Abstract
Background
Since late 2019, SARS-CoV-2 which leads to coronavirus disease 2019 (COVID-19), has caused thousands of deaths. There are some pieces of evidence that SARS-CoV-2 genome could be re-detectable in recovered patients.
Methods
We performed a systematic review in the PubMed/Medline database to address the risk of SARS-CoV-2 recurrence. The last update was for 20 November 2020. Among the 1178 initially found articles, 66 met the inclusion criteria and were considered.
Findings
In total, 1128 patients with at least one-time recurrence of SARS-CoV-2 were included. Recurrence rate has been reported between 2.3% and 21.4% in cohort studies, within a mean of 20 (ranged 1–98) days after discharge; younger patients are being affected more. Following the second course of disease, the disease severity decreased or remained unchanged in 97.3% while it increased in 2.6%. Anti-SARS-CoV-2 IgG and IgM were positive in 11–95% and 58.8–100%, respectively. Based on the literature, three possibilities include reactivation of previous disease, reinfection with the same virus, and false negative, which have been discussed in details.
Conclusion
There is a relatively notable risk of disease recurrence in previously recovered patients, even those who are immunised against the virus. More studies are required to clarify the underlying cause of this phenomenon.
Keywords: Coronavirus, SARS-CoV-2, COVID-19, recurrence, PCR, systematic review
Introduction
Since starting pandemic of the coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, by end of November 2020, more than 60 million people have been infected; near 1.5 million of them have died globally, although many of them have been recovered. Routine diagnosis of COVID-19 is based on the detection of SARS-CoV-2 virus genome using real-time reverse-transcription polymerase chain reaction (RT-PCR) test. Today, worldwide concerns about the recurrence of SARS-CoV-2 in recovered people are growing, given reports that up to one of the five discharged patients whose symptoms have resolved and have tested negative for COVID-19 turned positive again [1]. However, currently little is known about whether this phenomenon may be attributable to false-positive or false-negative results, the persistence of virus, or reinfection. It has been reported that up to 21.4% of discharged patients may experience becoming positive for viral genome within the days/weeks after discharge.
It is still unclear whether reinfection or reactivation of the previous infection is the reason for appearance of COVID-19 symptoms in the recovered patients. More precisely, distinguishing between the reactivation and reinfection is impossible with the currently available clinical and paraclinical settings [2–6]. Additionally, some studies have mentioned that the discharge criteria might not be rigorous enough [6,7], while others blame the low sensitivity of the nasopharyngeal swab test kits. Ignoring the important route of oral faecal transmission and the possibility of excretion of the virus from the faeces, and not considering it as a criterion for patient discharge is another case that highlights the importance of more sensitive tests to find the viral genome in the faeces [8–10].
Accepting either the hypothesis of reactivation of previous infection or de novo infection can pose many risks in the fight against the pandemic [7,11]. Recently, a meta-analysis has reported 7–23% as the SARS-CoV-2 recurrent RNA positivity rate among the previously recovered COVID-19 patients [12]. However, several other aspects of this phenomenon remained unknown. Considering the important nature of this issue and contradictory results in this regard, we have designed a systematic review to evaluate rate of SARS-CoV-2 recurrence, changes in disease severity, the interval between negative RT-PCR test for SARS-CoV-2 and disease recurrence, disease and patients’ characteristics, risk factors for predicting recurrence and outcomes, as well as the prognosis of recurrent patients.
Search strategy
The PubMed/Medline database was searched for any study associated with the recurrence of SARS-CoV-2 in those patients, who had been recovered and showed negative RT-PCR results. The employed keywords are brought in the Supplementary Table 1. The search results were updated on 20 November 2020. Data were extracted by two authors, independently. In the case of any discrepancy, the third independent reviewer was consulted to make the final decision.
Only journal articles published in English, those with the original data, and only human studies related to recurrence of SARS-CoV-2 in previously recovered patients were included. Recurrence was defined as a reappearance of the SARS-CoV-2 genome based on the RT-PCR test. More precisely, all the included patients should previously be diagnosed based on the RT-PCR test, and their tests became negative after disease recovery, and experience positivity of RT-PCR test for SARS-CoV-2 genome again after a while. Additionally, in the cases with positive RT-PCR test for more than 2 weeks, worsening of symptoms was considered as the disease recurrence, if RNA of virus was still detectable. After a primary screening, based on the titles and abstracts, we did the secondary screening, based on the full-text of articles; eligible studies were carefully read to extract any data regarding the rate of recurrence of SARS-CoV-2, characteristics of patients, disease features, risk factors, and outcomes.
