Abstract
Background
Percutaneous transforaminal endoscopic surgery has been used as a surgical measure for lumbar lateral recess stenosis. However, the necessary decompressive range has never been clearly documented in detail. Here, we discuss the effectiveness of a percutaneous transforaminal endoscopic procedure with clearly defined decompressive range.
Material/Methods
The relevant data were retrospectively collected from a series of degenerative lateral recess stenosis patients who acquired a prospectively designed percutaneous transforaminal endoscopic procedure in our department. The decompressive procedure mainly included undercutting of superior articular process and intervertebral disk annuloplasty. Leg pain and back pain was evaluated using visual analogue scale (VAS). The functional status was assessed using Oswestry disability index (ODI). The clinical results were also evaluated using MacNab criteria.
Results
From May 2014 to October 2018, a total of 33 patients who met our inclusion criteria were included for analysis. There were no perioperative complications. Leg pain VAS decreased from preoperative score of 6.18±2.38 to final follow-up score of 0.45±1.00 (P<0.01). Back pain VAS decreased from preoperative score of 1.88±2.19 to final follow-up score of 0.64±1.02 (P<0.01). ODI (%) decreased from preoperative score of 47.86±18.15 to final follow-up score of 6.29±6.75 (P<0.01). At the final follow-up, the results of MacNab criteria were excellent in 18 cases (54.55%), good in 14 cases (42.42%), fair in 1 case (3.03%) and poor in 0 cases. None of the patients complained of recurrence of the symptoms during follow-up.
Conclusions
Undercutting of “superior articular process neck” plus intervertebral disk annuloplasty is sufficient for lumbar lateral recess decompression in a transforaminal approach.
MeSH Keywords: Endoscopy, Foraminotomy, Lumbar Vertebrae, Spinal Stenosis
Background
Lateral lumbar stenosis is further divided into 3 subdivisions: entrance zone stenosis, mid zone stenosis, and exit zone stenosis [1]. Anatomically, lateral recess stenosis (LRS) refers to narrowing of the entrance zone, which is bordered laterally by the pedicle, posteriorly by the superior articular facet, and anteriorly by the posterolateral surface of the vertebral body and the adjacent intervertebral disk [2]. Laminectomy and a partial facetectomy was adopted to release a stenotic recess [2]. Yet, removal or damage of the posterior structures may lead to iatrogenic instability.
Less invasive direct decompressive techniques for LRS were designed to reserve as much stable structure as possible. These techniques may be classified into 3 categories: conventional open surgeries, tubular retractor surgeries, and percutaneous endoscopic surgeries through water media. In open surgeries, less invasive decompression is accomplished by partial undercutting facetectomy, with the assistance of special designed Kerrison rongeur or by iO-Flex system [3–6]. In tubular surgeries, the paraspinal muscles are protected to further decrease iatrogenic damage [7,8]. Percutaneous endoscopic surgery through water media, which would provide the smallest diameter instrument, is probably the least invasive methods theoretically and the decompressive procedures would be finished through transforaminal approach or posterior interlaminar approach.
In percutaneous endoscopic surgeries, although different approaches are applied for the interlaminar approach, such as unilateral approach [9], contralateral approach [10] and biportal approach [11,12], the results are generally positive. However, different voices came from the seemingly monotonous transforaminal approach. The effectiveness of transforaminal approach remains controversial [13–17]; in addition, the decompressive procedure details have not been sufficiently clarified. In this paper, we try to identify the necessary decompressive range by presenting clinical outcomes of a series of degenerative LRS cases that had percutaneous transforaminal endoscopic surgery (PTES) with clearly defined decompressive range.
Material and Methods
The present research plan followed the principles outlined in the Declaration of Helsinki, got approval from the Degree Awarding Committee of the author’s university and Ethics Committee of the author’s hospital.
Decompressive range definition
The decompressive procedure was designed to be performed from both the dorsal side and ventral side of an impinged nerve root (Figures 1–3). For dorsal decompression, the key steps of our technique are to do a “foraminoplasty” through resection of the lower part of the superior articular process (SAP), which is bordered cranially by the caudal attachment of capsule and caudally by superior edge of pedicle. We call this portion “superior articular process neck”, which looks like the “neck” of the SAP from a posterolateral view and is the main barrier for the endoscope to approach lateral recess in PTES. At the same time, the tip of the SAP and facet joint should be reserved intact. The ventral portion of the SAP neck is to be undercut and the corresponding ligamentum flavum is to be removed until the dorsal side of the nerve tissue is exposed. After dorsal decompression, annuloplasty of the bulged disk is performed as ventral decompression.
