Abstract
As the SARS-COV-2 becomes a global pandemic, many researchers have a concern about the long COVID-19 complications. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a persistent, debilitating, and unexplained fatigue disorder. We investigated psychological morbidities such as CFS and post-traumatic stress disorder (PTSD) among survivors of COVID-19 over 6 months. All COVID-19 survivors from the university-affiliated hospital of Tehran, Iran, were assessed 6 months after infection onset by a previously validated questionnaire based on the Fukuda guidelines for CFS/EM and DSM-5 Checklist for PTSD (The Post-traumatic Stress Disorder Checklist for DSM-5 or PCL-5) to determine the presence of stress disorder and chronic fatigue problems. A total of 120 patients were enrolled. The prevalence rate of fatigue symptoms was 17.5%. Twelve (10%) screened positive for chronic idiopathic fatigue (CIF), 6 (5%) for CFS-like with insufficient fatigue syndrome (CFSWIFS), and 3 (2.5%) for CFS. The mean total scores in PCL-5 were 9.27 ± 10.76 (range:0–44), and the prevalence rate of PTSD was 5.8%. There was no significant association after adjusting between CFS and PTSD, gender, comorbidities, and chloroquine phosphate administration. The obtained data revealed the prevalence of CFS among patients with COVID-19, which is almost similar to CFS prevalence in the general population. Moreover, PTSD in patients with COVID-19 is not associated with the increased risk of CFS. Our study suggested that medical institutions should pay attention to the psychological consequences of the COVID-19 outbreak.
Keywords: COVID-19, Chronic fatigue syndrome / myalgic encephalomyelitis, Post-traumatic stress disorder, Post-COVID morbidities
Introduction
Since the beginning of the novel coronavirus in China, it is noted that the long-term effects of the severe illness survivors remain unknown. As the virus pandemic is burning through the world and causing many mortalities and morbidities, researchers hypothesized that the COVID-19 could potentially cause post-viral complications, which may be lifelong and disabling (Perrin et al. 2020). Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is one of the well-known complications of some viruses which presented with prolonged relapse of exhaustion, cognitive dysfunction, depression, and other symptoms after a minimal amount of activity (Carruthers et al. 2011). Previous data showed that many survivors following the SARS-COV-1 outbreak developed CFS / ME-like symptoms (Moldofsky and Patcai 2011). There is currently no generally accepted diagnostic method for CFS/ME, so it is first necessary to rule out any disorders with similar symptoms. The literature indicates that several potential causes play a role in the pathophysiology of the disorder, including hormonal disturbances, immune system dysfunction, infection, and nervous system abnormalities (Shephard 2001). The precise explanation for what causes post-viral-fatigue remains uncertain. However, studies on the coronavirus and the influenza virus epidemics have suggested that these agents can alter the immune response. Moreover, the pro-inflammatory cytokines such as interferon-gamma and interleukins are released following the viral infection, passing through the blood-brain barrier and affecting the central nervous system (CNS) organs such as the hypothalamus (Hives et al. 2017). The autonomic alteration consequence of hypothalamus involvement may result in cognitive abnormality, sleep/wake cycle dysregulation, profound fatigue, and myalgia in the long term in favor of CSF/ME (Carruthers et al. 2011). Post-traumatic stress disorder (PTSD) refers to a category of psychiatric conditions triggered by trauma or other life-stressing factors (Organization 1992). People with stress-related disorders might represent a group of physiological dysregulation due to altered immune profiles (Glaser and Kiecolt-Glaser 2005; Passos, Vasconcelos-Moreno et al. 2015; Speer et al. 2018). Prior data indicated a correlation between PTSD and several infectious diseases (Jiang et al. 2019). According to the literature, PTSD could occur after recovery from a life-threatening illness. PTSD prevalence rates for a life-threatening condition in an intensive care unit (ICU) is 14–59% (Wu et al. 2005). Moreover, our previous work showed that stress and anxiety increased the vulnerability of COVID-19 infection (Ramezani et al. 2020). However, studies on the role of stress-related disorder in major life-threatening infections are still limited.
