Abstract
Bladder cancer is one of the most common and aggressive cancers and, regardless of the treatment, often recurs and metastasizes. Thus, a better understanding of the mechanisms regulating urothelial tumorigenesis is critical for the design and implementation of rational therapeutic strategies. We previously discovered that the IGF-IR axis is critical for bladder cancer cell motility and invasion, suggesting a possible role in bladder cancer progression. However, IGF-IR depletion in metastatic bladder cancer cells only partially inhibited anchorage-independent growth. Significantly, metastatic bladder cancer cells have decreased IGF-IR levels but overexpressed the insulin receptor isoform A (IR-A), suggesting that the latter may play a more prevalent role than the IGF-IR in bladder tumor progression. The collagen receptor DDR1 cross-talks with both the IGF-IR and IR in breast cancer, and previous data suggest a role of DDR1 in bladder cancer. Here, we show that DDR1 is expressed in invasive and metastatic, but not in papillary, non-invasive bladder cancer cells. DDR1 is phosphorylated upon stimulation with IGF-I, IGF-II, and insulin, co-precipitates with the IGF-IR, and the IR-A and transient DDR1 depletion severely inhibits IGF-I-induced motility. We further demonstrate that DDR1 interacts with Pyk2 and non-muscle myosin IIA in ligands-dependent fashion, suggesting that it may link the IGF-IR and IR-A to the regulation of F-actin cytoskeleton dynamics. Similarly to the IGF-IR, DDR1 is upregulated in bladder cancer tissues compared to healthy tissue controls. Thus, our findings provide the first characterization of the molecular cross-talk between DDR1 and the IGF-I system and could lead to the identification of novel targets for therapeutic intervention in bladder cancer. Moreover, the expression profiles of IGF-IR, IR-A, DDR1, and downstream effectors could serve as a novel biomarker signature with diagnostic and prognostic significance.
Keywords: Bladder cancer, DDR1, IGF system, Motility, IGF-IR, IR
Highlights
-
•
We discovered that the collagen receptor DDR1 cross-talks with insulin growth factor I (IGF-I) signaling in bladder cancer
-
•
DDR1 co-precipitates with the IGF-IR and the insulin receptor (IR), and is phosphorylated upon stimulation with IGF ligands
-
•
This collagen receptor modulates IGF-I-evoked motility and anchorage-independent growth
-
•
DDR1 complexes with Pyk2, myosin IIA, IGF-IR and/or IR and regulates actin dynamics
Introduction
Bladder cancer is one of the most prevalent cancers in the USA [1]. The prognosis for low-grade tumors is generally favorable, but ~15% of these patients will develop muscle-invasive disease, which has only a 50% survival rate at five years [2,3]. Compared to other cancers, bladder cancer has the highest cost/patient ratio due to disease prevalence and necessary long-term monitoring [4]. Surprisingly, this malignancy is considerably understudied and underfunded in spite of the high mortality [5,6].
The discoidin domain receptors, DDR1 and DDR2, are non-integrin collagen receptors under the auspices of the large receptor tyrosine kinase (RTK) family (RTKs) [[7], [8], [9]]. Both receptors bind triple-helical collagen. DDR1 and DDR2 bind and are mainly activated by fibrillar collagen type I but can also bind collagens type II, III and V. Collagen type IV interacts with DDR1 but not with DDR2, which is specifically activated by non-fibrillar collagen type X [10,11].
Structurally, DDRs possess a modular architecture characterized by an extracellular discoidin domain (ECD) and a juxta-membrane region [12,13]. DDR1 can be expressed as five isoforms by alternative splicing (DDR1a-e) and are widely distributed in normal epithelium. In contrast, DDR2 has no isoforms and is expressed primarily in stromal cells [9].
Interestingly, collagen evokes activation of DDR1 via lateral dimer association and phosphorylation between dimers [14]. In striking contrast with other RTKs, they have slow activation kinetics with Tyr-phosphorylation occurring after several h of ligand engagement [[14], [15], [16]]. It has been recently shown that DDR1 kinase activity is determined by its molecular density, and autophosphorylation of the receptor leads to aggregation into large clusters [17]. This discovery provides a plausible mechanism for well know slow kinetics of the receptor.
DDRs play essential roles throughout embryonic development [[7], [8], [9]]. DDR-modulated cellular functions include cell migration, cell survival, proliferation, and differentiation, as well as remodeling of extracellular matrices [[7], [8], [9]]. Aberrant receptor signaling is associated with the progression of various human diseases, including fibrosis, arthritis, and cancer [[7], [8], [9],18,19], where DDR1 may promote resistance to therapy [20].
Importantly, DDR1, although lacking a canonical nuclear localization signal, is present in the nucleus of injured renal tubules and evokes collagen Type IV transcription [21]. Notably, DDR1 nuclear translocation requires collagen-mediated receptor activation and interaction with SEC61B, non-muscle myosin IIA, and β-actin.
The type 1 IGF receptor (IGF-IR) is a hetero-tetrameric transmembrane tyrosine kinase receptor composed of two α and two β subunits linked by disulfide bridges [22]. The IGF-IR binds with high affinity to both insulin-like growth factors I and II (IGF-I and IGF-II) and plays an essential role in the regulation of mammalian growth [[23], [24], [25], [26], [27], [28]], and cell differentiation in several cellular models, including hemopoietic and neuronal cells [[29], [30], [31]]. In cancer, the expression levels of the receptor and its natural ligands are often altered and affect tumor initiation, progression, and resistance to therapy [[32], [33], [34], [35], [36]]. IGF-II, and to a lesser extent, IGF-I, binds to a second RTK, the isoform A of the insulin receptor (IR-A). The IR-A is highly homologous to the IGF-IR [22,37,38] and promotes mitogenic effects upon IGF-II or insulin binding [[38], [39], [40]], an action that may be important in several cancer models [22,[41], [42], [43]]. Moreover, the IR-A metabolically reprograms breast cancer cells in response to co-stimulation of IGF-II and insulin [11]. The second IR isoform (IR-B) is critical for glucose metabolism of insulin-responsive organs [22,38,41]. The IR-A is preferentially expressed over the IR-B, and an autocrine proliferative loop between IGF-II and the IR-A exists in malignant thyrocytes and breast cancer [22,[43], [44], [45], [46], [47], [48]].
Recent work from our laboratories has established a critical role of the IGF-IR in bladder cancer [[49], [50], [51]]. IGF-IR expression increases with tumor grade and stimulates the ability of urothelial cancer cells to migrate through and evade a 3D extracellular matrix [49]. Importantly, the extracellular matrix actively regulates IGF-IR, and IR-A [51] and a finely coordinated cross talk between IGF-IR and the epidermal growth factor receptor (EGFR) regulates the expression of matrix molecules such as proteoglycans [52]. Proteoglycan remodeling is emerging as a crucial step in regulating cancer phenotype and aggressiveness [53], vascular disease [54], neuro-inflammation [55], cardiac fibrosis [56,57], and tissue homeostasis and repair [58]. In cancer, the remodeling of multiple matrix proteins has been associated with the regulation of tumor neovascularization [59,60] and major biological processes [61,62], including tissue stiffness [63] and cardiac disease [64] For example, decorin, the most studied member of the small leucine-rich family of proteoglycans [[65], [66], [67], [68]] binds with high affinity to both IGF-IR and IGF-I and represses IGF-IR activity in bladder cancer cells [50]. Decorin expression is absent in bladder tumor tissues [50], suggesting that its loss is permissive for tumor growth and may modulate IGF-IR activity in urothelial carcinomas and perhaps other tumor types where IGF-IR plays a role. Further, decorin binds the IR-A and its associated ligands IGF-II, insulin, and proinsulin, although with different affinities [69]. Decorin does not affect ligand-mediated IR-A phosphorylation, but it attenuates IGF-II-induced Akt activation, promotes IGF-II-evoked IR-A downregulation and reduces IGF-II-mediated cell growth [69]. Importantly, decorin does not affect insulin or proinsulin-evoked biological responses downstream of the IR-A [69]. These discoveries support a new mechanism whereby decorin loss contributes to tumor formation in cancer systems addicted to an IGF-II/IR-A tumorigenic autocrine loop.
A putative oncogenic role for DDR1 [70] and DDR2 [71] was recently suggested in bladder cancer, and we demonstrated a functional interaction between DDR1 and both the IGF-IR and IR in breast cancer [11,[72], [73], [74], [75], [76]]. DDR1 potentiates the biological actions of insulin, IGF-I, and IGF-II. Conversely, DDR1 is upregulated by IGF-I, IGF-II, and insulin through the PI3K/AKT/miR-199a-5p signaling cascade [76]. These data suggest a more comprehensive role of DDR1 as a modulator of cell responses to hormones, growth factors, and signals from the extracellular matrix. However, whether DDR1 may functionally cross-talk with the IGF system and modulate IGF-evoked biological responses in bladder cancer has not been established.
Here we show that the IGF-IR is necessary but not sufficient for anchorage-independent growth of metastatic bladder cancer cells suggesting that the IR, and specifically the IR-A, may play a more critical role in bladder cancer progression. We further demonstrate that DDR1 modulates IGF-IR and IR-A-dependent bladder cancer cell motility by linking the IGF-IR and IR-A to the regulation of F-actin cytoskeleton dynamics. Untangling the intricacies of receptor cross-talk between the IGF-I system and DDR1 will significantly contribute to the identification of novel targets and biomarkers for advanced therapeutic intervention in bladder tumors.
