Abstract
PURPOSE:
To analyse the prognostic factors for eye salvage for eyes with intra-ocular retinoblastoma (RB) that is resistant to systemic chemotherapy and focal therapy by external beam radiation therapy (EBRT).
METHODS:
A retrospective analysis of 28 eyes with intra-ocular RB that was resistant for systemic chemotherapy and focal consolidation therapy and received EBRT. Outcome measures included tumor stage at diagnosis, stage migration, type of tumor seeds, treatment modalities, eye globe salvage, metastasis, and survival.
RESULTS:
Most of the patients (83%) had bilateral RB, and 42% were females. All eyes were treated initially by combination of systemic chemotherapy and focal consolidation therapy. The dose of EBRT was 45 Gy. The mean follow-up was 6.5 years, and the overall eye globe salvage rate post EBRT was 46%: 67% (2/3) for group B, 71% (5/7) for group C, and 33% (6/18) for group D. Patient's gender, tumor site, laterality, and tumor stage at diagnosis were not significant prognostic factors (p> 0.05) for final outcome. The significant poor prognostic factors were tumor stage migration during systemic chemotherapy (p= 0.03) and presence of vitreous seeds at time of EBRT (p=0.001). Post EBRT complication rate was 68% (19/28) including; retinal detachment (3), vitreous hemorrhage (4), neovascular glaucoma (1), cataract (16), radiation retinopathy (2), and second malignancy (2).
CONCLUSION:
EBRT is an alternative for enucleation when RB is resistant to combined chemoreduction/focal consolidation therapy in absence in vitreous seeds. The known risks for EBRT are not justified for patients with unilateral RB and for those who have functional vision in the other eye.
Keywords: Radiation therapy, retinoblastoma, stage migration, vitreous seeds
Introduction
Retinoblastoma is the most common primary intraocular malignancy in childhood and infancy. The incidence is estimated at about 1 in 15,000–20,000 live births.[1,2]
In 1954, external beam radiotherapy (EBRT) emerged as the first eye salvage therapy for advanced intraocular retinoblastoma and was used extensively as primary treatment.[3] Although EBRT was often effective to control tumor growth, it was associated with ocular complications, and therefore, the need for eye enucleation after radiotherapy was common because of these complications rather than because of failure of radiation to control the intraocular tumors. Moreover, EBRT greatly increased the lifelong risk of second cancers in children with a germline RB1 gene mutation and was associated with cosmetic problems due to orbital bone growth retardation, particularly in younger patients.[4,5] Therefore, systemic chemotherapy combined with focal therapy became the primary treatment for intraocular retinoblastoma in the 1990s[6] and achieved high globe salvage rates: 100% for International Intraocular Retinoblastoma Classification (IIRC) Group A, 93% for Group B, 90% for Group C, and 47% for Group D eyes.[7]
Despite the use of new treatment options, such as laser photocoagulation,[8] cryotherapy,[9] plaque brachytherapy,[10] and thermochemotherapy[11] with good success rates, EBRT (mainly with the new advances in types and techniques of radiation) is still one of the most effective therapies for resistant cases to chemotherapy, particularly in situations where globe salvage is desired, like for example when the opposite eye is enucleated.[12,13,14,15]
Herein, we are evaluating the outcome and the factors affecting the outcome of using EBRT in management of resistant intraocular retinoblastoma cases that failed treatment by combined chemotherapy and focal therapy.
Methods
This study was approved by the institutional review board. It was a retrospective case series of 28 eyes for 24 consecutive patients who had a clinical diagnosis of intraocular retinoblastoma and were treated with EBRT after failure of tumor control by combined chemotherapy and focal therapy. Selection required access to patients' medical records and RetCam images.
Data included patient's age, gender, laterality, age at diagnosis, initial IIRC stage,[16] initial Reese–Ellsworth (RE) group,[17] type of seeds (subretinal and vitreal), focal therapy, dose of radiation therapy, postradiation complications, eye salvage, visual acuity, metastasis, second malignancy, and mortality.