In the present study, we aimed to determine the presence of long-term adverse effects resembling CFS/ME symptoms such as persistent fatigue and PTSD in COVID-19 remitted patients who had no history of previous psychiatric problems or receiving psychiatric drugs. Moreover, the prevalence and predisposing factors for experiencing the mentioned symptoms were evaluated. To the best of our knowledge, it is the first study to assess PTSD and CFS in COVID-19 patients.
Patients and method
Study setting and population
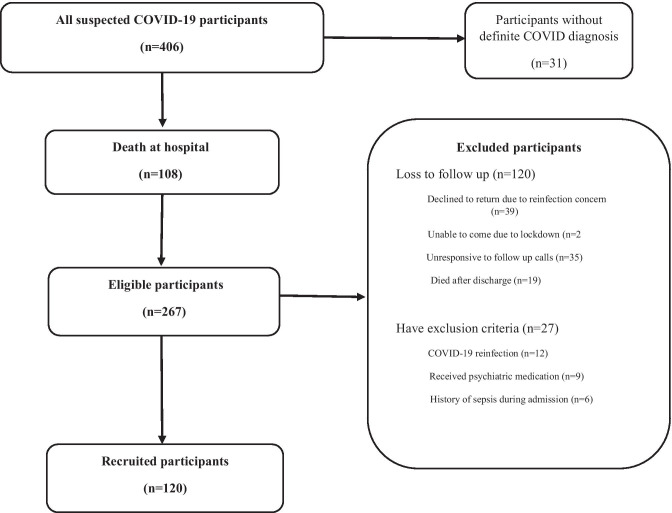
We evaluated all admitted patients diagnosed with COVID-19 between 20 February 2020 and 20 April 2020 (n = 406). History of previous medical and psychiatric illnesses, COVID-19 characteristics, viral markers, complete blood count, biochemistries, sedimentation rate, C-reactive protein was assessed. The exclusion criteria consisted of (1) patients who died at the hospital during the admission or in 6 months after the discharge; (2) patients infected with COVID-19 twice or more after discharge confirmed by positive polymerase chain reaction (PCR) test; (3) a history of previous influenza or any life-threatening infection (sepsis, endocarditis, and meningitis, or other central nervous system infections) before the diagnosis of COVID-19, (4) patients who have received psychiatric medication in the last year. These details and personal identifying information were stored. Out of 406 patients, 31 had no definitive diagnosis based on a spiral chest CT scan characteristics or positive COVID-PCR test and were removed from the study. Two hundred sixty-seven patients were eventually discharged, and 108 died. Eligible patients who had at least one negative PCR test during follow-up were contacted. At the time of the follow-up survey, 39 patients who declined to return to the hospital stated they were worried about COVID-reinfection. Twenty-seven residents in remote areas could not travel to our center due to lockdown. Nineteen patients died after discharge, and we failed to contact 35 patients by phone, in that they did not answer our phone call. The remainder had one of our exclusion criteria, so they were excluded, and ultimately, 120 patients were enrolled in our study (Fig. 1). All patients signed the informed consent forms and were evaluated by standard questionnaires at clinics 6 months after discharge. The study was approved by the ethical committee of the Shahid Beheshti University of Medical Science (IR.SBMU.RETECH.REC.1399. 104).
Fig. 1.
