Abstract
Background
A heterologous recombinant adenovirus (rAd)-based vaccine, Gam-COVID-Vac (Sputnik V), showed a good safety profile and induced strong humoral and cellular immune responses in participants in phase 1/2 clinical trials. Here, we report preliminary results on the efficacy and safety of Gam-COVID-Vac from the interim analysis of this phase 3 trial.
Methods
We did a randomised, double-blind, placebo-controlled, phase 3 trial at 25 hospitals and polyclinics in Moscow, Russia. We included participants aged at least 18 years, with negative SARS-CoV-2 PCR and IgG and IgM tests, no infectious diseases in the 14 days before enrolment, and no other vaccinations in the 30 days before enrolment. Participants were randomly assigned (3:1) to receive vaccine or placebo, with stratification by age group. Investigators, participants, and all study staff were masked to group assignment. The vaccine was administered (0·5 mL/dose) intramuscularly in a prime-boost regimen: a 21-day interval between the first dose (rAd26) and the second dose (rAd5), both vectors carrying the gene for the full-length SARS-CoV-2 glycoprotein S. The primary outcome was the proportion of participants with PCR-confirmed COVID-19 from day 21 after receiving the first dose. All analyses excluded participants with protocol violations: the primary outcome was assessed in participants who had received two doses of vaccine or placebo, serious adverse events were assessed in all participants who had received at least one dose at the time of database lock, and rare adverse events were assessed in all participants who had received two doses and for whom all available data were verified in the case report form at the time of database lock. The trial is registered at ClinicalTrials.gov (NCT04530396).
Findings
Between Sept 7 and Nov 24, 2020, 21 977 adults were randomly assigned to the vaccine group (n=16 501) or the placebo group (n=5476). 19 866 received two doses of vaccine or placebo and were included in the primary outcome analysis. From 21 days after the first dose of vaccine (the day of dose 2), 16 (0·1%) of 14 964 participants in the vaccine group and 62 (1·3%) of 4902 in the placebo group were confirmed to have COVID-19; vaccine efficacy was 91·6% (95% CI 85·6–95·2). Most reported adverse events were grade 1 (7485 [94·0%] of 7966 total events). 45 (0·3%) of 16 427 participants in the vaccine group and 23 (0·4%) of 5435 participants in the placebo group had serious adverse events; none were considered associated with vaccination, with confirmation from the independent data monitoring committee. Four deaths were reported during the study (three [<0·1%] of 16 427 participants in the vaccine group and one [<0·1%] of 5435 participants in the placebo group), none of which were considered related to the vaccine.
Interpretation
This interim analysis of the phase 3 trial of Gam-COVID-Vac showed 91·6% efficacy against COVID-19 and was well tolerated in a large cohort.
Funding
Moscow City Health Department, Russian Direct Investment Fund, and Sberbank.
Introduction
The COVID-19 pandemic has led to more than 98 million confirmed cases and more than 2 million deaths (at the time of publication). There are a few provisionally licensed vaccines against COVID-19, and global efforts are focusing on developing safe and efficacious vaccines for COVID-19 prevention. According to the WHO draft landscape of COVID-19 candidate vaccines,1 64 candidates are in clinical assessment (including 13 at phase 3) and 173 are in preclinical analyses. The phase 3 vaccine candidates include a variety of vaccine platforms: vector vaccines (Gamaleya National Research Centre for Epidemiology and Microbiology [NRCEM; this study], University of Oxford/AstraZeneca,2 CanSino Biological Inc/Beijing Institute of Biotechnology, and Janssen Pharmaceutical Companies), mRNA-based vaccines (Moderna/National Institute of Allergy and Infectious Diseases3 and BioNTech/Fosun Pharma/Pfizer4), inactivated vaccines (SinoVac, Wuhan Institute of Biological Products/Sinopharm, Beijing Institute of Biological Products/Sinopharm, and Bharat Biotech), and adjuvanted recombinant protein nanoparticles (Novavax).
Research in context.
Evidence before this study
We searched PubMed for research articles published up to Jan 25, 2021, with no language restrictions, using the terms “SARS-CoV-2” or “COVID-19”, “vaccine”, “clinical trial”, and “efficacy”. We found three peer-reviewed publications available on the efficacy of SARS-CoV-2 vaccines: AZD1222 (AstraZeneca/University of Oxford), a ChAdOx1-based vaccine with reported efficacy of 70·4% and two mRNA-based vaccines: BNT162b2, (Pfizer/BioNTech) with reported efficacy of 95%, and mRNA-1273 (Moderna/NIAID), with reported efficacy of 94·1%. We have previously published safety and immunogenicity results of Gam-COVID-Vac in phase 1/2 clinical trials.
Added value of this study
We report on the interim clinical efficacy results of the rAd26 and rAd5 vector-based COVID-19 vaccine Gam-COVID-Vac in a randomised, double-blind placebo-controlled multicentre phase 3 trial in Moscow, Russia, including 21 862 participants. We describe the first immunogenicity results of the trial, including receptor-binding domain-specific IgG titres, virus neutralising antibody titres, and IFN-γ response. The heterologous prime-boost regimen of vaccination provides robust humoral and cellular immune responses, with 91·6% (95% CI 85·6–95·2) efficacy against COVID-19. The vaccine is stored and distributed at –18°C, but storage at 2–8°C, a favourable temperature profile for global distribution, has also been approved by the Ministry of Health of the Russian Federation.
Implications of all the available evidence
A system-wide approach to stopping the COVID-19 pandemic requires the introduction of different vaccines based on different mechanisms of action to cover diverse global health demands with cost-effective and region-tailored methods. Our vaccine, along with other SARS-CoV-2 vaccines, helps to diversify the world SARS-CoV-2 vaccine pipeline.
The safety of adenoviral vector vaccines has been extensively studied, and adenoviral vector-based therapeutic drugs are used in clinical practice.5, 6, 7 Adenoviral vector-delivered antigens are known to induce both cellular and humoral immunity after a single immunisation, allowing their use as an emergency prophylaxis tool in a pandemic. Furthermore, the use of two immunisations gives a durable and long-lasting immune response.8, 9 These characteristics make recombinant replication-deficient adenovirus (rAd)-based vaccines suitable candidates for the WHO target product profiles for long-term protection of people at high risk of COVID-19 in outbreak settings because they stimulate rapid onset of protective immunity. Although adenoviral vectors might induce immune responses against vector components and attenuate antigen-induced responses, prime-boost heterologous vaccination with two different vectors allows minimisation of this effect.9, 10, 11 Thus, the most effective approach for generating a powerful and long-lasting immune response that does not depend on the presence of a pre-existing immune response to the vector is the heterologous prime-boost vaccination approach. We used this approach when developing a vaccine for the prevention of COVID-19.