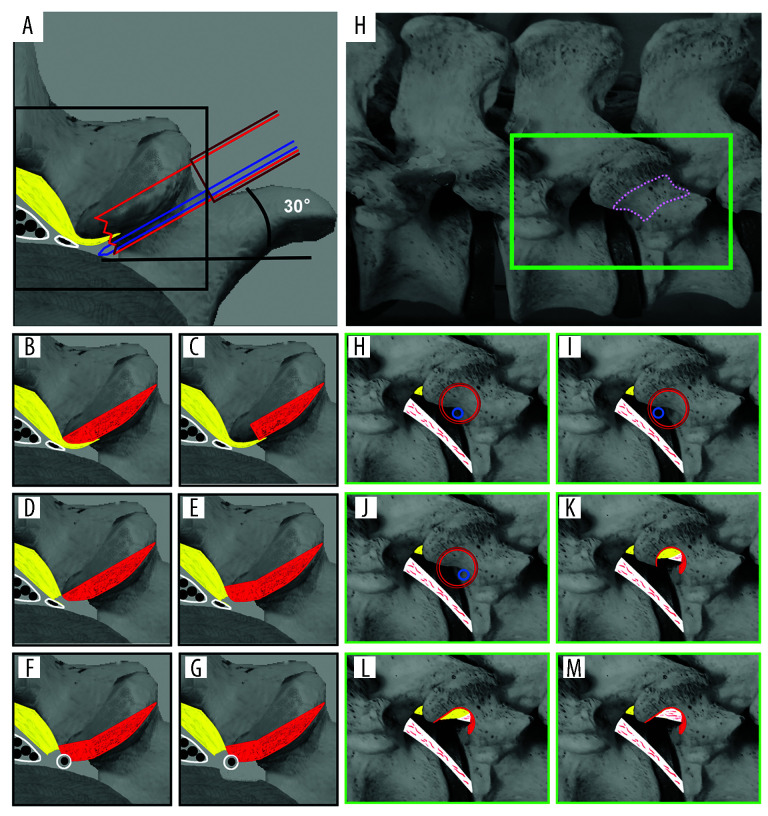
Figure 1.
Illustration of non-sequential reamer foraminoplasty. (A–G) Superior axial plane view. (A) Illustration shows the position of the guiding rod (blue), reamer (red), working tube (brown) and bone to be removed. (B) Complete removal of the bone en-bloc. (C) Incomplete removal of the bone. (D) Resection of ligamentum flavum. (E) Trimming under endoscope. (F) Further resection of ligamentum flavum. (G) Annuloplasty. (H–N) Posterolateral view of the neuroforamen in PTES. (H) Global view. Pink dotted line area is the “SAP neck”. (I) Illustration shows the position of the guiding rod (blue), reamer (red), working tube (brown) and bone to be removed. (J, K) The reamer can be adjusted to an ideal position by rotating around the guiding rod. (L) Ligamentum flavum exposure after removal of the bone. (M) Bone window is trimmed under endoscope. (N) Resection of ligamentum flavum and final exposure of the neural tissue.
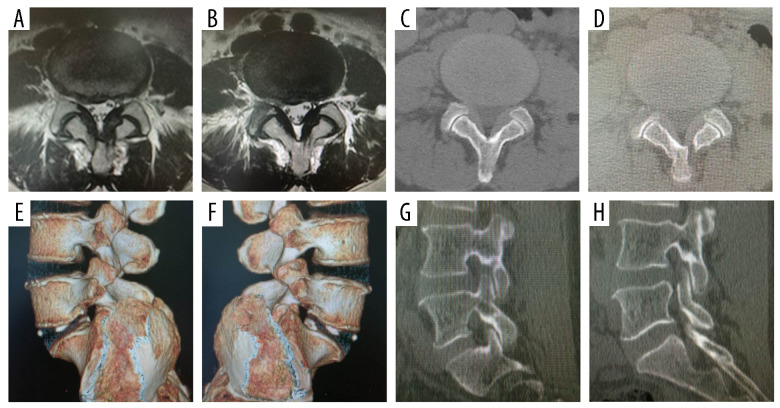
Figure 2.
Preoperative and 3-day postoperative images show the representative decompression range. (A) Preoperative axial magnetic resonance image (MRI). (B) Postoperative axial MRI image. (C) Preoperative axial computed tomography (CT) image. (D) Postoperative axial CT image. (E) Postoperative reconstruction CT image shows non-surgical side. (F) Postoperative reconstruction CT image shows surgical side. (G) Postoperative sagittal CT image shows non-surgical side. (H) Postoperative sagittal CT image shows surgical side.
