Abstract
A 15-year-old male was presented with blurred vision in his right eye for 2 weeks. The patient had a history of looking with the right eye for 5–6 s at a distance of 20 cm from green laser beam (class 3a, 5 mW, 532 nm). Dilated fundus examinations revealed a yellow lesion in the right eye, resulting in loss of foveal reflection at the fovea. Fundus fluorescein angiography (FFA) images and spectral-domain optical coherence tomography (OCT) scans were compatible with active classic choroidal neovascularization (CNV). A single dose of intravitreal aflibercept was performed to the right eye, and at the 1st month after the injection, the best-corrected visual acuity improved to 20/100 from 20/200. FFA showed staining of the scar with no leakage, and OCT revealed scar formation. At the follow-up visits, during 38-month follow-up, no CNV activity was observed. Intravitreal aflibercept may be an appropriate treatment option in cases with laser pointer injury-induced CNV.
Keywords: Aflibercept, blurred vision, choroidal neovascularization, laser pointer, scar formation
Introduction
Laser instruments are used in many areas of human activity including medicine, industry, laboratory research, entertainment, and military. In parallel with the widespread use of lasers, many accidents have resulted in ocular injury. Damage to the tissues occurs due to the wavelength, power, and contact time of the laser light.[1] Laser injuries can cause visual impairment by direct damage to the globe (retinal holes, scarring, or hemorrhages) or by long-term changes (i.e., cataract and choroidal neovascularization [CNV]).[2]
Herein, we describe the case of CNV secondary to the laser pointer injury treated with intravitreal aflibercept (Eylea®, Regeneron Pharmaceuticals Inc., Bayer) for the anatomic and functional results during 38-month follow-up. To our knowledge, this is the first reported case of laser pointer injury-associated CNV treated with intravitreal administration of aflibercept in the literature.
Case Report
A 15-year-old male patient complained of visual decrease in the right eye for 2 weeks. From the history, we learned that he had looked with the right eye for 5–6 s at a distance of 20 cm from green laser beam (class 3a, 5 mW, 532 nm) a month ago to play as a fun toy [Figure 1a].[3] Best-corrected visual acuity (BCVA) was 20/200 on the right and 20/20 on the left. Intraocular pressure was within normal limits in both eyes. Anterior segment examinations were normal in both eyes. Dilated fundus examination revealed that there was a ½ disk diameter dirty yellow lesion in the right eye, resulting in loss of foveal reflection at the macula, whereas the left eye was normal on examination [Figure 1b].
Figure 1.
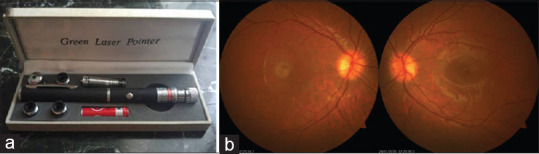
Green laser pointer to which the eye is exposed (a). A ½ disk diameter dirty yellow lesion in the right eye, resulting in loss of foveal reflection at the fovea, whereas the left eye was completely normal (b)
Optical coherence tomography (OCT) showed a hyperreflective lesion on the retinal pigment epithelium in the right eye and subretinal fluid (SRF) and subretinal hyperreflective material (SHRM) adjacent to the retinal thickening [Figure 2a]. Fundus fluorescein angiography (FFA) of the right eye illustrated a pattern of well-delineated diffuse fluorescence in the early phases of FFA increasing in late phases suggesting an active subfoveal CNV lesion [Figure 2b].
Figure 2.