Inclusion and exclusion criteria
The eligibility criteria for inclusion were children with intraocular retinoblastoma (as the presence of one or more retinal tumors detected on funduscopic examination using indirect ophthalmoscopy and scleral depression) who were initially treated with chemotherapy and focal treatment; however, these patients showed no response to these modalities since the beginning or showed favorable response initially but developed recurrence of the tumor during or after the treatment. Treatment was, therefore, followed by EBRT. Exclusion criteria included eyes that received plaque radiation therapy before EBRT and eyes followed for <12 months (unless there was recurrence).
Clinical characteristics and definitions
Tumors were staged at presentation according to IIRC[16] and RE[17] staging systems. At the time of radiation, tumors were divided into three groups based on the presence of tumor seeds as follows: no tumor seeds, subretinal seeds, and vitreous seeds. Stage migration was defined as tumor progression and development of new clinical features during management (by chemotherapy and focal therapy) that are features of more advanced IIRC stage. Failure of tumor control by combined chemotherapy and focal therapy was defined as tumor or seed recurrence with the need for external beam radiation therapy or enucleation. Eye salvage was defined as tumor control and avoidance of enucleation. The decision for enucleation was approved by two ocular oncologists and after consultation with an external reviewer.
Treatment methods
We performed a combination regimen of chemotherapy that consisted of carboplatin, vincristine, and etoposide (CVE). Each CVE cycle was repeated every 4 weeks for a total of 6–8 cycles according to patient's condition and tumor status. Ocular oncology follow-up was provided as examination under general anesthesia before each cycle of chemotherapy and every 4–8 weeks thereafter. Fundus photos were taken using a RetCam II (Clarity Medical System, Pleasanton, CA, USA), and focal therapy was provided using thermotherapy or cryotherapy until tumor control was achieved.
Radiation therapy was administered in a consistent fashion. Simulation and treatment were done for patients under general anesthesia while wearing a thermoplastic head mask immobilization device. All patients were treated in supine position using a linear accelerator at a photon energy of 6 millivolt (mV) (Elekta Synergy) through a three-dimensional conformal radiotherapy. The dose prescribed to the retinal target volume was 45 Gy in 25 fractions of 1.8 Gy per fraction.
Statistical analysis
Statistical analysis of tumor control and eye salvage was correlated to the gender, laterality, IIRC group, RE stage, type of seeds, stage migration, number of chemotherapy cycles, and dose of radiation. The P value was measured to test the predictive power of each factor using the Fisher's exact test.
Results
Between January 2003 and December 2018, there were 28 eyes for 24 patients with intraocular retinoblastoma (IIRC Group B, C, or D) resistant to treatment with combined chemotherapy and focal therapy and were secondarily treated by EBRT.
Demographics
The mean age at diagnosis was 19 months (median, 11 months; range, 2–48 months). There were 14 males (58%) and 10 females (42%). There were 4 (17%) unilateral and 20 (83%) bilateral cases. All unilateral cases and bilateral cases with good visual potential and tumor control in the other eye in this series refused the offered enucleation, therefore, they were treated by EBRT. The patients' demographics are listed in Table 1.
Table 1.
Demographic features for intraocular retinoblastoma cases that failed the combined treatment
| Factor | Number | % | 95% CI |
|---|---|---|---|
| Number of patients | 24 | ||
| Number of eyes | 28 | ||
| Male | 14 | 58% | 0.3880-0.7556 |
| Female | 10 | 42% | 0.2444-0.6120 |
| Bilateral | 20 | 83% | 0.6353-0.9393 |
| Unilateral | 4 | 17% | 0.0607-0.3647 |
| Right eye | 13 | 46% | 0.2953-0.6419 |
| Left eye | 15 | 54% | 0.3581-0.7047 |
| IIRC Group B | 3 | 11% | 0.0290-0.2801 |
| IIRC Group C | 7 | 25% | 0.1242-0.4361 |
| IIRC Group D | 18 | 64% | 0.4575-0.7937 |
| RE Stage I, II, III | 2 | 7% | 0.0090-0.2373 |
| RE Stage IV | 4 | 14% | 0.0508-0.3211 |
| RE Stage V | 22 | 79% | 0.6011-0.9014 |
| Stage Va | 6 | 21% | 0.0986-0.3989 |
| Stage Vb | 16 | 57% | 0.3905-0.7351 |
Treatment modalities
All cases received systemic chemotherapy (CVE) with a mean of 7 cycles (median, 8 cycles; range, 6–8 cycles), and 8 (33%) patients were further treated by 3 cycles of subtenon carboplatin injections. Consolidation therapy was applied as postchemoreduction transpupillary thermotherapy, cryotherapy, or both in each case. For EBRT, all treated eyes received the standard dose of 45 Gy in 25 fractions of 1.8 Gy per fraction.