Study selection flowchart
Screening questionnaire
CFS/EM symptoms’ severity was analyzed using a previously validated questionnaire based on the Fukuda guidelines (Fukuda et al. 1994). Participants were asked to rate the severity of fatigue and the eight additional criteria over the past 6 months using an ordinal scale of 0 (no symptoms), 1 (trivial), 2 (mild), 3 (moderate), and 4 (severe). The fatigue severity and the sum of the eight ancillary criteria (Sum8) were evaluated. The fatigue severity scales and Sum8 scores divided the graph into four quadrants consist of healthy controls (HC: fatigue = none, trivial, or mild; Sum8 < 14), chronic idiopathic fatigue (CIF: fatigue = moderate or severe; Sum8 < 14), CFS-like with insufficient fatigue syndrome (CFSLWIFS: fatigue = none, trivial, or mild; Sum8 ≥ 14), and chronic fatigue syndrome (CSF: fatigue = moderate or severe; Sum8 ≥ 14) (Baraniuk et al. 2013). The DSM-5 PTSD checklist (PCL-5) is a self-report assessment of 20 items that measure the presence and severity of PTSD symptoms (Weathers et al. 2013). It measures four symptom clusters B to E in DSM-5, including intrusion (five items; B), avoidance (two items; C), negative alterations in cognition and mood (seven items; D), and alterations in arousal and reactivity (six items; E). The items are rated from 0 (not at all) to 4 (extremely) and are summed for a total severity score. The total score ranges from 0 to 80, with a recommended cut-off of 33 for PTSD caseness (Blevins et al. 2015; Bovin et al. 2016). The Persian version of this scale was assessed in Iran for psychometric properties and yielded acceptable validity and reliability scores (Sadeghi et al. 2016).
Statistical analysis
All statistical analyses were performed using SSPS version 16.0 (SPSS, Inc., Chicago, IL, USA). Categorical variables are expressed as absolute values (percentage), and continuous variables were expressed as mean value ± standard deviation. Multivariable logistic regression was used to adjust for confounders’ effect when appropriate to determine independent associations of binary outcomes. The confidence interval of 95% was considered for the interpretation of the estimations.
Results
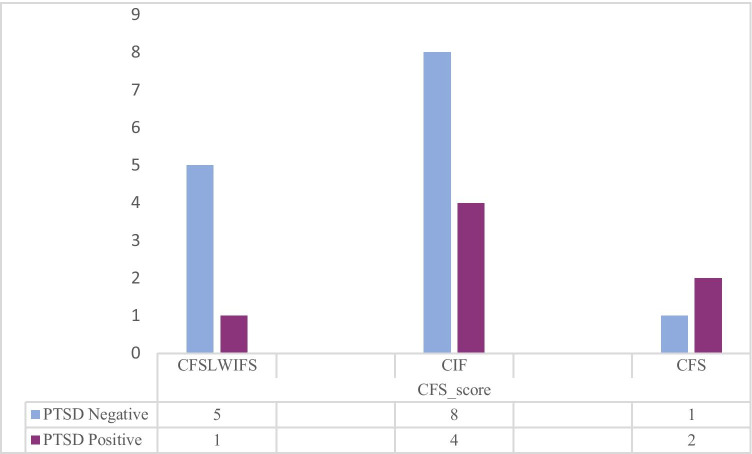
A total of 120 COVID-19 patients, 40 (33.3%) females and 80 (66.7) males, were included in our study. The mean age of patients was 54.62 ± 16.94 years. The mean length of hospital stay was 3.58 ± 2.52 days. The majority of the subjects had shortness of breath at the time of admission (72.2%); in addition, the most common comorbidity was hypertension (26.7%) (Table 1). The prevalence rate of CFS/ME was 21 (17.5%). While 99 (82.5%) respondents did not report any symptom within the preceding 6 months of the survey administration date, 12 (10%) reported CIF, 6 (5%) reported CFSLWIFS, and 3 (2.5%) reported CFS. Using our predefined cut-offs for the PTSD scoring system to screen the presence and severity of PTSD, we observed a total severity score in 7 (5.8%) study participants. The mean total score in PCL-5 was 9.27 ± 10.76 (range 0–44). Of the 7 participants who screened positive for PTSD, all (100%) of them were scored as CFSLWIFS 1 (14.3%), CIF 4 (57.1%), and CFS 2 (28.6%) (Fig. 2). According to univariate analysis, sex, PTSD, comorbidity, and chloroquine phosphate administration are significantly associated with asthenia score. Table 2 shows the logistic regression results for asthenia as the dependent variable and sex, PTSD, comorbidity, and chloroquine phosphate as independent variables. These variables did not reveal any significant predictive value for the severity and prevalence of asthenia in multivariate analysis.
