Abstract
The deleterious outcomes associated with exposure to childhood maltreatment (CM) are well known and may be at least partially mediated by self-harm behaviors. It has been suggested that these self-harm behaviors serve as a means of decreasing negative mood states but the effects of CM on health outcomes may be much more sinister. A wealth of data suggest that CM may lead to experience-dependent changes in neural circuits underlying reward processes; processes associated with many harmful behaviors. The present study examined the relationship between a history of CM and the microstructure of a white matter tract that may be central to reward processes. Healthy adults (N = 122) were assessed with a diffusion tensor imaging (DTI) exam and the Childhood Trauma Questionnaire (CTQ). Probabilistic tractography was used to delineate the accumbofrontal “reward” tract, connecting the orbitofrontal cortex and nucleus accumbens, and measures of white matter microstructure were extracted. We then examined whether variation in CTQ scores were associated with variation in the microstructure of this tract as measured by fractional anisotropy (FA). After accounting for the effects of age and sex, the CTQ total score accounted for approximately 6% of the variance of FA in the accumbofrontal tract (F(3, 121) = 5.74; p = .001). Post hoc analyses indicated that the overall severity of CM, rather than a specific type of maltreatment, drove this result. These findings indicate that CM influences white matter microstructure in a fiber tract that is likely central to reward processes and adds to a growing literature implicating CM in long-term health-related outcomes.
Keywords: Childhood maltreatment, CTQ, DTI, Reward, Accumbofrontal tract, Nucleus accumbens, Orbitofrontal cortex
Introduction
Childhood maltreatment (CM) encompasses all forms of physical and/or emotional ill-treatment, sexual abuse, neglect or exploitation that results in actual or potential harm to the child’s health, survival, development or dignity (WHO 2006). A wealth of evidence demonstrates that exposure to adverse childhood experiences, including CM, significantly increases the risk for a range of poor mental health outcomes (Green et al. 2010; McLaughlin et al. 2010). Data suggest that roughly one-third of all mental disorders worldwide are attributable to exposure to adverse childhood experiences (Kessler et al. 2010; McLaughlin et al. 2012) and this association is stable across prospective and retrospective measurements (Reuben et al. 2016). However, the impact of CM is not isolated to mental health outcomes. Indeed, CM is also recognized as a significant risk factor for a range of poor physical health outcomes including infectious diseases, pain disorders, cancer, and cardiovascular disease (Felitti et al. 1998).
Notably, many of the associations between CM and poor health outcomes may be at least partially mediated by harmful behaviors such as smoking, alcohol or drug abuse, overeating, or careless sexual behaviors (Felitti et al. 1998; Merrick et al. 2017). Although it has been suggested that these harmful behaviors serve as a means of decreasing negative mood states resulting from a history of CM (Dembo et al. 1992; Douglas et al. 2010), the effects of CM on health outcomes are likely much more complicated. Indeed, a wealth of recent data suggests that CM leads to experience-dependent changes in neural circuits and networks that may ultimately lead to dysfunction across a range of reward-based processes (Teicher et al. 2016a); processes that underlie a broad range of harmful behaviors.
For example, childhood maltreatment has been reported to be associated with reductions in size of the striatum (Baker et al. 2013; Dannlowski et al. 2012; Edmiston et al. 2011), alterations in the developmental trajectory of nucleus accumbens (NAcc) volume (Whittle et al. 2016), reduced volume and thickness of the orbitofrontal cortex (OFC) (Chaney et al. 2014; De Brito et al. 2013; Hanson et al. 2010; Hanson et al. 2015c; Kelly et al. 2013; Lim et al. 2018; Thomaes et al. 2010) as well as reduced connectivity of the OFC to other regions such as the amygdala (Hanson et al. 2015c). Data derived from task-based fMRI studies also provide consistent evidence that CM is associated with an attenuated striatal response to anticipation and/or receipt of reward (Boecker et al. 2014; Dillon et al. 2009; Hanson et al. 2015a; Hanson et al. 2015b; Mehta et al. 2010; Takiguchi et al. 2015) and more recent data have demonstrated that these effects may be related to the emergence of behaviors that increase the risk for self-harm. Specifically, a recent prospective study (Birn et al. 2017) found that relative to children who were in the lowest quartile of childhood stress exposure, those exposed to high levels of childhood stress evidenced altered brain activation within the reward network to a monetary incentive delay task 10 years later and this altered pattern of brain activation was associated with real-world measures of risk-taking.
