPTECs (×200). (B) Cells were infected with BTVs at MOIs between 0.001 and 10, and cell survival was measured by a CCK-8 assay at 24, 48, and 72 h after infection. For each cell line, the data were normalized to the uninfected control (no virus). Data are representative of at least 3 biological replicates. Columns, mean; bars, SEM.
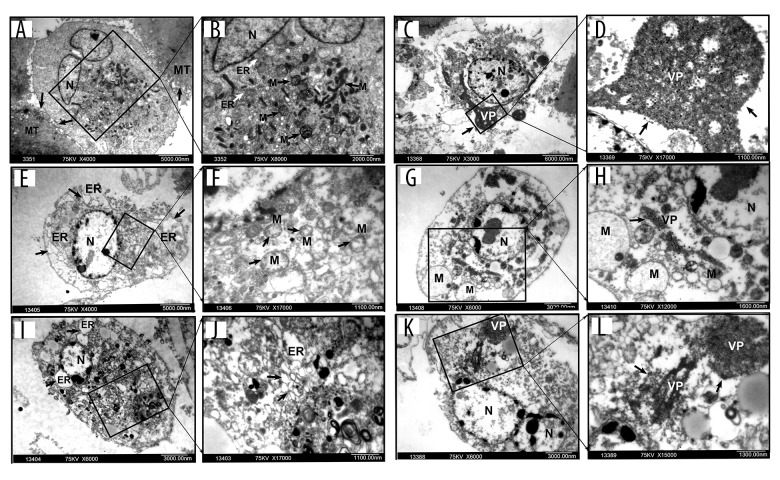
Ultramicroscopic structural changes in BTV-infected cells
BTV-infected cells were examined using electron microscopy to determine whether they could replicate selectively. The ultramicroscopic subcellular changes were also examined. The results indicated the presence of viral particles in all the RCC cell lines, but not in HPTEC cells (Figure 2A, 2B). The electron microscopic analysis revealed chromatin condensation along the periphery of the nucleus, mitochondrial membrane disintegration, an expansion of the rough endoplasmic reticulum, a decrease in mitochondrial matrix density, and balloon-like swelling in RCC cell lines at 72 h after BTV infection (Figure 2C–2L). However, nuclear membrane integrity and a cytoplasm enriched with organelles in HPTEC and the negative control were observed (data not shown). These findings indicate that BTVs selectively replicate and induce apoptosis in renal cancer cells.
Figure 2.
Electron microscopy of ultramicroscopic structure changes in 5 cell lines and HPTEC infected with BTV-10 72 hours after infection. HPTECs clearly show an unchanged ultramicroscopic structure compared to the untreated cells: microvilli (MT, arrows), chondriosome (M, arrows), rough endoplasmic reticulum (ER, arrows), nucleus (N) (A ×4000, B ×8000). Viral particles (VP) were observed in all the RCC cell lines, but not in the HPTECs (arrows) (B, C ×8000, D ×13000, G ×6000, H ×12000, K ×6000, L ×15000). Electron microscopy showed chromatin condensation along the periphery of the nucleus (C, E ×4000, G, I ×6000, K), expansion of the rough endoplasmic reticulum (arrows) (I, J ×17 000), decrease of mitochondrial matrix density, and balloon-like swelling (F ×17 000, G, H) in RCC cell lines. (A, B). HPTEC, (C, D). ACHN, (E, F). CAKI-1, (G, H). OR-SC-2, (I, J). 786-0, (K, L). A498.