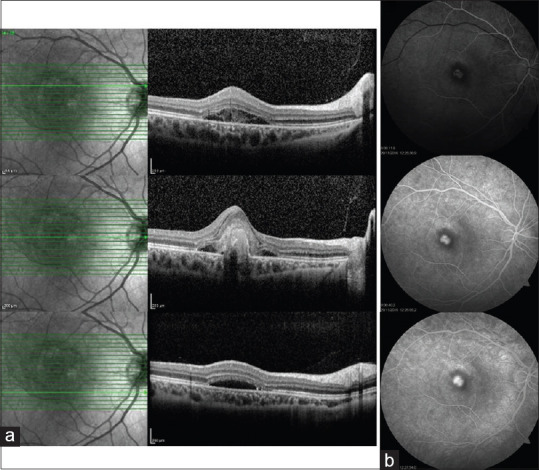
Optical coherence tomography revealed a hyperreflective lesion on the retinal pigment epithelium and subretinal fluid adjacent to the retinal thickening (a). Fundus fluorescein angiography demonstrates active subfoveal choroidal neovascularization lesion that hyperfluorescent in the early phases maintains well-demarcated borders and leaks (b)
A single dose of 2 mg intravitreal aflibercept injection (2 mg/0.05 ml) was performed to the right eye considering laser light injury-induced active classic CNV. Informed written consent was obtained from the patient and parents for the off-label use of aflibercept.
The 1st month after the injection, the right eye BCVA improved to 20/100, and the complaints of defective vision decreased. In the right eye, marked reduction of the SRF was observed, and FFA of the right eye showed staining of the scar with no leakage [Figure 3a] and OCT revealed contraction of the lesion with reduced SRF and SHRM [Figure 3b]. During the 38-month follow-up, no CNV activity was observed and no additional injections were needed. No recurrence was observed at the last examination, the BCVA was preserved, and the OCT showed a scarred CNVM which was similar to those at the 1st month postinjection [Figure 4a and b].
Figure 3.
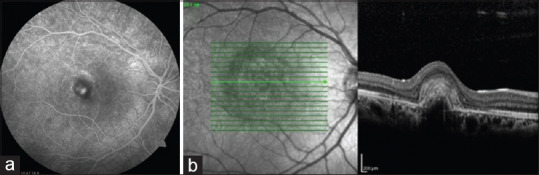
Fundus fluorescein angiography illustrates staining of the scar with no a b leakage 1 month after the aflibercept injection (a). Optical coherence tomography shows contraction of the lesion with scar formation and markedly reducing of the fluid 1 month after the aflibercept injection (b)
Figure 4.
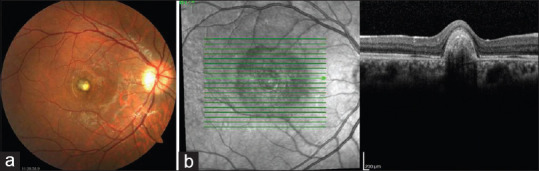
Thirty-eight months after the injection of intravitreal aflibercept, fundus photograph (a) and optical coherence tomography scans (b) are similar when compared to those at the first month postinjection
Discussion
Laser pointers are commonly used in presentations and in children's games. Exposure to these lasers can cause retinal damage. Macular hole, subhyaloid hemorrhage, premacular subinternal limiting membrane hemorrhage, outer retinal disruption, schisis-like cavity, epimacular membrane, vitreous or chorioretinal hemorrhage, retinal edema, scars in the pigment epithelium, foveal granulation, perifoveal drusen-like deposit, hypopigmented round lesions in the fovea, macular damage, and CNV have been reported associated with the laser pointer-induced retinal injury.[4,5,6,7]
The treatment options for retinal injury associated with a laser pointer are limited in the literature. Patients with laser pointer injuries were treated with corticosteroid drugs.[8] Xu et al. successfully treated laser pointer-induced CNV with intravitreal bevacizumab.[9]
Aflibercept is a recombinant fusion protein that inhibits the pathological angiogenic process. In contrast to other antivascular endothelial growth factor (VEGF) agents, aflibercept has a higher affinity for VEGF-A and additionally binds VEGF-B and placental growth factor.[10] As far as we know, cases of laser pointer-induced CNV treated with intravitreal administration of aflibercept has not been described to date.