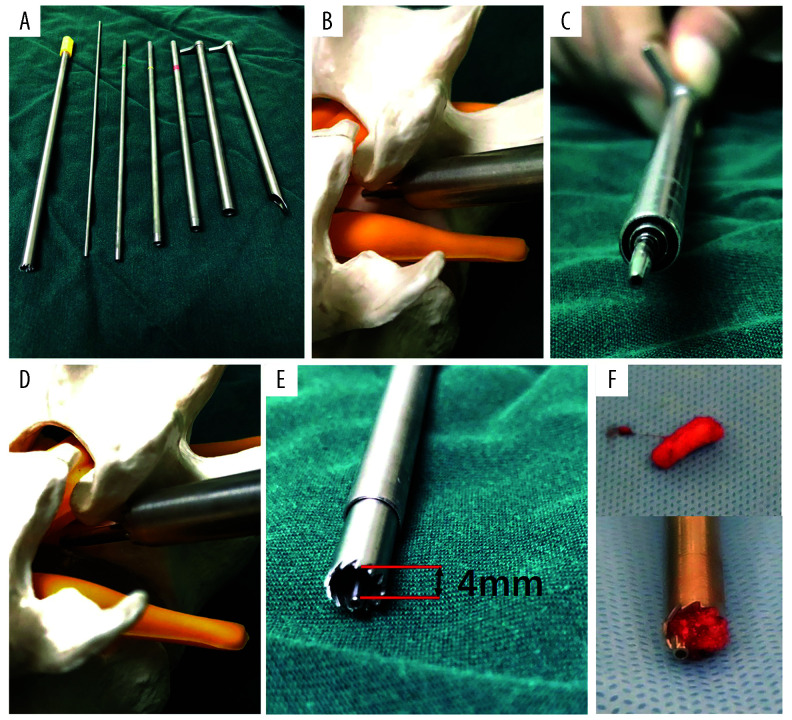
Figure 3.
Working principle of the instruments in a non-sequential reamer forminoplasty. (A) Main instruments. From left to right: 6.5 mm reamer, 2.5 mm guiding rod, 2.9 mm guiding tube, 5.2 mm guiding tube, 6.3 mm guiding tube, 7.5 mm working tube with straight 90° end without lips, 7.5 mm working tube with standard lip. (B) The guiding tubes and working tube with straight 90° end without lips stop on the SAP neck. (C) The arrangement of the instruments on the SAP neck. (D) 6.5 mm reamer working in the working tube. (E) The maximal bone removal is 4 mm thick. (F) Bone chip generated by the reamer.
Clinical data collection
Patient inclusion criteria were as follows: 1) unilateral intractable radiculopathy with or without low back pain; and 2) mono-segmental degenerative lateral recess stenosis. Patient exclusion criteria were as follows: 1) concomitant lumbar disc extrusion or sequestration; 2) concomitant foraminal stenosis; 3) concomitant scoliosis; 4) patients with segmental instability at the time of operation; 5) procedures were not accomplished according to the decompressive design; 6) cases who had not reached 1-year follow-up. The data from our department, including leg pain and back pain visual analogue scale (VAS) scores, Oswestry Disability Index (ODI) scores, and MacNab criteria were used for analysis.
Surgical procedures
A spinal endoscope system (Joimax HD Foraminoscope for TESSYS, L171 mm/Ø 6.3 mm/30°/3.7 mm WCh, joimax® GmbH, Karlsruhe, Germany) and relative instruments were used in all the cases.
Patient position and intervertebral foramen puncture
The patient was placed in a prone position. The ideal puncture trajectory was 30° to the coronal plane and 20–30° to the axial plane of the target segment (Figures 1, 4). The entrance point was selected based on the our targeted ideal foraminal acupuncture trajectory. An 18-gauge spine needle was used for puncture and local anesthesia. The skin, subcutaneous tissue, deep fascia in the trajectory, and SAP were anesthetized with 1% lidocaine. The needle was inserted downward into neuroforamen with the tip slipping along the SAP neck. Then, the spine needle was replaced with a 2.5 mm guiding rod. Sometimes, a guiding rod was used directly to puncture neural foramen for easier manipulation. Ideally, the rod should touch the SAP neck after stopping in lateral recess and the position of the rod should be confirmed on the AP and lateral view (Figure 4).