Results
Search results
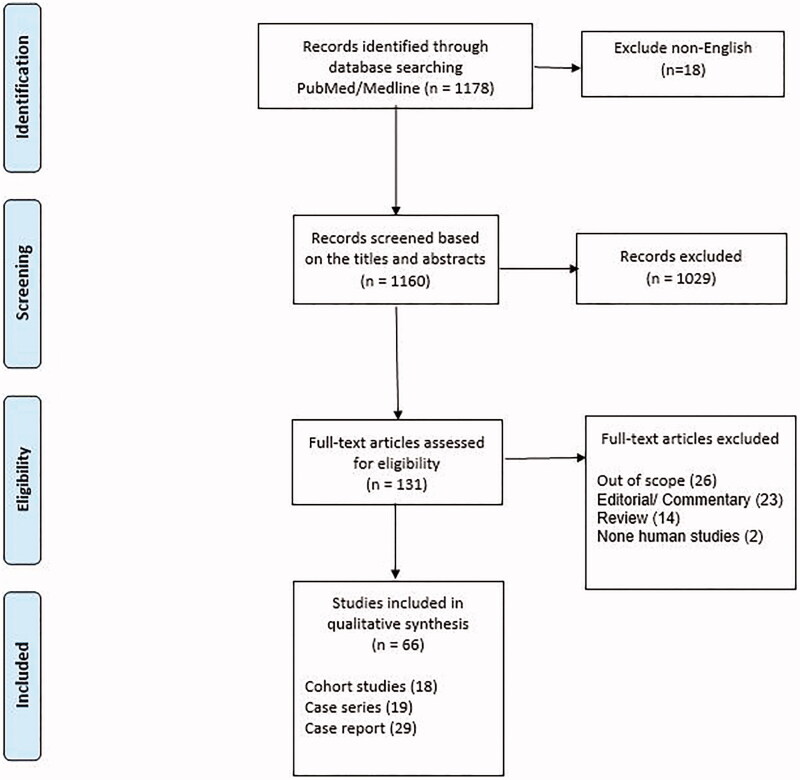
Among the 1178 initially found records, after excluding non-English studies (18 articles), 1160 records had been selected for initial screening, according to the titles and abstracts, in which 1029 of them were irrelevant. Subsequently, among 131 article selected to assessing eligibility for full-text reading, after excluding two non-human studies, and 63 other articles for different reasons including being out of scope or not meeting the inclusion criteria regarding PCR-based diagnosis of patients (n = 26), and lack of original data (23 editorial letters/commentary/Correspondence and 14 review articles). Finally, 66 articles have been selected for evaluation. The most important findings of such studies have been categorised into the rate of recurrence, risk factors, and outcomes, which have been discussed in detail. The study selection process and reasons for exclusions are presented in Figure 1.
Figure 1.
PRISMA 2009 flow diagram.
Patients’ characteristics and recurrence rate
Reviewing cohort studies, case series, and case reports led to finding a total number of 1128 patients with the recurrence of SARS-CoV-2; 957 in cohort studies [1,13–28], 142 in case series [3,17,29–45] and 29 in case report studies [46–74]. The recurrence rate, which has been defined as positive SARS-CoV-2 RNA detection by RT-PCR test in patients who were recovered (showed negative RT-PCR test), was reported between 2.3% and 21.4% in cohort studies [1,13–19,21–28,75]. As an exception, a cohort study on paediatrics reported that seven patients out of 14 (50%) experienced recurrence [20]. Regardless of study type, based on 57 studies with available data (1019 patients), the first detection of recurrence of SARS-CoV-2 had been reported within a mean of 20 days, with a range of 1–140 days after meeting criteria for discharge [3,13,15–17,19–23,26–33,35–39,41–74].
Among the reported patients, eight cases were reported that had experienced recurrence more than once; two times in four patients [20,58,69], three times in two patients [14,50], and four times in two patients [48,62]. In a more detailed review of five cases with positive RT-PCR test for repeated times, most of them were asymptomatic or presented mild symptoms [14,48,50,58,69]. Among them, an eight year-old boy showed the second positive RT-PCR test after 17 days of discharge, and his test remained positive for the next 20 days [14]. In addition, two men, 33 and 35-year old showed the second positive RT-PCR at nearly 2 weeks after discharge. They tested positive for the fourth and third times on days 111 and 49 days of hospital discharge, respectively [48,50].
The most critical data from cohort studies are summarised in Table 1.
Table 1.
The details of included cohort studies.