Results
IGF-IR loss partially inhibits migration and anchorage-independent growth
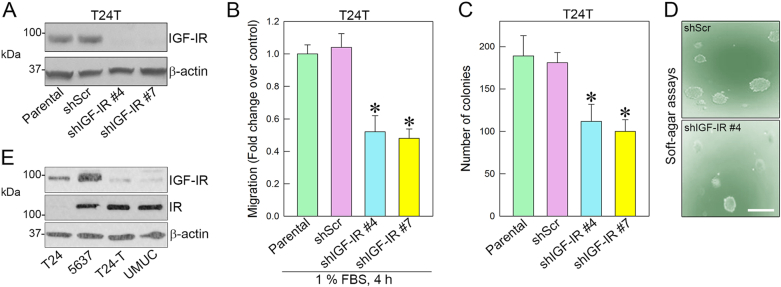
We previously demonstrated that activation of the IGF-IR pathway promotes motility and invasion of bladder cancer cells [[49], [50], [51]]. These results provided the first evidence that the IGF-I system plays an essential role in bladder cancer and may drive the transition to the invasive phenotype. To determine whether the IGF-IR may be critical for bladder cancer formation, we stably depleted endogenous IGF-IR in T24T urothelial cancer cells. T24T cells are transforming isogenic derivatives of T24 cells and grow in soft-agar, generate tumors, and form metastases in nude mice [[77], [78], [79]]. We discovered that IGF-IR depletion (Fig. 1A) only partially reduced cell migration in 1% serum-condition (Fig. 1B) and colony formation in anchorage-independent growth (Fig. 1C). Representative images depict colony formation from shScr control or shIGF-IR#4 T24T cells (Fig. 1D).
Fig. 1.
IGF-IR depletion in metastatic T24T cells only partially inhibits motility and anchorage-independent growth. (A) IGF-IR depletion in T24T cells was obtained by stable transfection of the pRS/shIGF-IR plasmid or control and subsequent selection in puromycin-containing media. (B) Migration of T24T cells was assessed in Boyden chambers as described [49,80,81].*p < 0.05. (C) Anchorage-independent growth was measured by soft-agar assays. Colonies ≥150 μM were counted [82]. Experiments are the average of three independent experiments ±SD. Bar~200 μm. *p < 0.05. (D) Representative colonies generated from shScr or shIGF-IR#4 transfected T24T cells. (E) IGF-IR and IR expression levels in various urothelial carcinoma-derived cells were assessed by immunoblot with anti-IGF-IR and anti-IR polyclonal antibodies. β-Actin served as the loading control.
As mentioned above, increasing evidence indicates that the IR-A may be involved in the pathogenesis of cancer [41,42]. Notably, the IR-A is the dominant IR isoform present in several bladder cancer cells [83]. Thus, we evaluated the relative protein expression of IGF-IR and IR in various bladder cancer cell lines with diverse malignant behavior. Surprisingly, IGF-IR levels were low in tumorigenic and metastatic T24T and UMUC3 cells, which instead expressed high levels of the IR (Fig. 1E), presumably ~70% is the IR-A isoform [83]. T24 cells, which are invasive but not tumorigenic in vivo, express the IGF-IR and undetectable levels of the IR, whereas 5637 cells are tumorigenic in vivo but do not form metastases, and express high levels of IGF-IR and IR (Fig. 1E). However, in 5637 cells, the ratio IR-A vs. IR-B is 50:50, as previously demonstrated [83]. These findings suggest that IGF-IR is important but not sufficient for migration and anchorage-independent growth.
IGF-IR and IR-A differentially contribute to urothelial cancer cell motility
To assess the contribution of the IGF-IR and IR-A to the migratory ability of urothelial cancer cells, we compared the migration of T24 and T24T cells after stimulation with equimolar physiological concentrations of IGF-I, IGF-II, and insulin [69,84]. While T24 cells preferentially responded to IGF-I and a lesser extent to IGF-II and insulin (Fig. 2), IGF-II and insulin evoked a stronger migratory response in T24T cells compared to IGF-I (Fig. 2).
Fig. 2.
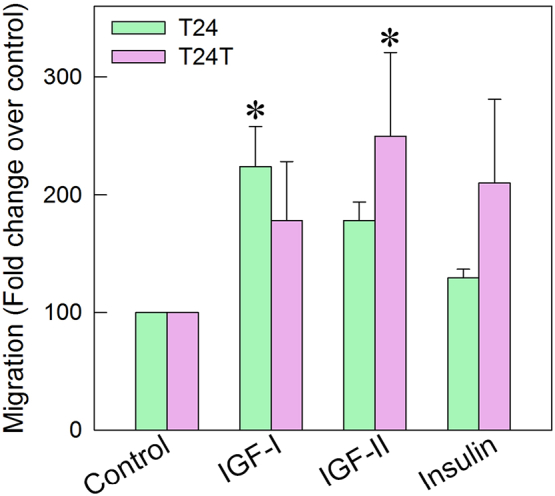
IR-A plays a critical role in modulating bladder cancer cell motility. Analysis of IGF-I, IGF-II, or insulin-induced migration of T24 or T24T cells. Migration of serum-starved T24 or T24T cells after 18 h of stimulation with ligand was assessed in Boyden chambers as described [49,80,81]. Experiments are the average of three independent experiments. *p < 0.05.
These findings suggest that in metastatic bladder cancer cells, the IR-A, activated by both IGF-II and insulin, may play a more prominent role than the IGF-IR in modulating cancer cell motility.
DDR1 is expressed in bladder cancer cells and interacts with the IGF-IR and IR
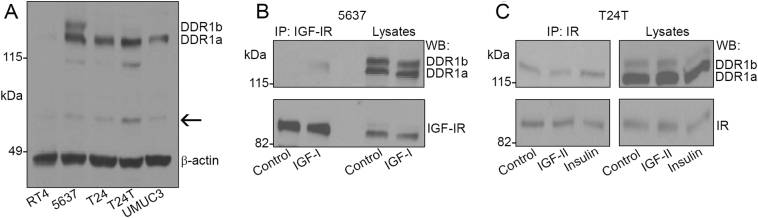
Proteomic studies have recently identified DDR1 as a novel substrate of the IR-A [72]. We recently established that this receptor functionally modulates IGF-IR and IR-A action in breast cancer [48,[73], [74], [75], [76]], suggesting that a functional cross-talk between DDR1 and the IGF system may occur in bladder cancer, where both IGF-IR [[49], [50], [51]] and DDR1 play an important role [70]. To investigate whether DDR1 cross-talks with the IGF-I signaling axis, we first assessed baseline expression levels of this RTK in various bladder cancer cells. DDR1 was expressed in invasive (T24 and 5367) and metastatic (T24T and UMUC3), but not in papillary non-invasive (RT4) cells (Fig. 3A). 5637 cells expressed both DDR1a and DDR1b isoforms, whereas T24, T24T, and UMUC3 cells mainly expressed DDR1a (Fig. 3A) [7,9], with a slight amount of DDR1b in T24T. We found that DDR1b was detectable only after overexposure of the film. Whether the bands detectable around 60 kDa are soluble, partially processed receptor forms [12], remain to be established (Fig. 3A, arrow). To determine if DDR1 may contribute to the IGF-I system to regulate cell motility, we determined whether it might associate with the IGF-IR and/or the IR. In 5637 cells, DDR1a was not detectable in immunoprecipitates from serum-starved cell lysates but did co-immunoprecipitate with the IGF-IR after 5 min of IGF-I stimulation (Fig. 3B). In T24T cells, instead, DDR1a was constitutively associated with the IR and this association increased after insulin stimulation (Fig. 3C).
Fig. 3.
DDR1 is expressed in bladder cancer cells and interacts with the IGF-IR and IR. (A) Immunoblotting of DDR1 using an anti-DDR1 polyclonal antibody towards its C-terminus.DDR1c was not detectable in these experimental conditions (50 μg of lysate). (B) 2.5 mg of 5637 cell lysates were immunoprecipitated with anti-IGF-IR monoclonal antibodies after 5 min stimulation with 10 nM of IGF-I. Total IGF-IR was detected using an anti-IGF-IR polyclonal antibody. (C) 2 mg of T24T cell lysates were immunoprecipitated with anti-IR monoclonal antibodies after 5-min stimulation with 10 nM of IGF-II or insulin. Total IR was detected using an anti-IR polyclonal antibody.
Collectively, these findings suggest that DDR1a interacts with the IGF-IR and IR in bladder cancer cells. However, the kinetics of association between DDR1a and the IGF-IR in invasive cells (e.g., 5637) may differ from the interaction with the IR in metastatic cells (e.g., T24T).