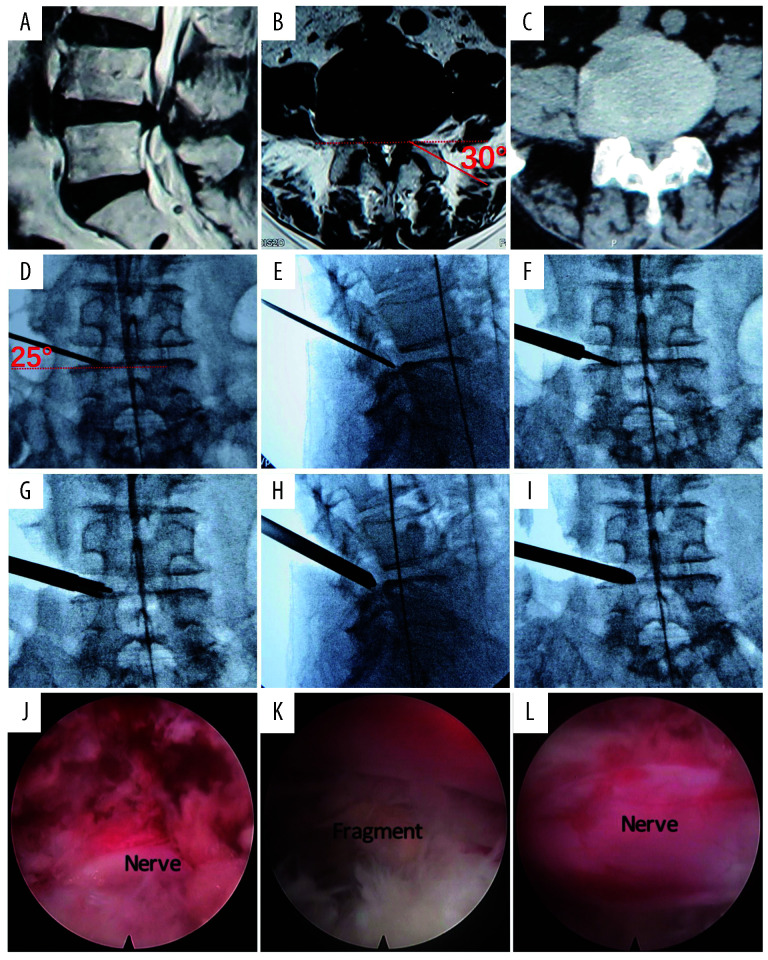
Figure 4.
Key surgical procedure of a lateral recess stenosis (LRS) case with left radiculopathy. (A–C) Image studies. (A) Sagittal magnetic resonance image (MRI) shows L4/5 stenosis. (B) Axial MRI of L4/5. Red line shows the designed foraminoplasty trajectory. (C) Axial computed tomography (CT) of L4/5. (D–I) Intraoperative x-ray images. (D) Guiding rod on AP view. The rod is 30° to axial plane. (E) Guiding rod on lateral view. (F) Insertion of the guiding tubes and straight 90° end working tube. (G) Reaming under x-ray guidance. (H) Lateral view shows insertion of the working tube with standard lip. (I) AP view shows insertion of the working tube with standard lip. (J–L) Endoscopic images. (J) Image after dorsal decompression. (K) Annuloplasty on the bulged disk and degenerative fragment tissue is shown. (L) Freed traversing nerve after dorsal and ventral decompression.
Foraminoplasty under fluoroscopy guidance
The reamer was used in a sequential manner or a non-sequential manner (Figures 1, 3). The former has been fully introduced in a previous study [18]. For the later, a reamer (ID 5.5 mm/OD 6.5 mm) was used directly to do resection on the SAP. Briefly, a working tube with straight 90° end without lips (ID 6.5 mm/OD 7.5 mm) was introduced after soft tissue dilation with sequential guiding tubes. The tubes all stopped on the SAP neck right at the gate of intervertebral foramen. The guiding tubes were removed, and the working tube was moved dorsally on the SAP. The reamer was introduced through the working tube to remove the bones after confirming the position of the reamer on AP and lateral view. Theoretically, a maximal 4 mm bone chip would be removed by one reamer cutting. Then the reamer and the guiding rod were rotated out together with the resected bone chips. In some cases, the reamer could not cut through all the bones in its trajectory and the reamer was stopped by the stiff medial part of the SAP. If so, the reamer and the guiding rod were held together and twisted up and down to break the bones in the reamer from the SAP and the remnant medial part of the SAP in the reamer trajectory would be removed under endoscope.
Endoscopic procedures
The straight 90° end without lips working tube was replaced with a working tube with standard lip and the endoscope was introduced. The foraminoplasty window, together with the residual medial part of the SAP (if exists), were trimmed with an endoscopic reamer, chisel or high-speed drill. The exposed ligamentum flavum was removed until dorsal part of the traversing nerve root was exposed. Then, ventral annuloplasty on the bulged disk was performed until the bulging disk was flattened. The loosened annular tissues should be removed as thoroughly as possible once the annular fibrosus was broken through. The pulse of the nerve should be observed, and the nerve should move with irrigation at the end of decompression (Video 1).
Video 1.
Video shows key endoscopic procedures and the decompressive range.
Postoperative protocol
Ambulation was permitted under the protection of an orthosis the next day after operation. The orthosis was suggested to be worn in the following 3 to 4 weeks. The follow-up was performed in clinic or through telephone and internet.