Ocular outcomes
Thirteen eyes (46%) were salvaged by EBRT. In particular, the salvage rate was 67% (2/3) for IIRC Group B, 71% (5/7) for Group C, and 33% (6/18) for Group D eyes [Figure 1]. The mean follow-up time after radiation therapy was 75 months (median, 62 months; range, 12–160 months).
Figure 1.
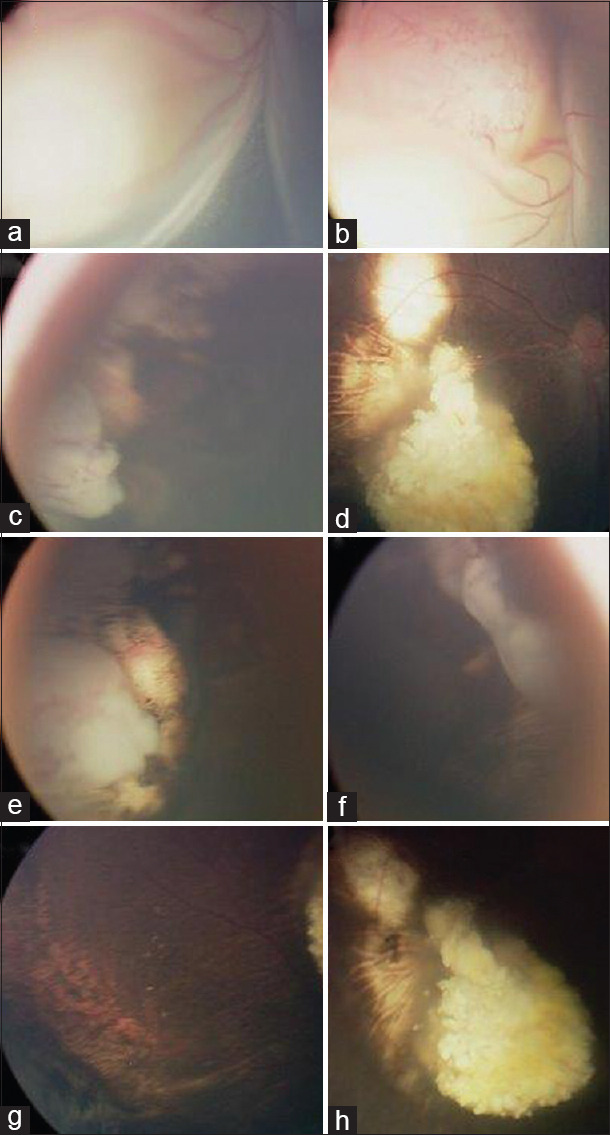
Clinical images of an eye with intraocular retinoblastoma (in a patient who had the left eye enucleated for advanced intraocular retinoblastoma) that was salvaged by EBRT after failure of combined chemotherapy and focal therapy. Fundus examination of the right eye at diagnosis showed intraocular retinal tumor with total retinal detachment (a + b). After 6 cycles of carboplatin, vincristine, and etoposide chemotherapy combined with transpupillary thermotherapy and cryotherapy, the tumor responded by shrinkage and calcifications (c + d). Residual active tumors were not controlled at 2 different quadrants; inferotemporal (e) and superonasal (f) after additional 3 sessions of focal treatment by triple freeze-thaw cryotherapy, therefore, EBRT was applied. No tumor activity was seen over 2-year follow-up postsalvage EBRT (g + h)
Of the 13 eyes salvaged by EBRT, 3 eyes developed recurrence of intraocular RB after initial control for at least 3 months and 1 eye developed a new intraocular tumor, all of which were controlled by focal therapy (thermal therapy and/or cryotherapy).