DDR1 is phosphorylated by IGF-I, IGF-II, and insulin in bladder cancer cells and is required for IGF-I-induced migration
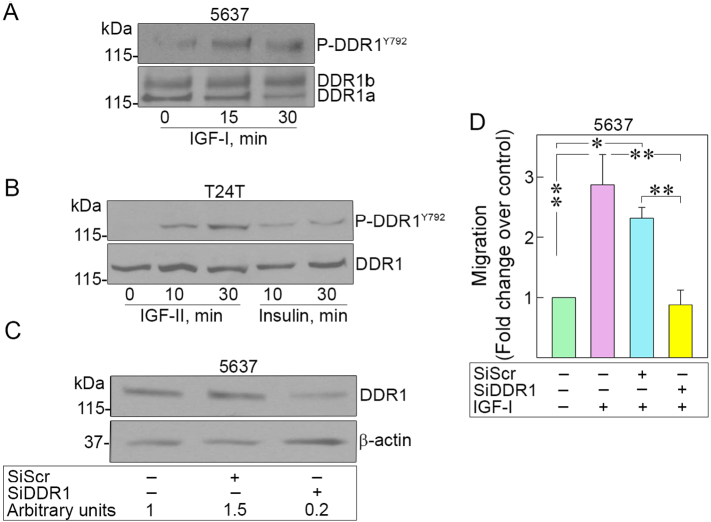
Next, we tested whether DDR1 would be phosphorylated by IGF-I in 5637 or by IGF-II and insulin in T24T cells. Notably, IGF-I, IGF-II, and insulin-induced phosphorylation of DDR1 as early as 10 min and remains activated up to 30 min (Fig. 4A, B). In agreement with our previous results [76], this observation suggests very different kinetics from collagen-dependent receptor activation, which is very slow and requires several h of collagen stimulation [7,15,16,85]. We then established whether DDR1 is necessary for IGF-IR-dependent motility of 5637 cells. Specific RNAi-mediated depletion of endogenous DDR1 (Fig. 4C) severely inhibited IGF-I-induced migration of invasive 5637 cells as compared to siRNA-control-transfected cells (*p < 0.05, **p < 0.01) (Fig. 4D).
Fig. 4.
DDR1 is phosphorylated by IGF-I, IGF-II, and insulin in 5637 and T24T bladder cancer cells and is required for IGF-I-induced migration of 5637 cells. (A) DDR1 phosphorylation after stimulation with 10 nM IGF-I (5637), (B) IGF-II, and insulin (T24T) for 10 or 30 min was assessed by immunoblot using Phospho-DDR1 antibodies (Tyr792). Total DDR1 expression was determined using anti-DDR1 polyclonal antibodies. (C) DDR1 was depleted by siRNA and DDR1 levels were assessed by immunoblot with anti-DDR1 polyclonal antibodies and normalized to β-actin. Densitometric analysis was performed using ImageJ (National Institute of Health) and expressed as arbitrary units. (D) Migration of serum-starved and DDR1-depleted 5637 cells after stimulation with IGF-I (10 nM) was assessed at 18 h as previously described. *p < 0.05, **p < 0.01.
These results indicate that DDR1 is necessary for IGF-I-induced motility, corroborating the notion that cross-talk between DDR1 and IGF-I system is biologically relevant in bladder cancer progression.
Stable depletion of DDR1 in UMUC-3 cells inhibits anchorage-independent growth
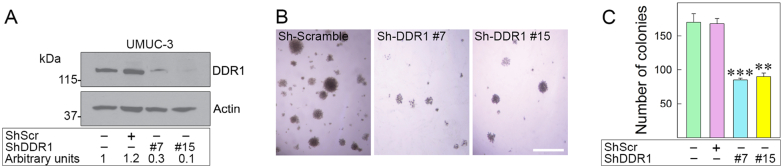
To determine whether DDR1 is critical for anchorage-independent growth, we used shRNA to stably deplete it in invasive UMUC-3 cells, which forms colonies in soft-agar assays [80,81]. We isolated two pools of UMUC-3 cells showing significant DDR1 depletion (~80–90%) (Fig. 5A). Notably, DDR1-deficient cells produced very few colonies in soft-agar (**p < 0.01, ***p < 0.001, Fig. 5B, C).
Fig. 5.
DDR1 depletion inhibits anchorage-independent growth of UMUC-3 cells. (A) DDR1 depletion was achieved by shRNA. Stably-depleted DDR1 UMUC-3 cells were analyzed for DDR1 expression by immunoblot with anti-DDR1 antibodies. DDR1 expression was quantified to β-actin by densitometric analysis using ImageJ. (B) (C) Anchorage-independent growth was assessed by colony formation via soft-agar assays [80,81]. Colonies ≥150 μm were counted [82]. Mean ± SD of three biological replicates; **p < 0.01, ***p < 0.001.
These results imply a critical role for DDR1 in regulating anchorage-independent growth of urothelial cancer cells.
IGF-I and IGF-II regulate F-actin networks of bladder cancer cells
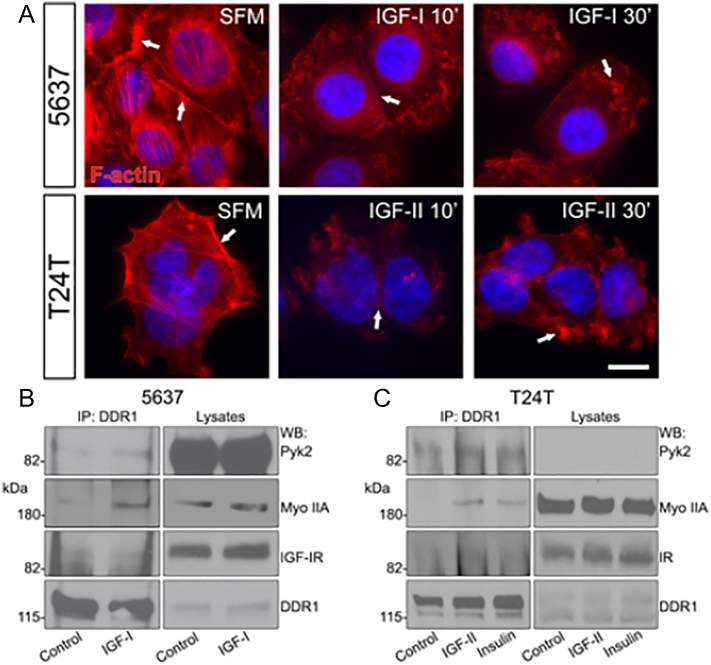
Our work has established the role of the IGF-IR in the motility of bladder cancer cells [49,50], and additional experiments have demonstrated an interaction between DDR1 and myosin IIA, which impact cell spreading and migration by regulating adhesive contacts with collagen [86]. Based on these observations, we hypothesize that a specific interaction between DDR1 and the IGF-IR/IR would regulate cell migration by controlling actomyosin and focal adhesion dynamics. Thus, we assayed whether IGF-IR or IR-A activation would affect cytoskeletal structures. Bladder cancer cells were stimulated in serum-free media (SFM) with physiological concentrations of IGF-I or IGF-II (10 nM) and F-actin dynamics visualized with Alexa Fluor® 594-phalloidin. In unchallenged conditions, both 5637 and T24T showed intense cortical F-actin staining at cell-cell contacts and in the organized network of the cytoskeleton (Fig. 6A, SFM arrows). In contrast, the F-actin cytoskeletal network was significantly disrupted after 10 min of IGF-I or IGF-II stimulation (Fig. 6A, IGF-I or IGF-II 10′), with progressive disruption of F-actin cortical structures at cell-cell contacts (Fig. 6A, IGF-I or IGF-II 30′).
Fig. 6.
The IGF-system and DDR1 modulate F-actin networks. (A) 5637 and T24T cells were serum-starved for 18 h, and then stimulated with 10 nM IGF-I or IGF-II for 10 or 30 min. F-actin was detected by staining with Alexa Fluor® 594-phalloidin, followed by confocal analysis. Images are representative of 10 independent fields from three independent experiments. An average of 100 cells/condition was examined. White arrows point to reorganization and remodeling of the actin filaments and loss of cortical actin upon stimulation with the growth factors. Nuclei were stained with DAPI. Bar ~10 μm. (B) 5637 and (C) T24T cells were serum-starved for 18 h and stimulated for 5 min with 10 nM of IGF-I, IGF-II, or insulin. Lysates were immunoprecipitated with anti-DDR1 polyclonal antibodies. Immunoblots were performed using specific antibodies for Pyk2, myosin IIA heavy chain, IGF-IR or IR, and DDR1.
Collectively, these results indicate that IGF-IR and IR-A activation might regulate actomyosin turnover and support our hypothesis that DDR1 may regulate bladder cancer cell motility by linking the IGF-IR and IR-A to the regulation of the F-actin network.