Table 1.
Baseline characteristics of study participants (120)
| Characteristic | N(%) |
|---|---|
| Gender, (%) | |
| Female | 40(33.3%) |
| Male | 80(66.7%) |
| Age (Mean ± SD) | 54.62 ± 16.94 |
| Comorbidity | 51(44.3%) |
| Medical History, n (%) | |
| Diabetes | 28(23.3%) |
| Hypertension | 32(26.7%) |
| Cardiovascular disease | 20(16.7%) |
| Rheumatologic condition | 2(1.7%) |
| COPD | 6(5%) |
| Chronic liver disease | 5(4.2%) |
| Malignancy | 4(3.3%) |
| Hepatitis-B | 5(4.2%) |
| Immunodeficiency | 3(2.5%) |
| Fever ≥ 37.5 | 54(47%) |
| Shortness of breath Dyspnea | 88(72.2%) |
| Anorexia | 13(11.3%) |
| Myalgia | 27(23.5%) |
| Headache | 11(9.2%) |
| ICU admission | 9(7.5%) |
| Length of hospital stay | 3.58 ± 2.52 |
| O2 Saturation Pulseoxymeter | 87.5 ± 7.04 |
| Laboratory test | |
| First WBC | 8.09 ± 4.78 |
| First Neutrophil percentage | 75.09 ± 11.87 |
| Lymphocyte Percentage | 18.11 ± 10.59 |
| ESR | 46.45 ± 25.83 |
| CRP | 63.75 ± 41.80 |
| Bilateral distribution | 78(83.9%) |
| Unilateral distribution | 1(1.1%) |
| Bilateral consolidation CT | 14(15.1%) |
| Unilateral consolidation CT | 1(1.1%) |
| Abnormalities on chest CT | 91(97.8%) |
| Antibiotic | 72(62.6%) |
| Drug regimen | |
| Monotherapy | 9(7.9%) |
| Two drug | 35(30.7%) |
| Three drug | 49(43%) |
| Four drug | 1(0.9%) |
| Oseltamivir | 28(25%) |
| hydroxy_chloroquine | 67(58.8%) |
| chloroquine phosphate | 19(17%) |
| Kaletra | 92(80%) |
| Ribavirin | 52(45.2%) |
| Glucocorticoids therapy | 3(2.6%) |
| Interferon_Beta_1a | 10(9%) |
| Interferon_Beta_1b | 9(8%) |
| PTSD | |
| PCL-5 | 9.27 ± 10.76 |
| Positive | 7(5.8%) |
Fig. 2.
Number of participants experiencing PTSD and CFS
Table 2.
The estimation of Asthenia odds ratio for study-specific variables. Univariate & multivariate logistic regression for eight variables. Significant level < 0.05
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95%CI | P-value | Odds ratio | 95%CI | P-value | |
| Sex | 2.655 | 1.017–6.931 | 0.046 | 0.563 | 0.164–1.929 | 0.360 |
| PTSD | 1.552 | 1.237–1.948 | 0.001 | 2.341 | 0.700–1.963 | 0.999 |
| Comorbidity | 0.359 | 0.131–0.982 | 0.046 | 0.140 | 0.230–1.314 | 0.996 |
| chloroquine phosphate | 3.937 | 1.295–11.971 | 0.016 | 1.545 | 0.331–7.221 | 0.580 |
Discussion
COVID-19 has affected many people throughout the world. In light of public health concerns about the COVID-19 infection’s adverse effects, and according to the available evidence for SARS-COV-1 and H1N1 fatigue-related symptoms (Islam et al. 2020), we assessed the prevalence of CFS/EM following COVID-19 infection. This study found that 17.5% of patients experienced various fatigue levels; only 14.2% qualified for CFS criteria. According to the literature, CFS/EM prevalence rates following viral infections were varied by population group, case definitions, and diagnostic techniques (Lim et al. 2020).