Gam-COVID-Vac is a combined vector vaccine, based on rAd type 26 (rAd26) and rAd type 5 (rAd5)—both of which carry the gene for SARS-CoV-2 full-length glycoprotein S (rAd26-S and rAd5-S). rAd26-S and rAd5-S are administered intramuscularly separately with a 21-day interval. The phase 1/2 clinical trials of the vaccine were completed in August, 2020.12 The results showed that the vaccine was well tolerated and highly immunogenic in healthy participants. As a result, the vaccine candidate was provisionally approved in Russia according to national legislation. Such registration allows the vaccine to be used in high-risk groups, with enhanced pharmacovigilance, while a post-marketing efficacy study is conducted. Here, we present preliminary efficacy and safety results of a phase 3 multicentre study using Gam-COVID-Vac in adults, with subanalysis of adults older than 60 years.
Methods
Study design and participants
This is a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial to assess efficacy, immunogenicity, and safety of the Gam-COVID-Vac combined vector vaccine against SARS-CoV-2-induced COVID-19 in adults, done at 25 hospitals and polyclinics in Moscow, Russia (appendix pp 14–15). Only sites accredited by the Ministry of Health of the Russian Federation for the conduct of clinical research were approved for participation. The trial protocol was reviewed and approved by appropriate competent authorities, including the Department of State Regulation for Circulation of Medicines of the Ministry of Health of the Russian Federation (approval number 450 from Aug 25, 2020), Moscow City Independent Ethics Committee, and independent local ethics committees of clinical sites.
The study used recruitment strategies that included use of the online platform of the Moscow Government and its call centres, community outreach, and recruitment efforts by approved clinical sites to achieve a high level of participation in the study. The study involved everyone who signed informed consent and passed screening.
Eligibility criteria were age 18 years or older; negative HIV, hepatitis B and C, and syphilis test results; negative anti-SARS-CoV-2 IgM and IgG antibody and SARS-CoV-2 PCR tests; no history of COVID-19; no contact with anyone with COVID-19 in the preceding 14 days; consent to use effective contraceptive methods; negative urine pregnancy test (for women of child-bearing potential); negative drug and alcohol tests at screening visit; no history of vaccine-induced reactions; and no acute infectious or respiratory disease in the 14 days before enrolment.
Exclusion criteria were any vaccination in the 30 days before enrolment; steroids or immunoglobulins in the 30 days before enrolment; immunosuppression in the 3 months before enrolment; pregnancy or breastfeeding; acute coronary syndrome or stroke in the year before enrolment; tuberculosis or chronic systemic infections; allergy or hypersensitivity to the drug or components; neoplasms; blood donation in the 2 months before enrolment; splenectomy; neutropenia, agranulocytosis, significant blood loss, severe anaemia, or immunodeficiency in the 6 months before enrolment; active form of a disease caused by HIV, syphilis, or hepatitis B or C; anorexia or protein deficiency; large tattoos at the injection site; history of alcohol or drug addiction; participation in any other clinical trial; study centre staff or other employees directly involved in the trial or their families; or any other condition deemed a problem by the study physician. All participants provided signed informed consent to be included in the database for study participation.
Randomisation and masking
Enrolled participants were divided into five age strata (18–30 years, 31–40 years, 41–50 years, 51–60 years, and >60 years) and were assigned to two study groups using stratified (block size 4) interactive web response system (IWRS) randomisation in a ratio of 3:1 to the vaccine group or the placebo group. Study participants were assigned unique randomisation numbers that remained unchanged throughout the study. The statistician generated a sequence, according to which the drug was labelled. The drug and placebo were outwardly indistinguishable (packaging, label, and content). Investigators, participants, and all study staff were masked to group assignment.
Procedures
All participants who consented to participate attended a screening visit for physical examination, checks of vital signs (eg, blood pressure, heart rate, and temperature), and blood tests for infections (HIV, hepatitis B and C, and syphilis) and collection of baseline immunogenicity characteristics. Urine tests for drugs and alcohol were done in all volunteers and pregnancy tests were done in women. PCR SARS-CoV-2 swab tests were also done at screening by the central laboratory in Moscow to exclude participants with COVID-19. At screening, information on the presence of concomitant diseases and SARS-CoV-2 infection risk group was entered into the case report forms of the participants. High risk denotes those whose work involves interaction with patients with a confirmed diagnosis of COVID-19; medium risk is those who have professional contact with a large number of people, such as general practitioners, social workers, and shop assistants; and general risk denotes those with no additional risks associated with their professional activities. Intended duration of participation of individuals in the trial was 180 days after the first dose of the vaccine or placebo. One screening visit and five on-site visits to a clinical site over the course of the trial were planned.
The vaccine comprises two vector components, rAd26-S and rAd5-S. A full dose of the vaccine was 1011 viral particles per dose for each recombinant adenovirus; 0·5 mL/dose for intramuscular injection. The placebo consists of the vaccine buffer composition, but without the recombinant adenoviruses, made up to equal the vaccine volume. Vaccine and placebo were developed, manufactured, and stored by Gamaleya NRCEM (Moscow, Russia) according to Good Manufacturing Practices. The vaccine and placebo were used in liquid form (frozen). The compositions of the vaccine and placebo are described in the appendix (p 1). The vaccine (first dose rAd26, second dose rAd5) or placebo were administered intramuscularly into the deltoid muscle with a 21-day interval between doses.
Subsequent observation visits were planned for day 28 (±2 days), day 42 (±2 days), and day 180 (±14 days). During the observation visits, vital signs were assessed in all trial participants and changes in the participants’ condition and wellbeing compared with the previous visit were recorded. A PCR test was done in combination with the clinical examination on the day of the second dose (day 21) for the diagnosis of symptomatic and asymptomatic COVID-19 cases. In the presence of clinical signs of respiratory infection and a positive PCR test, the participant would not be vaccinated with the second dose and was referred to medical staff for treatment of COVID-19. Participants without signs of respiratory infection were vaccinated before PCR results were received. In the case of a positive PCR test result, participants were classified as asymptomatic and were not counted as COVID-19 cases in the efficacy analysis, according to protocol. During the trial, apart from the screening visit and day of the second dose, no additional PCR tests were done, except when COVID-19 symptoms were reported by participants.
The sponsor arranged some additional observation visits remotely as telemedicine consultations. Unscheduled telemedicine consultations were encouraged for complaints or questions from participants about study procedures. All participants were given study team contacts at signing of informed consent and were instructed to contact the team on an as-needed basis, but primarily to report any signs or symptoms that could be indicative of an adverse event. All participants were also offered electronic diaries to be installed on their smartphone devices to monitor their health status. Information from those participants who chose not to use e-diaries was collected by site staff via teleconsultation technology. Data collected from these telemedicine consultations were entered by the site investigators directly into the participant's medical record.