| First author | Total number | Number of patients with recurrence | Follow-up duration (days) | Details (if any) | |
|---|---|---|---|---|---|
| Li Y | – | 13 | 28 | – | |
| Wang X | 131 | 8 | 28 | Four re-positive patients were readmitted to hospital. | |
| Zhu H | – | 17 | 14 | Disease duration was significantly longer in re-positive group than other group. The level of natural killer cells was higher in re-positive group than in other group. |
|
| Zhao W | 14 | 7 | 14 | Two of 7 re-positive patients experienced the second reactivation after being discharged. | |
| Kang YJ | 8922 | 292 | 21 | Positive cases were isolated and the virus was detected again in a PCR test within a very short time. | |
| Zou Y | 257 | 53 | 1–12 | N/A | |
| Yuan B | 182 | 20 | 21 | Twelve cases with negative RT-PCR, 2 cases with positive RT-PCR at the end of follow-up. | |
| Xiao AT | 70 | 15 | 10–20 | N/A | |
| Wu J | 60 | 10 | 10–20 | N/A | |
| Chen JX | 1087 | 81 | 15–50 | Increased serum IL-6, increasing of lymphocytes counts and CT imaging features of lung consolidation during hospitalisation. | |
| Chen LZL | – | 44 | 14 | Concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were noticeably increased in re-positive patients. | |
| Chen SLX | 1282 | 189 | 28 | Re-positive patients were considerably younger with a higher proportion of moderate symptoms in the first hospitalisation than in the other group | |
| Du HWC | 126 | 3 | 60 | N/A | |
| He ST | 420 | 24 | 14 | N/A | |
| Lu JP | 619 | 87 | 14 | N/A | |
| Shui TJL | 758 | 59 | 13 | N/A | |
| Zheng JZ | 285 | 27 | 26–44 | N/A | |
| Liu BS | 47 | 8 | 39 | N/A |
Characteristics and clinical manifestations of patients with SARS-CoV-2 recurrence
Based on the 1077 patients in 62 studies, the mean age of patients with disease recurrence was 44.25 years with a minimum and maximum of 3 months and 93 years, respectively. Among the 790 patients with the reported data, there were 377 (47.7%) men and 413 (52.3%) women. Considering 28 studies that have examined comorbidities in detail, the most commonly reported underlying medical conditions in patients with recurrence were hypertension (46.7%), diabetes (21.9%), and coronary heart disease (11.3%). In some patients, lung diseases (8%), hepatopathy (4.8%), cerebrovascular disease (3%), and malignancy (3.6%) have also been reported. Considering the fact that the most common comorbidities were reported as hypertension followed by diabetes mellitus in COVID-19 patients, regardless of disease recurrence, such comorbidities might not be considered as the independent risk factors for the disease recurrence in COVID-19 patients [76].
Within the first admission, considering 1064 patients, the majority of re-positive cases (n = 987; 92.7%) were asymptomatic or had mild to moderate symptoms [1,3,13,14,16,17,19,21–24,26–28,30–33,36–45,47,48,50–52,54,56,58,59,61,62,64–67,69–73,75,77], but some of them had severe or critical disease (n = 77, 7.3%) [1,13,18,21,23,24,27,30,31,35,36,39,42,43,46,49,55,60,63,68,74]. To compare the severity of disease during the first and second courses of illness in re-positive patients, 57 studies including 858 re-positive patients, it was reported that the disease severity decreased or remained unchanged in 835 cases (97.3%) [13,15–20,23,24,26,28–31,33,36–48,50,52–54,58–61,65–68,70,71,74,75], and increased in 23 cases (2.6%) [18,19,32,35,36,39,43,49,51,55–57,62–64,69,72,73]. In a study [55], it was reported that in an 11 month old boy, despite the asymptomatic course of disease in the first phase, the disease was severe in the second phase. Meanwhile, 39 studies reported concerning temporal changes in the manifestations of chest CT scans of 375 re-positive patients. Among them, 290 and 65 cases presented improved [17,18,20,23,26,28–31,36,38,43,47,50,52–54,58–61,67] and unchanged lesions [17,23,30,40,48], respectively. Also, CT worsening was observed in 20 patients [18,19,32,35,39,46,49,51,55,57,62,63,69,72]. Based on the available data, not in all, but in most of the studies, the duration of being positive for the SARS-CoV-2 genome in the second course of the disease was shorter as compared to the first one.
Risk factors
Demographic data and patients’ characteristics
In most of the studies, no significant association between the gender and susceptibility of disease recurrence has been found [1,13,14,19,20,34]. Besides, age was not associated with the risk of SARS-CoV-2 recurrence [1,13,14,19,20,34], although in a number of studies, some evidence regarding the increased risk among the younger patients has been shown [1,14]. In a study, it has been suggested that patients in some specific age ranges are at the higher risk of recurrence, in this case, 25% of those with disease recurrence were between 20 and 29 years old [16]. It has also been reported that patients aged under 18 years old had a higher rate of recurrence as compared to patients aged over 18 (4 of 13, 30.8% vs. 16 of 169, 9.5%) [14]. Regarding the disease severity, Wang et al. [18] have reported that the median age of patients with severe disease was significantly higher than non-severe patients. No correlation between body mass index (BMI) and smoking habit with recurrence was reported so far [19]. In addition, some studies evaluated the association between exposure to someone with confirmed COVID-19 and recurrence risk and found no significant association, which suggest the possibility of viral reactivation instead of reinfection [19,20]. It is worthy to note that some evidence represented that the rate of SARS-CoV-2 recurrence did not differ by the comorbidities [13,14,18,19].
Disease’s characteristics
There are some associations between the disease severity on first admission and recurrence risk. As an example, in a relatively large cohort study, Yuan et al. reported a statistically significant association between disease severity and recurrence risk. They showed that all of the patients with recurrence were non-severe [14]. This association would raise the assumption of a more likelihood of virus persistence after discharge in non-severe cases due to their faster reach to the discharge criteria, and shorter hospital stays [14,18,20]. In addition, another speculation is the more robust immune response in severely ill patients leading to the more effective elimination of viruses and reducing the risk of recurrence in these patients [14]. However, this evidence is not sufficient to prove a lower risk of recurrence in severely and critically ill patients. In contrast, two cohort studies on 53 and 15 patients with recurrence have found contradictory results, no significant association between the severity of disease and recurrence susceptibility was reported [1,13].