DDR1 associates with the IGF-IR or IR, Pyk2 and non-muscle myosin IIA
To further define the molecular mechanisms by which IGF-IR (or IR) activation may regulate the actomyosin cytoskeleton, we performed co-immunoprecipitation experiments assessing whether DDR1 and IGF-IR/IR could interact with Pyk2 or myosin IIA, which regulates focal adhesions [51]. DDR1 and myosin IIA cooperate to regulate collagen-dependent cell spreading and motility [86]. Serum-starved 5637 and T24T cells were stimulated with IGF-I (5637 cells), IGF-II or insulin (T24T cells), and lysates immunoprecipitated with anti-DDR1 antibodies. In both 5637 (Fig. 6B) and T24T cells (Fig. 6C), DDR1 associated with Pyk2 and myosin IIA and the interactions were increased after IGF-I (Fig. 6B), IGF-II or insulin (Fig. 6C) stimulation. Pyk2 expression was significantly lower in T24T cells and, therefore not detectable (Fig. 6C). We also detected the IGF-IR (Fig. 6B) and IR (Fig. 6C) in DDR1 co-immunoprecipitates, confirming our previous experiments (Fig. 3) and the association between this receptor and the IGF-IR (in 5637 cells) and the IR (in T24T cells). These data indicate that DDR1 may be a part of a multi-protein complex including Pyk2, myosin IIA, and the IGF-IR or IR. This complex may be critical for bladder cancer cell motility by regulating actin skeleton dynamics.
Analysis of DDR1 expression in a bladder cancer tissue microarray
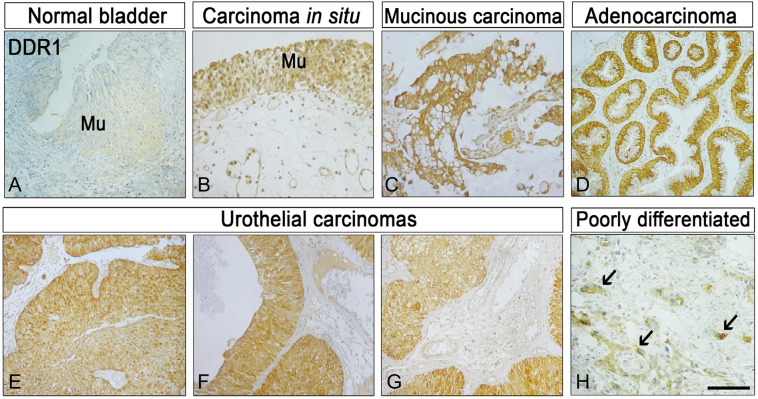
To investigate whether DDR1 may work in conjunction with the IGF-IR as a diagnostic or prognostic biomarker for bladder cancer, we performed immunohistochemical analysis of DDR1 expression in an AccuMax™ bladder cancer tissue microarray. This tissue array contained 43 cases of urothelial carcinoma, 7 adenocarcinoma, 6 squamous cell carcinoma, 4 metastatic carcinoma and 9 bladder tissue, triplicate cores per case. In contrast to normal bladder mucosa (Fig. 7A), DDR1 was highly expressed in both carcinoma in situ (Fig. 7B) as well as in all the forms of bladder cancer studied (Fig. 7C–H). Notably, bladder cancer cells expressed DDR1 both at the cell surface and within the cytoplasm. DDR1 positivity was noted in nests of poorly differentiated urothelial carcinoma cells (arrows, Fig. 7H).
Fig. 7.
DDR1 expression in bladder cancer tissue microarrays. DDR1 expression was determined by immunohistochemistry using a specific anti-DDR1 rabbit monoclonal antibody on a Biomax human bladder cancer tissue microarray. Mu, mucosa. The arrows in panel H point to nests of poorly differentiated, urothelial carcinoma cells (brown) infiltrating the negative stroma. Bar ~200 μm.
These results suggest that DDR1, IGF-IR, and possibly IR levels, may constitute a novel biosignature predictive of bladder cancer progression.
Discussion
Bladder cancer is among the most common solid tumors in the United States, with an alarming 80,470 new cases and 17,670 predicted deaths in 2019 [1]; however, a profound lack of knowledge on the molecular mechanisms that govern tumor formation and metastasis persists in the field.
Our laboratories demonstrated that activated IGF-IR functions as a “scatter factor” and induces motility and invasion without affecting cell proliferation [49]. IGF-IR-evoked Akt and MAPK pathways and IGF-I-induced Akt- and MAPK-dependent phosphorylation of paxillin, which relocated to focal adhesions, and was necessary for promoting motility of bladder cancer cells. These results are innovative, considering that the IGF-IR in most other cancers promotes cell proliferation and survival [33]. Furthermore, while IGF-IR action and regulation are well characterized in cell survival [87], the mechanisms modulating IGF-IR activity in motility and invasion are still very poorly defined. Moreover, whether the IR may crosstalk with the IGF-IR in bladder cancer progression and dissemination has not been previously established.
In the present study, we show that: (A) Stable IGF-IR depletion in metastatic bladder cancer cells only partially inhibits motility and anchorage-independent growth. (B) Tumorigenic and metastatic bladder cancer cells express lower levels of IGF-IR but overexpress the IR-A. (C) Expression of the collagen receptor DDR1 enhances bladder cancer progression. (D) DDR1 is phosphorylated by IGF ligands and interacts with IGF-IR and IR. (E) IGF-I and IGF-II regulate F-actin dynamics and the formation of a multi-protein complex, which includes IGF-IR/DDR1 or IR-A/DDR1, Pyk2, and non-muscle myosin IIA, and (F) DDR1 expression is upregulated in human bladder cancer tissues compared to healthy controls.
The extracellular matrix regulates IGF signaling in physiology and disease. In particular, the small leucine rich proteoglycan decorin [53,58,[66], [67], [68],88,89] modulates IGF-IR activity by regulating IGF-IR phosphorylation and activation of downstream effectors [90]. In non-transformed cells, decorin mimics IGF-I, thereby functioning as an IGF-IR agonist to regulate specific biological responses [51]. Conversely, decorin counteracts IGF-IR action in bladder cancer cells and negatively regulates IGF-I-evoked physiological reactions [50]. As an additional layer of regulation, decorin binds the IR-A and IR-A ligands, IGF-II, insulin, and proinsulin, with a hierarchy of affinities [69], but preferentially suppresses IGF-II-dependent IR-A activation of downstream signaling [69]. Collectively, these results suggest an exciting, functional dichotomy of decorin towards the IGF-I system in non-transformed versus cancer cells and the ligand-specific regulation of IR-A [51]. Cancer cells often produce IGF-II, and an autocrine loop involving IGF-II and the IR-A stimulates thyroid cancer growth [44]. Additionally, DDR1 regulates thyroid cancer cell differentiation via an IGF-2/IR-A autocrine signaling loop [11]. Importantly, urothelial bladder carcinomas produce IGF-II [91,92] suggesting that decorin loss in the tumor microenvironment may enhance IGF-II-evoked and DDR1-regulated IR-A for bladder cancer progression.
To further elucidate the role of the IGF-IR in regulating urothelial tumorigenesis, we initially tested IGF-IR function in anchorage-independent growth. Surprisingly, IGF-IR depletion only partially reduced the ability of bladder cancer cells to grow in a soft-agar assay. Thus, we hypothesize that the IR-A, which is the IR isoform preferentially expressed in metastatic bladder cancer [83], may work in conjunction with the IGF-IR in driving bladder cancer progression. Indeed, recent studies support the concept that both IR isoforms are broadly overexpressed in human tumors and that the IR-A/IR-B ratio is skewed to favor the IR-A isoform [22]. Studies have also demonstrated that IR isoforms act in strict connection with the IGF-IR, often forming heterodimeric receptor complexes [22], which are activated by multiple IGF ligands, usually produced by cancer cells [22]. However, whether IGF-IR/IR-A hybrids are present and activated in an autocrine or paracrine manner in bladder cancer cells has not yet been determined.
Based on our previous observations that the collagen receptor DDR1 functionally interacts with both the IGF-IR and IR in breast cancer [11,[72], [73], [74], [75], [76]] and prior reports on the relevance of DDR1 [70] and DDR2 [71] in bladder cancer, we determined whether DDR1 may regulate IGF-IR/IR in bladder cancer. Our results suggest that this RTK is critical in regulating IGF ligand-evoked cell motility and anchorage-independent growth by functionally interacting with the IGF-IR and IR. DDR1 is expressed in five different isoforms, three of which are full-length, functional RTKs (DDR1a, DDR1b, and DDR1c), while DDR1d and DDR1e are truncated or kinase-inactive [12,13]. We discovered that DDR1a is the isoform prominently expressed in the majority of invasive and metastatic bladder cancer, while 5637 cells express a considerable amount of DDR1b as well, albeit at lower levels. These data suggest that DDR1a is likely the active isoform in bladder cancer cells, but further studies are required to determine whether multiple isoforms may also contribute to its oncogenic function. We hypothesize that different isoforms may have opposite effects by acting in a dominant negative manner towards DDR1a, thus negatively regulating IGF-evoked bladder cancer cell motility. Recent data indicate that DDR1 translocates to the nucleus, where it regulates collagen transcription [21]. Significantly, there is strong evidence that both the IGF-IR and the IR also translocate to the nucleus [[93], [94], [95], [96], [97], [98], [99]], but whether DDR1 translocation may be mechanistically linked with the IGF-IR or IR remains to be elucidated.