Indication for enucleation in the 15 failed eyes was persistent tumor activity in 73% (11/15) of eyes, total retinal detachment (RD) and vitreous hemorrhage in 20% (3/15), and neovascular glaucoma in 6% (1/15) of treated eyes.
The overall ocular complication rate was 68% (19/28) of treated eyes: controllable tumor recurrence or new tumor (4), uncontrollable residual tumor (9), RD (3), vitreous hemorrhage (4), neovascular glaucoma (1), cataract (16), radiation retinopathy (2), and eventually enucleated (13).
Visual acuity in the salvaged 13 eyes was better than 20/100 in 0 (0%), 20/100–20/200 in 4 (31%), 20/200–20/400 in 4 (31%), counting fingers to 20/400 in 3 (27%), light perception in 1 (10%), and no light perception in 1 (10%). Postenucleation high-risk pathological features were seen in 3 eyes, and all were treated by adjuvant systemic chemotherapy. One patient died secondary to bone marrow metastasis. We have 2 cases who developed secondary sarcomas during the follow-up period: one patient had osteosarcoma of the thigh and the other osteosarcoma of the backbones. One passed away, and the second is still alive.
Predictive factors of ocular outcome
Patient's gender, tumor site, laterality, number of cycles of chemotherapy, and tumor stage at diagnosis did not show a difference in the control rate of tumors treated by EBRT after failure of chemotherapy and focal therapy [Table 2]. Of note, out of 4 unilateral cases in this series, 3 (75%) were consecutively enucleated; 2 had uncontrolled vitreous seeds and 1 had subretinal seeds and had massive hemorrhage post-EBRT.
Table 2.
Predictive factors of eye salvage by external beam radiation therapy after failure of chemotherapy and focal therapy
| n | Success rate | Percentage | 95% CI | P | |
|---|---|---|---|---|---|
| Salvaged | 13 | - | 46 | 0.2953-0.6419 | - |
| Enucleated | 15 | - | - | 0.3581-0.7047 | - |
| Male | 14 | 8 | 57 | 0.3255-0.7866 | 1.00 |
| Female | 10 | 5 | 50 | 0.2366-0.7634 | |
| Bilateral | 20 patients, 24 eyes | 12 | 50 | 0.3143-0.6857 | |
| Unilateral | 4 | 1 | 25 | 0.0341-0.7109 | |
| Right eye | 13 | 8 | 61 | 0.3541-0.8240 | 0.1228 |
| Left eye | 15 | 5 | 33 | 0.1496-0.5850 | |
| No vitreous seeds | 10 | 9 | 90 | 0.5740->0.9999 | 0.0011 |
| Vitreous seeds | 18 | 4 | 22 | 0.0847-0.4575 | |
| IIRC (B, C) | 10 | 7 | 70 | 0.3923-0.8967 | 0.1141 |
| IIRC D | 18 | 6 | 33 | 0.1610-0.5643 | |
| RE (I-IV) | 6 | 4 | 67 | 0.2957-0.9075 | 0.6703 |
| RE V | 22 | 9 | 4 | 0.2321-0.6131 | |
| RE Va | 6 | 5 | 83 | 0.4178-0.9886 | 0.0464 |
| RE Vb | 16 | 4 | 25 | 0.0971-0.4997 | |
| No Stage migration 9 (B, C) | 6 | 6 | 100 | 0.5572-1.0000 | 0.0333 |
| Stage migration (B, C) | 4 | 1 | 25 | 0.0341-0.7109 | |
| 8 cycles of CVE | 14 | 7 | 50 | 0.2680-0.7320 | 1.00 |
| 6 cycles CVE | 14 | 6 | 43 | 0.2134-0.6745 |
Vitreous seeds at the time of EBRT were associated with a significantly higher risk of failure of tumor control (P = 0.001). Eye salvage was achieved in 90% of eyes with no seeds; in contrast, 22% of eyes with seeds could be salvaged. Stage migration was associated with a significantly higher risk of failure of tumor control (P = 0.03). In our series, stage migration was seen in 4 eyes (1 B eye and 3 C eyes). All showed IIRC Group D features at the time of radiation therapy, and only one eye of those was well controlled by EBRT [Figure 2]; meanwhile, all the 6 eyes in Groups B and C that did not show stage migration were controlled by EBRT (100%). The initial RE stage was not predictive of the outcome in this series (P = 0.67).