Although several white matter fiber tracts serve to connect brain regions comprising the reward network, to our knowledge no studies have sought to examine structural connectivity of reward regions in relation to CM. One pathway that may be of particular interest is the connection between the OFC and the NAcc, as evidence suggests that the neurodevelopmental trajectory of these regions is related to risk-taking behavior (Galvan et al. 2006). Recently, Karlsgodt et al. (2015b) successfully isolated the white matter tract connecting these regions, the accumbofrontal tract and examined its developmental trajectory in a large cross-sectional sample ranging in age from 8 to 68 years old. This work demonstrated that FA within this tract was highest at around the age of 14 years old suggesting that the development of this tract may be more susceptible to early stress exposure, including CM, than other white matter tracts which tend to peak in late adolescence or adulthood (Peters et al. 2012). Critically, Karlsgodt et al. (2015a) also demonstrated that the accumbofrontal tract was distinct from the uncinate fasciculus, a large white matter tract connecting the OFC and amygdala which has previously been associated with exposure to CM (Hanson et al. 2015c).
The present study sought to examine the relationship between CM and variation in the microstructure of the accumbofrontal tract using diffusion tensor imaging (DTI), which is often used to estimate microstructural characteristics of brain white matter in humans (Assaf and Pasternak 2008). The most commonly used measure derived from DTI is fractional anisotropy (FA), which provides an index of the degree of anisotropic diffusion along a fiber tract and is presumed to reflect different characteristics of axonal microstructure like extent of myelination or axonal size, commonly described together as white matter microstructure or integrity (Mori and Zhang 2006). Given the extent of prior data implicating CM in the structure and function of reward-related brain regions, in the present study we hypothesized that the microstructure of the accumbofrontal ‘reward’ tract would be directly impacted by the history and severity of CM in otherwise healthy adults.
Methods
Participants
The present sample is comprised of 122 healthy adult volunteers (54% male; 57% White, 27% Black, 16% Other race; Mage = 35.72 ± 12.92) recruited from the general population via word of mouth, newspaper and internet advertisements and posted flyers for an NIMH-funded study of subclinical psychopathology (MH086756 to PD). Full demographic details on the sample are provided in Table 1. Participants were excluded from the study if they had a current or past psychiatric disorder, recent illicit substance use (determined by urine toxicology) or any disorder known to affect the brain. Approximately 6% of the healthy participants screened for this project were excluded; most of whom either met for a past affective disorder or tested positive for illicit substance use.
Table 1.
Descriptives for sample included in all analyses (N = 122; 45.90% Female)
| Mean | SD | Range | |
|---|---|---|---|
| Age | 35.72 | 12.92 | 18.89–68.11 |
| PSES | 2.46 | 1.07 | 1–5 |
| Education years | 15.05 | 2.15 | 11–20 |
| CTQ total score | 30.60 | 7.64 | 25–76 |
| Emotional abuse | 6.89 | 2.91 | 5–20 |
| Physical abuse | 6.12 | 2.21 | 5–24 |
| Sexual abuse | 5.23 | 1.11 | 5–12 |
| Emotional neglect | 7.92 | 3.45 | 5–19 |
| Physical neglect | 9.40 | 1.52 | 5–17 |
| Minimization/Denial | 0.66 | 1.01 | 0–3 |
SD: standard deviation; PSES: Parental Socioeconomic Status; CTQ: Childhood Trauma Questionnaire (Bernstein et al. 2003)
Diagnostic assessments
Participants were initially administered the Structured Clinical Interview for the DSM-IV, Non-Patient edition (SCID-I/NP) (First et al. 1995) by Ph.D. or Master’s level psychometricians. Information obtained from the SCID was compiled into a narrative case summary and presented to two senior Zucker Hillside Hospital faculty. Absence of pathology was determined by consensus after the presentation of the narrative case summary and discussion of any relevant symptomatology.
Assessment of childhood maltreatment
To assess the history of childhood maltreatment we utilized the 28-item Childhood Trauma Questionnaire (CTQ) (Bernstein et al. 2003). The CTQ is a Likert-type self-report questionnaire that measures five dimensions of maltreatment during childhood including emotional (EA), physical (PA) and sexual abuse (SA) and emotional (EN) and physical neglect (PN). All items are rated on a 5-point frequency scale in which 1 = never true, 2 = rarely true, 3 = sometimes true, 4 = often true, and 5 = very often true. Each subscale score ranges from 5 (no history of abuse or neglect) to 25 (very extreme history of abuse and neglect) and summing across all 5 subscales provides a total score, ranging from 25 to 125, representing the severity of overall maltreatment experienced by an individual during childhood. Additionally, a 3-item minimization/denial (M/D) score, ranging from 0 (no minimization) to 3 (substantial minimization), is also calculated to detect a response bias that minimizes the extent of childhood trauma experienced.