A city-wide electronic health record (EHR) platform—the Unified Medical Information and Analytical System (UMIAS) is in place in Moscow. The UMIAS EHR is a controlled electronic medical record used by all Moscow health-care institutions for care provision to Moscow residents. EHRs of the trial participants were updated to indicate their participation in the trial and were used as a source for electronic data capture and source data verification by contract research organisation monitors. In addition to protocol-defined visits and teleconsultations, principal investigators and study teams were able to track patient status through this city-wide EHR platform, including possible hospital admission and use of ambulatory services. Electronic diaries from participants who agreed to use e-diaries were also integrated within the UMIAS EHR. For those participants who chose not to use e-diaries, data on participant status were collected by site staff via teleconsultations and entered into the EHR by site investigators. All adverse events were followed up by a clinical investigator until resolution and were reviewed by the data safety and monitoring board and verified by the trial monitor. When COVID-19 was suspected, participants were assessed according to COVID-19 diagnostic protocols, including PCR testing at a central laboratory in Moscow. Severity of disease was established upon confirmation of the COVID-19 diagnosis by site investigators. A description of the assessment criteria for severity of COVID-19 is in the appendix (p 2).
The study was organised and monitored by the Moscow branch of the Dutch contract research organisation Crocus Medical. Data management is done through the DM 365 MainEDC system (developed by Data Management 365), a powerful cloud-based platform integrated to comprise the functions of data collection, advanced randomisation techniques, full control over drug supply and dispensing, and patient e-diaries (electronic data capture, IWRS, drug supply, and electronic patient-reported outcomes). The system complies with all applicable international regulations, including Code of Federal Regulations Title 21 Part 11, Good Clinical Practice, Good Automated Manufacturing Practice 5, Health Insurance Portability and Accountability, and General Data Protection Regulation. The system allows collection and validation of clinical data in high-load clinical trials and supports central monitoring and risk-based monitoring processes, automated coding with the Medical Dictionary for Regulatory Activities (MedDRA), WHODrug, and Logical Observation Identifiers Names and Codes, and instant mapping of the exported data to the standard Clinical Data Interchange Standards Consortium Study Data Tabulation Model.
Blood sampling was done on the day of vaccination immediately before study drug administration. Blood sampling for assessment of immunogenicity parameters was only done in some study centres, selected on the basis of the logistics chain for the delivery of biomaterial to the central laboratory where primary blood processing was done (sera collection, aliquoting, and freezing). Blood samples for antigen-specific IgG analysis are planned to be taken from up to 9520 trial participants before completion of the trial.
Immunogenicity was analysed as described previously.12 In brief, antigen-specific humoral immune response was analysed on the day of first vaccination and day 42. The titre of glycoprotein-specific antibodies in serum was ascertained by ELISA. To test anti-SARS-CoV-2 IgG, we used an ELISA that was developed at Gamaleya NRCEM and registered for clinical use in Russia (P3H 2020/10393 2020-05-18). The ELISA measures IgGs specific to the receptor-binding domain (RBD) of SARS-CoV-2 glycoprotein S. The titre of neutralising antibodies was measured on the day of first vaccination and day 42 by microneutralisation assay using SARS-CoV-2 (hCoV-19/Russia/Moscow_PMVL-1/2020) in a 96-well plate and a 50% tissue culture infective dose (TCID50) of 100. The seroconversion rate was calculated as a four-fold increase in titre at 42 days compared with the day before first vaccination. Cell-mediated immune response was measured on the day of first vaccination and day 28 by quantification of IFN-γ secretion upon antigen restimulation in peripheral blood mononuclear cell culture.
Outcomes
The primary outcome was the proportion of participants with COVID-19 confirmed by PCR from day 21 after receiving the first dose. The secondary outcomes were severity of COVID-19; changes in antibody levels against SARS-CoV-2 glycoprotein S; proportion of participants with antibodies against SARS-CoV-2 N-protein; changes in SARS-CoV-2 neutralising antibody titres (increase of titres); changes in antigen-specific cellular immunity level (increase of cell-mediated immune response to antigen); and incidence and severity of adverse events. Serious adverse events were diagnosed on the basis of the event requiring hospital admission. Here, we report preliminary results on the primary outcome measure, incidence and severity of adverse events, immunogenicity, and safety.
Statistical analysis
In this interim analysis, we present efficacy data at the point of confirmation of 78 COVID-19 cases in participants after receiving the second dose, as stipulated by the protocol.
In this study, the primary endpoint is the proportion of participants without COVID-19 confirmed by laboratory tests during the study. The frequency of COVID-19 in the general population, and thus the expected frequency in our placebo group, is 20 people per 1000 or 2·0%. The study aims to show that the proportion of participants with COVID-19 will be at least a third lower in the intervention group than the control group (odds ratio [OR] for the null hypothesis of 0·67)—ie, the upper limit of the 95% CI for the OR should not exceed 0·67. The expected value of the effect is about 0·500 (OR for the alternative hypothesis of 0·500). With a planned study population of 40 000 participants and randomisation 3:1 vaccine to placebo, the study power will be 85%, with a unilateral statistical significance level of 0·025.
The study protocol did not originally prespecify a target number of events in this trial. However, because of the increase in the incidence of COVID-19 in Russia, changes were made to the clinical trial protocol on Nov 5, 2020, including an interim analysis to preliminarily calculate the vaccine efficacy and to establish ethical appropriateness of further inclusion of the placebo group in the trial in the context of a growing pandemic if the vaccine is effective. Three interim analyses were completed when 20, 39, and 78 documented cases of COVID-19 had occurred across both groups combined. Our original conservative estimate of efficacy was 50%. If the efficacy was at least 70%, then a statistically significant difference between the groups would be detected when at least 20 events were reached across the two groups. If efficacy was 65%, then the number of cases required would be 39. 60% efficacy would be statistically significant when 78 cases had been reported.
The OR and 95% CI were calculated according to previously described methods.13 The primary endpoint was calculated using the following formula: vaccine efficacy (%)=(1 – OR) × 100, where the OR is as follows:
where a is the number of vaccinated participants with COVID-19, b is the number of vaccinated participants without COVID-19, c is the number of unvaccinated participants with COVID-19, and d is the number of unvaccinated participants without COVID-19.
ORs and 95% CIs were obtained by the Baptista-Pike method, p values were obtained by χ2 test or Fisher's exact test (if the expected frequency in any cell is <5). Cumulative incidence is presented using the Kaplan-Meier method.
In the safety analysis, adverse events were coded using MedDRA, version 23.0. Adverse events were presented by group, system organ and class, and preferred term. Normality of the data distribution was assessed with the d’Agostino-Pearson test in the analysis of quantitative data (immunogenicity analyses). In the analysis of immunogenicity (analysis of parametric data) in the case when two groups of data were compared, the Mann-Whitney U test was used (eg, the vaccine group vs placebo group or men vs women) for unpaired samples and the Wilcoxon signed rank test for paired samples (eg, cellular response data on days before and after vaccination). When comparing several groups of data (eg, age strata), the Kruskal-Wallis test was used. To compare the frequency indicators between groups, the χ2 test and, if necessary, Fisher's exact test were used (if the expected frequency in any of the cells was <5).