Figure 2.
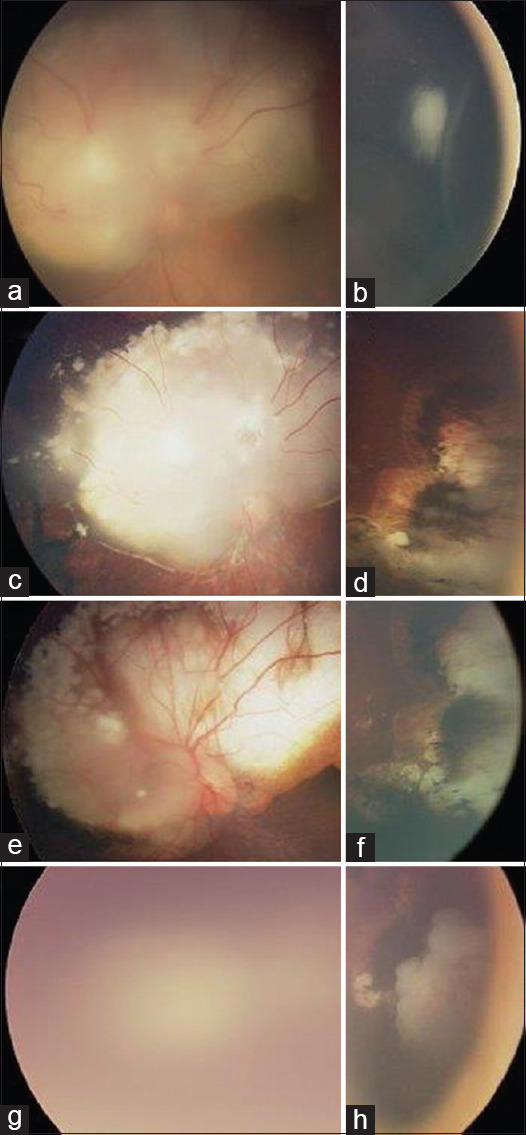
Clinical images of an eye with intraocular retinoblastoma (in a patient who had bilateral retinoblastoma) that showed stage migration during chemotherapy and focal therapy and consequentially failed treatment by EBRT. Fundus examination of the left eye at diagnosis showed 2 retinal tumors (a + b) with no subretinal or vitreous seeds (staged as IIRC group B). After 6 cycles of carboplatin, vincristine, and etoposide chemotherapy combined with transpupillary thermotherapy and cryotherapy, the tumors responded partially by shrinkage, scaring, and calcifications but showed stage migration where new vitreous seeds (features of International Intraocular Retinoblastoma Classification Group D tumor) appeared (c + d) that are features of more advanced tumor than the initial presentation. Since the vitreous seeds were not controlled by additional 2 cycles of carboplatin, vincristine, and etoposide and 3 cycles of subtenon carboplatin injections, salvage EBRT was applied. Initially, the tumor was controlled by EBRT (e + f), but at 6 months postradiation, the main tumor around the optic disc could not be assessed well secondary to cataract (g), and tumor recurrence was seen close to the oral in the inferotemporal quadrant (h). Since cataract surgery was unsafe for the risk of metastasis, and the tumor in the other eye was well controlled with good visual potential, the eye was enucleated. Pathologically, no high-risk features were seen in the enucleated eye
Discussion
Over many years, EBRT was the treatment of choice for retinoblastoma, but because of the associated side effects, chemotherapy followed by focal therapy became the primary treatment of choice for retinoblastoma in most of the developed countries worldwide.[3,4,5,6,7] In spite of the limitation of EBRT, it is still used at certain circumstances, particularly after failure of combined chemotherapy with focal therapy.