Parental socioeconomic status (PSES)
Because childhood maltreatment may be more common in low socioeconomic status households (Hussey et al. 2006), we also assessed parental socioeconomic status using the Hollingshead and Redlich Two-Factor Social Position Index (Hollingshead 1975). This index utilizes measures of parental educational attainment and occupational prestige to estimate a social position index (SPI). SPI classifies individuals into one of five potential classes ranging from the highest (Class 1) to lowest (Class 5) socioeconomic classes. In the present study, we utilized the rating for the parent that produced the highest class as the primary measure of PSES.
Imaging
Image acquisition
MR imaging exams were conducted at the North Shore University Medical Center on a General Electric 3 Tesla whole body superconducting imaging system. A radiologist reviewed all scans for gross anatomic pathology that would preclude participation in this study. Scans with significant artifacts were repeated. We minimized movement by stabilizing the head with cushions prior to scanning. Diffusion tensor imaging (DTI) data were acquired using single shot echo planar imaging, and a double spin echo to decrease distortions due to eddy currents, with the following parameters: repetition time = 14,000 ms, echo time = minimum, matrix = 128 × 128, field of view = 240 mm, slice thickness = 2.5 mm, and 51 contiguous axial slices aligned to the anterior and posterior commissures. A total of 36 DTI volumes were obtained for each subject that included 31 volumes with diffusion gradients applied along 31 non-collinear directions (b = 1000 s/mm2) and 5 volumes without diffusion weighting. To address motion, all scans were carefully examined by both the radiologist and the lab technician prior to conducting standard automated corrections. Additionally, prior to calculating diffusion metrics, we calculate head displacement for each subject, estimated as a displacement distance between two DTI volumes; the root mean square deviation is calculated from intra-subject registration (eddy-current and motion correction) parameters, at an r = 40 mm spherical surface using FSL’s rmsdiff tool (http://www.fmrib.ox.ac.uk/fsl/flirt/overview.html). For each subject, the sum of displacement distances between each consecutive pair of 31 DTI volumes (i.e., 30 displacement distances) is computed as the total head displacement for the subject. The distribution of total displacement distances in the sample is then examined and any outliers are removed prior to initiating probabilistic tractography.
Diffusion tensor imaging analysis
Images were processed using the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL version 5.1; Oxford, United Kingdom; http://fsl.fmrib.ox.ac.uk/fsl). Eddy-current distortions and head displacements were corrected through affine registration of the 31 diffusion volumes to the first b0 volume using FSL’s Linear Registration Tool. The b-vector table (i.e., gradient directions) for each participant was then adjusted according to the rotation parameters of this linear correction. Non-brain tissue was removed using FSL’s Brain Extraction Tool. Fractional anisotropy (FA) and diffusivity measures, including axial, radial, and mean diffusivities, were then calculated at each voxel of the brain by fitting a diffusion tensor model to the raw diffusion data using weighted least squares in FSL’s Diffusion Toolbox. FA was chosen as the primary measure for analysis because it has been the most widely used measure in DTI studies. Ancillary analyses investigated axial, radial, and mean diffusivity.