The primary outcome analysis included all participants who had received at least two doses at the time of database lock and followed protocol without violations. The analysis of serious adverse events included all participants who had received at least one dose at the time of database lock and followed protocol without violations. The safety analysis (including rare adverse events) included all participants who had received two doses and for whom all available data were verified in the case report form at the time of database lock. The statistical analysis was done using Stata, version 14, and GraphPad Prism, version 9.0. This trial is registered with ClinicalTrials.gov (NCT04530396).
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
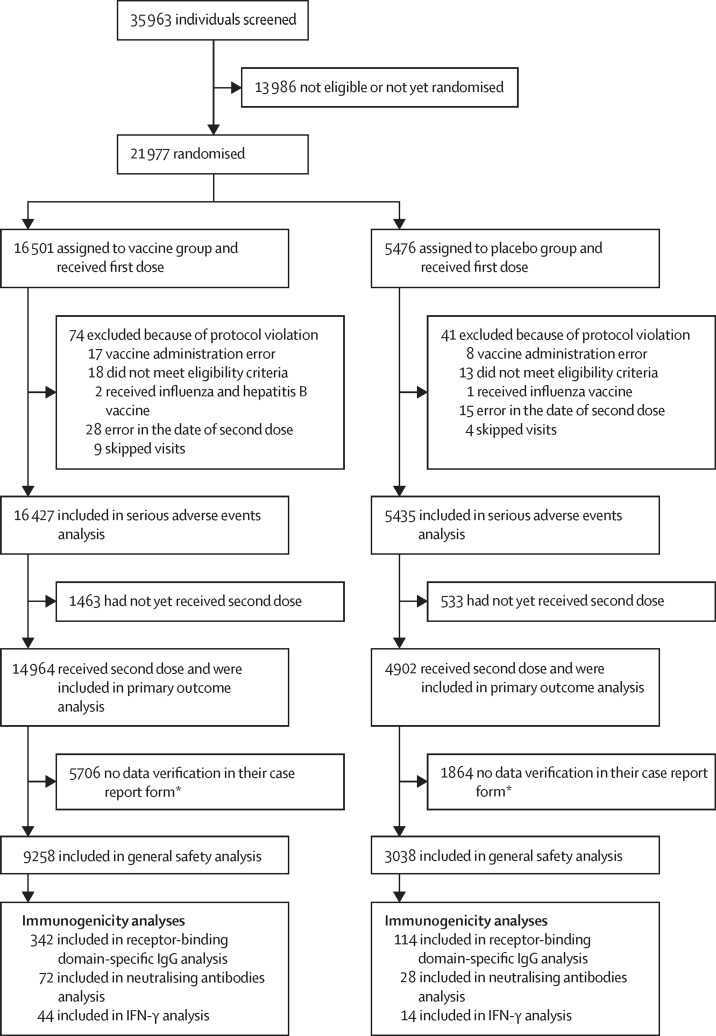
Between Sept 7 and Nov 24, 2020, 21 977 adults were eligible and randomly assigned to receive placebo (n=5476) or vaccine (n=16 501; figure 1 ).
Figure 1.
Trial profile
*At the time the database was locked, the data on adverse events in the case report form had not yet been verified in these participants; the data verification procedure can be done with a slight delay, thus, participants whose data were not verified were not included in this analysis.
The first database lock was on Nov 18, 2020, when 20 cases of COVID-19 had been reported. The interim safety analysis (analysis of rare adverse events) was done with data up to the first database lock. Since there was an increase in COVID-19 incidence in Moscow during November, the second database lock was done on Nov 24, 2020, when 78 COVID-19 cases had been reported. Data for the interim efficacy analysis and serious adverse events analysis are presented up to the second database lock.
74 participants from the vaccine group and 41 from the placebo group were excluded from analyses (figure 1). This preliminary analysis included 16 427 participants in the vaccine group and 5435 in the placebo group, who received at least one dose and continued participation in the trial. 14 964 in the vaccine group and 4902 in the placebo group had received two doses at the time of database lock (Nov 24, 2020) and were included in the primary outcome analysis (table 1 ). Median time from participants receiving the first dose to the date of database lock was 48 days (IQR 39–58). Among the participants who received two doses, the mean age was 45·3 years (SD 12·0) in the vaccine group and 45·3 years (SD 11·9) in the placebo group; the distribution by sex (p=0·619), incidence of concomitant diseases (p=0·420), and infection risk (p=0·851) were similar between the two groups (table 1).
Table 1.
Baseline characteristics of participants who received two doses of assigned treatment and were included in primary outcome analysis
| Vaccine (n=14 964) | Placebo (n=4902) | ||
|---|---|---|---|
| Sex | |||
| Female | 5821 (38·9%) | 1887 (38·5%) | |
| Male | 9143 (61·1%) | 3015 (61·5%) | |
| Race | |||
| White | 14 741 (98·5%) | 4830 (98·5%) | |
| Asian | 217 (1·5%) | 69 (1·4%) | |
| Other* | 6 (<0·1%) | 3 (<0·1%) | |
| Age group, years | |||
| 18–30 | 1596 (10·7%) | 521 (10·6%) | |
| 31–40 | 3848 (25·7%) | 1259 (25·7%) | |
| 41–50 | 4399 (29·4%) | 1443 (29·4%) | |
| 51–60 | 3510 (23·5%) | 1146 (23·4%) | |
| >60 | 1611 (10·8%) | 533 (10·9%) | |
| Age, years | 45·3 (12·0) | 45·3 (11·9) | |
| Bodyweight, kg | 81·3 (17·5) | 81·6 (17·7) | |
| Height, cm | 173·1 (9·1) | 173·3 (9·0) | |
| Body-mass index, kg/m2 | 26·75 (4·56) | 26·75 (4·55) | |
| Concomitant diseases (diabetes, hypertension, ischaemic heart disease, obesity)† | 3687/14 944 (24·7%) | 1235/4892 (25·2%) | |
| Risk of infection in volunteers†‡ | |||
| High | 65/14 567 (0·4%) | 23/4778 (0·5%) | |
| Medium | 3853/14 567 (26·5%) | 1280/4778 (26·8%) | |
| General | 10649/14 567 (73·1%) | 3475/4778 (72·7%) | |
Data are n (%) and mean (SD).
Includes Black or African American, Native Hawaiian or other Pacific Islander, or undefined.
Denominator shows number of participants for whom these data were available.
High risk denotes those whose work involves interaction with patients with a confirmed diagnosis of COVID-19; medium risk is those who have professional contact with a large number of people, such as general practitioners, social workers, and shop assistants; and general risk denotes those with no additional risks associated with their professional activities.