Our series showed that the significant predictors for failure of EBRT for the treatment of intraocular retinoblastoma after failure of chemotherapy and focal therapy were vitreous seeds as the indication for EBRT and tumor stage migration during treatment by chemotherapy. Failure of tumor control was not correlated to patient's gender, tumor site, laterality, and number of cycles of chemotherapy. The initial RE stage was not a predictive factor of the outcome as well. In this study, we included patients with vitreous seeds who were offered EBRT, and this was before the introduction of intravitreal injections of melphalan to treat those cases, which we do for our patients nowadays.
The adverse effects associated with EBRT are major issues. Radiation is associated with a 36%–51% risk of second cancers in heritable retinoblastoma that increases with patient's age, which is more than three times the risk in nonirradiated heritable retinoblastoma patients.[18,19] In addition, EBRT is associated with ocular complications including cataract formation, blinding and painful anterior segment complications, vitreous hemorrhage, and orbital deformities.[20] Herein, we tried to highlight the predictive factors of tumor control by EBRT for retinoblastoma that was resistant to chemotherapy and focal therapy to determine whether the expected outcome justifies the known risks of EBRT.
The overall ocular salvage rate for eyes with retinoblastoma treated initially by EBRT with no previous chemotherapy was 72%–81%,[12,13,21] while the ocular salvage rate in our series was 46%. Our lower salvage rate may be due to more tumor resistance in eyes that failed chemotherapy than eyes that were treated initially by EBRT or due to the more advanced cases in our series where more than half (57%) of our patients were Group Vb. Chan et al.[22] studied the rate of eye salvage by EBRT after failure of chemotherapy and focal therapy in 36 eyes and reported an 83.3% rate of eye salvage, ranging from 29% to 33% for Groups Va and IIIa consecutively to 100% for Groups I and II. Our success rate was 46%, ranging from 25% for Group Vb to 100% for Group I, which is still less than Chan's salvage rate. Our relatively low overall success rate was due to the more advanced tumors in our series and therefore less favorable outcome. In fact, 57% of our patients were Group Vb (the most advanced stage) and only 7% were Groups I to III, while only 11% were Group Vb and 55% were Groups I to III in Chan's series.
Few reports focused on the salvage rate by radiation therapy as initial treatment for Group Vb eyes.[14,15,20] Abramson et al.[20] described 63 Group Vb eyes treated initially by EBRT, and the ocular survival rate was 53.4% at 10 years, which is still higher than our 25% control rate for the same group. The difference indicates that tumors uncontrolled by chemotherapy are the tumors that are originally highly resistant and therefore were more resistant for EBRT than tumors that were treated initially by EBRT with no history of previous therapy.
Even the RE[17] RB classification system was not intended to be a traditional cancer staging scheme initially.[20] Most reports revealed the strength of this scheme and found that Group V tumors are less often cured with radiation as initial therapy.[23,24,25,26] In our series, the initial RE stage was not a significant predictive factor for the tumor control rate in eyes that failed chemotherapy (P = 0.67). That was expected as some eyes showed features of more advanced tumor stage during management process as vitreous seeds, which may not correlate with the initial tumor stage. Vitreous seeds at the time of radiation therapy were associated with unfavorable tumor control (P = 0.001). Of note, the initial staging was not predictive of the outcome of management by EBRT, while tumor deterioration and development of more advanced tumor features during chemotherapy (stage migration) was a significant poor predictive factor for tumor control (P = 0.03).
