Abstract
The adaptive immune response relies on specific apoptotic programs to maintain homeostasis. Conventional effector T cell (Tcon) expansion is constrained by both forkhead box P3 (FOXP3)+-regulatory T cells (Tregs) and restimulation-induced cell death (RICD), a propriocidal apoptosis pathway triggered by repeated stimulation through the T-cell receptor (TCR). Constitutive FOXP3 expression protects Tregs from RICD by suppressing SLAM-associated protein (SAP), a key adaptor protein that amplifies TCR signaling strength. The role of transient FOXP3 induction in activated human CD4 and CD8 Tcons remains unresolved, but its expression is inversely correlated with acquired RICD sensitivity. Here, we describe a novel role for FOXP3 in protecting human Tcons from premature RICD during expansion. Unlike FOXP3-mediated protection from RICD in Tregs, FOXP3 protects Tcons through a distinct mechanism requiring de novo transcription that does not require SAP suppression. Transcriptome profiling and functional analyses of expanding Tcons revealed that FOXP3 enhances expression of the SLAM family receptor CD48, which in turn sustains basal autophagy and suppresses pro-apoptotic p53 signaling. Both CD48 and FOXP3 expression reduced p53 accumulation upon TCR restimulation. Furthermore, silencing FOXP3 expression or blocking CD48 decreased the mitochondrial membrane potential in expanding Tcons with a concomitant reduction in basal autophagy. Our findings suggest that FOXP3 governs a distinct transcriptional program in early-stage effector Tcons that maintains RICD resistance via CD48-dependent protective autophagy and p53 suppression.
Keywords: conventional T cells, RICD, FOXP3, autophagy, CD48
Subject terms: Immune cell death, Cell death and immune response, Macroautophagy
Introduction
Rapid clonal T-cell expansion must be restrained to ensure that the adaptive immune response is sufficient for pathogen clearance without overtly damaging the host. To maintain homeostasis, cytokine withdrawal-induced cell death (CWID) and restimulation-induced cell death (RICD) are critical apoptosis mechanisms that balance robust T-cell expansion by efficiently restraining it and by inducing subsequent memory T-cell formation.1,2 RICD is especially important for preventing excessive T-cell responses; the massive overaccumulation of RICD-resistant effector T cells in X-linked lymphoproliferative disorder (XLP-1) patients causes life-threatening immunopathology during Epstein–Barr virus infection.3–5 Although unrestrained T-cell expansion is dangerous, a robust effector T-cell response remains critical for controlling certain infections.6 Hence, understanding the factors that modify RICD sensitivity throughout the entire T-cell response may reveal intriguing strategies for increasing or decreasing effector T-cell numbers as needed.
Activated T cells remain relatively resistant to RICD during the initial rounds of clonal expansion, whereas RICD susceptibility emerges in differentiated effector T cells over several days. Apoptosis is triggered through avid T-cell receptor (TCR) restimulation7,8 as effectors consume interleukin-2 (IL-2)9 and cycle through S-phase.10 Although changes in FAS-dependent apoptotic signaling help to render effector T cells vulnerable to RICD,11,12 it remains relatively unclear how RICD resistance is enforced in early expanding T cells.
SLAM-associated protein (SAP) is an adaptor that partners with the signaling lymphocyte activation molecule (SLAM) family receptor NK, T-, B-cell antigen (NTB-A) to amplify the TCR signal strength and induce RICD.13,14 XLP-1 patients harbor null mutations in the SAP-encoding gene SH2D1A, which results in attenuated TCR signaling and impaired RICD.14 Remarkably, inhibition of diacylglycerol kinase alpha (DGKα), an enzyme that significantly modulates TCR signaling in the absence of SAP, can restore RICD in XLP-1 patient T cells and reduce effector CD8 T-cell accumulation to limit immunopathology.15 These findings collectively suggest that SAP upregulation in activated effector T cells16,17 is a critical molecular determinant that distinguishes TCR restimulation signaling for apoptosis.