From 21 days after the first dose of vaccine (the day of dose 2), 16 COVID-19 cases were confirmed in the vaccine group (of 14 964 participants; 0·1%) and 62 cases were confirmed in the placebo group (of 4902 participants; 1·3%); vaccine efficacy was 91·6% (95% CI 85·6–95·2; table 2 ). The observed vaccine efficacy was greater than 87% in all age and sex subgroups. Notably, vaccine efficacy was 91·8% (67·1–98·3) in participants older than 60 years. There were no cases (vaccine group) and 20 cases (placebo group) of moderate or severe COVID-19 confirmed at least 21 days after dose 1; thus, vaccine efficacy against moderate or severe COVID-19 was 100% (94·4–100·0). From 15 to 21 days after the first dose, efficacy was 73·6% (p=0·048), then from day 21, efficacy was 100% (p<0·0001; appendix p 11).
Table 2.
Interim results on vaccine efficacy
| Total cases | Vaccine group | Placebo group | Vaccine efficacy (95% CI) | p value | ||
|---|---|---|---|---|---|---|
| First COVID-19 occurrence from 21 days after dose 1 (day of dose 2)* | ||||||
| Overall | 78 | 16/14 964 (0·1%) | 62/4902 (1·3%) | 91·6% (85·6–95·2) | <0·0001 | |
| Age group (years) | ||||||
| 18–30 | 5 | 1/1596 (0·1%) | 4/521 (0·8%) | 91·9% (51·2–99·3) | 0·0146 | |
| 31–40 | 17 | 4/3848 (0·1%) | 13/1259 (1·0%) | 90·0% (71·1–96·5) | <0·0001 | |
| 41–50 | 19 | 4/4399 (0·1%) | 15/1443 (1·0%) | 91·3% (73·7–96·9) | <0·0001 | |
| 51–60 | 27 | 5/3510 (0·1%) | 22/1146 (1·9%) | 92·7% (81·1–97·0) | <0·0001 | |
| >60 | 10 | 2/1611 (0·1%) | 8/533 (1·5%) | 91·8% (67·1–98·3) | 0·0004 | |
| Sex | ||||||
| Female | 32 | 9/5821 (0·2%) | 23/1887 (1·2%) | 87·5% (73·4–94·2) | <0·0001 | |
| Male | 46 | 7/9143 (0·1%) | 39/3015 (1·3%) | 94·2% (87·2–97·4) | <0·0001 | |
| Moderate or severe cases | 20 | 0/14 964 | 20/4902 (0·4%) | 100% (94·4–100·0) | <0·0001 | |
| First COVID-19 occurrence after dose 1† | ||||||
| Any time after dose 1 | 175 | 79/16 427 (0·5%) | 96/5435 (1·8%) | 73·1% (63·7–80·1) | <0·0001 | |
| From 14 days after dose 1 | 109 | 30/14 999 (0·2%) | 79/4950 (1·6%) | 87·6% (81·1–91·8) | <0·0001 | |
| First COVID-19 occurrence after dose 2 (28 days after dose 1)* | ||||||
| All | 60 | 13/14 094 (0·1%) | 47/4601 (1·0%) | 91·1% (83·8–95·1) | <0·0001 | |
Data are n/N (%), unless otherwise stated.
Includes those who received both doses.
Includes participants who received at least one dose.
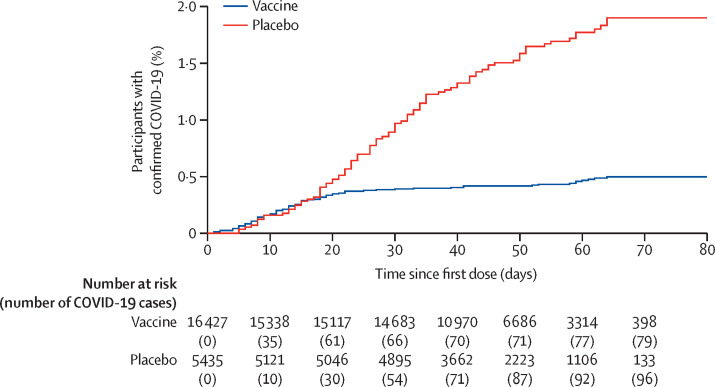
97 confirmed cases of COVID-19 (63 in the vaccine group and 34 in the placebo group) are not reflected in the analysis of the primary endpoint because they occurred fewer than 21 days after dose 1 (ie, before dose 2; table 2, figure 2 ). Estimated vaccine efficacy against confirmed COVID-19 occurring at any time after dose 1 was 73·1% (95% CI 63·7–80·1). Notably, in the vaccine group, most cases of COVID-19 occurred before dose 2. Rates of disease onset were similar for the vaccine and placebo groups until about 16–18 days after dose 1, after which, early onset of protection led to the number of cases in the vaccine group increasing much more slowly than in the placebo group (figure 2).
Figure 2.
Kaplan-Meier cumulative incidence curves for the first symptomatic, PCR-positive COVID-19 after dose 1, in participants who received at least one dose of vaccine or placebo
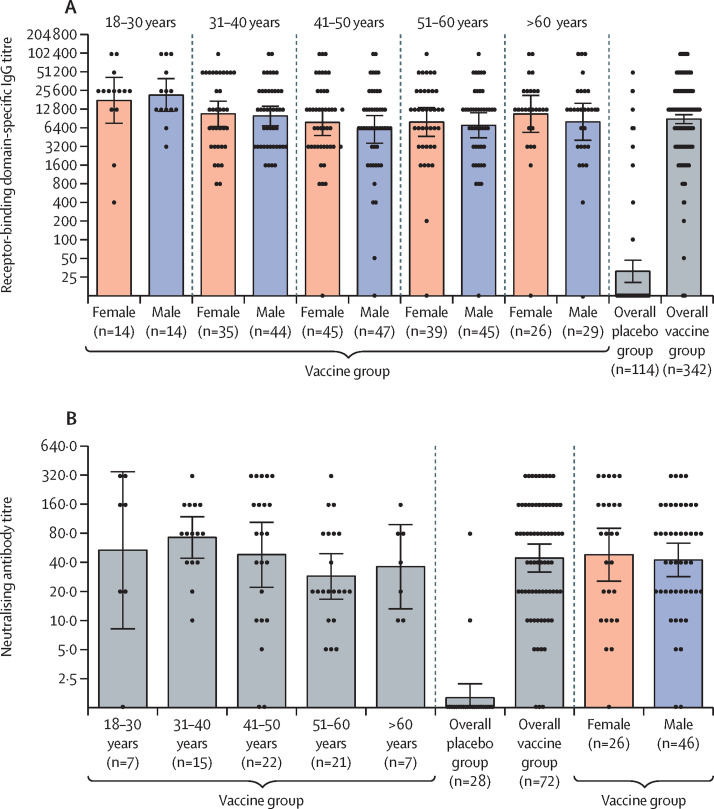
The interim immunogenicity analysis included samples transferred from the central laboratory that were collected before Nov 30, 2020, and showed that the vaccine induces an immune response in participants. Before first vaccination, no RBD-specific antibodies (of 456 participants tested) or virus-neutralising antibodies (of 100 participants tested) were detected in the blood serum of participants. In the analysis of humoral immune response, serum samples of 456 participants (342 from the vaccine group and 114 from the placebo group) were analysed for the presence of antibodies specific to the receptor-binding domain of SARS-CoV-2 glycoprotein S 42 days from the start of vaccination (figure 3 A). In the vaccine group, RBD-specific IgG was detected in 336 (98%) of 342 samples, with a geometric mean titre (GMT) of 8996 (95% CI 7610–10 635), and a seroconversion rate of 98·25%. In the placebo group, RBD-specific IgG was detected in 17 (15%) of 114 samples, with a GMT of 30·55 (20·18–46·26), and a seroconversion rate of 14·91% (p<0·0001 vs the vaccine group). When comparing the level of RBD-specific antibodies between age strata, we noted that the age 18–30 years group (combined male and female) had a significantly higher GMT than the other age groups (p=0·0065). There were no differences between the other age groups (p=0·343). Antibody levels did not differ significantly between men (n=179) and women (n=159; p=0·258). Descriptive statistics by age stratum and sex are in the appendix (p 3).