Tansey et al. in their study, investigated survived patients following the SARS-COV-1 outbreak. They found that 64% of subjects reported fatigue and sleep disturbances at 3 months, while after 6 months and 12 months, the numbers were 54% and 60% (Tansey et al. 2007). In 2009, Lam et al. evaluated patients recovering from SARS in Hong Kong over 4 years. They noticed that 27.1% met the criteria for CFS/ME. Moreover, 42.5% had experienced at least one active psychiatric illness at the follow-up, and the most common problem was PTSD (54.5%) (Lam et al. 2009). In a case-control study, Moldofsky et al. examined people about 19 months after the onset of infection following the SARS outbreak. They demonstrated fatigue, myalgia and pain, depression, and sleep difficulties in subjects similar to post-febrile CFS symptoms. They also showed that only 2 of 22 patients had scores suggestive of PTSD (Moldofsky and Patcai 2011). In addition, earlier post-H1N1 pandemic experiments had similar findings. A study by Magnus et al. about the H1N1 pandemic in Norway suggested that H1N1 infection was associated with an increased CFS/EM risk more than twofold. Moreover, the younger population would be at more risk for post-infection CFS/EM (Magnus et al. 2015). Our study demonstrated that the measured CFS prevalence following COVID-19 is 2.5% (3 out of 120); on the other hand, the estimated prevalence of chronic fatigue syndrome in a general population using the Fukuda criteria is around 2% (Baraniuk 2017). Therefore, there is well within this ballpark of population prevalence. This finding would suggest that the contribution of COVID-19 disease to the future risk of CFS is minimal.
We showed 5.8% of subjects suffered from PTSD after 6 months of infection onset. A systematic analysis of the psychological consequences of SARS and the H1N1 pandemic indicated that the average prevalence of PTSD among healthcare workers (HCWs) was approximately 21% (Vyas et al. 2016). However, this proportion was variable among the general population following the infectious disease outbreak (Lam et al. 2009; Vyas et al. 2016).
We noticed that variables such as oxygen saturation at the time of admission, primary symptoms, admission in the ICU, and lab test parameters were not associated with asthenia occurrence. Female sex was associated with an increased risk of CFS/ME. This finding is in line with the previous data, which showed that women are susceptible to CFS/ME approximately 1.5- to 2-fold higher than men (Lim et al. 2020). However, after adjustment, the mentioned result did not remain.
Our study has several limitations. The sample size was small because many survivors were reluctant to attend the hospital. Patients’ premorbid psychiatric status was not documented; additionally, we did not evaluate the patients’ depression and quality of life scores at the survey time, which may cause some of the functional limitations. We assessed patients only for 6 months post-discharge; no special groups such as HCW enrolled in our study. Therefore, further study with a larger sample size, including ethnically and professionally diverse groups and longer follow-up duration, should be accomplished.
Conclusion
Our findings illustrated the incidence of CFS/EM and associated factors in non-HCW COVID-19 recovered participants. This is the first study to discover the long-term psychological adverse effects in COVID patients. Considering CFS/ME clinical heterogeneity, longitudinal and standardized studies to determine CFS/ME courses with treatment intervention and follow-up assessments are recommended.
Acknowledgements
The authors would like to thank the Clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran, for their support, cooperation, and assistance throughout the period of study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Leila Simani, Email: l.simani62@gmail.com.
Mahtab Ramezani, Email: drramezani23@gmail.com.
Ilad Alavi Darazam, Email: ilad13@yahoo.com.
Mastooreh Sagharichi, Email: Mas.saghirich@gmail.com.
Mohammad Amin Aalipour, Email: Aalipour000@gmail.com.
Hossein Pakdaman, Email: Hpakdaman20@gmail.com.