Forkhead box P3 (FOXP3)+-regulatory CD4 T cells (Tregs) express low levels of SAP, and are extremely resistant to RICD compared with conventional T cells (Tcons; i.e., non-Tregs),18 in part because of TGF-β1 signaling.19,20 FOXP3 induces RICD resistance in activated human Tregs,18,21 and we recently showed that FOXP3 directly represses SAP transcription to confer resistance.18 Human Tcons also express FOXP3 transiently after TCR activation,22 but FOXP3 function in this context remains mysterious, as it does not confer a Treg-like suppressive phenotype.23,24 FOXP3 may curb proliferation and cytokine production in human Tcons,25,26 but studies have shown inconsistent results.27,28 Therefore, it is likely that transient FOXP3 expression serves additional, unappreciated functions in Tcons. Intriguingly, transient FOXP3 induction in activated Tcons is correlated inversely with RICD sensitization. We therefore hypothesized that transient FOXP3 expression in activated Tcons helps to protect expanding T cells from RICD by suppressing SAP expression.
In this study, we elucidated a novel intrinsic mechanism involved in T-cell homeostasis by which FOXP3 expression protects newly activated CD4 and CD8 Tcons from premature RICD during clonal expansion. Unlike Tregs, FOXP3 expression protected Tcons through a unique, SAP-independent mechanism involving protective autophagy and the suppression of p53 signaling through the glycosylphosphatidylinositol (GPI)-anchored SLAM family receptor CD48. These findings illuminate important differences in the factors that govern RICD sensitivity in newly activated vs. late-stage effector T cells, indicating that FOXP3 can shield both Tregs and Tcons from RICD via distinct mechanisms.
Results
Induced FOXP3 expression protects newly activated T cells from RICD
TCR-dependent FOXP3 expression in human Tcons varies in different in vitro activation conditions.22,24,29 Therefore, we first measured FOXP3 expression in isolated CD4 and CD8 T cells (Supplementary Fig. 1A) from multiple healthy blood donors after activation with CD2/3/28 beads. FOXP3 was strongly induced in both CD4 and CD8 T cells, 1–2 days post activation (Supplementary Fig. 1B). Using intracellular flow cytometry, we observed that FOXP3 was prominently expressed in a subset of cells (Supplementary Fig. 1C), which is consistent with other observations.24 To exclude the possibility that the FOXP3hi population originated from contaminating Tregs in our CD4 Tcon population, we purified naive CD4+CD25− T cells and assessed FOXP3 expression using the same activation conditions. Indeed, FOXP3 induction in naive T cells was similar on day 4 post activation (Supplementary Fig. 1D).
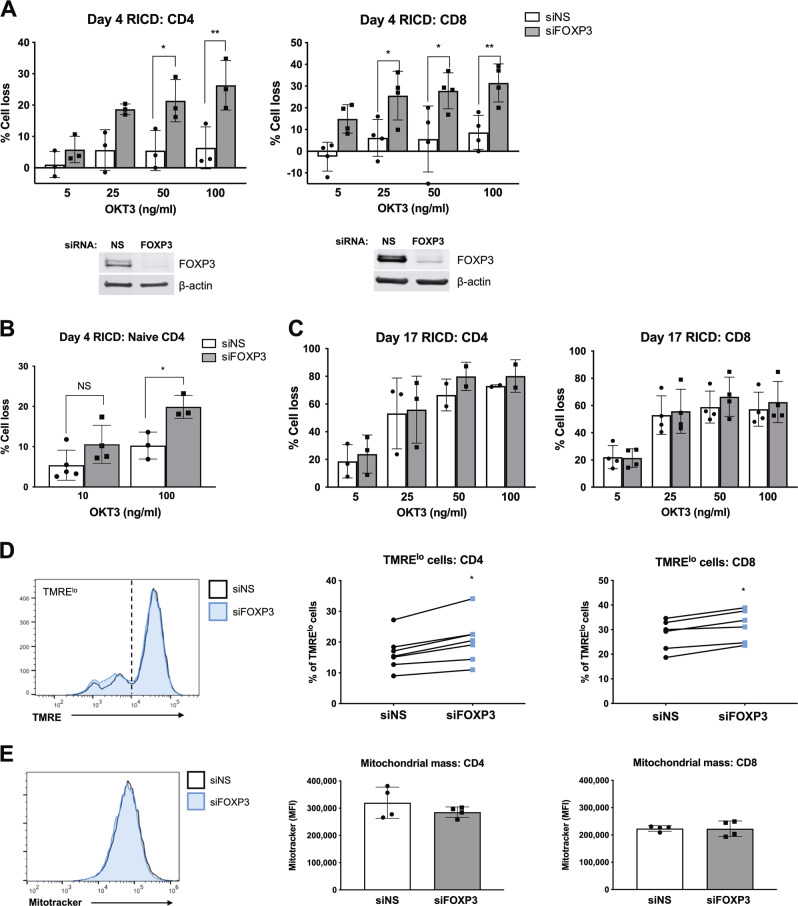