Evidence could not reach any rigorous conclusion regarding the impact of disease duration and hospital stays at first admission on the recurrence rate. Yuan et al. have found a significant correlation between hospital stays and the risk of recurrence. Patients with recurrence stayed for a shorter time in hospitals, suggesting that probably the virus was not eradicated before discharge in such patients [14]. By contrast, Xiao et al. mentioned that patients who experienced a recurrence of SARS-CoV-2 have a longer disease duration [1]. On the other hand, multiple studies have shown no statistically significant correlation between disease duration and hospital stays on first admission and recurrence rate [13,19,20,30].
Three studies revealed no significant correlation between the time interval of onset of symptoms to hospital admission and recurrence risk [13,14,20]. However, the time interval between onset symptoms and final negative RT-PCR test has been reported considerably longer in the patients who had experienced disease recurrence compared to the others [13,19].
Although no significant association between initial symptoms and CT results, on first admission and susceptibility of recurrence was found in different studies [13,19,20], in one study, the possible association between the number of initial symptoms, fatigue, and creatine kinase levels with a re-positive RT-PCR test has been reported [37].
Treatments
Györfi et al. [51] showed that despite the usefulness of specific immunosuppressive drugs in the treatment of COVID-19, non-selective immunosuppressive drugs, such as prednisolone, may cause recurrence, even with first clinical improvement. It has been reported that corticosteroids were used in three of seven patients with disease recurrence [39]. Two of the three patients who did not generate any anti-SARS-CoV-2 antibodies more than 21 days after symptoms received chemotherapy treatments and/or Rituximab. However, no statistically significant difference was found regarding treatment with corticosteroids in their therapeutic schedule between the patients with/without recurrence [13].
Regarding antiviral therapies, no association was found between the risk of recurrence and disease duration, or viral shedding [3,19,20,30,79,80].
Anti-SARS-CoV-2 serological antibodies
In eleven cohort studies and case series, positive rates of serum-specific IgM and IgG against SARS-CoV-2 in patients with recurrence ranged 11–95% and 58.8–100%, respectively [13,14,19,21–23,26,27,31,34,45,75]. Although timing of sampling could affect, some pieces of evidence have cast doubt on the protective role of the specific antibodies, concentrations of antibodies needed for conferring protection, and their protection duration in patients who experienced recurrence. It is worthy to note that Zhu et al. [19] showed no significant differences in the dynamics of specific antibodies that were observed between the re-positive group and non-re-positive group groups [19].
Different studies observed no significant association between the recurrence susceptibility and the presence of these serum-specific antibodies [13,14,34]. Several cases with recurrence have been tested positive for the IgG test [31,34,49]. According to this evidence, it could be speculated that antibody presence does not necessarily prevent disease recurrence. Therefore, physicians need to consider positive antibodies tests as immune certificate and hospital discharge criteria cautiously. On the other hand, some patients with recurrence may produce lower specific antibody titres in comparison to those without recurrence, suggesting that the virus may not confer immunity sufficiently due to limited antibody levels and after that causes recurrence [46,48]. In a case series study, a 33-year old man who experienced recurrence two times did not develop IgM ever during the follow-up period [45].
Conversely, an experimental study on Chinese-origin rhesus macaques could prove the protective role of antibodies. That study demonstrated a gradual increase in the specific antibody levels following primary infection and reinfection with SARS-CoV-2 and a significantly higher level on 28 days after reinfection compared to the primary infection. The study presented that viral replication was not detected upon reinfection, indicating the protective role of the specific antibodies following primary infection [77].
Secondary positive SARS-CoV-2 PCR test: is it a sampling issue, false-negative results, the persistence of virus, or reinfection?
There have been many hypotheses about the explanation and interpretation of secondary positive SARS-CoV-2 PCR tests, but none of them has been conclusively proven. Based on our review, in addition to the sampling issue, three major assumptions for the cause of the secondary positive SARS-CoV-2 PCR test exist, including false-negative results, the persistence of shedding of virus, and reinfection.
The gold standard diagnostic test for SARS-CoV-2 infection is RT-PCR. Failing of appropriate sampling might result in false negative and also missing patients who may still be carriers. Testing a swab from the oropharynx, nose, saliva, or blood is also likely to reduce sensitivity [81–83]. Li et al. [17] found the sputum sample to be more sensitive than the other two sample types. For example, oral swabs have been reported to be 30–50% sensitive. Thus, locations of sampling may play a significant role. Indeed, one study found that the high sensitivity of the nasal test versus the throat test was due to the higher viral load in the nose than in the throat [81]. Also, the sensitivity of diagnostic kits, technical differences in sample collection and preparation, human error, and pre-analytical variables could lead to false-negative results [8]. The other thing is that the RT-PCR may represent false-negative results due to low viral load or sampling errors [30,31,46,49,50]. According to current guidelines for COVID-19 management, one of the main criteria of hospital discharge is two consecutively negative RT-PCR tests. Two studies presented that more than two consecutive RT-PCR tests, which were taken with longer than 24 h intervals, can more accurately identify recurrence potential and reduce the recurrence rate substantially (20.4% versus 5.4% and 0%) [13,30]. Another suggested reason for false-negative tests is the problem with laboratory test kits due to their low cut-off assay [17] or even operator technical error. Aside from the false-negative issue, some studies have even shown that the RNA-based SARS-CoV-2 PCR test can be positive again after one or two consecutive negative tests [3,17,53,84].