Chan et al.[22] concluded that salvage EBRT was highly effective in preserving eyes with useful vision in bilateral retinoblastoma after failed chemotherapy and focal treatments without evaluating the predictive factors of the outcome. Our study highlighted the poor outcome of eyes that had vitreous seeds and/or tumor stage migration before EBRT and showed that patient's gender, tumor site, laterality, and number of cycles of chemotherapy were not significantly associated with difference in the control rate of tumors treated.
Conclusion
Our results showed that eyes with retinoblastoma treated by EBRT after failure of chemotherapy may be more resistant than eyes treated initially by EBRT. Even it has a role to salvage eyes with no vitreous seeds (mainly when there is good visual potential), EBRT has a low ocular salvage rate for eyes with vitreous seeds and eyes that deteriorated during chemotherapy, and therefore, the radiation side effects may not be justified for this group mainly if the other eye is either normal or has good vision and tumor control. Nowadays, with the introduction of intravitreal injections to control vitreous seeds, the need to refer patients with active seeds for EBRT and it is known limitations is no longer needed since those eyes will achieve tumor control with repeated intravitreal injections.
This study examined the outcome and the predictive factors for tumor control by EBRT for retinoblastoma in eyes that was resistant to chemotherapy and focal therapy. Although this is unique work, it is retrospective and of limited size. Therefore, a larger and more comprehensive multicenter study should be performed for better evaluation of the efficacy and tumor-specific factors to expect the outcome of radiation therapy and to justify the possible associated risks.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Incidence of Childhood Cancer. IARC Sci Publ. 1998;2:1–391. [PubMed] [Google Scholar]
- 2.Jaradat I, Yousef YA, Mehyar M, Sultan I, Khurma S, Al-Rawashded K, et al. Retinoblastoma in Jordan: An epidemiological study (2006-2010) Hematol Oncol Stem Cell Ther. 2011;4:126–31. doi: 10.5144/1658-3876.2011.126. [DOI] [PubMed] [Google Scholar]
- 3.Reese AB, Hyman GA, Merriam GR, Jr, Forrest AW, Kligerman MM. Treatment of retinoblastoma by radiation and triethylenemelamine. AMA Arch Ophthalmol. 1954;53:505–13. doi: 10.1001/archopht.1955.00930010507007. [DOI] [PubMed] [Google Scholar]
- 4.Servodidio CA, Abramson DH. Acute and long-term effects of radiation therapy to the eye in children. Cancer Nurs. 1993;16:371–81. [PubMed] [Google Scholar]
- 5.Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, Goldman MB, et al. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85:1121–8. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- 6.Ferris FL, 3rd, Chew EY. A new era for the treatment of retinoblastoma. Arch Ophthalmol. 1996;114:1412. doi: 10.1001/archopht.1996.01100140612015. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Hopping W, Bunke-Schmidt A. Light coagulation and cryotherapy. In: Blodi FC, editor. Retinoblastoma. New York: Churchill Livingstone; 1985. pp. 95–110. [Google Scholar]
- 9.Abramson DH, Ellsworth RM, Rozakis GW. Cryotherapy for retinoblastoma. Arch Ophthalmol. 1982;100:1253–6. doi: 10.1001/archopht.1982.01030040231003. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Shields JA, De Potter P, Minelli S, Hernandez C, Brady LW, et al. Plaque radiotherapy in the management of retinoblastoma. Use as a primary and secondary treatment. Ophthalmology. 1993 Feb;100(2):216–24. doi: 10.1016/s0161-6420(93)31667-2. [DOI] [PubMed] [Google Scholar]