In human T cells, FOXP3 expression is induced upon TCR activation, and subsequently diminishes as T-cell expansion slows and RICD sensitivity peaks. To determine whether activation-induced FOXP3 expression protects expanding T cells from RICD, we performed siRNA transfections of purified CD4 and CD8 T cells to knockdown (KD) FOXP3 expression during the initial activation. Activated T cells were then restimulated with anti-CD3 antibody (OKT3) on day 4 post activation, and RICD was measured by propidium iodide (PI) staining 24 h later.30 Despite variable FOXP3 expression among different human donors (Supplementary Fig. 1C), FOXP3 KD significantly increased RICD sensitivity in both CD4 and CD8 T cells (Fig. 1a). The enhancement of RICD in CD4 T cells was not due to the silencing of FOXP3 expression in residual contaminating Tregs, because naive FOXP3 KD CD4 T cells demonstrated a comparable increase in RICD sensitivity on day 4 post activation (Fig. 1b). In contrast, FOXP3 KD did not impact RICD on day 17 post activation, when effector Tcons had fully expanded in vitro and reached peak RICD sensitivity (Fig. 1c); this correlated with markedly decreased levels of FOXP3 expression (Supplementary Fig. 1E). These data suggest that transient FOXP3 expression in activated Tcons helps to protect them from premature RICD during early expansion.
Fig. 1.
FOXP3 protects expanding T cells from RICD. a Purified CD4 and CD8 T cells were electroporated with nonspecific (NS) scrambled siRNA or FOXP3-specific siRNA. The activated cells were then restimulated with OKT3 on day 4, and RICD was measured by PI staining and flow cytometry. FOXP3 knockdown was verified by western blotting. Statistical significance was tested by one-way ANOVA with Sidak’s multiple comparisons test. CD4: *p = 0.017, **p = 0.003. CD8: *p = 0.031, *p = 0.012, **p = 0.009. Each symbol represents a different human donor. b Naive CD4 T cells were purified from 3–5 healthy donors and electroporated with siRNA as in (a). Cells were restimulated with OKT3 on day 4 post activation to induce RICD. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test, *p = 0.021. c Activated CD4 and CD8 T cells were transfected with siRNAs on day 13 post activation and restimulated prior to RICD assays on day 17. Statistical significance was assessed by one-way ANOVA; all tests showed no significance. d Activated T cells on day 4 post activation were stained with tetramethylrhodamine, ethyl ester, and perchlorate (TMRE), and assessed by flow cytometry. The lines indicate connected data from individual donors. Statistical significance was determined with a paired Wilcoxon test. *p < 0.05. e Activated T cells on day 4 post activation were stained with MitoTracker Green and assessed by flow cytometry. MFI: mean fluorescence intensity
Some have suggested that the altered cellular localization of FOXP3 may explain why it cannot confer suppressive function in Tcons under physiological conditions.31 In both expanding T cells (day 4) and late-stage effectors (day 17), we detected FOXP3 exclusively in the nucleus, where it was unaffected by TCR restimulation (Supplementary Fig. 1E). These data suggest that FOXP3 likely acts as a functional transcription factor in activated Tcons. Intriguingly, FOXP3 KD resulted in a higher percentage of T cells with a small but significant reduction in the mitochondrial outer membrane potential (MOMP) (Fig. 1d), but no difference in the overall mitochondrial mass (Fig. 1e). Collectively, these results connect nuclear FOXP3 expression to RICD resistance in human Tcons and suggest that FOXP3 expression aids in maintaining healthy, polarized mitochondria.