Probabilistic Tractography
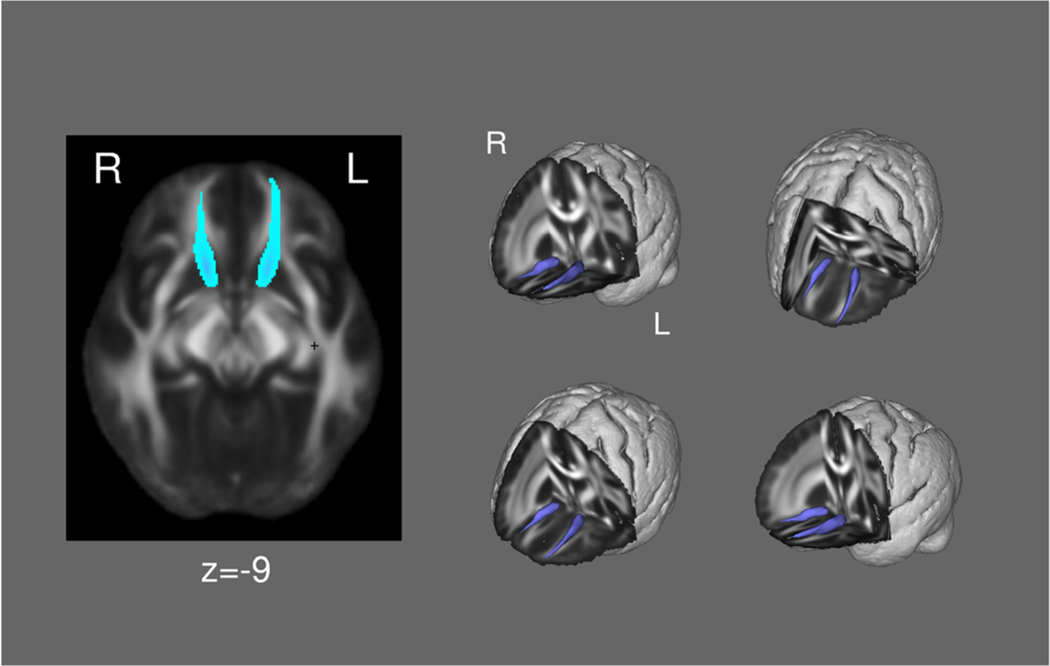
To segment the accumbofrontal tract (Karlsgodt et al. 2015b), within-voxel probability density functions of the principal diffusion direction were estimated using Markov Chain Monte Carlo sampling in FSL’s BEDPOSTX tool (Behrens et al. 2003). A spatial probability density function was then estimated across voxels based on these local probability density functions using FSL’s PROBTRACKX tool, in which 5000 samples were taken for each input voxel with a 0.2 curvature threshold, 0.5-mm step length, and 2000 steps per sample. For each tract, seed and exclusion masks were defined on the MNI152 T1 1-mm template. The exclusion masks included the entire contralateral hemisphere, superior frontal regions, and regions posterior to the striatum, and the seed masks (NAcc and OFC) were defined by Harvard-Oxford atlas. Masks were normalized to each subjects’ diffusion space using FSL’s Linear Registration Tool (Jenkinson and Smith 2001) applying the affine parameters obtained by co-registering the first b0 volume to the MNI152 T1 1-mm template. The resulting bilateral tracts were thresholded at a normalized probability value and visually inspected to confirm successful tracing in each individual subject. The tract is illustrated in Fig. 1. Mean FA and diffusivity measures of the entire tract was then extracted for analysis.
Fig. 1.
The accumbofrontal tract (group mean)
Statistical analysis
To assess the effect of a history of childhood maltreatment on white matter microstructure within the accumbofrontal tract, we utilized a linear regression model. We used a block-wise approach to account for variation in age and sex (block 1), which can impact FA measures in the accumbofrontal tract (Karlsgodt et al. 2015b), as well as parental socioeconomic status (block 2), which is often associated with childhood maltreatment (Hussey et al. 2006). Finally, total CTQ score was entered into the third block of the model and bilateral FA in the accumbofrontal tract was entered as the outcome variable. To further examine the relationship between a history of CM and microstructure of the accumbofrontal tract, bilateral axial, radial, and mean diffusivities were independently examined using a similar block-wise approach.
Results
Mean scores on each CTQ subscale as well as on the total score in the present sample are presented in Table 1. Comparison of males and females across each of the CTQ measures did not reveal any significant sex differences (all p’s > .20) and no significant differences in minimization/denial were observed (p = .38).
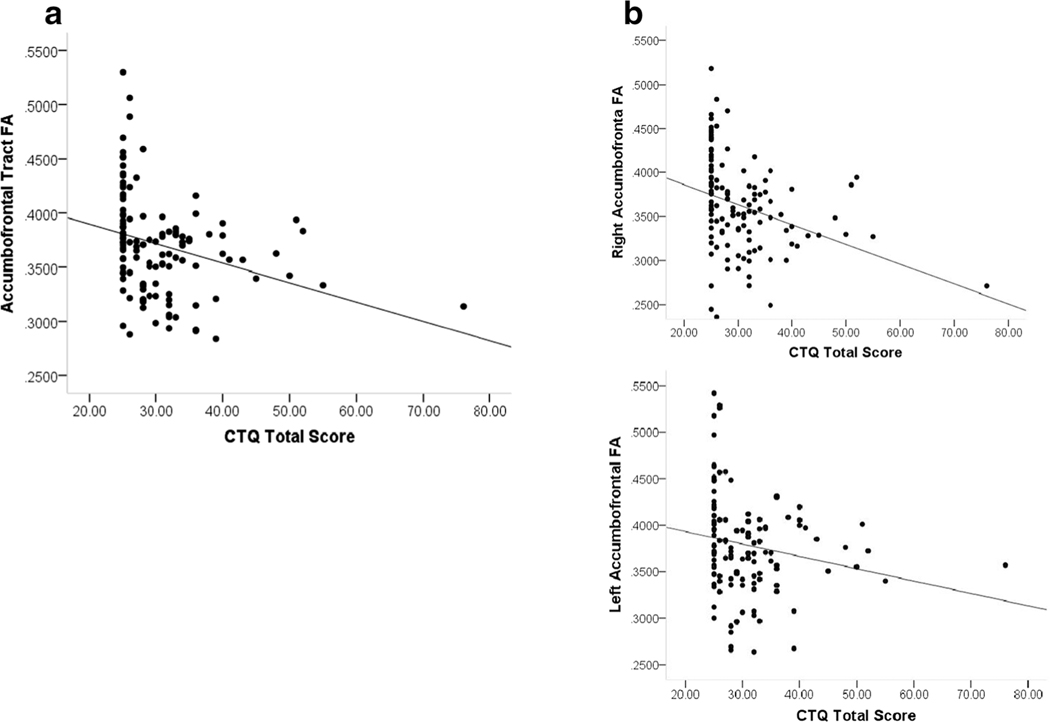