Figure 3.
Humoral immune response
(A) Receptor-binding domain-specific antibodies on day 42, as measured by ELISA, in participants administered with vaccine, by age group and overall, or placebo overall. Four participants are not included in the subgroup analysis by age because of missing date of birth on the case report form for this analysis. (B) Neutralising antibodies on day 42, as measured by neutralisation assay with 100 TCID50, in participants administered with vaccine or placebo. Data are divided by age strata and by sex. Data of the overall vaccine group and placebo group are also presented. Dots show individual datapoints, bars show geometric mean titres, and whiskers show 95% CI. TCID50=50% tissue culture infective dose.
To assess the induction of a humoral immune response, serum samples from 100 participants were analysed for the presence of neutralising antibodies on day 42 after first vaccination (figure 3B); the GMT of neutralising antibodies was 44·5 (95% CI 31·8–62·2) and the seroconversion level was 95·83% in the vaccine group. GMT in the placebo group was 1·6 (1·12–2·19) and the seroconversion rate was 7·14%, which was significantly lower than that in the vaccine group (p<0·0001). Levels of neutralising antibodies were similar between age strata (p=0·222) and between men and women (p=0·639). Descriptive statistics by age stratum and sex are in the appendix (p 3).
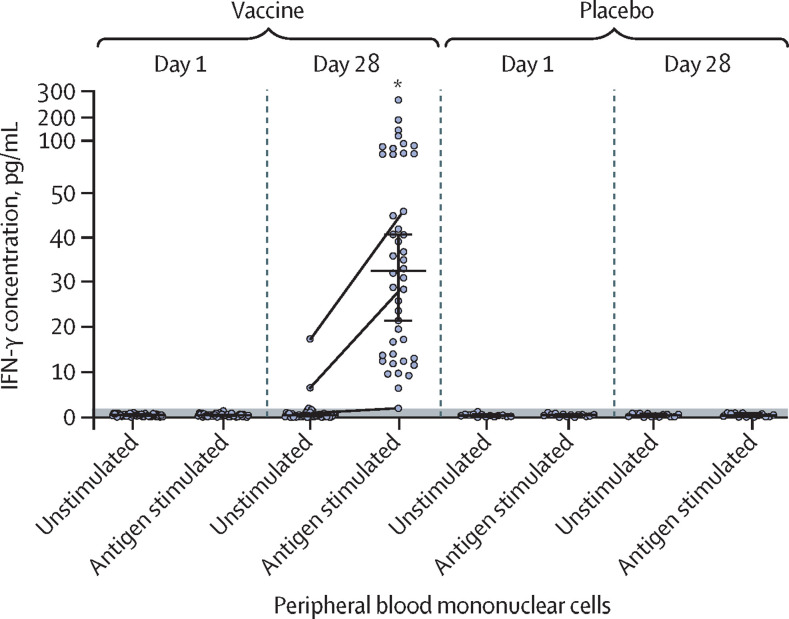
Cellular immune response in participants was characterised by secretion of IFN-γ of peripheral blood mononuclear cells upon SARS-CoV-2 glycoprotein S restimulation in culture. To assess cellular immune response, serum samples from 58 participants (44 from the vaccine group and 14 from the placebo group) were analysed. By day 28 after first vaccination, all participants in the vaccine group had significantly higher levels of IFN-γ secretion upon antigen restimulation (median 32·77 pg/mL [IQR 13·94–50·76]) compared with the day of administration of the first dose (figure 4 ). Descriptive statistics for IFN-γ immune responses are in the appendix (p 4).
Figure 4.
IFN-γ response to SARS-CoV-2 glycoprotein S of peripheral blood mononuclear cells of participants who received two doses of vaccine (n=44) or placebo (n=14)
Dots show individual datapoints of intact (unstimulated) cells and cells stimulated with SARS-CoV-2 glycoprotein S (antigen stimulated). Horizontal lines show median values, whiskers show 95% Cl. The threshold of detection (<2 pg/mL) is indicated with the grey shaded area. The grey lines connecting dots between unstimulated and antigen-stimulated cells show changes in IFN-γ response in some representative individuals. *p<0·0001 for day 28 antigen-stimulated cells versus day 1 antigen-stimulated cells, in the vaccine group.
The general safety and rare adverse event analyses included 12 296 participants who received both doses up to the database lock on Nov 18, 2020. The most common adverse events were flu-like illness, injection site reactions, headache, and asthenia. Most of the reported adverse events (7485 [94·0%] of 7966) were grade 1; 451 were grade 2 (5·66%) and 30 were grade 3 (0·38%). 122 rare adverse events were reported in the study (91 in the vaccine group and 31 in the placebo group; appendix pp 8–9).
The analysis of serious adverse events included 21 862 participants who received at least one dose (of whom 19 866 received two doses) up to database lock on Nov 24, 2020. 70 episodes of serious adverse events, considered not related to COVID-19, were recorded in 68 participants: in 45 (0·3%) of 16 427 participants from the vaccine group and 23 (0·4%) of 5435 participants from the placebo group (appendix pp 5–7). None of the serious adverse events were considered associated with vaccination, as confirmed by the independent data monitoring committee (IDMC).
Because there were few serious adverse events, it was possible to process and verify the serious adverse events data up to the second database lock; however, full adverse events data, which has not yet been processed, will be provided in a later publication to avoid discrepancies with the final report after full data processing is complete.
During the study, four deaths were recorded: three (<0·1%) of 16 427 participants in the vaccine group and one (<0·1%) of 5435 participants in the placebo group. No vaccine-related deaths were reported. In the vaccine group, one death was associated with fracture of the thoracic vertebra and the other two were associated with COVID-19 (one patient with a severe cardiovascular background who developed symptoms on day 4 after first dose and one patient with a background of endocrinological comorbidities who developed symptoms on day 5 after first dose; appendix p 12). Based on the incubation period of the disease, both participants were deemed to be already infected before being included in the study, despite a negative PCR test. In the placebo group, the death was associated with haemorrhagic stroke.