FOXP3 knockdown does not impact T-cell proliferation or SAP expression
To investigate how FOXP3 mediates RICD resistance in early Tcons, we first assessed whether FOXP3 KD affected T-cell proliferation. Although conflicting reports have implied that FOXP3 may restrain T-cell proliferation under certain conditions,25,27 we did not observe any differences between control and FOXP3 KD cells using either 5-ethynyl-2′-deoxyuridine (EdU) labeling or carboxyfluorescein succinimidyl ester (CFSE) dilution (Supplementary Fig. 2A–C). Moreover, silencing FOXP3 expression did not alter cell cycle status (Supplementary Fig. 2D), which influences the likelihood of effector T cells to die by RICD.10
We next asked whether traditional pro-apoptotic effector molecules could be responsible for increased RICD in FOXP3 KD cells. FAS ligand (FASL) upregulation is intimately associated with RICD in late-stage effector T cells.32,33 Intriguingly, TCR restimulation in both CD4 and CD8 T cells induced robust FASL expression and cleavage at the plasma membrane, as indicated by the amount of cytoplasmic N-terminal fragment (NTF), which was slightly reduced with FOXP3 KD (Supplementary Fig. 2E). However, FAS receptor engagement in newly activated T cells generally results in non-apoptotic signals important for cell differentiation.34 We also noted no significant differences between control and FOXP3 KD cells in terms of the expression of BIM (Supplementary Fig. 2E, F), a pro-apoptotic BCL2 family protein that facilitates RICD in late-stage effectors after TCR-induced upregulation.35 Regardless of FOXP3 status, BIM expression varied considerably between donors before and after TCR restimulation (Supplementary Fig. 2F).
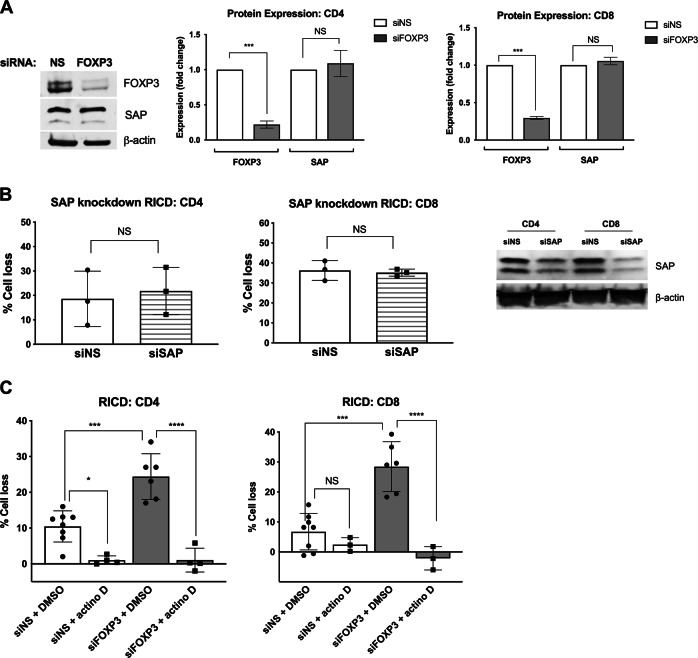
We initially hypothesized that FOXP3 protects expanding Tcons from RICD by suppressing SAP expression, similar to Tregs.18 Surprisingly, FOXP3 KD cells showed no increase in SAP expression relative to controls (Fig. 2a). Although silencing SAP decreased RICD sensitivity in late-stage effectors,3,14 we found that SAP KD was not sufficient to protect T cells from RICD at day 4 post activation (Fig. 2b), suggesting that RICD susceptibility is regulated differentially in the early and late stages of the effector T-cell response.
Fig. 2.