The block-wise regression analysis examining the effect of CM on FA within the accumbofrontal tract revealed a significant negative relationship. Specifically, the first block of the model, which included sex and age as predictors of FA was significant (F(2, 121) = 4.06; p = .02) and accounted for approximately 5% of the variance in FA (r2 = .053) with age (β = −.26; p = .005), but not sex, significant in the model. There was no significant change in r2 from the first to the second block of the model (F(3,120) = 0.31; p = .579) suggesting that parental socioeconomic status did not account for any meaningful variance in FA. The r2 change from the second to the third block was significant (F(1, 118) = 8.59; p = .004). This block of the model, which included CTQ total score as well as all prior predictors, was significant (F(4, 119) = 4.56; p = .002) and accounted for 11% of the variance in accumbofrontal FA (r2 = .108). The effect of age remained significant and there was a significant effect of CTQ total score (β = −.27; p = .004). The relationship between CTQ total score and bilateral accumbofrontal tract FA is shown in Fig. 2a. Notably, when we examined the left and right accumbofrontal tracts separately, there was evidence of a lateralized effect. Specifically, only the analysis examining the right accumbofrontal tract was significant (Final Model F(4,119) = 5.89; p < .001). In this case, the results of the analysis mirrored the results examining bilateral FA indicating that age (β = −.21; p = .02) and CTQ total score (β = −.30; p = .001) were significant predictors of FA in the right accumbofrontal tract. This model accounted for 14% of the variance in FA (r2 = .143). The relationship between CTQ total score and FA in the right and left accumbofrontal tract are shown in Fig. 2b. It should be noted that one of our participants had a severe history of CM as indexed by a score of 76 on the CTQ, which was confirmed by clinical interview. To ensure that this individual was not skewing the results of our analyses, we repeated the analyses leaving that participant out; this did not result in any substantial changes to the results and the findings remained significant.
Fig. 2.
Association between the severity of childhood maltreatment as measured by the Childhood Trauma Questionnaire (CTQ) and a bilateral fractional anisotropy (FA) of the Accumbofrontal Tract and b FA of the Right (top panel) and Left (bottom panel) Accumbofrontal Tract in 122 Healthy Adults
Post hoc analyses, which aimed to examine whether a specific dimension of childhood maltreatment could account for the relationship between CTQ total score and FA, were carried out using the same block-wise structure as the primary analyses except that CTQ total score was replaced by scores on all of the subscales (emotional abuse and neglect, physical abuse and neglect, and sexual abuse). In this analysis, the final model was significant (F(7,119) = 3.22; p = .004), but only age was a significant predictor of FA (β = −.22; p = .02). Finally, to further investigate the relationship between the severity of childhood maltreatment and white matter microstructure in the accumbofrontal tract, axial (AD), radial (RD) and mean (MD) diffusivities were also examined. Although significant final models were produced for both RD (F(4, 119) = 5.32; p = .001) and MD (F(4, 119) = 3.20; p = .016), only age was identified as a significant predictor in these models (RD: β = .36; p < .001; MD: β = .29; p = .002). The model examining AD was not significant (F(4, 119) = 1.82; p = .13).