Efficient cancer cell migration depends on dynamic interactions between F-actin, myosin II, microtubules, and focal adhesions [[100], [101], [102]]. The ATPase function of non-muscle myosin IIA (NMHC-IIA) allows transient binding to the actin cytoskeleton, which generates mechanical forces essential to maintain cellular architecture or to initiate cell motility [[101], [102], [103], [104]]. Mouse embryos lacking Nmhc-IIA die at an early stage of development, confirming the fundamental role of myosin IIA in cellular function [105]. Recent data have demonstrated an interaction between DDR1 and myosin IIA, which impacts cell spreading and migration by regulating adhesive contacts with collagen [86]. We discovered that IGF-I-induced paxillin-regulated focal adhesion dynamics of invasive bladder cancer cells [49]. Significantly, DDR1 and Pyk2 contribute to collagen-dependent up-regulation of N-cadherin and enhanced motility [106]. Based on these observations, we hypothesize that a specific RTK interaction between DDR1 and the IGF-IR/IR would regulate cell migration by controlling actomyosin and focal adhesion dynamics. We believe that the IGF-IR/DDR1 and IR-A/DDR1 complexes recruit Pyk2 and non-muscle myosin IIA in a ligand-dependent manner is an innovative and posits a novel, functional link to the F-actin cytoskeleton as a regulatory mechanism driving bladder cancer cell motility.
Growth factor-dependent phosphorylation of myosin IIA heavy chain plays an essential regulatory role in mediating cell motility and chemotaxis of breast cancer cells [107]. Specifically, myosin IIA phosphorylation at Ser1293 is a critical step in regulating locomotion of mammary carcinoma cells [107]. However, we did not determine in this study whether IGF ligands may promote myosin IIA phosphorylation, and further experiments are required to address this issue. As non-muscle myosin IIA affects not only motility, but also the adhesive ability of migrating cells [[101], [102], [103], [104]], we postulate that myosin IIA may not regulate cell motility, but instead modulate IGF-regulated cell adhesion, a critical step for metastatic cells to colonize distant organs.
In conclusion, our data provide essential information to understand better the molecular mechanisms that regulate bladder cancer progression and offer valuable insight for translational research. Once fully characterized, the roles of the IGF-IR, IR, and DDR1 in bladder cancer could contribute to the identification of novel targets for therapeutic intervention. Furthermore, the IGFI-R, IR, and/or DDR1 may prove useful as clinical biomarkers for detecting bladder cancer.
Materials and methods
Cell lines
Urothelial carcinoma-derived human RT4, 5637, T24, and UMUC-3 cells were obtained from the ATCC. T24T cells (a kind gift from Dr. Dan Theodorescu, University of Colorado Cancer Center) are more aggressive isogenic derivatives of T24 cells that grow in soft-agar, [[77], [78], [79]]. RT4, 5637 and T24 cells were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS). UMUC-3 cells were maintained in MEM with EARL medium with 10% fetal bovine serum (FBS), while T24T cells were maintained in DMEM/F12 medium supplemented with 10% FBS. Serum-free medium (SFM) is DMEM supplemented with 0.1% bovine serum albumin and 50 μg/ml of transferrin (Sigma-Aldrich). Recombinant IGF-I was purchased from Calbiochem (San Diego, CA). Recombinant bovine insulin was from Sigma-Aldrich, while recombinant IGF-II was from PreproTech.
Generation of IGF-IR- and DDR1-depleted bladder cancer cells
Transient DDR1 depletion in 5637 cells was achieved by siRNA. DDR1-specific siRNA were SMART pool oligos designed by Dharmacon. Cells were transfected using the TransIT®-Prostate Transfection Kit (Mirus), and cells untreated or treated with scrambled siRNA were used as controls. Motility was assessed 72 h post-transfection as described below. Cell lines stably depleted of endogenous IGF-IR (T24T) or DDR1 (UMUC-3) were generated by transfecting the pRS-sh-Scr (scrambled shRNAs) and pRS/shIGF-IR or shDDR1 plasmids expressing shRNAs against human IGF-IR or DDR1 (OriGene Technologies, Inc.) using the TransIT®-Prostate Transfection Kit (Mirus). Cells were selected in medium supplemented with 2 μg/ml of Puromycin. After selection, pools of either IGF-IR or DDR1-depleted T24T or UMUC-3 cells respectively were tested for IGF-IR or DDR1 expression levels by immunoblot using specific polyclonal antibodies as previously described [69,73,84].
Co-immunoprecipitation and Western immunoblots
Serum-starved 5637 and T24T cells were stimulated with 10 nM of either IGF-I (5637), IGF-II or insulin (T24T), for 5 min, lysed and 2 mg of proteins immunoprecipitated with either IGF-IR or IR using a monoclonal antibody against the IGF-IR (Oncogene Sciences) or IR α-subunits (Novus Biologicals) as previously described [84,108]. After SDS-PAGE, membranes were immunoblotted with β-subunit-specific anti-IGF-IR or anti-IR polyclonal antibodies (Santa Cruz Biotechnology). DDR1 was immunoprecipitated using anti-DDR1 polyclonal antibodies (C-20, sc-532, Santa Cruz Biotechnology) and detected by immunoblot with anti-DDR1 antibodies (C-20, sc-532, Santa Cruz Biotechnology) as described [[73], [74], [75]]. Phospho-DDR1 (Tyr792), Pyk2, and myosin IIA antibodies were from Cell Signaling Technology.
Migration and colony formation assays
Cell motility was assessed in Boyden chambers as described [80,81,109]. Briefly, HTS FluoroBloks™ inserts (Becton Dickinson) were saturated with PBS-1% bovine serum albumin for 2 h at room temperature. Serum-starved cells were labeled with DiI (Molecular Probes) for 20 min at 37 °C and then seeded in the HTS FluoroBloks™ upper chamber in either SFM or SFM supplemented with either 1% FBS, 10 nM of IGF-I, IGF-II or insulin and incubated at 37 °C for either 4 (1% serum) or 18 h. After fixing in 4% paraformaldehyde, membranes were mounted on a slide, and migrated cells were counted and photographed with a Zeiss Axiovert 200M live-cell microscope at the Kimmel Cancer Center Confocal Microscopy Core Facility. Anchorage-independent grow was measured by colony formation in soft-agar as previously described [80,81,110,111]. Briefly, cells were seeded in soft-agar at a density of 5 × 103 cells/35 mm plate and counted after three weeks in culture. Colonies > 150 μm were scored as positive.
Confocal microscopy and immunohistochemistry
Confocal microscopy analysis was performed as previously described [112]. Serum-starved 5637 and T24T cells plated on 0.2% gelatin-coated four-well chamber slides (Nunc, Thermo Fisher Scientific) were stimulated with either IGF-I or IGF-II (10 nM) for 10 or 30 min. After treatment, cells were rinsed twice with cold DPBS (Thermo Fisher Scientific) and fixed in 4% (wt/vol) paraformaldehyde for 30 min. After washing, F-actin was detected using Alexa Fluor® 594-phalloidin followed by confocal analysis. Images are representative of 10 independent fields from three independent experiments. Confocal analysis was performed using a 63×, 1.3 oil-immersion objective of an LSM-780 confocal laser-scanning microscope (ZEISS) with filters set at 450/594 nm for dual-channel imaging. All images were analyzed using ImageJ (National Institute of Health) and Adobe Photoshop CS6 (Adobe Systems).
DDR1 expression was analyzed by immunohistochemistry as described [80,81,113] on a Biomax human bladder cancer tissue microarray (BL208) at the Translational Core Facility of the Sidney Kimmel Cancer Center. The antibody used was an anti-DDR1 rabbit monoclonal antibody (Abcam, ab108608) at a dilution of 1:400. Detailed product specifications of the bladder tissue array can be found at http://www.biomax.us/tissue-arrays/Bladder/BL208. The array contains 43 cases of urothelial carcinoma, 7 adenocarcinoma, 6 carcinoma in situ, 4 metastatic carcinoma and 9 bladder tissue, with triplicate cores per case. Quantification of DDR1 staining in the microarray was done utilizing ImageJ (NIH). The threshold of staining of each image acquired was adjusted in order to show only the specific staining.
Statistical analysis
Results of multiple experiments are expressed as mean ± SD. All statistical analyses were carried out with SigmaStat for Windows v3.10 (Systat Software, Inc., Port Richmond, CA). Significance of the differences was evaluated by Student's t-test with significance at p < 0.05. All data presented herein were collected from a minimum of three independent, biological experiments. Where more than two experimental conditions were compared, one-way ANOVA was performed.
Abbreviations
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgments
Acknowledgments
This work was supported in part by the Benjamin Perkins Bladder Cancer Fund and The Schorsch Family Fund for Innovative Medical Research and Care (A. Morr.), National Institutes of Health Grants RO1 CA164462 (A. Morr., R.V.I.), CA39481 (RVI) and grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) (grant n. AIRC IG 19242) (A.B.). The Bioimaging Core Facility of the Sidney Kimmel Cancer Center is supported by NIH/NCI (P30CA056036).
Author contributions
Conceptualization: AM, SB, A Morr., B and RVIO and AN; Data acquisition: SB, AM, MS, CP, S X; Data curation: A Morr., SB, AB, and RVIO; Supervision: A Morr., AB and RVI; Writing - review & editing: SB, A Morr., TN, and RVI.
Contributor Information
Renato V. Iozzo, Email: renato.iozzo@jefferson.edu.