RICD in expanding T cells is SAP-independent, but requires de novo transcription. a Day 4 FOXP3-KD cells were analyzed for FOXP3 and SAP expression by western blotting (left panel). Protein expression was quantified after normalization to β-actin (right panels). The data represent three independent experiments. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test. CD4: ***p < 0.001, NS not significant. CD8: ***p < 0.001, NS not significant. b Purified CD4 and CD8 T cells were electroporated with SAP-specific siRNA or a nonspecific (NS) control. Activated T cells were restimulated on day 4 with 100 ng/mL OKT3, and RICD was assessed by PI staining and flow cytometry. Gene knockdown was verified by western blotting (right). Statistical significance was assessed with paired t tests, NS not significant. c Day 4 KD T cells were pretreated with 100 ng/mL actinomycin D (actino D) or a DMSO solvent control for 30 min before restimulation with 100 ng/mL OKT3. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test. CD4: *p = 0.0198, ***p = 0.001, ****p < 0.0001. CD8: ***p = 0.001, ****p < 0.0001
RICD in expanding T cells is dependent on de novo transcription
FOXP3 nuclear localization in expanding Tcons (Supplementary Fig. 1E) implied that FOXP3 can act as a transcriptional activator or repressor. We determined whether FOXP3-mediated RICD suppression was dependent on transcriptional changes by pretreating control or FOXP3 KD cells with actinomycin D to inhibit de novo RNA transcription during TCR restimulation. Surprisingly, actinomycin D treatment completely suppressed RICD regardless of FOXP3 status (Fig. 2c). RICD sensitivity in expanding Tcons was therefore dependent on de novo transcription, unlike that in late-stage effector T cells, which readily succumb to RICD via both preformed apoptotic molecules and de novo transcription.36
FOXP3 elevates CD48 expression in activated T cells
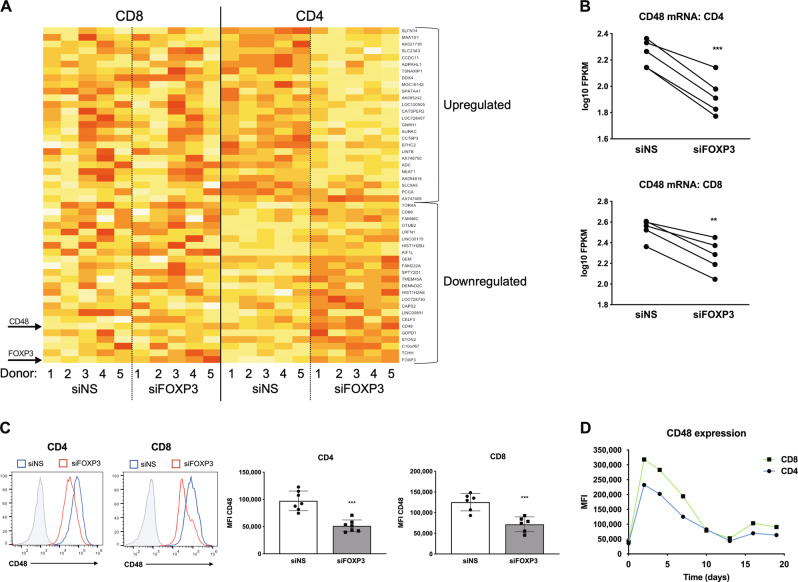
Based on these results, we used an unbiased approach to ascertain the global transcriptional changes associated with FOXP3 expression in T cells and identify the candidate genes involved in FOXP3-mediated RICD protection. Nonspecific (NS) siRNA control and FOXP3 KD CD4 and CD8 T cells from five healthy human donors were profiled by mRNA sequencing (RNA-Seq). Comparative gene expression analysis revealed 110 differentially expressed transcripts in both CD4 and CD8 T cells in the NS and FOXP3 KD treatment groups (Fig. 3a), with FOXP3 as the most greatly affected transcript (2.93-fold decrease in the FPKM value in FOXP3-KD CD4 T cells). Interestingly, the highest abundance transcript that was significantly decreased by FOXP3 KD in every anonymous human donor sample profiled was CD48 (SLAMF2), which encodes a SLAM family-related receptor (Fig. 3b).37 We verified that CD48 protein expression was significantly decreased in FOXP3 KD T cells (Fig. 3c), suggesting that FOXP3 upregulates CD48 expression in Tcons after TCR activation. Although typically thought of as a repressor, FOXP3 also activates gene transcription in certain contexts.38 Indeed, chromatin immunoprecipitation (ChIP) assays identified CD48 as a target gene for FOXP3 binding in human Tregs,39 and CD48 protein expression was elevated in FOXP3+ Tregs.40 Mirroring that of FOXP3, CD48 expression was rapidly induced in activated T cells and peaked on days 4–5 before declining over time (Fig. 3d). Although CD48 expression was not strictly dependent on FOXP3 (Fig. 3c), these data show that FOXP3 enhances CD48 expression in activated Tcons during the early expansion window, which is characterized by relative RICD resistance.