References
- Baraniuk JN (2017) Chronic fatigue syndrome prevalence is grossly overestimated using Oxford criteria compared to Centers for Disease Control (Fukuda) criteria in a U.S. population study. Fatigue: Biomedicine, Health & Behavior 5(4):215–230 [DOI] [PMC free article] [PubMed]
- Baraniuk JN, Adewuyi O, Merck SJ, Ali M, Ravindran MK, Timbol CR, Rayhan R, Zheng Y, Le U, Esteitie R. A chronic fatigue syndrome (CFS) severity score based on case designation criteria. Am J Transl Res. 2013;5(1):53. [PMC free article] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, Keane TM. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379. doi: 10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AP, Speight N, Vallings R. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Hives L, Bradley A, Richards J, Sutton C, Selfe J, Basu B, Maguire K, Sumner G, Gaber T, Mukherjee A. Can physical assessment techniques aid diagnosis in people with chronic fatigue syndrome/myalgic encephalomyelitis? A diagnostic accuracy study. BMJ Open. 2017;7(11):e017521. doi: 10.1136/bmjopen-2017-017521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MF, Cotler J, Jason LA (2020) Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue: Biomedicine, Health & Behavior 1–9
- Jiang T, Farkas DK, Ahern TP, Lash TL, Sørensen HT, Gradus JL. Posttraumatic stress disorder and incident infections: a nationwide cohort study. Epidemiology. 2019;30(6):911–917. doi: 10.1097/EDE.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MH-B, Wing Y-K, Yu MW-M, Leung C-M, Ma RC, Kong AP, So W, Fong SY-Y, Lam S-P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Ahn YC, Jang ES, Lee SW, Lee S-H, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) J Transl Med. 2020;18(1):1–15. doi: 10.1186/s12967-019-02189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Gunnes N, Tveito K, Bakken IJ, Ghaderi S, Stoltenberg C, Hornig M, Lipkin WI, Trogstad L, Håberg SE. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine. 2015;33(46):6173–6177. doi: 10.1016/j.vaccine.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11(1):37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH (1992) The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire 67(30):227
- Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhães PV, Kapczinski F, Kauer-Sant'Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. The Lancet Psychiatry. 2015;2(11):1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses. 2020;144:110055. doi: 10.1016/j.mehy.2020.110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani M, Simani L, Karimialavijeh E, Rezaei O, Hajiesmaeili M, Pakdaman H. The role of anxiety and cortisol in outcomes of patients with Covid-19. Basic and Clinical Neuroscience. 2020;11(2):179. doi: 10.32598/bcn.11.covid19.1168.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi M, Taghva A, Goudarzi N, Rah Nejat A. Validity and reliability of persian version of “post-traumatic stress disorder scale” in war veterans. Iranian Journal of War and Public Health. 2016;8(4):243–249. [Google Scholar]
- Shephard RJ. Chronic fatigue syndrome. Sports Med. 2001;31(3):167–194. doi: 10.2165/00007256-200131030-00003. [DOI] [PubMed] [Google Scholar]
- Speer K, Upton D, Semple S, McKune A. Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res. 2018;11:111. doi: 10.2147/JIR.S155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey CM, Louie M, Loeb M, Gold WL, Muller MP, de Jager J, Cameron JI, Tomlinson G, Mazzulli T, Walmsley SL. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167(12):1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- Vyas KJ, Delaney EM, Webb-Murphy JA, Johnston SL. Psychological impact of deploying in support of the US response to Ebola: a systematic review and meta-analysis of past outbreaks. Mil Med. 2016;181(11–12):e1515–e1531. doi: 10.7205/MILMED-D-15-00473. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP (2013) The ptsd checklist for dsm-5 (pcl-5). Scale available from the National Center for PTSD at https://www.ptsd.va.gov10
- Wu KK, Chan SK, Ma TM. Posttraumatic stress after SARS. Emerg Infect Dis. 2005;11(8):1297. doi: 10.3201/eid1108.041083. [DOI] [PMC free article] [PubMed] [Google Scholar]