Andrea Morrione, Email: andrea.morrione@jefferson.edu, andrea.morrione@temple.edu.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Mitra A.P., Cote R.J. Molecular pathogenesis and diagnostics of bladder cancer. Annu. Rev. Pathol. 2009;4:251–285. doi: 10.1146/annurev.pathol.4.110807.092230. [DOI] [PubMed] [Google Scholar]
- 3.Knowles M.A. Molecular pathogenesis of bladder cancer. Int. J. Clin. Oncol. 2008;13(4):287–297. doi: 10.1007/s10147-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 4.Botteman M.F., Pashos C.L., Redaelli A., Laskin B., Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 5.Boormans J.L., Zwarthoff E.C. Limited funds for bladder cancer research and what can we do about it. Bladder Cancer. 2016;2(1):49–51. doi: 10.3233/BLC-150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.H., Hu W., Matulay J.T., Silva M.V., Owczarek T.B., Kim K., Chua C.W., Barlow L.J., Kandoth C., Williams A.B., Bergren S.K., Pietzak E.J., Anderson C.B., Benson M.C., Coleman J.A., Taylor B.S., Abate-Shen C., McKiernan J.M., Al-Ahmadie H., Solit D.B., Shen M.M. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173(2):515–528. doi: 10.1016/j.cell.2018.03.017. (e17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitinger B., Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26(3):146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Borza C.M., Pozzi A. Discoidin domain receptors in disease. Matrix Biol. 2014;34:185–192. doi: 10.1016/j.matbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 2014;310:39–87. doi: 10.1016/B978-0-12-800180-6.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H., Raynal N., Stathopoulos S., Myllyharju J., Farndale R.W., Leitinger B. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011;30(1):16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vella V., Malaguarnera R., Nicolosi M.L., Morrione A., Belfiore A. Insulin/IGF signaling and discoidin domain receptors: an emerging functional connection. Biochim Biophys Acta Mol Cell Res. 2019;1866(11):118522. doi: 10.1016/j.bbamcr.2019.118522. [DOI] [PubMed] [Google Scholar]
- 12.Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 1999;13(Suppl):S77–S82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- 13.Valiathan R.R., Marco M., Leitinger B., Kleer C.G., Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metas Rev. 2012;31(1–2):295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juskaite V., Corcoran D.S., Leitinger B. Collagen induces activation of DDR1 through lateral dimer association and phosphorylation between dimers. Elife. 2017;6 doi: 10.7554/eLife.25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrivastava A., Radziejewski C., Campbell E., Kovac L., McGlynn M., Ryan T.E., Davis S., Goldfarb M.P., Glass D.J., Lemke G., Yancopoulos G.D. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell. 1997;1(1):25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 16.Vogel W., Gish G.D., Alves F., Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell. 1997;1(1):13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran D.S., Juskaite V., Xu Y., Gorlitz F., Alexandrov Y., Dunsby C., French P.M.W., Leitinger B. DDR1 autophosphorylation is a result of aggregation into dense clusters. Sci. Rep. 2019;9(1):17104. doi: 10.1038/s41598-019-53176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitinger B., Saltel F. Discoidin domain receptors: multitaskers for physiological and pathological processes. Cell Adhes. Migr. 2018:1–2. doi: 10.1080/19336918.2018.1491495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadiya M., Chakraborty G. Signaling by discoidin domain receptor 1 in cancer metastasis. Cell Adhes. Migr. 2018;12(4):315–323. doi: 10.1080/19336918.2018.1520556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vehlow A., Klapproth E., Jin S., Hannen R., Hauswald M., Bartsch J.W., Nimsky C., Temme A., Leitinger B., Cordes N. Interaction of discoidin domain receptor 1 with a 14-3-3-Beclin-1-Akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. 2019;26(13):3672–3683. doi: 10.1016/j.celrep.2019.02.096. (e7) [DOI] [PubMed] [Google Scholar]
- 21.Chiusa M., Hu W., Liao H.J., Su Y., Borza C.M., de Caestecker M.P., Skrypnyk N.I., Fogo A.B., Pedchenko V., Li X., Zhang M.Z., Hudson B.G., Basak T., Vanacore R.M., Zent R., Pozzi A. The extracellular matrix receptor discoidin domain receptor 1 regulates collagen transcription by translocating to the nucleus. J. Am. Soc. Nephrol. 2019;30(9):1605–1624. doi: 10.1681/ASN.2018111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belfiore A., Malaguarnera R., Vella V., Lawrence M.C., Sciacca L., Frasca F., Morrione A., Vigneri R. Insulin receptor isoforms in physiology and disease: an updated view. Endocr. Rev. 2017;38(5):379–431. doi: 10.1210/er.2017-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher C.D., Stone M.E., Stiles C.D. Platelet-derived growth factor prevents G0 growth arrest. Nature. 1979;281(5730):390–392. doi: 10.1038/281390a0. [DOI] [PubMed] [Google Scholar]
- 24.Stiles C.D., Isberg R.R., Pledger W.J., Antoniades H.N., Scher C.D. Control of the Balb/c-3T3 cell cycle by nutrients and serum factors: analysis using platelet-derived growth factor and platelet-poor plasma. J. Cell. Physiol. 1979;99(3):395–405. doi: 10.1002/jcp.1040990314. [DOI] [PubMed] [Google Scholar]
- 25.Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 26.Liu J.P., Baker J., Perkins A.S., Robertson E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75(1):59–72. [PubMed] [Google Scholar]
- 27.Eggenschwiler J., Ludwig T., Fisher P., Leighton P.A., Tilghman S.M., Efstratiadis A. Mouse mutant embryos overexpressing IGF-II exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes. Genes Dev. 1997;11(23):3128–3142. doi: 10.1101/gad.11.23.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrione A. Grb10 adapter protein as regulator of insulin-like growth factor receptor signaling. J. Cell. Physiol. 2003;197(3):307–311. doi: 10.1002/jcp.10363. [DOI] [PubMed] [Google Scholar]
- 29.Morrione A., Romano G., Navarro M., Reiss K., Valentinis B., Dews M., Eves E., Rosner M.R., Baserga R. Insulin-like growth factor I receptor signaling in differentiation of neuronal H19-7 cells. Cancer Res. 2000;60(8):2263–2272. [PubMed] [Google Scholar]
- 30.Morrione A., Navarro M., Romano G., Dews M., Reiss K., Valentinis B., Belletti B., Baserga R. The role of the insulin receptor substrate-1 in the differentiation of rat hippocampal neuronal cells. Oncogene. 2001;20(35):4842–4852. doi: 10.1038/sj.onc.1204649. [DOI] [PubMed] [Google Scholar]
- 31.Valentinis B., Navarro M., Zanocco-Marani T., Edmonds P., McCormick J., Morrione A., Sacchi A., Romano G., Reiss K., Baserga R. Insulin receptor substrate-1, p70S6K, and cell size in transformation and differentiation of hemopoietic cells. J. Biol. Chem. 2000;275(33):25451–25459. doi: 10.1074/jbc.M002271200. [DOI] [PubMed] [Google Scholar]
- 32.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55(2):249–252. [PubMed] [Google Scholar]
- 33.Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19(49):5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- 34.Baserga R., Hongo A., Rubini M., Prisco M., Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332(3):F105–F126. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 35.LeRoith D., Roberts C.T., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195(2):127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 36.R. Ramcharan, T. Aleksic, W.P. Kamdoum, S. Gao, S.X. Pfister, J. Tanner, E. Bridges, R. Asher, A.J. Watson, G.P. Margison, M. Woodcock, E. Repapi, J. Li, M. Middleton, V.M. Macaulay, IGF-1R inhibition induces schedule-dependent sensitization of human melanoma to temozolomide, Oncotarget 6(36) 39877–39890. [DOI] [PMC free article] [PubMed]
- 37.Krywicki R.F., Yee D. The insulin-like growth factor family of ligands, receptors, and binding proteins. Breast Cancer Res. Treat. 1992;22(1):7–19. doi: 10.1007/BF01833329. [DOI] [PubMed] [Google Scholar]
- 38.Frasca F., Pandini G., Scalia P., Sciacca L., Mineo R., Costantino A., Goldfine I.D., Belfiore A., Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 1999;19(5):3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrione A., Valentinis B., Xu S.Q., Yumet G., Louvi A., Efstratiadis A., Baserga R. Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc. Natl. Acad. Sci. U. S. A. 1997;94(8):3777–3782. doi: 10.1073/pnas.94.8.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandini G., Frasca F., Mineo R., Sciacca L., Vigneri R., Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J. Biol. Chem. 2002;277(42):39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 41.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr. Pharm. Des. 2007;13(7):671–686. doi: 10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- 42.Belfiore A., Frasca F., Pandini G., Sciacca L., Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009;30(6):586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 43.Malaguarnera R., Belfiore A. The insulin receptor: a new target for cancer therapy. Front. Endocrinol. 2011;2:93. doi: 10.3389/fendo.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vella V., Pandini G., Sciacca L., Mineo R., Vigneri R., Pezzino V., Belfiore A. A novel autocrine loop involving IGF-II and the insulin receptor isoform-a stimulates growth of thyroid cancer. J. Clin. Endocrinol. Metab. 2002;87(1):245–254. doi: 10.1210/jcem.87.1.8142. [DOI] [PubMed] [Google Scholar]