Fig. 3.
CD48 expression is impaired in FOXP3-KD T cells. a Activated CD4 and CD8 KD T cells on day 4 post activation (NS vs. FOXP3) from five anonymous healthy blood donors were subjected to transcriptome profiling by RNA-seq. Normalized mean-centered log base 10 FPKM values were used for the heatmap visualization of the expression levels (the yellow to red color scale represents higher to lower expression levels, respectively). Arrows indicate CD48 and FOXP3. b CD48 transcript levels (fragments per kilobase of transcript per million reads (FPKM)) in each donor are displayed. Statistical significance was determined by a paired t test. CD4: ***p = 0.0008. CD8: **p = 0.0025. c Cell surface expression of CD48 on NS (blue) vs. FOXP3-KD (red) T cells was measured by flow cytometry on day 4 post activation. Isotype control Ab staining is shown in light gray (left). CD48 expression was quantified according to the mean fluorescence intensity (MFI) in multiple donors (right panels). Statistical significance was determined by unpaired t tests. CD4: ***p = 0.0001; CD8: ***p = 0.0008. d CD48 expression (MFI) was quantified over time on purified CD4 and CD8 T cells before and after activation. The data are representative of three separate experiments using different donors
CD48 protects activated CD4 and CD8 T cells from RICD
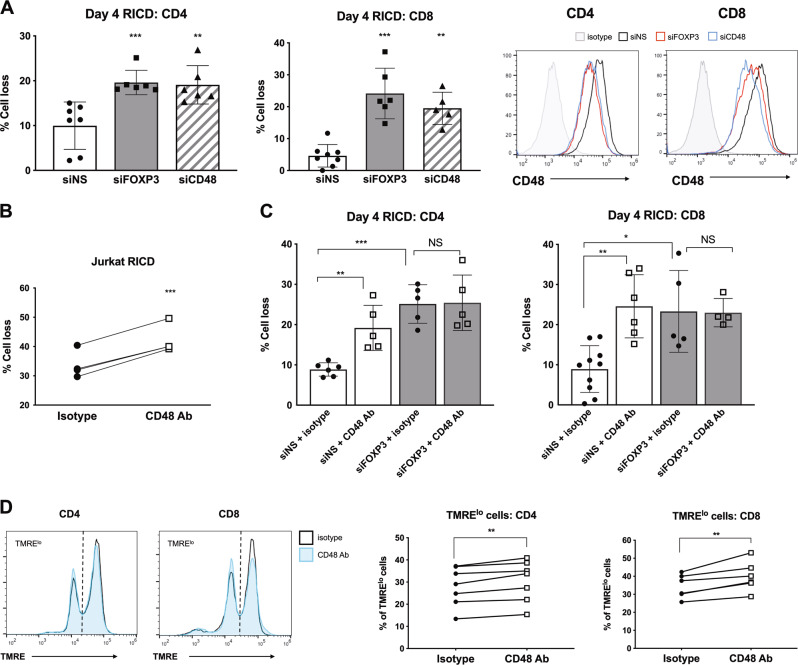
To determine if CD48 helped to enforce FOXP3-dependent RICD resistance in early effectors, we first knocked down CD48 in CD4 and CD8 T cells during activation and measured RICD. Similar to FOXP3-KD T cells, CD48-KD T cells exhibited an analogous increase in RICD sensitivity (Fig. 4a). Moreover, pretreatment with a CD48-blocking antibody (Ab) also enhanced RICD in both Jurkat (Fig. 4b) and primary T cells (Fig. 4c). CD48 blockade also slightly reduced TMRE staining in primary T cells at day 4 post activation (Fig. 4d), which was consistent with observations in FOXP3 KD cells (Fig. 1d). Importantly, CD48 blockade did not further boost RICD levels in FOXP3-KD cells (Fig. 4c), implying that FOXP3 exerts RICD suppression through CD48 upregulation in early activated Tcons.