Discussion
The present findings suggest that CM contributes to variation in the microstructure of white matter in a fiber tract connecting the NAcc and OFC, which is likely central to reward processes. These findings may have substantial implications for elucidating how CM contributes to risk for psychiatric disorders that are characterized by deficits in reward processing. Critically, the sample included in the present study was comprised of healthy adults who were, on average, well beyond the age of risk for the development of serious mental illness and who evidenced no present or past psychiatric disorders. Thus, although none of our participants would be expected to show the type of severe deficits in reward processing that are typically observed in psychiatric disorders, the microstructure of the brain in regions central to reward processing evidenced a significant impact of CM. Given these findings, it seems likely that CM may, at least in part, contribute to vulnerability to psychopathology by disrupting the normal trajectory of neurodevelopment in key nodes of the reward system. Notably, this effect was not specific to a type of maltreatment but rather, to the total severity of CM. Thus, it appears that the type of CM is not as relevant as the overall severity of CM when seeking to elucidate the impact of CM on this fiber tract. This is broadly consistent with prior findings suggesting a cumulative effect of environmental stressors, including CM, on the developing brain (Anda et al. 2006; DeRosse et al. 2014).
It should be noted that when we examined the left and right accumbofrontal tract individually, the effects we observed appeared to be driven primarily by the right tract. This laterality is noteworthy as prior work (Clark et al. 2003) has demonstrated that relative to patients with left frontal lesions, those with right frontal lesions were significantly more likely to make risky decisions on the Iowa Gambling Task. Thus, our findings of an association between lower FA in the accumbofrontal tract and severity of CM, might point to a potential mechanism for the increased risk-behavior often observed in those exposed to maltreatment during childhood and adolescence. Although we did not directly assess reward processing in this sample, the role of this tract in reward processing is supported by prior work demonstrating a significant inverse relationship between FA in a fronto-striatal tract originating in the NAcc with probabilistic reward learning (Samanez-Larkin et al. 2012) as well as findings that variation in frontal white matter encompassed by the accumbofrontal tract, is negatively associated with trait-based reward sensitivity (Bjornebekk et al. 2012). Moreover, both the NAcc and OFC are central nodes in the reward network and likely work in concert to drive reward-related decision-making though their involvement in reward prediction and reward valuation, respectively (Kring and Barch 2014). Finally, recent findings derived from resting state functional MRI demonstrate that the NAcc and the OFC, along with the ventromedial prefrontal cortex comprise a distinct system that is stable in the brain at rest and overlaps with data derived from meta-analyses of task-based reward studies (Huckins et al. 2019).
Although prospective measures of CM have a greater potential for elucidating the role it may play in altering neurodevelopmental trajectories, in the present study we did not prospectively assess CM but rather, relied on data derived from a retrospective, self-report measure. Thus, it could be argued that healthy adults over-report CM and that the present results are inflated by this over-reporting. However, several studies have demonstrated that adults under-report such experiences (Shaffer et al. 2008; Williams 1994) and this is at least partially supported by the minimization/denial scores in the present sample. Specifically, roughly 40% of our sample showed evidence of minimization and denial (M/D score > 0). This might suggest that the effect of CM on the microstructure of the accumbofrontal tract detected in the present study is attenuated by under-reporting (MacDonald et al. 2016).
Moreover, the retrospective assessment used in the current study did not take into consideration the timing of the maltreatment being reported, which may be critical for elucidating the impact of CM on neurodevelopment. For example, data suggest that the developmental peak of this tract occurs earlier in males relative to females and thus, exposure to CM at any given age may differentially impact this tract in males and females. Indeed, post-hoc examination of sex differences in the present study indicated that while the effect of CM on FA was highly significant in males (p = .001), it was only nominally significant in females (p = .10). Given that males may experience CM earlier than females (Stevens et al. 2018), it seems plausible that the sex difference we observed is related to sex differences in the timing of CM exposure. Unfortunately, the CTQ does not provide data related to the timing of CM. However, more recent measures such as the Maltreatment and Abuse Chronology of Exposure (MACE) Scale (Teicher and Parigger 2015) may allow for further examination of how the timing of CM might impact the development of the accumbofrontal tract. It should be noted that several alternative explanations may also account for the sex difference observed. First, it is possible that the brains of males are simply more susceptible to the effects of CM. This would be consistent with our prior findings that a history of CM is associated with reduced hippocampus volume in males, but not in females (Samplin et al. 2013). However, it is also possible that because females exposed to CM are more likely to develop a psychiatric disorder (Zlotnick et al. 2008), the sample of females in the current study is inherently biased.
Finally, although FA is thought to index white matter microstructure, reflecting both myelination and organization of fiber tracts that form the basis of inter-regional brain connection (Assaf and Pasternak 2008), it is by nature an inferential measure. Nevertheless, the present findings are consistent with data derived from a variety of brain imaging studies, including structural as well as task-based and resting-state fMRI, demonstrating that CM significantly impacts brain development (Teicher et al. 2016b). Thus, these findings add to a growing literature implicating CM in altering the developmental trajectory and function of brain regions that play a critical role in reward-related processes and may provide further insight into the mechanism underlying the association between CM and poor health outcomes.