The effective oncolytic activity of BTVs in a xenograft tumor model
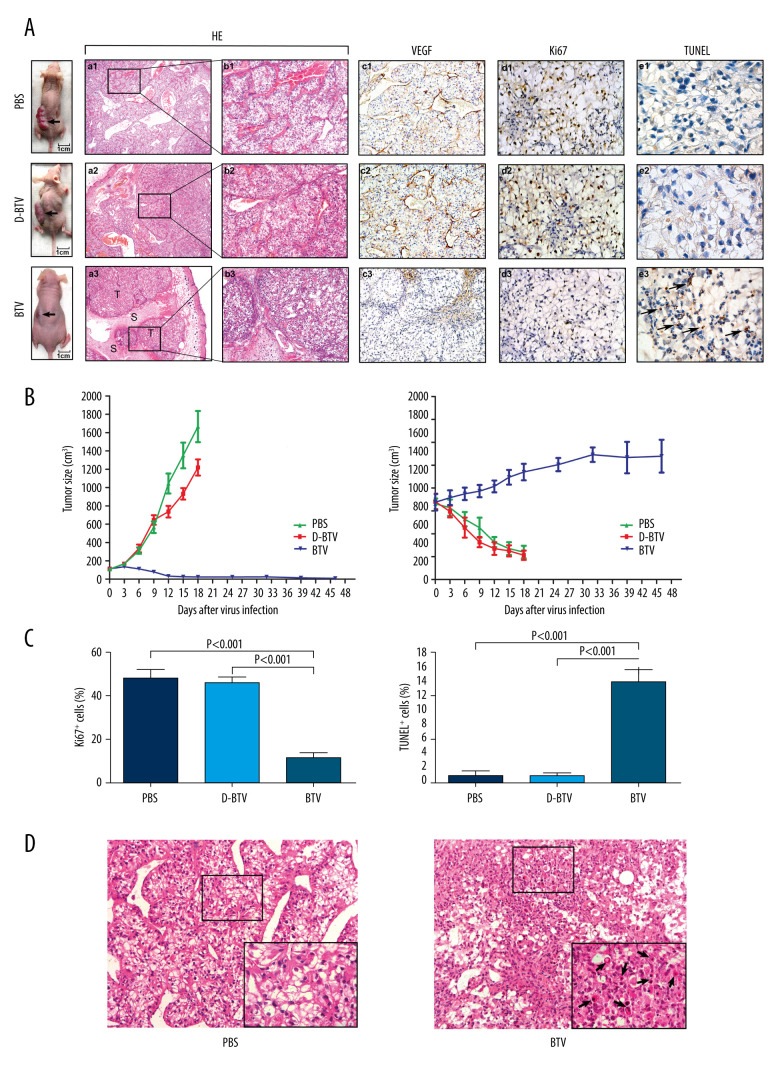
To determine whether BTVs could be used to treat established xenograft tumors in vivo, we used an intratumoral injection of the virus to treat established subcutaneous OS-RC-2 tumors (Figure 3A). The D-BTV- and PBS-treated mice were subsequently sacrificed under anesthesia after 18 days of treatment because of weight loss. Moreover, the BTV-treated mice were examined 4 weeks after BTV treatment. It was found that all BTV-treated mice survived after 6 intratumoral administrations of BTV and 67% (4/6) of BTV-treated animals were apparently cured.
Figure 3.
BTVs exert antitumor effects in a renal cancer OS-RC-2 xenograft mouse model. (A) Representative tumor sections from treated mice were harvested after treatment and analyzed by H&E (×40 and×100), cell proliferation (Ki67 ×400), apoptosis (TUNEL ×1000), or angiogenesis (VEGF ×200), by immunohistochemistry. The H&E staining shows that the tumor tissue had disintegrated into smaller components. T – tumor tissue, S – tumor stroma. Sinusoid-like blood vessels were occlusive, degenerated, or absent entirely. The VEGF staining showed less immunoreactivity in BTV-treated tumors than in control groups. (B) Tumor volume (mm3) was measured and the data are presented as mean±SD. Body weight was monitored continuously, and the data shown represent the mean±SD. (C) Quantification of mitotic index (left), and apoptosis (right). Labeled cells were counted in an average of 6 high-power fields. Data represent means±SEM. (D) H&E staining also revealed apoptotic-type cells, such as condensed nuclei and cytoplasm (arrow), in BTV-treated tumors.
As shown in Figure 3A, direct, intratumoral injections of BTVs significantly decreased the tumor volume compared to animals that received no virus treatment. Differences in tumor size were most dramatic at later time points. The BTV-treated animals continued to gain weight, while the D-BTV and PBS groups showed significant weight loss (Figure 3B). H&E staining and immunohistochemical analysis were performed to examine the histological sections. Macroscopically, a profuse network of small, thin-walled, sinusoid-like blood vessels were observed in the D-BTV and PBS groups (Figure 3A). The VEGF was expressed predominantly in the cytoplasm of tumor cells and vascular endothelial cells (Figure 3A). In contrast, the VEGF showed less immunoreactivity in the BTV-treated tumor compared to the 2 control groups (Figure 3A). Furthermore, the BTV-treated group also showed degeneration of the vasculature and tumor tissue as assessed by H&E staining. These differences included tissues that had degraded. The sinusoid-like blood vessels had been occlusive, degenerated, or absent entirely (Figure 3A). Additionally, apoptotic cells were also found in BTV-treated histological sections. In Figure 3C, tumor sections collected from BTV-treated mice showed significantly (P<0.001) enhanced apoptosis as detected by TUNEL assay and significant (P<0.001) inhibition of proliferation in vivo as detected by Ki67 staining. Figure 3D is a photomicrograph of a section of tissue from BTV-treated mice. The arrows depict apoptotic cells that are shrunken and show a condensed cytoplasm. The nuclei are pyknotic and fragmented.