- 45.Kalli K.R., Falowo O.I., Bale L.K., Zschunke M.A., Roche P.C., Conover C.A. Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology. 2002;143(9):3259–3267. doi: 10.1210/en.2001-211408. [DOI] [PubMed] [Google Scholar]
- 46.Sciacca L., Costantino A., Pandini G., Mineo R., Frasca F., Scalia P., Sbraccia P., Goldfine I.D., Vigneri R., Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18(15):2471–2479. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 47.Sciacca L., Mineo R., Pandini G., Murabito A., Vigneri R., Belfiore A. In IGF-I receptor-deficient leiomyosarcoma cells autocrine IGF-II induces cell invasion and protection from apoptosis via the insulin receptor isoform A. Oncogene. 2002;21(54):8240–8250. doi: 10.1038/sj.onc.1206058. [DOI] [PubMed] [Google Scholar]
- 48.Vella V., Nicolosi M.L., Cantafio P., Massimino M., Lappano R., Vigneri P., Ciuni R., Gangemi P., Morrione A., Malaguarnera R., Belfiore A. DDR1 regulates thyroid cancer cell differentiation via IGF-2/IR-A autocrine signaling loop. Endocr. Relat. Cancer. 2019;26(1):197–214. doi: 10.1530/ERC-18-0310. [DOI] [PubMed] [Google Scholar]
- 49.Metalli D., Lovat F., Tripodi F., Genua M., Xu S.Q., Spinelli M., Alberghina L., Vanoni M., Baffa R., Gomella L.G., Iozzo R.V., Morrione A. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am. J. Pathol. 2010;176(6):2997–3006. doi: 10.2353/ajpath.2010.090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iozzo R.V., Buraschi S., Genua M., Xu S.Q., Solomides C.C., Peiper S.C., Gomella L.G., Owens R.C., Morrione A. Decorin antagonizes IGF-IR function by interfering with IGF-IR activity and attenuating downstream signaling. J. Biol. Chem. 2011;286(40):34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrione A., Neill T., Iozzo R.V. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013;280(10):2138–2149. doi: 10.1111/febs.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skandalis S.S., Afratis N., Smirlaki G., Nikitovic D., Theocharis A.D., Tzanakakis G.N., Karamanos N.K. Cross-talk between estradiol receptor and EGFR/IGF-IR signaling pathways in estrogen-responsive breast cancers: focus on the role and impact of proteoglycans. Matrix Biol. 2014;35:182–193. doi: 10.1016/j.matbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Theocharis A.D., Karamanos N.K. Proteoglycans remodeling in cancer: underlying molecular mechanisms. Matrix Biol. 2019;75–76:220–259. doi: 10.1016/j.matbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Wight T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018;71–72:396–420. doi: 10.1016/j.matbio.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heindryckx F., Li J.P. Role of proteoglycans in neuro-inflammation and central nervous system fibrosis. Matrix Biol. 2018;68–69:589–601. doi: 10.1016/j.matbio.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Li L., Zhao Q., Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018;68–69:490–506. doi: 10.1016/j.matbio.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Walraven M., Hinz B. Therapeutic approaches to control tissue repair and fibrosis: extracellular matrix as a game changer. Matrix Biol. 2018;71–72:205–224. doi: 10.1016/j.matbio.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Karamanos N.K., Theocharis A.D., Neill T., Iozzo R.V. Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019;75–76:1–11. doi: 10.1016/j.matbio.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mongiat M., Buraschi S., Andreuzzi E., Neill T., Iozzo R.V. Extracellular matrix: the gatekeeper of tumor angiogenesis. Biochem. Soc. Trans. 2019;47(5):1543–1555. doi: 10.1042/BST20190653. [DOI] [PubMed] [Google Scholar]
- 60.Andreuzzi E., Capuano A., Pellicani R., Poletto E., Doliana R., Maiero S., Fornasarig M., Magris R., Colombatti A., Cannizzaro R., Spessotto P., Mongiat M. Loss of multimerin-2 and EMILIN-2 expression in gastric cancer associate with altered angiogenesis. Int. J. Mol. Sci. 2018;19(12) doi: 10.3390/ijms19123983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricard-Blum S., Vallet S.D. Fragments generated upon extracellular matrix remodeling: biological regulators and potential drugs. Matrix Biol. 2019;75–76:170–189. doi: 10.1016/j.matbio.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Gotte M., Kovalszky I. Extracellular matrix functions in lung cancer. Matrix Biol. 2018;73:105–121. doi: 10.1016/j.matbio.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Brauchle E., Kasper J., Daum R., Schierbaum N., Falch C., Kirschniak A., Schaffer T.E., Schenke-Layland K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018;68–69:180–193. doi: 10.1016/j.matbio.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Christensen G., Herum K.M., Lunde I.G. Sweet, yet underappreciated: proteoglycans and extracellular matrix remodeling in heart disease. Matrix Biol. 2019;75–76:286–299. doi: 10.1016/j.matbio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Neill T., Schaefer L., Iozzo R.V. Decorin: a guardian from the matrix. Am. J. Pathol. 2012;181(2):380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iozzo R.V., Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iozzo R.V., Sanderson R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011;15(5):1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iozzo R.V., Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277(19):3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morcavallo A., Buraschi S., Xu S.Q., Belfiore A., Schaefer L., Iozzo R.V., Morrione A. Decorin differentially modulates the activity of insulin receptor isoform A ligands. Matrix Biol. 2014;35:82–90. doi: 10.1016/j.matbio.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie X., Rui W., He W., Shao Y., Sun F., Zhou W., Wu Y., Zhu Y. Discoidin domain receptor 1 activity drives an aggressive phenotype in bladder cancer. Am. J. Transl. Res. 2017;9(5):2500–2507. [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai M., Li W., Huang C., Ke H., Li C., Yeh H., Chan T., Liang P., Yeh B., Wu W., Lim S., Li C. DDR2 overexpression in urothelial carcinoma indicates an unfavorable prognosis: a large cohort study. Oncotarget. 2016;7(48):78918–78931. doi: 10.18632/oncotarget.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morcavallo A., Gaspari M., Pandini G., Palummo A., Cuda G., Larsen M.R., Vigneri R., Belfiore A. Research resource: new and diverse substrates for the insulin receptor isoform A revealed by quantitative proteomics after stimulation with IGF-II or insulin. Mol. Endocrinol. 2011;25(8):1456–1468. doi: 10.1210/me.2010-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malaguarnera R., Nicolosi M.L., Sacco A., Morcavallo A., Vella V., Voci C., Spatuzza M., Xu S.Q., Iozzo R.V., Vigneri R., Morrione A., Belfiore A. Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget. 2015;6(18):16084–16105. doi: 10.18632/oncotarget.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mata R., Palladino C., Nicolosi M.L., Lo Presti A.R., Malaguarnera R., Ragusa M., Sciortino D., Morrione A., Maggiolini M., Vella V., Belfiore A. IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget. 2016;7(7):7683–7700. doi: 10.18632/oncotarget.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vella V., Malaguarnera R., Nicolosi M.L., Palladino C., Spoleti C., Massimino M., Vigneri P., Purrello M., Ragusa M., Morrione A., Belfiore A. Discoidin domain receptor 1 modulates insulin receptor signaling and biological responses in breast cancer cells. Oncotarget. 2017;8(26):43248–43270. doi: 10.18632/oncotarget.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belfiore A., Malaguarnera R., Nicolosi M.L., Lappano R., Ragusa M., Morrione A., Vella V. A novel functional crosstalk between DDR1 and the IGF axis and its relevance for breast cancer. Cell Adhes. Migr. 2018;12(4):305–314. doi: 10.1080/19336918.2018.1445953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gildea J.J., Golden W.L., Harding M.A., Theodorescu D. Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer. Genes Chromosomes Canc. 2000;27(3):252–263. doi: 10.1002/(sici)1098-2264(200003)27:3<252::aid-gcc5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 78.Gildea J.J., Harding M.A., Seraj M.J., Gulding K.M., Theodorescu D. The role of Ral A in epidermal growth factor receptor-regulated cell motility. Cancer Res. 2002;62(4):982–985. [PubMed] [Google Scholar]
- 79.Gildea J.J., Seraj M.J., Oxford G., Harding M.A., Hampton G.M., Moskaluk C.A., Frierson H.F., Conaway M.R., Theodorescu D. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62(22):6418–6423. [PubMed] [Google Scholar]