It has been speculated that incomplete elimination of the virus after discharge is the main reasons for recurrence; however, definitive data whether the virus is viable infectious or not is lacking [1,3,13–15,29,34,46]. Some studies suggested that positive RT-PCR could be due to shedding of virus residue, which does not result in transmission. Another hypothesis proposed for cause of recurrence has been raised, which mentioned the hypothesis of presence of a latent SARS-CoV-2 infection within immune cells [11]. Others have suggested that SARS-CoV-2 could multiply inside the peripheral blood mononuclear cell (PBMC) through interaction with ACE2 receptor on the surface of human monocytes [36,85,86]. In a way, there may be a hidden infection of SARS inside the cells of the body, and the cause of viral recurrence after a negative test is the presence of these hidden viruses [11] and this could be called a malaria-like action. Also the study by Wang et al. claimed that the entry of the virus into T lymphocytes is not accompanied by proliferation, and the virus somehow settles in the cell. In confirmation of their hypothesis, they showed that the concentration of the viral genome in lymphocytes is higher than its concentration in plasma [87]. This could be another hypothesis for repetitive positive test after consecutive negative test.
It should be mentioned that finding RNA in a sample does not necessarily mean the presence of a complete or active virus in it, so it does not indicate that the virus is alive in the sample [88]. In fact, because the epithelial cell half-life in the respiratory system is 3 months, the virus genome could be detected even after elimination during this time. The mean time when the virus RNA PCR test of first SARS-Cov-2 infection became negative, it was 34 days in faecal specimens compared to 9 days in the respiratory samples, indicating that RNA could persist more in the gastrointestinal tract than respiratory tract [55] and may be mistaken for reinfection. Monitoring the disease symptoms could be helpful in this regard. For example, the unchanged or improvement in CT after recurrence might suggest that a new infection has not occurred.
In the case of disease recurrence, the detection of a positive test might be interpreted as a new infection. Studies mostly have shown no agreement on the third major assumption, which is reinfection. Although the likelihood of reinfection after the first discharge was not completely ruled out, in late of August 2020, following confirmation of the reinfection documented in Hong Kong [89], and also some subsequent cases in Belgium, Netherlands [90], and in the United States [91], it could be speculated that that humans may become infected more than one time by SARS-CoV-2.
In addition to the three mentioned possibilities, one of the possible reasons for reactivation and subsequent positive re-testing is considered to be the antibody dependent enhancement (ADE) phenomenon; a phenomenon that has also been described in coronavirus [92] and probably can lead to more severe disease due to causing an unnecessary immune response [93].
In terms of the impact of immunity on recurrence, there are some controversies. In a study on rhesus monkeys, it has been shown that monkeys conferring sufficient immunity after the first discharge did not show any evidence of reinfection [77]. However, another study proposes that the immunity failure might cause not only the persistence of the virus but also reinfection [34]. Although most the rate of positive IgG antibodies against the SARS-CoV-2 has been reported between 52.8% and 95%, the absence of sufficient immunity in patients with re-positive SARS-CoV-2 test was observed in multiple studies [14,31,34,46]. Robbiani et al. [94] state that plasma obtained from COVID 19-positive individuals did not have high levels of neutralising antibodies but had receptor-binding domain-specific antibodies with strong antiviral activity. This had led to the hypothesis that high affinity versus low avidity could cause the virus to escape the immune system due to low levels of antibodies. However, over the time, the avidity of the antibodies increases, and diagnostic tests detect the viral remnants.
The failure of immunity for protection may lead to the prolonged viral shedding of the non-viable virus, replication of a viable virus, or reinfection. Three studies asserted that non-strong immune response and insufficient immunity led to the persistence of shedding of the virus, but not a replication of a viable virus [14,31,34]. In this regard, a case report study on a patient with four times recurrence within 137 days assumed that the antibody levels are just sufficient for preventing transmission of disease considering negative RT-PCR tests of the patient's parents, not for full clearance of virus [48]. In contrast, in a case series, four patients from one family experienced recurrence [32]. However, Lafaie et al. reported inconsistent evidence with these studies; the reason for the recurrence of COVID-19 in three women who have died was described as the persistence of a viable virus and viral replication [35].
In conclusion, although the underlying cause is not clear, recovered patients, even those with detectable anti-SARS-CoV-2 IgG may show disease recurrence. More studies in this regard and modification of management’s protocols for COVID-19 patients might be required.