To determine if BTVs are safe when administered in immunocompromised (nude) mice, we delivered BTV doses as high as 6×106 PFUs in this study. During the experiment, all mice survived until sacrifice. A histologic examination of the main organs (brain, liver, spleen, lung, and kidneys) showed normal histological features and no evidence of diffuse inflammation.
Induction of apoptosis by BTVs through a mitochondria-mediated pathway
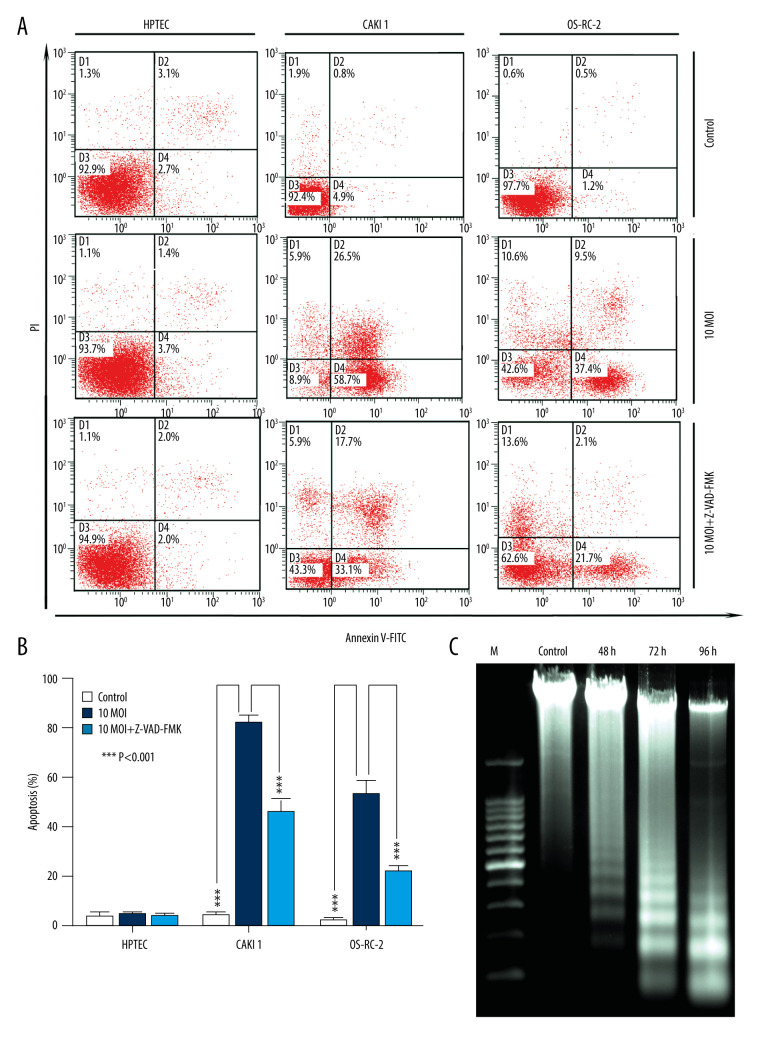
As shown in Figure 4A, infection with BTVs for 24 h significantly (*** P<0.001) increased the percentage of apoptotic cells (annexin V-FITC-positive) CAKI-1 and OR-SC-2 cells but not HPTEC cells. Moreover, to further confirm if BTV-induced apoptosis is caspase-dependent, cancer cells were pre-treated with a pan-caspase inhibitor, Z-VAD-FMK (10 μM), and apoptosis was analyzed by flow cytometry. The results showed marked inhibition of apoptosis in the pre-treated cells at 24 h, indicating that caspase activity is required for BTV-triggered apoptosis in renal cancer cells (*** P<0.001) (Figure 4B). The degradation of DNA into a specific fragmentation pattern (consisting of DNA ladders) is a characteristic feature of apoptosis. Agarose gel electrophoresis of the DNA from CAKI-1 cells revealed the formation of clear DNA fragmentation ladders in a time-dependent manner (Figure 4C).
Figure 4.