The study included 2144 participants older than 60 years (1611 in the vaccine group and 533 in the placebo group). The mean age in this subgroup was 65·7 years (SD 4·5) in the vaccine group and 65·3 years (4·3) in the placebo group (appendix p 10). The maximum ages of the participants were 87 years in the vaccine group and 84 years in the placebo group. Proportions of participants by sex (p=0·378), incidence of concomitant diseases (p=0·774), and risk of infection (p=0·090) were similar between the vaccine and placebo groups. The vaccine was well tolerated in these participants. 1369 participants older than 60 years (who received two doses and for whom data in the case report form was verified at the time of database lock [Nov 18, 2020]) were included in the safety analysis. The most common adverse events were flu-like illness in 156 (15·2%) and local reaction in 56 (5·4%) of 1029 participants in the vaccine group and 30 (8·8%) and four (1·2%) of 340 participants in the placebo group (appendix p 11). There were three episodes of adverse events of grade 3 or worse, considered not associated with vaccination: an exacerbation of urolithiasis and acute sinusitis in the vaccine group and a flu-like illness in the placebo group. All these adverse events were resolved. In the participants older than 60 years, there were three serious adverse events reported in the vaccine group: renal colic and deep vein thrombosis (both associated with pre-existing comorbidities) and extremity abscess (due to physical injury and subsequent infection of the wound surface of the soft tissues of the finger). No association was found between serious adverse events and vaccine administration.
Discussion
Our interim results of the phase 3 Gam-COVID-Vac trial show that the vaccine is 91·6% (95% CI 85·6–95·2) efficacious against COVID-19 (from day 21 after first dose, the day of receiving second dose). Our results also showed that the vaccine was 100% (95% CI 94·4–100) efficacious against severe COVID-19, although this was a secondary outcome so the results are preliminary. The vaccine was well tolerated, with 45 (0·3%) of 16 427 participants in the vaccine group reporting serious adverse events, all of which were considered not related to the vaccine. According to the study design, the starting point for counting COVID-19 cases for estimation of vaccine efficacy was 21 days after dose 1 (day of dose 2 administration). Although the study was not designed to assess the efficacy of a single-dose regimen, our early starting point allows us to observe a possible partial protective effect of a single dose. The cumulative COVID-19 incidence curves of COVID-19 cases among the placebo and vaccine groups begin to diverge 16–18 days after the first immunisation, showing early onset of a partially protective effect after a single-dose immunisation; however, the study design does not allow us to draw conclusions from these observations.
The vaccine induced robust humoral (n=342) and cellular (n=44) immune responses in all age strata. Notably, there were a few non-responders in the vaccine group (six of 342), possibly due to immunosenescence in older people, individual characteristics of the formation of an immune response, or concomitant immunological disorders.
17 (15%) of 114 participants in the placebo group had RBD-specific antibodies on day 42, probably associated with asymptomatic COVID-19; however, none of these participants were SARS-CoV-2 PCR positive, nor did they report the onset of respiratory symptoms in the electronic diary or when interviewed as part of the telemedicine follow-up.
Given the importance of protecting populations at risk because of older age, we assessed the ability of the vaccine to induce an immune response and protect against COVID-19 in individuals older than 60 years. Our results show that the two-component vaccine Gam-COVID-Vac was able to induce a virus-neutralising humoral response in participants older than 60 years. Furthermore, vaccine efficacy in this group of participants did not differ significantly from the efficacy of the age 18–60 years group.
The limitations of the interim analysis of efficacy include the small sample sizes within age strata. Further data collection will allow for clarification of efficacy data within age groups. Furthermore, COVID-19 cases were detected through self report of symptoms by participants, followed by a PCR test, so only symptomatic cases of COVID-19 are included in the efficacy analyses.
Initially, we developed a vaccine in two forms: liquid (which is stored at –18°C) and freeze dried (which is stored at 2–8°C). In this study, we studied the liquid form of the vaccine that requires storage at –18°C. Storage at 2–8°C, a favourable temperature profile for global distribution, has been approved by the Ministry of Health of the Russian Federation.
We previously reported on the local and systemic post-vaccine adverse reactions of the Gam-COVID-Vac vaccine in a small sample of participants.12 In this interim analysis, we report serious adverse events in more than 21 000 participants (of whom more than 16 000 received the vaccine).
70 episodes of serious adverse events were recorded in 68 participants across the two groups; none of these events were considered related to the vaccine. During the study, four deaths were recorded: three in the vaccine group and one in the placebo group. None were considered related to the vaccine, with confirmation by the IDMC. No post-vaccination adverse events were reported in any of these participants after vaccination.
The two COVID-19-related deaths were due to pre-existing cardiovascular and endocrinological conditions exacerbated by COVID-19. Taking into account the length of the incubation period described by WHO and the Centers for Disease Control and Prevention (CDC),14, 15 these two participants were probably already infected with SARS-CoV-2 at the time of randomisation and vaccination. On the basis of WHO and CDC guidelines and review of the underlying clinical data, the IDMC confirmed that participants were infected and disease had progressed before any immunity from the vaccine developed. A detailed description of the condition of participants with COVID-19 is in the appendix (p 12). Among the seven participants assigned to the placebo group who were confirmed to have COVID-19 within the first 7 days after the first dose, there were no comorbid conditions, in contrast to the vaccine group, in which there were three participants with a comorbidity among 25 who were confirmed to have COVID-19 within the first 7 days.
In summary, both COVID-19-related deaths have several principal points to be considered. First, despite the negative PCR test at screening and absence of increased temperature at the time of first vaccine dose administration, the onset of the first COVID-19 symptoms (4–5 days after first dose, similar to the average COVID-19 incubation period) testifies that participants had been infected with SARS-CoV-2 before or near the vaccination day, which was additionally confirmed by the IDMC, on the basis of WHO and CDC guidelines and review of underlying clinical data. Second, both participants self-administered non-steroidal anti-inflammatory drugs without informing clinicians, which interfered with diagnosis and receipt of medical help upon hospital admission. Third, because of limited diagnostics at screening (limitations of medical examination and testing and patient unaware of comorbidities) each participant had comorbidities that were only known after hospital admission. Participants who have not developed protective immunity to SARS-CoV-2 (ie, were infected before vaccination or early after vaccination) showed the natural clinical course of COVID-19. We did another analysis of the severity of the course of COVID-19 in the two groups, which showed that in the first 2 weeks after the first dose, there was no significant difference in the severity of the course of COVID-19 between the vaccine and placebo groups. From 15 to 21 days after the first dose, efficacy was 73·6% (p=0·048), then from day 21, efficacy was 100% (p<0·0001; appendix p 11). Therefore, in this study, the efficacy analysis was done 21 days after the first dose, because by that time, the immune response is formed.