Statistical methods
The data were analyzed with a GB-Stat v70 statistical software (Dynamic Microsystems. Inc. Silver Spring, MD, USA). VAS and ODI were expressed as χ̆±SD. Paired t-test was used to compare preoperative with last follow-up data and P<0.01 was considered a significant difference. The 2-sample t-test was used for comparison of symptom improvement data from sequential reamer patients with non-sequential reamer patients and P<0.05 was considered a significant difference.
Results
Demographics and perioperative data
From May 2014 to October 2018, there were a total of 38 patients who met our inclusion criteria. Five of them were lost during follow-up. So, the data for analysis were from 33 patients who had finished 1-year follow-up (20 males, 13 females, 29–75 years old, average age 56.58 years). There were 25 patients (75.76%) who finished the 2-year follow-up and 5 patients (20%) who finished the 3-year follow-up. All the procedures were performed at L4/5 level by the first author. There were 13 patients who had sequential reamer technique and 20 patients who had non-sequential reamer technique. In all the surgeries, there was no over resection of the SAP and the facet articular surface remained intact. There were no perioperative complications such as neural function deterioration, infection, dural tear, or hematoma.
Clinical outcomes
Leg pain VAS score decreased significantly from preoperative score of 6.18±2.38 to final follow-up score of 0.45±1.00 (P<0.01). The surgical procedure also had an effect on back pain with preoperative VAS score of 1.88±2.19 decreasing to final follow-up score of 0.64±1.02 (P<0.01). Significant functional improvement could also be found with the ODI (%) score decrease from preoperative score of 47.86±18.15 to final follow-up score of 6.29±6.75 (P < 0.01) (Table 1). At the final follow-up, the results of MacNab criteria were excellent in 18 cases (54.55%), good in 14 cases (42.42%), fair in 1 case (3.03%), and poor in 0 case (Table 2). So, excellent and good results were obtained in 96.97% of the patients. The 1 case with a fair MacNab score was a patient who at her 1-year follow-up felt better with her leg pain score decrease from 6 to 4 and her ODI score decrease from 60 to 22.2, although her back pain score increased from 0 to 3. None of the patients complained of recurrence of symptoms during follow-up. There was no significant change of VAS or ODI scores when the data from the patients who had sequential reamer technique and the patients who had non-sequential technique were compared (Table 3).
Table 1.
VAS Score and ODI index of preoperative and last follow-up (χ̆±SD).
| Outcomes | Pre-operative | Last follow-up | P value |
|---|---|---|---|
| VAS (back pain) | 1.88±2.19 | 0.64±1.02 | 0.0017 |
| VAS (leg pain) | 6.18±2.38 | 0.45±1.00 | <.001 |
| ODI (%) | 47.86±18.15 | 6.29±6.75 | <.001 |
VAS – visual analogue scale; ODI – Oswestry disability index; SD – standard deviation.
Table 2.
Outcome of MacNab criteria.
| Outcomes | Number/ total number of patients |
|---|---|
| Excellent | 18/33 |
| Good | 14/33 |
| Fair | 1/33 |
| Poor | 0/33 |
Table 3.
VAS Score and ODI index improvement of 2 foraminoplasty group (χ̆±SD).
| Outcomes | Sequential | Non-sequential | P value |
|---|---|---|---|
| VAS decrease (back pain) | 1.92±2.47 | 0.80±1.70 | 0.13 |
| VAS decrease (leg pain) | 6.38±2.50 | 5.35±2.80 | 0.29 |
| ODI decrease (%) | 46.79±19.24 | 38.17±18.14 | 0.20 |
VAS – visual analogue scale; ODI – Oswestry disability index; SD – standard deviation.
Discussion
Is PTES an effective treatment for LRS?
Controversy exists about the efficiency of PTES in the treatment of LRS (Table 4 [13–16]). Kambin et al. [13] acquired satisfactory results by using the mildest decompression, but cases of severe intervertebral disk narrowing and bony LRS were excluded. Li et al. [14] and Wang et al. [15] reported good clinical results by more aggressive dorsal and caudal bony structure removal. However, the power of persuasion was weakened by mixing or not clearly excluding the cases with concomitant disk herniation. Disk herniation tends to be responsible for the symptoms in concurrent LRS, because surgical outcomes of LRS with concomitant extruded disk herniation were more favorable when being compared with that of LRS with concomitant contained disk bulges [17]. Lewandrowski [17] reported negative results, although aggressive decompression was used, but detailed range of decompression was not reported. Therefore, it is hard to draw a meaningful conclusion from the current results in published literature due to the inconsistency or obscurity of the main components of these study reports, such as inclusion and exclusion criteria, surgical techniques, etc. Probably, data from “classic” degenerative LRS cases [1,2] with clear depiction of decompression details are appropriate for verifying the effectiveness of PTES on degenerative LRS.