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Xiao AT, Tong YX, Zhang S.. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020;92(10):1755–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou L, Liu K, Liu HG.. [Cause analysis and treatment strategies of “recurrence” with novel coronavirus pneumonia (COVID-19) patients after discharge from hospital]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(4):281–284. [DOI] [PubMed] [Google Scholar]
- 3.Ye G, Pan Z, Pan Y, et al. . Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. 2020;80(5):e14–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing Y, Mo P, Xiao Y, et al. . Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill. 2020;25(10):2000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu Y-M, Kang E-M, Cong H-Y.. Positive result of Sars-Cov-2 in sputum from a cured patient with COVID-19. Travel Med Infect Dis. 2020;34:101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan L, Xu D, Ye G, et al. . Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323(15):1502–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Zhang C, Wu Z, et al. . The mechanism and clinical outcome of patients with corona virus disease 2019 whose nucleic acid test has changed from negative to positive, and the therapeutic efficacy of Favipiravir: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance, 17 January 2020. 2020. [Google Scholar]
- 9.Xia J, Tong J, Liu M, et al. . Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Du R-H, Li B, et al. . Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elberry MH, Ahmed H.. Occult SARS-CoV-2 infection; a possible hypothesis for viral relapse. Med Hypotheses. 2020;144:109980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattiuzzi C, Henry BM, Sanchis-Gomar F, et al. . SARS-CoV-2 recurrent RNA positivity after recovering from coronavirus disease 2019 (COVID-19): a meta-analysis. Acta Bio-Medica: Atenei Parmensis. 2020;91(3):e2020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou Y, Wang BR, Sun L, et al. . The issue of recurrently positive patients who recovered from COVID-19 according to the current discharge criteria: investigation of patients from multiple medical institutions in Wuhan, China. J Infect Dis. 2020;222(11):1784–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan B, Liu HQ, Yang ZR, et al. . Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation. Sci Rep. 2020;10(1):11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Liu X, Liu J, et al. . Coronavirus disease 2019 test results after clinical recovery and hospital discharge among patients in China. JAMA Netw Open. 2020;3(5):e209759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang YJ. South Korea’s COVID-19 infection status: from the perspective of re-positive test results after viral clearance evidenced by negative test results. Disaster Med Public Health Prep. 2020. DOI: 10.1017/dmp.2020.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Hu Y, Yu Y, et al. . Positive result of Sars-Cov-2 in faeces and sputum from discharged patient with COVID-19 in Yiwu. J Med Virol. 2020;92(10):1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Xu H, Jiang H, et al. . The clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM. 2020;hcaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Fu L, Jin Y, et al. . Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. J Clin Lab Anal. 2020;34(7):e23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Chen Z, Azman AS, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. medRxiv. 2020. DOI: 10.1101/2020.09.11.20192773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JX, Hu X, Chen J, et al. . Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: a retrospective cohort study from Wuhan, China. Aging (Albany NY). 2020;12(17):16675–16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LZL, Chen ZH, Liu J, et al. . Can elevated concentrations of ALT and AST predict the risk of ‘recurrence’ of COVID-19? Epidemiol Infect. 2020;148:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SLX, Feng H, Sun HY, et al. . Epidemiological and clinical findings of short-term recurrence of severe acute respiratory syndrome coronavirus 2 ribonucleic acid polymerase chain reaction positivity in 1282 discharged coronavirus disease 2019 cases: a multicenter, retrospective, observational study. Open Forum Infect Dis. 2020;7(10):ofaa432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du H-W, Chen J-N, Pan X-B, et al. . Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. Eur J Clin Microbiol Infect Dis. 2020. DOI: 10.1007/s10096-020-04024-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He ST, Li J, Zhou X, et al. . Positive RT-PCR test results in 420 patients recovered from COVID-19 in Wuhan: an observational study. Front Pharmacol. 2020;11:549117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu JP, Xiong J, Liu Q, et al. . Ke, C. Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR. EBioMedicine. 2020;59:102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shui T-J, Li C, Liu H-B, et al. . Characteristics of recovered COVID-19 patients with recurrent positive RT-PCR findings in Wuhan, China: a retrospective study. BMC Infect Dis. 2020;20(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng JZ, Chen R, Tang F, et al. . Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID-19 patients in Guangzhou, China: a prospective cohort study. PLoS Negl Trop Dis. 2020;14(8):e0008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng KI, Wang XB, Jin XH, et al. . A case series of recurrent viral RNA positivity in recovered COVID-19 Chinese patients. J Gen Intern Med. 2020;35(7):2205–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan J, Kou S, Liang Y, et al. Polymerase chain reaction assays reverted to positive in 25 discharged patients with COVID-19. Clin Infect Dis. 2020;71(16):2230–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian M, Long Y, Hong Y, et al. . The treatment and follow-up of “recurrence” with discharged COVID-19 patients: data from Guizhou, China. Environ Microbiol. 2020;22(8):3588–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravioli S, Ochsner H, Lindner G.. Reactivation of COVID-19 pneumonia: a report of two cases. J Infect. 2020;81(2):e72–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng J, Wang M, Zhang G, et al. . Seven discharged patients turning positive again for SARS-CoV-2 on quantitative RT-PCR. Am J Infect Control. 2020;48(6):725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenner J, Min MS, Liu S, et al. . A case of neonatal pemphigus vulgaris with co-existing BP180 autoantibodies. Pediatr Dermatol. 2020;37(1):241–243. [DOI] [PubMed] [Google Scholar]