Currently, scrupulous monitoring continues, in particular for cases of COVID-19. All safety data will be provided to the regulator for analysis.
In this interim analysis, we have not been able to assess duration of protection; median follow-up time was 48 days after first dose. Although the study enrolled participants with comorbidities, not all risk groups are represented. There is a need to further investigate the vaccine in adolescents and children under Pediatric Investigational Plans, as well as pregnant and lactating women. Most participants in our trial were white, so we welcome further investigation in a more diverse cohort.
Interim results on efficacy have been announced for several vaccine candidates against SARS-CoV-2. The safety and efficacy study of the ChAdOx1 nCoV-19 vaccine (AZD1222) provides an analysis of data from four randomised controlled trials in Brazil, South Africa, and the UK. 11 636 participants were included in the primary efficacy analysis. Among participants who received two standard doses, the reported efficacy of the vaccine was 62·1%, and in participants who received a low dose followed by a standard dose, efficacy was 90·0%, resulting in overall efficacy of 70·4%.2 BNT162b2, an mRNA-based vaccine developed by Pfizer/BioNTech has reported 95% efficacy against COVID-19 in a multinational, placebo-controlled, observer-blinded, pivotal efficacy trial.4 36 523 participants without baseline infection were included in the primary efficacy analysis. Eight cases of COVID-19 with onset at least 7 days after the second dose were observed in the study group and 162 cases were observed in the placebo group. There was one case of severe COVID-19 in a study group with onset at least 7 days after the second dose of BNT162b2.4 A phase 3, randomised, stratified, observer-blind, placebo-controlled study of an mRNA-1273 vaccine has enrolled 30 000 participants, 25% of whom are age 65 years or older. Interim results of the trial suggest efficacy of 94·1% based on 95 cases of asymptomatic COVID-19 among participants: 90 in the placebo group and five in the study group.3 The results of this Gam-COVID-Vac trial are not dissimilar to those reported for the other vaccines.
Vaccination strategies should account for a number of concerns regarding the priority of access to COVID-19 vaccines in various population groups, reliable risk assessment of adverse effects of vaccination in population groups with increased risk of severe COVID-19 (older adults and individuals with comorbidities), vaccine logistics (cold chain supply), sufficient coverage of immunisation, and duration of protective immune response. According to WHO target product profiles for COVID-19 vaccines,16 the characteristics required for emergency use during an outbreak include efficacy of at least 50%, suitability for use in older adults, maximum of two-dose regimen, and protection for at least 6 months. Further studies of candidate vaccines are needed to obtain information on duration of post-vaccine protective immune response. Yet, the results on efficacy and safety of COVID-19 vaccine candidates thus far are promising.
Our interim analysis of this phase 3 trial of Gam-COVID-Vac has shown promising results. In parallel with implementation of multiple clinical trials (in Russia, Belarus, United Arab Emirates, and India), the vaccine has already been released in Russia for use by the public, largely in at-risk populations, medical workers, and teachers, and as of Jan 23, 2021, more than 2 million doses of Gam-COVID-Vac have already been administered to the public (pharmacovigilance and monitoring of the incidence of rare adverse events is controlled by the Federal Service for Surveillance in Healthcare).
We are conducting research to investigate a single-dose regimen of the vaccine (the clinical trial was approved by the Regulator and Ethics committee on Jan 8, 2021, number 1). Our interim analysis of the randomised, controlled, phase 3 trial of Gam-COVID-Vac in Russia has shown high efficacy, immunogenicity, and a good tolerability profile in participants aged 18 years or older.
This online publication has been corrected. The corrected version first appeared at thelancet.com on February 18, 2021
Data sharing
Anonymous participant data will be available upon completion of clinical trials and publication of the results of the completed study upon request to the corresponding author. Proposals will be reviewed and approved by the sponsor, security department, researcher, and staff on the basis of scientific merit and absence of competing interests. Once the proposal has been approved, data can be transferred through a secure online platform after the signing of a data access agreement and a confidentiality agreement.
Acknowledgments
Acknowledgments
We would like to thank the study participants, site research staff, and members of the trial management groups, trial steering committee, and IDMC.
Contributors
DYL is the principal investigator, performed research, and coordinated the study. IVD, DVS, AIT, and AVK drafted the manuscript. IVD, NLL, YVS, and EAT coordinated the study. IVD, OVZ, AIT, ASD, DMG, ASE, AVK, AGB, FMI, OP, TAO, IBE, IAF, DIZ, DVV, DNS, and ASS collected data. IVD, OVZ, AIT, YVS, EAT, NLL, DAE, NAN, MMS, and VAG contributed to the analysis and interpretation of the data. DYL, IVD, DVS, SVB, BSN, and ALG edited the manuscript. IVD and DVS did the statistical analysis. EAS and SKZ were involved in organisation, coordination, conduct, and technical support of the study. ALG was involved in organisation of research and the final decision to publish. All authors critically reviewed the manuscript and approved the final version. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Declaration of interests
OVZ, TAO, IVD, OP, DVS, DMG, ASD, AIT, DNS, IBE, EAT, AGB, ASE, ASS, SVB, DYL, BSN, and ALG report patents for an immunobiological expression vector, pharmaceutical agent, and its method of use to prevent COVID-19. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO Draft landscape of COVID-19 candidate vaccines. Jan 22, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 2.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2035389. published online Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Zhou D. Adenoviral vector-based strategies against infectious disease and cancer. Hum Vaccin Immunother. 2016;12:2064–2074. doi: 10.1080/21645515.2016.1165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpers C, Kochanek S. Adenoviral vectors for gene transfer and therapy. J Gene Med. 2004;6(suppl 1):S164–S171. doi: 10.1002/jgm.496. [DOI] [PubMed] [Google Scholar]
- 8.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolzhikova IV, Zubkova OV, Tukhvatulin AI, et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother. 2017;13:613–620. doi: 10.1080/21645515.2016.1238535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovyrshina AV, Dolzhikova IV, Grousova DM, et al. A heterologous virus-vectored vaccine for prevention of Middle East respiratory syndrome induces long protective immune response against MERS-CoV. Immunologiya. 2020;41:135–143. (in Russian). [Google Scholar]
- 12.Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman DG. Chapman and Hall; London: 1991. Practical statistics for medical research. [Google Scholar]
- 14.WHO Transmission of SARS-CoV-2: implications for infection prevention precautions. July 9, 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions
- 15.Centers for Disease Control and Prevention Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated Dec 8, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
- 16.WHO Target product profiles for COVID-19 vaccines. April 9, 2020. https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymous participant data will be available upon completion of clinical trials and publication of the results of the completed study upon request to the corresponding author. Proposals will be reviewed and approved by the sponsor, security department, researcher, and staff on the basis of scientific merit and absence of competing interests. Once the proposal has been approved, data can be transferred through a secure online platform after the signing of a data access agreement and a confidentiality agreement.