Acknowledgements
This work was supported in part by grants from the National Institute of Mental Health to Dr. DeRosse (MH086756) and Dr. Karlsgodt (MH101506) and the NSLIJ Research Institute General Clinical Research Center (M01 RR018535).
Funding information This study was funded by grants from the National Institute of Mental Health to Dr. DeRosse (MH086756) and Dr. Karlsgodt (MH101506) and the NSLIJ Research Institute General Clinical Research Center (M01 RR018535).
Footnotes
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest Dr.’s DeRosse, Ikuta, Karlsgodt and Szeszko report no competing interests. Dr. Malhotra has served as consultant or speaker for Bristol-Myers Squibb, Astra Zeneca, Vanda Pharmaceuticals and Clinical Data, Inc., and has received research support from Pfizer, Janssen Pharmaceuticals, Bristol-Myers Squibb, and Eli Lilly.
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, & Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, & Pasternak O. (2008). Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. Journal of Molecular Neuroscience, 34(1), 51–61. [DOI] [PubMed] [Google Scholar]
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, & Paul RH (2013). Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging and Behavior, 7(2), 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich M, Smith S, Wheeler-Kingshott C, Boulby P, Barker G, Sillery E, Sheehan K, & Ciccarelli O. (2003). Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6(7), 750–757. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, & Desmond D. (2003). Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse & Neglect, 27(2), 169–190. [DOI] [PubMed] [Google Scholar]
- Birn RM, Roeber BJ, Pollak SD (2017). Early childhood stress exposure, reward pathways, and adult decision making. Proceedings of the National Academy of Sciences, 201708791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornebekk A, Westlye LT, Fjell AM, Grydeland H, & Walhovd KB (2012). Social reward dependence and brain white matter microstructure. Cerebral cortex (New York, N.Y. : 1991), 22(11), 2672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Baumeister S, Meyer-Lindenberg A, Banaschewski T, & Brandeis D. (2014). Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS One, 9(8), e104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney A, Carballedo A, Amico F, Fagan A, Skokauskas N, Meaney J, & Frodl T. (2014). Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. Journal of psychiatry & neuroscience : JPN, 39(1), 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, & Robbins TW (2003). The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia, 41(11), 1474–1483. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, & Kugel H. (2012). Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, & McCrory EJ (2013). Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54(1), 105–112. [DOI] [PubMed] [Google Scholar]
- Dembo R, Williams L, Wothke W, Schmeidler J, & Brown CH (1992). The role of family factors, physical abuse, and sexual victimization experiences in high-risk youths’ alcohol and other drug use and delinquency: A longitudinal model. Violence and Victims, 7(3), 245–266. [PubMed] [Google Scholar]
- DeRosse P, Ikuta T, Peters BD, Karlsgodt KH, Szeszko PR, & Malhotra AK (2014). Adding insult to injury: Childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry Research, 224(3), 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, & Pizzagalli DA (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry, 66(3), 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas KR, Chan G, Gelernter J, Arias AJ, Anton RF, Weiss RD, Brady K, Poling J, Farrer L, & Kranzler HR (2010). Adverse childhood events as risk factors for substance dependence: Partial mediation by mood and anxiety disorders. Addictive Behaviors, 35(1), 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, & Blumberg HP (2011). Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics & Adolescent Medicine, 165(12), 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, & Gibbon M. (1995). Structured clinical interview for DSM-IV-non-patient edition. New York: New York State Psychiatric Institute. [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, & Casey B. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience, 26(25), 6885–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, & Pollak SD (2010). Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30(22), 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Albert D, Iselin A-MR, Carre JM, Dodge KA, & Hariri AR (2015a). Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Social Cognitive and Affective Neuroscience, 11(3), 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, & Williamson DE (2015b). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78(9), 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD, & Hariri AR (2015c). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Development and Psychopathology, 27(4 Pt 2), 1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, 1975. Four factor index of social status. [Google Scholar]