- 35.Lafaie L, Célarier T, Goethals L, et al. . Recurrence or relapse of COVID-19 in older patients: a description of three cases. J Am Geriatr Soc. 2020;68(10):2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang M, Li Y, Han M, et al. . Recurrent PCR positivity after hospital discharge of people with coronavirus disease 2019 (COVID-19). J Infect. 2020;81(1):147–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu R, Jiang Z, Gao H, et al. . Recurrent positive reverse transcriptase-polymerase chain reaction results for coronavirus disease 2019 in patients discharged from a hospital in China. JAMA Netw Open. 2020;3(5):e2010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Bai W, Liu B, et al. . Re-evaluation of retested nucleic acid-positive cases in recovered COVID-19 patients: report from a designated transfer hospital in Chongqing, China. J Infect Public Health. 2020;13(7):932–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batisse D, Benech N, Botelho-Nevers E, et al. . Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81:816–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B, Liu S, Dong Y, et al. . Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19). J Infect. 2020;81(2):e49–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bongiovanni M, Basile F.. Re-infection by COVID-19: a real threat for the future management of pandemia? Infect Dis (Lond). 2020;52(8):581–582. [DOI] [PubMed] [Google Scholar]
- 42.Tomassini S, Kotecha D, Bird PW, et al. . Setting the criteria for SARS-CoV-2 reinfection – six possible cases. J Infect. 2020. DOI: 10.1016/j.jinf.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gidari A, Nofri M, Saccarelli L, et al. . Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature. Eur J Clin Microbiol Infect Dis. 2021;40(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta V, Bhoyar RC, Jain A, et al. . Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song KH, Kim DM, Lee H, et al. . Dynamics of viral load and anti-SARS-CoV-2 antibodies in patients with positive RT-PCR results after recovery from COVID-19. Kor J Intern Med. 2020. DOI: 10.3904/kjim.2020.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X, Zhou J, Zhao J.. Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect Dis. 2020;20(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo SY, Lee Y, Lee GH, et al. . Reactivation of SARS-CoV-2 after recovery. Pediatr Int. 2020;62(7):879–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang P. Recurrent presence of SARS-CoV-2 RNA in a 33-year-old man. J Med Virol. 2020;93(2):592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loconsole D, Passerini F, Palmieri VO, et al. . Recurrence of COVID-19 after recovery: a case report from Italy. Infection. 2020;48(6):965–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Cai ZB, Huang JS, et al. . Positive SARS-CoV-2 RNA recurs repeatedly in a case recovered from COVID-19: dynamic results from 108 days of follow-up. Pathog Dis. 2020;78(4):ftaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Györfi AH, Kopp M, May M, et al. . Glucocorticoid-induced relapse of COVID-19 in a patient with sarcoidosis. Ann Rheumat Dis. 2020. DOI: 10.1136/annrheumdis-2020-218258 [DOI] [PubMed] [Google Scholar]
- 52.Dou C, Xie X, Peng Z, et al. . A case presentation for positive SARS-CoV-2 RNA recurrence in a patient with a history of type 2 diabetes that had recovered from severe COVID-19. Diabetes Res Clin Pract. 2020;166:108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen D, Xu W, Lei Z, et al. . Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020;93:297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentivegna E, Sentimentale A, Luciani M, et al. . New IgM seroconversion and positive RT-PCR test after exposure to the virus in recovered COVID-19 patient. J Med Virol. 2020;93(1):97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du W, Yu J, Liu X, et al. . Persistence of SARS-CoV-2 virus RNA in feces: a case series of children. J Infect Public Health. 2020;13(7):926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alonso FOM, Sabino BD, Guimarães MAAM, et al. Recurrence of SARS-CoV-2 infection with a more severe case after mild COVID-19, reversion of RT-qPCR for positive and late antibody response: Case report. J Med Virol. 2021;93(2):655–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duggan NM, Ludy SM, Shannon BC, et al. . A case report of possible novel coronavirus 2019 reinfection. Am J Emerg Med. 2020;39:256.e1-256.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu S, Gao D, Zhang Y, et al. . Follicular lymphoma with paraneoplastic pemphigus as the first symptom: a case report and review of the literature. Int J Clin Exp Pathol. 2020;13(7):1915–1923. [PMC free article] [PubMed] [Google Scholar]
- 59.Geling T, Huaizheng G, Ying C, et al. . Recurrent positive nucleic acid detection in a recovered COVID-19 patient: a case report and literature review. Respir Med Case Rep. 2020;31:101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He FL, Lei Q, Fan M, et al. . Successful recovery of recurrence of positive SARS-CoV-2 RNA in COVID-19 patient with systemic lupus erythematosus: a case report and review. Clin Rheumatol. 2020;39(9):2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Beek N, Schumacher N, Rose C, et al. . [Modern diagnostics of autoimmune bullous diseases]. Pathologe. 2020;41(4):317–325. [DOI] [PubMed] [Google Scholar]