Table 4.
Overview of PTES surgical techniques on LRS.
| Research | Inclusion/Exclusion | Measures for decompression | Case number | Time of evaluation | Evaluation methods | Outcomes/Number | Complication/Number |
|---|---|---|---|---|---|---|---|
| Kambin et al. 1996 [13] | Unilateral or bilateral radicular pain/ Severe intervertebral disc narrowing and bony LRS | Annulectomy and nuclear fragments in the disk | 38 | Not exactly mentioned | Leg pain; Back pain; Function | Excellent and good/ 31 | Causalgic-type pain/4; Disk infection/1 |
| Ahn et al. 2004 [16] | Recurrent disc herniation concomitant LRS | Annulectomy and nuclear fragments in the disk; Tip of SAP | 6 | Not mentioned | MacNab; VAS | Successful/ 2 | Not mentioned |
| Lewandrowski 2014 [17] | Monoradiculopathy/ Segmental instability; Severe central stenosis | Ventral SAP; Ventral IAP; Herniated disk; Annulectomy and nuclear fragments in the disk | 32 | 24 months | MacNab; Leg VAS | Fair and poor/32 | None |
| Li et al. 2016 [14] | LRS with or without disk herniation/ Segmental instability; Severe central stenosis | Ventral SAP; Ventral IAP; Intradiscal decompression | 85 | 24 months | VAS; ODI; MacNab | Excellent and good/ 77 | Dysesthesia/3; Herniation recurrence/2 |
| Wang et al. 2016 [15] | Unilateral radicular pain/Concomitant foraminal stenosis | Facet; Upper-edge portion of pedicle | 52 | 24 months | VAS; JOA; ODI; MacNab | Excellent and good/ 44 | Dural laceration/1 |
PTES – percutaneous transforaminal endoscopic surgery; LRS – lateral recess stenosis; VAS – visual analogue scale; IAP – inferior articular process; SAP – superior articular process.
Thus, in the present study, data of “classic” degenerative LRS cases who accepted PTES with the same decompression range performed by a single surgeon were collected and analyzed. All the patients reported improvement, and excellent and good results were obtained in 96.97% of the patients. In addition, there was no complaint of recurrence from the patients at their 2-year or 3-year follow-up. Thus, our data showed that the present PTES technique is an effective treatment for degenerative LRS.
What is the necessary decompressive range for LRS?
The identification of necessary decompression is important for minimally invasive surgery, because invalid structure removal is not only time-consuming but also has the risk for complications. In posterior approach LRS decompression, the decompression territory has been shown to have a decreased tendency as minimally invasive surgery became popular [2–4,6,9] (Table 5). But the smallest necessary range of depression has not been defined. Except for iO-Flex system, the authors tended to free the nerve at least from the origin to intervertebral foramen, anatomically which goes beyond the entrance zone into the mid zone [1].
Table 5.
Overview of LRS decompression from posterior approach.
| Research | Type of surgery | Range of key decompression |
|---|---|---|
| Ciric et al. 1980 [2] | Conventional open: Wide laminectomy and partial facetectomy | Overhanging portion of the superior articular facet; Especially at the level of superior pedicle border; Lateral till the medial surface of the corresponding pedicle; The entire course of the nerve in the lateral recess |
| Sanderson et al. 1996 [3] | Less invasive open: Laminotomy fenestration and partial facet undercut | Part of pars interarticularis and lamina of the upper vertebra; Whole length of the facet joint complex in a cephalocaudal direction; From nerve root origin to passage out through the foramen |
| Çolak et al. 2008 [4] | Less invasive open: Small laminotomy fenestration and partial facetectomy | Part of pars interarticularis and medial facetectomy; Lateral till the medial wall of the pedicle |
| Dickinson et al. 2013 [6] | Less invasive open: Small laminotomy fenestration and partial facet undercut with iO-Flex | Small laminotomy followed by undercutting the superior articular process from ventral side to dorsal side; Caudal till the superior border of pedicle |
| Birjandian et al. 2017 [9] | Percutaneous endoscopy: partial facetectomy | Part of pars interarticularis and medial facetectomy; Partial facetectomy of superior articular process from tip to mid pedicle plane |
Should PTES reach the same territory?
In PTES, enlargement of lateral recess can be achieved through not only dorsal structures removal but also ventral structures removal. Partial removal of the SAP, partial removal of the inferior articular process (IAP), and even complete removal of the SAP have been achieved in dorsal decompression [14,17,19]. For ventral decompression, removal of fibrotic bulging annulus, contained disk, posterolateral marginal osteophytosis, or even removal of endplate and posterior part of vertebral body have been reported [13,20,21]. In addition, caudal pedicle has been reported to be partially resected for more caudal decompression [15]. But the necessary range of decompression has not been clearly documented in the literature.