We injected a single-dose intravitreal aflibercept to our patient, and at the 1st month of the injection, involution of the CNV and improvement in BCVA from 20/200 to 20/100 was obtained. No recurrence was observed at 38-month follow-up. Final visual recovery was low due to the subfoveal location and scarring of the lesion. Laser injury-induced CNV involves rupture of Bruch's membrane which caused a local inflammatory response resulting in angiogenesis.[11] Experimental and clinical evidence has shown that VEGF is the key component in the pathogenesis of CNV.[12] A single dose of aflibercept injection may have been sufficient due to its higher binding affinity for VEGF in comparison with other anti-VEGFs. Furthermore, the absence of a provocative disease on the base that stimulates CNV progression contributes predominantly, as well as the classic CNV nature similar to myopic CNV[13] may be among the possible mechanisms of less treatment required.
In conclusion, intravitreal aflibercept injection may be an effective and safe treatment alternative that can be considered in cases with laser pointer injury-induced CNV.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ajudua S, Mello MJ. Shedding some light on laser pointer eye injuries. Pediatr Emerg Care. 2007;23:669–72. doi: 10.1097/PEC.0b013e31814b2dc4. [DOI] [PubMed] [Google Scholar]
- 2.Link B, Michelson G, Horn FK, Jünemann A. Accidental focal laser injury – A correlation of electrophysiology, perimetry and clinical findings. Doc Ophthalmol. 2008;117:69–72. doi: 10.1007/s10633-007-9104-7. [DOI] [PubMed] [Google Scholar]
- 3.Mainster MA, Timberlake GT, Warren KA, Sliney DH. Pointers on laser pointers. Ophthalmology. 1997;104:1213–4. doi: 10.1016/s0161-6420(97)30156-0. [DOI] [PubMed] [Google Scholar]
- 4.Alsulaiman SM, Alrushood AA, Almasaud J, Alzaaidi S, Alzahrani Y, Arevalo JF, et al. High-power handheld blue laser-induced maculopathy: The results of the King Khaled Eye Specialist Hospital Collaborative Retina Study Group. Ophthalmology. 2014;121:566–72. doi: 10.1016/j.ophtha.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Wyrsch S, Baenninger PB, Schmid MK. Retinal injuries from a handheld laser pointer. N Engl J Med. 2010;363:1089–91. doi: 10.1056/NEJMc1005818. [DOI] [PubMed] [Google Scholar]
- 6.Turaka K, Bryan JS, Gordon AJ, Reddy R, Kwong HM, Jr, Sell CH. Laser pointer induced macular damage: Case report and mini review. Int Ophthalmol. 2012;32:293–7. doi: 10.1007/s10792-012-9555-z. [DOI] [PubMed] [Google Scholar]
- 7.Fujinami K, Yokoi T, Hiraoka M, Nishina S, Azuma N. Choroidal neovascularization in a child following laser pointer-induced macular injury. Jpn J Ophthalmol. 2010;54:631–3. doi: 10.1007/s10384-010-0876-z. [DOI] [PubMed] [Google Scholar]
- 8.Birtel J, Harmening WM, Krohne TU, Holz FG, Charbel Issa P, Herrmann P. Retinal ınjury following laser pointer exposure-a systematic review and case series. Dtsch Arztebl Int. 2017;114:831–7. doi: 10.3238/arztebl.2017.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu K, Chin EK, Quiram PA, Davies JB, Parke DW, Almeida DR. Retinal ınjury secondary to laser pointers in pediatric patients. Pediatrics. 2016;138:e20161188. doi: 10.1542/peds.2016-1188. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah RS, Soetikno BT, Lajko M, Fawzi AA. A mouse model for laser-induced choroidal neovascularization. J Vis Exp. 2015;106:e53502. doi: 10.3791/53502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–44. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong TY, Ohno-Matsui K, Leveziel N, Holz FG, Lai TY, Yu HG, et al. Myopic choroidal neovascularisation: Current concepts and update on clinical management. Br J Ophthalmol. 2015;99:289–96. doi: 10.1136/bjophthalmol-2014-305131. [DOI] [PMC free article] [PubMed] [Google Scholar]