- 62.Liu F, Cai ZB, Huang JS, et al. . Repeated COVID-19 relapse during post-discharge surveillance with viral shedding lasting for 67 days in a recovered patient infected with SARS-CoV-2. J Microbiol Immunol Infect. 2020. DOI: 10.1016/j.jmii.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mardani M, Nadji SA, Sarhangipor KA, et al. . COVID-19 infection recurrence presenting with meningoencephalitis. New Microbes New Infect. 2020;37:100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.AlFehaidi A, Ahmad SA, Hamed E.. SARS-CoV-2 re-infection: a case report from Qatar. J Infect. 2020. DOI: 10.1016/j.jinf.2020.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bongiovanni M. COVID-19 re-infection in an healthcare worker. J Med Virol. 2020. DOI: 10.1002/jmv.26565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colson P, Finaud M, Levy N, et al. . Evidence of SARS-CoV-2 re-infection with a different genotype. J Infect. 2020. DOI: 10.1016/j.jinf.2020.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coppola A, Annunziata A, Carannante N, et al. . Late reactivation of SARS-CoV-2: a case report. Front Med (Lausanne). 2020;7:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldman JD, Wang K, Roltgen K, et al. Reinfection with SARS-CoV-2 and failure of humoral immunity: a case report. medRxiv. 2020. DOI: 10.1101/2020.09.22.20192443 [DOI] [Google Scholar]
- 69.Lancman G, Mascarenhas J, Bar-Natan M.. Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma BMH, Chan IFN, Tam GCW, et al. . Case of “relapsing” COVID-19 in a kidney transplant recipient. Nephrology (Carlton, Vic). 2020;25(12):933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nachmias V, Fusman R, Mann S, et al. . The first case of documented Covid-19 reinfection in Israel. IDCases. 2020;22:e00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patrocínio de Jesus RS, Aliyeva R, Lopes E, et al. . Reactivation of SARS-CoV-2 after asymptomatic infection while on high-dose corticosteroids. SN Compr Clin Med. 2020;2(11):2402–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tillett RL, Sevinsky JR, Hartley PD, et al. . Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2021;21(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.To KK-W, Hung IF-N, Ip JD, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu BS, Zhang Y, Li W, et al. . Recovered COVID-19 patients with recurrent viral RNA exhibit lower levels of anti-RBD antibodies. Cell Mol Immunol. 2020;17(10):1098–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaur N, Gupta I, Singh H, et al. . Epidemiological and clinical characteristics of 6635 COVID-19 patients: a pooled analysis. SN Compr Clin Med. 2020. DOI: 10.1007/s42399-020-00393-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ota M. Will we see protection or reinfection in COVID-19? Nat Rev Immunol. 2020;20(6):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ling Y, Xu S-B, Lin Y-X, et al. . Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133(9):1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young BE, Ong SWX, Kalimuddin S, et al. . Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zou L, Ruan F, Huang M, et al. . SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carver C, Jones N. Comparative accuracy of oropharyngeal and nasopharyngeal swabs for diagnosis of COVID-19 - CEBM [Internet]. CEBM 2020 [cited 20 Nov 2020]. Available from: https://www.cebm.net/covid-19/comparative-accuracy-of-oropharyngeal-and-nasopharyngeal-swabs-for-diagnosis-of-covid-19/ [Google Scholar]
- 83.Wang W, Xu Y, Gao R, et al. . Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18):1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J-f, Yan K, Ye H-h, et al. . SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standards for discharge. Int J Infect Dis. 2020;97:212–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yilla M, Harcourt BH, Hickman CJ, et al. . SARS-coronavirus replication in human peripheral monocytes/macrophages. Virus Res. 2005;107(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L, Wo J, Shao J, et al. . SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J Clin Virol. 2003;28(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H, Mao Y, Ju L, et al. . Detection and monitoring of SARS coronavirus in the plasma and peripheral blood lymphocytes of patients with severe acute respiratory syndrome. Clin Chem. 2004;50(7):1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ravi D, Prabhu SS, Rao R, et al. . Comparison of immunofluorescence and desmoglein enzyme-linked immunosorbent assay in the diagnosis of pemphigus: a prospective, cross-sectional study in a tertiary care hospital. Indian J Dermatol. 2017;62(2):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong Kong man was reinfected with Covid: study 2020. Available from: https://news.rthk.hk/rthk/en/component/k2/1545589-20200824.htm.
- 90.Joseph A. Scientists are reporting several cases of Covid-19 reinfection — but the implications are complicated; 2020. Available from: https://www.statnews.com/2020/08/28/covid-19-reinfection-implications/
- 91.Tillett R, Sevinsky J, Hartley P, et al. Genomic evidence for a case of reinfection with SARS-CoV-2: THELANCETID-D-20-05376; 2020. Available from: https://ssrn.com/abstract=3681489
- 92.Tirado SMC, Yoon K-J.. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69–86. [DOI] [PubMed] [Google Scholar]
- 93.Galanti M, Shaman J.. Direct observation of repeated infections with endemic coronaviruses. J Infect Dis. 2020. DOI: 10.1093/infdis/jiaa392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 infection in convalescent individuals. Nature 2020;584:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.