- Huckins JF, Adeyemo B, Miezin FM, Power JD, Gordon EM, Laumann TO, Heatherton TF, Petersen SE, & Kelley WM (2019). Reward-related regions form a preferentially coupled system at rest. Human Brain Mapping, 40(2), 361–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey JM, Chang JJ, & Kotch JB (2006). Child maltreatment in the United States: Prevalence, risk factors, and adolescent health consequences. Pediatrics, 118(3), 933–942. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Bato AA, Blair MA, DeRosse P, Szeszko PR, & Malhotra AK (2015a). White matter microstructure in the executive network associated with aggression in healthy adolescents and young adults. Social Cognitive and Affective Neuroscience, 10(9), 1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, John M, Ikuta T, Rigoard P, Peters BD, Derosse P, Malhotra AK, & Szeszko PR (2015b). The accumbofrontal tract: Diffusion tensor imaging characterization and developmental change from childhood to adulthood. Human Brain Mapping, 36(12), 4954–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, & McCrory EJ (2013). Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: Neural markers of vulnerability? Biological Psychiatry, 74(11), 845–852. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, & Angermeyer M. (2010). Childhood adversities and adult psychopathology in the WHO world mental health surveys. The British Journal of Psychiatry, 197(5), 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, & Barch DM (2014). The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. European Neuropsychopharmacology, 24(5), 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Hart H, Mehta M, Worker A, Simmons A, Mirza K, & Rubia K. (2018). Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychological Medicine, 48(6), 1034–1046. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Thomas ML, Sciolla AF, Schneider B, Pappas K, Bleijenberg G, Bohus M, Bekh B, Carpenter L, Carr A, Dannlowski U, Dorahy M, Fahlke C, Finzi-Dottan R, Karu T, Gerdner A, Glaesmer H, Grabe HJ, Heins M, Kenny DT, Kim D, Knoop H, Lobbestael J, Lochner C, Lauritzen G, Ravndal E, Riggs S, Sar V, Schäfer I, Schlosser N, Schwandt ML, Stein MB, Subic-Wrana C, Vogel M, & Wingenfeld K. (2016). Minimization of childhood maltreatment is common and consequential: Results from a large, multinational sample using the childhood trauma questionnaire. PLoS One, 11(1), e0146058–e0146058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green J, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication ii: Associations with persistence of dsm-iv disorders. Archives of General Psychiatry, 67(2), 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69(11), 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, & Sonuga-Barke E. (2010). Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience, 22(10), 2316–2325. [DOI] [PubMed] [Google Scholar]
- Merrick MT, Ports KA, Ford DC, Afifi TO, Gershoff ET, & Grogan-Kaylor A. (2017). Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse & Neglect, 69, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, & Zhang J. (2006). Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron, 51(5), 527–539. [DOI] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, & Malhotra AK (2012). White matter development in adolescence: Diffusion tensor imaging and meta-analytic results. Schizophrenia Bulletin, 38(6), 1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, Hogan S, Ramrakha S, Poulton R, & Danese A. (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57(10), 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Levens SM, Perry LM, Dougherty RF, & Knutson B. (2012). Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. Journal of Neuroscience, 32(15), 5333–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samplin E, Ikuta T, Malhotra AK, Szeszko PR, & Derosse P. (2013). Sex differences in resilience to childhood maltreatment: Effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. Journal of Psychiatric Research, 47(9), 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer A, Huston L, & Egeland B. (2008). Identification of child maltreatment using prospective and self-report methodologies: A comparison of maltreatment incidence and relation to later psychopathology. Child Abuse & Neglect, 32(7), 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, van Rooij SJH, & Jovanovic T. (2018). Developmental contributors to trauma response: The importance of sensitive periods, early environment, and sex differences. Current Topics in Behavioral Neurosciences, 38, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi S, Fujisawa TX, Mizushima S, Saito DN, Okamoto Y, Shimada K, Koizumi M, Kumazaki H, Jung M, Kosaka H, Hiratani M, Ohshima Y, Teicher MH, & Tomoda A. (2015). Ventral striatum dysfunction in children and adolescents with reactive attachment disorder: Functional MRI study. BJPsych open, 1(2), 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Teicher MH, & Parigger A. (2015). The ‘maltreatment and abuse chronology of exposure’(MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One, 10(2), e0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K. (2016a). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K. (2016b). The effects of childhood maltreatment on brain structure, function and connectivity. Nature reviews. Neuroscience, 17(10), 652–666. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, & Veltman DJ (2010). Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. Journal of Clinical Psychiatry, 71(12), 1636–1644. [DOI] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Dennison M, Schwartz O, Simmons JG, Sheeber L, & Allen NB (2016). Observed measures of negative parenting predict brain development during adolescence. PLoS One, 11(1), e0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO T.I.S.f.P.o.A.N.a. (2006). Preventing child maltreatment: A guide to taking action and generating evidence. WHO Press, France. [Google Scholar]
- Williams LM (1994). Recall of childhood trauma: A prospective study of women’s memories of child sexual abuse. Journal of Consulting and Clinical Psychology, 62(6), 1167–1176. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Johnson J, Kohn R, Vicente B, Rioseco P, & Saldivia S. (2008). Childhood trauma, trauma in adulthood, and psychiatric diagnoses: Results from a community sample. Comprehensive Psychiatry, 49(2), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]