BTV induces apoptosis in renal cancer cells. (A) Apoptosis was analyzed by flow cytometry. The HPTEC, CAKI-1, and OR-SC-2 cells were pre-treated with a pan-caspase inhibitor Z-VAD-FMK (10 μM) for 24 h before BTV infection (MOI=10, 24 h). The untreated cells served as control. (B). Quantification of apoptosis. The cell population in the right 2 quadrants (Annexin V-positive) correspond to the apoptotic cells. Columns, mean; bars, SEM. (C) The ladder-like pattern of DNA fragmentation was observed in BTV-infected CAKI-1 cells at 48, 72, and 96 h after BTV infection (MOI=10).
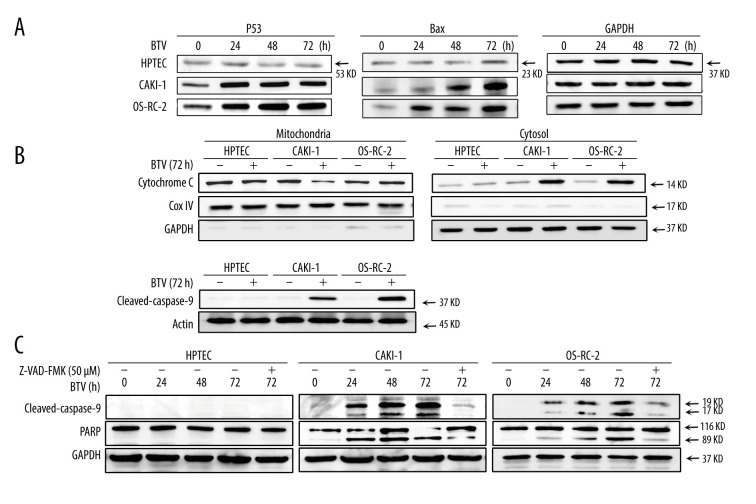
The processes of apoptosis require p53, which also plays an important role in the pathogenesis of many infectious diseases, including those caused by viruses [19]. Hence, we examined the activation of p53 in cells. As shown in Figure 5A, the p53 protein expression was markedly upregulated after infection in BTV-infected cancer cells in a time-dependent manner. Compared to mock infected cells, the Bax protein levels were also elevated after infection (Figure 5A). During the intrinsic apoptotic process, Bax is translocated from the cytoplasm to mitochondria, leading to the discharge of cytochrome c. This process prompted us to further investigate the activation of the mitochondria apoptotic pathways. Figure 5B shows an increase in cytochrome c in the cytosol 72 h after BTV infection in all cancer cell lines.
Figure 5.
BTV induces mitochondria-mediated apoptosis in renal cancer cells. GAPDH and β-actin were used as loading control for cytosolic fraction and COX IV was used for mitochondrial fraction. (A). Cells were treated as described previously. Detection of P53, Bax expression. The p53 and Bax protein expression levels were upregulated in cancer cells but not in HPTEC. (B) The cytochrome c protein level was elevated in the cytosolic fractions in cancer cells. (C) Detection of the activated form of caspase-3, -9, and cleaved poly (ADP-ribose) polymerase (PARP). Pre-treatment of CAKI-1 and OS-RC-2 cells with Z-VAD-FMK (10 μM) for 24 h before BTV infection (MOI=10) markedly inhibited the expression of the activated form of caspase-3 and PARP.
We also analyzed the activation of caspase-3 and -9 and the PARP cleavage in BTV-infected cells. As shown in Figure 5B, BTV infection in cancer cells resulted in the proteolytic cleavage of procaspase-9 to the active cleaved products. This cleavage indicated that the effector caspase-9 was activated during BTV-triggered apoptosis. To confirm the involvement of caspases in BTV-triggered apoptosis, the cells were pre-treated with Z-VAD-FMK. The results showed that pre-treating the CAKI-1 and OS-RC-2 cells with 10 μM Z-VAD-FMK for 24 h markedly prevented the BTV-triggered apoptosis as well as the cleavage of procaspase-3 and PARP (Figure 5C). These finding indicate that the inactivation of caspase-3 attenuates BTV-triggered apoptosis in renal cancer cells, which was consistent with the results of the annexin V staining (Figure 4A).