- 11.Schueler AO, Jurklies C, Heimann H, Wieland R, Havers W, Bornfeld N. Thermochemotherapy in hereditary retinoblastoma. Br J Ophthalmol. 2003;87:90–5. doi: 10.1136/bjo.87.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schipper J. An accurate and simple method for megavoltage radiation therapy of retinoblastoma. Radiother Oncol. 1983;1:31–41. doi: 10.1016/s0167-8140(83)80005-x. [DOI] [PubMed] [Google Scholar]
- 13.Choi SY, Kim MS, Yoo S, Cho C, Ji Y, Kim K, et al. Long term follow-up results of external beam radiotherapy as primary treatment for retinoblastoma. J Korean Med Sci. 2010;25:546–51. doi: 10.3346/jkms.2010.25.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao LY, Tsang NM. Advanced bilateral retinoblastoma treated conservatively with lens sparing external beam radiation therapy: Report of three cases. Changgeng Yi Xue Za Zhi. 1999;22:100–5. [PubMed] [Google Scholar]
- 15.Yousef YA, Al-Nawaiseh I, Mehyar M, Sultan I, Al-Hussaini M, Jaradat I, et al. How Telemedicine and Centralized Care Changed the Natural History of Retinoblastoma in a Developing Country: Analysis of 478 Patients. Ophthalmology. 2020 Jul;16:S0161. doi: 10.1016/j.ophtha.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Linn Murphree A. Intraocular retinoblastoma: The case for a new group classification. Ophthalmol Clin North Am. 2005;18:41–53. doi: 10.1016/j.ohc.2004.11.003. viii. [DOI] [PubMed] [Google Scholar]
- 17.Reese AB, Ellsworth RM. The evaluation and current concept of retinoblastoma therapy. Trans Am Acad Ophthalmol Otolaryngol. 1963;67:164–72. [PubMed] [Google Scholar]
- 18.Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J Clin Oncol. 2005;23:2272–9. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 19.Wong FL, Boice JD, Jr, Abramson DH, Tarone RE, Kleinerman RA, Stovall M. Cancer incidence after retinoblastoma Radiation dose and sarcoma risk. JAMA. 1997;278:1262–7. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 20.Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol. 2004;122:1316–23. doi: 10.1001/archopht.122.9.1316. [DOI] [PubMed] [Google Scholar]
- 21.Phillips C, Sexton M, Wheeler G, McKenzie J. Retinoblastoma: Review of 30 years' experience with external beam radiotherapy. Australas Radiol. 2003;47:226–30. doi: 10.1046/j.1440-1673.2003.01167.x. [DOI] [PubMed] [Google Scholar]
- 22.Chan MP, Hungerford JL, Kingston JE, Plowman PN. Salvage external beam radiotherapy after failed primary chemotherapy for bilateral retinoblastoma: Rate of eye and vision preservation. Br J Ophthalmol. 2009;93:891–4. doi: 10.1136/bjo.2007.129981. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez JC, Brady LW, Shields JA, Shields CL, DePotter P, Karlsson UL, et al. External beam radiation for retinoblastoma: Results, patterns of failure, and a proposal for treatment guidelines. Int J Radiat Oncol Biol Phys. 1996;35:125–32. doi: 10.1016/s0360-3016(96)85020-6. [DOI] [PubMed] [Google Scholar]
- 24.Blach LE, McCormick B, Abramson DH. External beam radiation therapy and retinoblastoma: Long-term results in the comparison of two techniques. Int J Radiat Oncol Biol Phys. 1996;35:45–51. doi: 10.1016/s0360-3016(96)85010-3. [DOI] [PubMed] [Google Scholar]
- 25.Amendola BE, Lamm FR, Markoe AM, Karlsson UL, Shields J, Shields CL, et al. Radiotherapy of retinoblastoma. A review of 63 children treated with different irradiation techniques. Cancer. 1990;66:21–6. doi: 10.1002/1097-0142(19900701)66:1<21::aid-cncr2820660105>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Fontanesi J, Pratt CB, Hustu HO, Coffey D, Kun LE, Meyer D. Use of irradiation for therapy of retinoblastoma in children more than 1 year old: The St. Jude Children's Research Hospital experience and review of literature. Med Pediatr Oncol. 1995;24:321–6. doi: 10.1002/mpo.2950240510. [DOI] [PubMed] [Google Scholar]