- 80.Xu S.Q., Buraschi S., Morcavallo A., Genua M., Shirao T., Peiper S.C., Gomella L.G., Birbe R., Belfiore A., Iozzo R.V., Morrione A. A novel role for drebrin in regulating progranulin bioactivity in bladder cancer. Oncotarget. 2015;6(13):10825–10839. doi: 10.18632/oncotarget.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buraschi S., Xu S.Q., Stefanello M., Moskalev I., Morcavallo A., Genua M., Tanimoto R., Birbe R., Peiper S.C., Gomella L.G., Belfiore A., Black P.C., Iozzo R.V., Morrione A. Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin. Oncotarget. 2016;7(26):39980–39995. doi: 10.18632/oncotarget.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrione A., Valentinis B., Resnicoff M., Xu S., Baserga R. The role of mGrb10alpha in insulin-like growth factor I-mediated growth. J. Biol. Chem. 1997;272(42):26382–26387. doi: 10.1074/jbc.272.42.26382. [DOI] [PubMed] [Google Scholar]
- 83.Gao J., Chesebrough J.W., Cartlidge S.A., Ricketts S.A., Incognito L., Veldman-Jones M., Blakey D.C., Tabrizi M., Jallal B., Trail P.A., Coats S., Bosslet K., Chang Y.S. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71(3):1029–1040. doi: 10.1158/0008-5472.CAN-10-2274. [DOI] [PubMed] [Google Scholar]
- 84.Morcavallo A., Genua M., Palummo A., Kletvikova E., Jiracek J., Brzozowski A.M., Iozzo R.V., Belfiore A., Morrione A. Insulin and insulin-like growth factor II differentially regulate endocytic sorting and stability of insulin receptor isoform A. J. Biol. Chem. 2012;287(14):11422–11436. doi: 10.1074/jbc.M111.252478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carafoli F., Mayer M.C., Shiraishi K., Pecheva M.A., Chan L.Y., Nan R., Leitinger B., Hohenester E. Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure. 2012;20(4):688–697. doi: 10.1016/j.str.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang Y., Arora P., McCulloch C.A., Vogel W.F. The collagen receptor DDR1 regulates cell spreading and motility by associating with myosin IIA. J. Cell Sci. 2009;122(Pt 10):1637–1646. doi: 10.1242/jcs.046219. [DOI] [PubMed] [Google Scholar]
- 87.Morcavallo A., Stefanello M., Iozzo R.V., Belfiore A., Morrione A. Ligand-mediated endocytosis and trafficking of the insulin-like growth factor receptor I and insulin receptor modulate receptor function. Front. Endocrinol. (Lausanne) 2014;5:220. doi: 10.3389/fendo.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iozzo R.V., Gubbiotti M.A. Extracellular matrix: the driving force of mammalian diseases. Matrix Biol. 2018;71–72:1–9. doi: 10.1016/j.matbio.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Afratis N.A., Bouris P., Skandalis S.S., Multhaupt H.A., Couchman J.R., Theocharis A.D., Karamanos N.K. IGF-IR cooperates with ERalpha to inhibit breast cancer cell aggressiveness by regulating the expression and localisation of ECM molecules. Sci. Rep. 2017;7:40138. doi: 10.1038/srep40138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schonherr E., Levkau B., Schaefer L., Kresse H., Walsh K. Decorin affects endothelial cells by Akt-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2002;973:149–152. doi: 10.1111/j.1749-6632.2002.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 91.Byun H.M., Wong H.L., Birnstein E.A., Wolff E.M., Liang G., Yang A.S. Examination of IGF2 and H19 loss of imprinting in bladder cancer. Cancer Res. 2007;67(22):10753–10758. doi: 10.1158/0008-5472.CAN-07-0329. [DOI] [PubMed] [Google Scholar]
- 92.Wu H., Li Y., Cui H., Wu W., Yang H., Yang X., Wang Y., Li P. IGF2/IGF2R expression in urothelial bladder cancer and its implications for tumor recurrence and prognosis. Int. J. Clin. Exp. Med. 2017;10(1):881–888. [Google Scholar]
- 93.Aleksic T., Chitnis M.M., Perestenko O.V., Gao S., Thomas P.H., Turner G.D., Protheroe A.S., Howarth M., Macaulay V.M. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70(16):6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aleksic T., Gray N., Wu X., Rieunier G., Osher E., Mills J., Verrill C., Bryant R.J., Han C., Hutchinson K., Lambert A.G., Kumar R., Hamdy F.C., Weyer-Czernilofsky U., Sanderson M.P., Bogenrieder T., Taylor S., Macaulay V.M. Nuclear IGF1R interacts with regulatory regions of chromatin to promote RNA polymerase II recruitment and gene expression associated with advanced tumor stage. Cancer Res. 2018;78(13):3497–3509. doi: 10.1158/0008-5472.CAN-17-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asmane I., Watkin E., Alberti L., Duc A., Marec-Berard P., Ray-Coquard I., Cassier P., Decouvelaere A.V., Ranchere D., Kurtz J.E., Bergerat J.P., Blay J.Y. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur. J. Cancer. 2012;48(16):3027–3035. doi: 10.1016/j.ejca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Codony-Servat J., Cuatrecasas M., Asensio E., Montironi C., Martinez-Cardus A., Marin-Aguilera M., Horndler C., Martinez-Balibrea E., Rubini M., Jares P., Reig O., Victoria I., Gaba L., Martin-Richard M., Alonso V., Escudero P., Fernandez-Martos C., Feliu J., Mendez J.C., Mendez M., Gallego J., Salud A., Rojo F., Castells A., Prat A., Rosell R., Garcia-Albeniz X., Camps J., Maurel J. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br. J. Cancer. 2017;117(12):1777–1786. doi: 10.1038/bjc.2017.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hancock M.L., Meyer R.C., Mistry M., Khetani R.S., Wagschal A., Shin T., Ho Sui S.J., Naar A.M., Flanagan J.G. Insulin receptor associates with promoters genome-wide and regulates gene expression. Cell. 2019;177(3):722–736. doi: 10.1016/j.cell.2019.02.030. (e22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Titone R., Zhu M., Robertson D.M. Insulin mediates de novo nuclear accumulation of the IGF-1/insulin hybrid receptor in corneal epithelial cells. Sci. Rep. 2018;8(1):4378. doi: 10.1038/s41598-018-21031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sehat B., Tofigh A., Lin Y., Trocme E., Liljedahl U., Lagergren J., Larsson O. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Sci Signal. 2010;3(108) doi: 10.1126/scisignal.2000628. (ra10) [DOI] [PubMed] [Google Scholar]
- 100.Gupton S.L., Waterman-Storer C.M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125(7):1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 101.Even-Ram S., Doyle A.D., Conti M.A., Matsumoto K., Adelstein R.S., Yamada K.M. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat. Cell Biol. 2007;9(3):299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 102.Even-Ram S., Yamada K.M. Of mice and men: relevance of cellular and molecular characterizations of myosin IIA to MYH9-related human disease. Cell Adhes. Migr. 2007;1(3):152–155. doi: 10.4161/cam.1.3.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Even-Ram S., Yamada K.M. Cell migration in 3D matrix. Curr. Opin. Cell Biol. 2005;17(5):524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 104.Giannone G., Dubin-Thaler B.J., Rossier O., Cai Y., Chaga O., Jiang G., Beaver W., Dobereiner H.G., Freund Y., Borisy G., Sheetz M.P. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128(3):561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Conti M.A., Even-Ram S., Liu C., Yamada K.M., Adelstein R.S. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 2004;279(40):41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 106.Shintani Y., Fukumoto Y., Chaika N., Svoboda R., Wheelock M.J., Johnson K.R. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J. Cell Biol. 2008;180(6):1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dulyaninova N.G., House R.P., Betapudi V., Bresnick A.R. Myosin-IIA heavy-chain phosphorylation regulates the motility of MDA-MB-231 carcinoma cells. Mol. Biol. Cell. 2007;18(8):3144–3155. doi: 10.1091/mbc.E06-11-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vecchione A., Marchese A., Henry P., Rotin D., Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol. Cell. Biol. 2003;23(9):3363–3372. doi: 10.1128/MCB.23.9.3363-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lovat F., Bitto A., Xu S.Q., Fassan M., Goldoni S., Metalli D., Wubah V., McCue P., Serrero G., Gomella L.G., Baffa R., Iozzo R.V., Morrione A. Proepithelin is an autocrine growth factor for bladder cancer. Carcinogenesis. 2009;30(5):861–868. doi: 10.1093/carcin/bgp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monami G., Emiliozzi V., Bitto A., Lovat F., Xu S.Q., Goldoni S., Fassan M., Serrero G., Gomella L.G., Baffa R., Iozzo R.V., Morrione A. Proepithelin regulates prostate cancer cell biology by promoting cell growth, migration, and anchorage-independent growth. Am. J. Pathol. 2009;174(3):1037–1047. doi: 10.2353/ajpath.2009.080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tanimoto R., Morcavallo A., Terracciano M., Xu S.Q., Stefanello M., Buraschi S., Lu K.G., Bagley D.H., Gomella L.G., Scotlandi K., Belfiore A., Iozzo R.V., Morrione A. Sortilin regulates progranulin action in castration-resistant prostate cancer cells. Endocrinology. 2015;156(1):58–70. doi: 10.1210/en.2014-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neill T., Buraschi S., Goyal A., Sharpe C., Natkanski E., Schaefer L., Morrione A., Iozzo R.V. EphA2 is a functional receptor for the growth factor progranulin. J. Cell Biol. 2016;215(5):687–703. doi: 10.1083/jcb.201603079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vecchione A., Fassan M., Anesti V., Morrione A., Goldoni S., Baldassarre G., Byrne D., D’Arca D., Palazzo J.P., Lloyd J., Scorrano L., Gomella L.G., Iozzo R.V., Baffa R. MITOSTATIN, a putative tumor suppressor on chromosome 12q24.1, is downregulated in human bladder and breast cancer. Oncogene. 2009;28(2):257–269. doi: 10.1038/onc.2008.381. [DOI] [PMC free article] [PubMed] [Google Scholar]