The anatomy of lateral recess was depicted as an area bordered laterally by the pedicle, posteriorly by the superior articular facet, and anteriorly by the posterolateral surface of the vertebral body and the adjacent intervertebral disk [2]. Degenerative lateral recess stenosis results from degenerative bulged disk, thickened ligamentum flavum, hypertrophic osteophyte of vertebral body and articular process [1,2]. Although it is difficult to correlate clinical manifestations with anatomical anomalies [22] and the diagnostic criteria differ a little from each other, most authors agree on lateral recess stenosis >5 mm in height as normal [2,22]. Thus, our surgical plan was designed to restore lateral recess to this capacity while avoiding unnecessary resection.
Dorsal structure removal was the first step to reach and decompress lateral recess, the ventral portion of the SAP neck was resected. The failure of undercutting this structure has been reported to be the cause of unsuccessful outcomes [16]. Cranially, we reserved the tip of the SAP, which was to be removed in PTES for lumbar foraminal stenosis [23], to avoid invasion to facet. Caudally, we did not resect pedicle, because the narrowest part is at superior pedicle border level [2] rather than mid-pedicular level [9]. Ventrally, we did annuloplasty despite the consideration that stand-alone posterior lateral recess decompression without discectomy is clinically effective for a large majority of patients [8]. Neural canal volume would decrease when the patient was permitted to change posture from supine to upright [24]. Posterior approach surgery has a wider decompression range than the present PTES, which could provide the nerve root a better buffer room to avoid nerve impingement. That was the rational to design a ventral decompression for the present technique. Although aggressive discectomy would lead to unpleasant prognosis [25], none of the patient encounter a recurrent disk problem during follow-up.
Technical consideration
In addition to sequential reamer foraminoplasty technique, we also used a non-sequential reamer foraminoplasty technique which was modified from techniques used by the pioneers in PTES [16,18,23]. In our non-sequential technique, the guiding rod and working tube worked like a Vernier caliper. Theoretically, a maximal 4 mm bone chip would be removed through one reamer cutting. The 5 mm goal would be achieved if the ligamentum flavum resection and ventral decompression was included. Thus, the endoscopic instruments were used for trimming, instead of further extensive bone removal. In addition, the reamer did not cut into the facet articular space as it was designed when 4 mm thick ventral bone was removed with a 30° angle. At the same time, the present trajectory could provide a good view angle to explore the epidural space. Although with more efficiency, the non-sequential technique did not show a superior clinical result than sequential technique. It seems that decompression range plays the key role rather than technique.
Limitations and prospect
The present study was designed without a control series and the sample size was relatively small. Smaller “necessary” decompressive range may exist. In fact, we did find that the patients usually could achieve relief from radiculopathy immediately after dorsal decompression during the operation. Can we just do dorsal decompression alone while leaving the ventral bulged disk intact to further simplify the procedure? This hypothesis should be tested in the future.
Conclusions
Undercutting of “superior articular process neck” plus intervertebral disk annuloplasty is sufficient for lumbar lateral recess decompression in a transforaminal approach.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: Classification, pathologic anatomy and surgical decompression. Spine (Phila Pa 1976) 1976;13:313–20. doi: 10.1097/00007632-198803000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Ciric I, Mikhael MA, Tarkington JA, Vick NA. The lateral recess syndrome A variant of spinal stenosis. J Neurosurg. 1980;53:433–43. doi: 10.3171/jns.1980.53.4.0433. [DOI] [PubMed] [Google Scholar]
- 3.Sanderson PL, Getty CJ. Long term results of undercutting facetectomy of lumbar lateral recess stenosis. Spine (Phila Pa 1976) 1996;21:1352–56. doi: 10.1097/00007632-199606010-00015. [DOI] [PubMed] [Google Scholar]
- 4.Çolak A, Topuz K, Kutlay M, et al. A less invasive surgical approach in the lumbar lateral recess stenosis: Direct approach to the medial wall of the pedicle. Eur Spine J. 2008;17:1745–51. doi: 10.1007/s00586-008-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank EH, Martin J, Hsu FPK. An endoscopic curved Kerrison rongeur for spinal stenosis surgery. Minim Invasive Neurosurg. 2002;45:254–56. doi: 10.1055/s-2002-36199. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson LD, Phelps J, Summa CD, et al. Facet-sparing decompression with a minimally invasive flexible microblade shaver: A prospective operative analysis. J Spinal Disord Tech. 2013;26:427–36. doi: 10.1097/BSD.0b013e318290fc62. [DOI] [PubMed] [Google Scholar]