Discussion
A previous experimental study demonstrated the oncolytic potential of BTV-10 against human hepatic cancer and lung cancer cell lines [17]. Our current study for the first time investigated the potential utility of BTVs against human renal cancer in vitro and in vivo. This potential is particularly surprising because BTV infection was previously considered to be ruminant-specific, and BTV was thought to be incapable of infecting human cells. Here, we made several observations. Firstly, BTV selectively infects and kills renal cancer cells in vitro as opposed to normal cells. Secondly, BTV exhibits efficacy in oncotherapy without significant toxicities in vivo in an immunocompromised nude mouse host. Thirdly, BTV triggers apoptosis through a mitochondria-mediated pathway. These observations suggest that BTV warrants further evaluation as a novel natural oncolytic virus against human renal cancer.
Renal cell carcinoma (RCC), the most lethal type of genitourinary cancer, is generally resistant to chemotherapy and radiation therapy. Therefore, novel therapeutic approaches are urgently needed for the management of RCC. Several virotherapy candidates have been tested in preclinical and clinical trials to date to evaluate their safety and efficacy [20,21]. Using viruses in conjunction with other forms of treatment could prove to be a useful strategy for the treatment of human cancers. Oncolytic virotherapy employing nature’s own agents to find and destroy malignant cells is a promising prospective treatment option that can be applied in the treatment of RCC [22]. The natural tropism of BTVs occurs in domestic animals, including sheep and cattle, as well as wild ruminants. The BTV has never been demonstrated to infect humans until now. Consequently, we confirm that BTV is a newly discovered member of natural oncolytic viruses.
A previous study found that the molecular basis for this selective oncolysis is based on activation of the Ras/RalGEF/p38 pathway, similar to that of a reovirus [23]. However, oncogenic Ras mutations that activate downstream signaling pathways are rare and are found in only 1% of kidney tumors [24]. Our ultramicroscopic analysis did not reveal viral particles in normal cells, which clearly revealed the selective cytotoxic effect of BTVs to human. Hence, these data and those of previous studies suggest the involvement of an unknown pathway. Successful viral replication requires not only the efficient production and spread of progeny, but also evasion of the host defense mechanisms that limit replication by killing the infected cells. The BTVs are double-stranded (ds) RNA viruses, which are good inducers of interferons (IFN). Therefore, we hypothesized that an impaired anti-viral response may be attributed to the failure of critical components of the interferon (IFN) response system, which likely contributes to this oncolytic capability. The virus-triggered apoptosis plays an important part in the oncolytic capability [25]. In the present study, we first investigated the BTV-triggered apoptosis in renal cancer cell lines through a mitochondria-mediated pathway. The immunoblot analyses revealed considerable upregulation of p53 and Bax after BTV infection. The induction of apoptosis facilitates the dissemination of BTVs and is an essential step in the viral life cycle. Here, we showed that BTV-induced apoptosis in RCC cell lines is facilitated by signaling through a mitochondria-mediated pathway. Subsequent flow cytometry and DNA fragmentation analyses revealed that BTV-triggered apoptosis depends on activation of caspases. Nonetheless, the apoptotic phenomenon was not completely inhibited by the pan-caspase inhibitor Z-VAD-FMK. Accumulating evidence in the literature supports the existence of caspase-independent programmed cell death pathways (CI-PCD) [26]. Our results suggest that apoptosis and CI-PCD can occur concurrently in BTV-infected cancer cells. The identification of these selective replication mechanisms and potential CI-PCD mechanisms is currently in progress.
To the best of our knowledge, BTVs have not been previously demonstrated to exhibit a therapeutic effect on human cancers in vivo. Here, we demonstrated that BTVs exhibit an oncolytic effect on the OS-RC-2 renal cancer cells transplanted in nude mice. Importantly, the intratumoral administration of BTVs dramatically improved the survival rate, and 67% (4/6) of BTV-treated animals were apparently cured. Consistent with our in vitro study, BTVs effectively inhibited the human renal cancer growth in vivo. Taken together, the evidence shows that BTVs are effective and non-pathogenic against renal cancer in our animal model. Furthermore, high doses of BTVs and other administration routes, such intraperitoneal or intravenous injection, should be evaluated in future studies. In the present study, we report BTV as a novel oncolytic virus that selectively inhibits human renal cancer cell growth. We strongly believe that our findings will reinforce further research endeavors on this non-pathogenic virus to treat human malignant neoplasms. Future research should focus on the applicability of this treatment in a clinical setting.
Conclusions
Collectively, the findings of the present study for the first time demonstrate the oncolytic potential of BTV in experimental models of human renal cancer. Our study further indicated that BTV triggers apoptosis in renal cancer cells via a mitochondria-mediated pathway.