- 7.Mikami Y, Nagae M, Ikeda T, et al. Tubular surgery with the assistance of endoscopic surgery via midline approach for lumbar spinal canal stenosis: A technical note. Eur Spine J. 2013;22:2105–12. doi: 10.1007/s00586-013-2806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulkarni AG, Patel R, Dutta S, Patil V. Stand-alone lateral recess decompression without discectomy in patients presenting with claudicant radicular pain and MRI evidence of lumbar disc herniation. Spine (Phila Pa 1976) 2017;42:984–91. doi: 10.1097/BRS.0000000000001944. [DOI] [PubMed] [Google Scholar]
- 9.Birjandian Z, Emerson S, Telfeian AE, Hofstetter CP. Interlaminar endoscopic lateral recess decompression – surgical technique and early clinical results. J Spine Surg. 2017;3:123–32. doi: 10.21037/jss.2017.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HS, Patel R, Paudel B, et al. Early outcomes of endoscopic contralateral foraminal and lateral recess decompression via an interlaminar approach in patients with unilateral radiculopathy from unilateral foraminal stenosis. World Neurosurg. 2017;108:763–73. doi: 10.1016/j.wneu.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Soliman HM. Irrigation endoscopic decompressive laminotomy. A new endoscopic approach for spinal stenosis decompression. Spine J. 2015;15:2282–89. doi: 10.1016/j.spinee.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Liu X. A novel biportal full endoscopy technique for lumbar lateral recess stenosis: Technical report. Clin Spine Surg. 2019;32:51–56. doi: 10.1097/BSD.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 13.Kambin P, Casey K, O’Brien E, Zhou L. Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg. 1996;84:462–67. doi: 10.3171/jns.1996.84.3.0462. [DOI] [PubMed] [Google Scholar]
- 14.Li ZZ, Hou SX, Shang WL, et al. Percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar decompression for lateral recess stenosis through transforaminal approach: Technique notes and 2 years follow-up. Clin Neurol Neurosurg. 2016;143:90–94. doi: 10.1016/j.clineuro.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y-P, Zhang W, Li B-L, et al. Suprapedicular foraminal endoscopic approach to lumbar lateral recess decompression surgery to treat degenerative lumbar spinal stenosis. Med Sci Monit. 2016;22:4604–11. doi: 10.12659/MSM.901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn Y, Lee S-H, Park W-M, et al. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine (Phila Pa 1976) 2004;29:E326–32. doi: 10.1097/01.brs.0000134591.32462.98. [DOI] [PubMed] [Google Scholar]
- 17.Lewandrowski K-U. “Outside-in” technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: A retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg. 2014:8. doi: 10.14444/1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol. 2005;17:641–61. doi: 10.1007/s00064-005-1156-9. [DOI] [PubMed] [Google Scholar]
- 19.Sairyo K, Chikawa T, Nagamachi A. State-of-the-art transforaminal percutaneous endoscopic lumbar surgery under local anesthesia: Discectomy, foraminoplasty, and ventral facetectomy. J Orthop Sci. 2018;23:229–36. doi: 10.1016/j.jos.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Kitahama Y, Sairyo K, Dezawa A. Percutaneous endoscopic transforaminal approach to decompress the lateral recess in an elderly patient with spinal canal stenosis, herniated nucleus pulposus and pulmonary comorbidities. Asian J Endosc Sur. 2013;6:130–33. doi: 10.1111/ases.12004. [DOI] [PubMed] [Google Scholar]
- 21.Kambin P, Zhou L. Arthroscopic discectomy of the lumbar spine. Clin Orthop Relat Res. 1997;(337):49–57. doi: 10.1097/00003086-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Steurer J, Roner S, Gnannt R, Hodler J. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: A systematic literature review. BMC Musculoskelet Disord. 2011;12:175. doi: 10.1186/1471-2474-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn Y, Oh HK, Kim H, et al. Percutaneous endoscopic lumbar foraminotomy: An advanced surgical technique and clinical outcomes. Neurosurgery. 2014;75:124–32. doi: 10.1227/NEU.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanno H, Endo T. Does it reproduce the positional change of the dural sac detected by upright myelography? Spine (Phila Pa 1976) 2012;37:E985–92. doi: 10.1097/BRS.0b013e31821038f2. [DOI] [PubMed] [Google Scholar]
- 25.Barth M, Diepers M, Weiss C, Thome C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 2: Radiographic evaluation and correlation with clinical outcome. Spine (Phila Pa 1976) 2008;33:273–79. doi: 10.1097/BRS.0b013e31816201a6. [DOI] [PubMed] [Google Scholar]