Footnotes
Conflict of Interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China (Grant No. 81502196)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Harrington K, Freeman DJ, Kelly B, et al. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019;18(9):689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 3.Yoon AR, Hong J, Li Y, et al. Mesenchymal stem cell-mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Cancer Res. 2019;79(17):4503–14. doi: 10.1158/0008-5472.CAN-18-3900. [DOI] [PubMed] [Google Scholar]
- 4.Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: Replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–27. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aref S, Bailey K, Fielding A. Measles to the rescue: A review of oncolytic measles virus. Viruses. 2016;8(10):294. doi: 10.3390/v8100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twumasi-Boateng K, Pettigrew JL, Kwok YYE, et al. Publisher Correction: Oncolytic viruses as engineering platforms for combination immunotherapy. Nat Rev Cancer. 2018;18(8):526. doi: 10.1038/s41568-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 7.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–19.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly K, Nawrocki S, Mita A, et al. Reovirus-based therapy for cancer. Expert Opin Biol Ther. 2009;9(7):817–30. doi: 10.1517/14712590903002039. [DOI] [PubMed] [Google Scholar]
- 10.Benencia F, Courreges MC, Fraser NW, Coukos G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol Ther. 2008;7(8):1194–205. doi: 10.4161/cbt.7.8.6216. [DOI] [PubMed] [Google Scholar]
- 11.Breitbach CJ, Paterson JM, Lemay CG, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15(9):1686–93. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 12.Li JKKL. Bluetongue virus (BTV): propagation, quantification, and storage. Curr Protoc Microbiol. 2012;Chapter 15(Unit15C.4) doi: 10.1002/9780471729259.mc15c04s24. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Dong CY, Li JK, et al. Selective in vitro cytotoxic effect of human cancer cells by bluetongue virus-10. Acta Oncol. 2008;47(1):124–34. doi: 10.1080/02841860701403038. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Chen MN, Cheng K, et al. Cytotoxic effect of a combination of bluetongue virus and radiation on prostate cancer. Exp Ther Med. 2014;8(2):635–41. doi: 10.3892/etm.2014.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valente MJ, Henrique R, Costa VL, et al. A rapid and simple procedure for the establishment of human normal and cancer renal primary cell cultures from surgical specimens. PLoS One. 2011;6(5):e19337. doi: 10.1371/journal.pone.0019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi W, Johnson DW, Vesey DA, et al. Isolation, propagation and characterization of primary tubule cell culture from human kidney. Nephrology (Carlton) 2007;12(2):155–59. doi: 10.1111/j.1440-1797.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 17.Avia M, Rojas JM, Miorin L, et al. Virus-induced autophagic degradation of STAT2 as a mechanism for interferon signaling blockade. EMBO Rep. 2019;20(11):e48766. doi: 10.15252/embr.201948766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Tolstov Y, Arslan A, et al. Harnessing the p53-PUMA axis to overcome DNA damage resistance in renal cell carcinoma. Neoplasia. 2014;16(12):1028–35. doi: 10.1016/j.neo.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turpin E, Luke K, Jones J, et al. Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J Virol. 2005;79(14):8802–11. doi: 10.1128/JVI.79.14.8802-8811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JW, Kang E, Kwon OJ, et al. Local sustained delivery of oncolytic adenovirus with injectable alginate gel for cancer virotherapy. Gene Ther. 2013;20(9):880–92. doi: 10.1038/gt.2013.10. [DOI] [PubMed] [Google Scholar]
- 21.Cheema TA, Wakimoto H, Fecci PE, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci USA. 2013;110(29):12006–11. doi: 10.1073/pnas.1307935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheema TA, Kanai R, Kim GW, et al. Enhanced antitumor efficacy of low-dose Etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin Cancer Res. 2011;17(23):7383–93. doi: 10.1158/1078-0432.CCR-11-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norman KL, Hirasawa K, Yang AD, et al. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci USA. 2004;101(30):11099–104. doi: 10.1073/pnas.0404310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanus DM, Mentle IR, Motzer RJ, et al. Infrequent ras oncogene point mutations in renal cell carcinoma. J Urol. 1990;143(1):175–78. doi: 10.1016/s0022-5347(17)39905-6. [DOI] [PubMed] [Google Scholar]
- 25.Nuovo GJ, Garofalo M, Valeri N, et al. Reovirus-associated reduction of microRNA-let-7d is related to the increased apoptotic death of cancer cells in clinical samples. Mod Pathol. 2012;25(10):1333–44. doi: 10.1038/modpathol.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–98. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]