Fig. 4.
CD48 expression protects expanding T cells from RICD. a Purified CD4 and CD8 T cells were electroporated with siRNA against FOXP3, CD48, or a nonspecific (NS) control. Activated T cells were restimulated with 100 ng/mL OKT3 on day 4 for RICD assays. Statistical significance was determined by one-way ANOVA with Dunnett’s multiple comparisons test. CD4: ***p = 0.0019, **p = 0.003. CD8: ***p = 0.001, **p = 0.0005. Gene knockdown was verified by flow cytometry (right). b Jurkat T cells were incubated with 10 μg/mL anti-CD48-blocking Ab or an isotype control Ab for 1 h before restimulation with 500 ng/mL OKT3 to induce RICD. Statistical significance was determined by a paired t test, ***p = 0.0003. c Day 4 activated CD4 and CD8 T cells were incubated with 10 μg/mL anti-CD48-blocking Ab or an isotype control Ab for 6 h and then stained with TMRE. The data from independent donors are connected by lines (right). Statistical significance was determined by paired t tests. CD4: **p = 0.0033. CD8: **p = 0.0057. d NS control or FOXP3 KD cells were incubated with 10 μg/mL anti-CD48-blocking Ab or an isotype control Ab for 1 h and then restimulated with 100 ng/mL OKT3 to induce RICD. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test. CD4: **p = 0.0097, ***p = 0.0001. CD8: **p = 0.0011, *p = 0.0042
CD48 ligand interactions have variable effects on RICD sensitivity
CD48 is a GPI-anchored surface receptor with two known binding partners: CD2 and 2B4.41 Only CD48 expression was reduced by FOXP3 KD, whereas CD2 and 2B4 expression remained unaffected (Supplementary Fig. 3A). To investigate whether CD48-mediated RICD protection was dependent on specific ligand interactions, we employed CD2-specific or 2B4-specific blocking antibodies in RICD assays at day 4 post activation. Although CD48 blockade consistently increased RICD (relative to isotype control Ab), CD2 and 2B4 blockade did not significantly alter RICD sensitivity (Supplementary Fig. 3B). We noted highly variable effects among the different donors; for example, ~50% showed increased RICD sensitivity upon 2B4 blockade, whereas other donors showed no change. Collectively, this variability could reflect the complex, bimodal nature of CD2 and 2B4 signaling,42 which may vary as a result of CD48 abundance, ligand-independent signaling, or unknown CD48 interaction partners. Another source of donor variability could be the differences in the naive vs. memory T-cell populations at the time of T-cell isolation and activation. Based on the strong and consistent effects, we observed in multiple human donors resulting from CD48 KD- or Ab-mediated blockade, we focused on CD48 disruption alone to further delineate the mechanisms involved in RICD protection.
FOXP3 and CD48 expression in memory vs. naive T cells
Another reason for donor variability in the RICD assays could be related to the different proportions of naive vs. memory T cells in the experiments. To further characterize FOXP3 and CD48 expression with these cell subsets in mind, we sorted naïve and memory T cells from multiple donors (Supplementary Fig. 4A). Unexpectedly, FOXP3 expression was consistently higher in memory T cells on day 4 post activation compared with that in naive T cells (Supplementary Fig. 4B). However, the surface expression of CD48 was lower on memory T cells (Supplementary Fig. 4C). Furthermore, the RICD sensitivity of activated memory T cells was considerably higher than that of naive cells (Supplementary Fig. 4D), which is consistent with the findings of a previous report.43 Collectively, these data imply that FOXP3 and CD48 expression may be uncoupled and differentially regulated in memory T cells. Although our preliminary results suggest that FOXP3 may also be protective (data not shown), the role of FOXP3 in regulating RICD sensitivity in memory T cells requires further investigation.
CD48-mediated RICD protection involves a reduction in p53 signaling
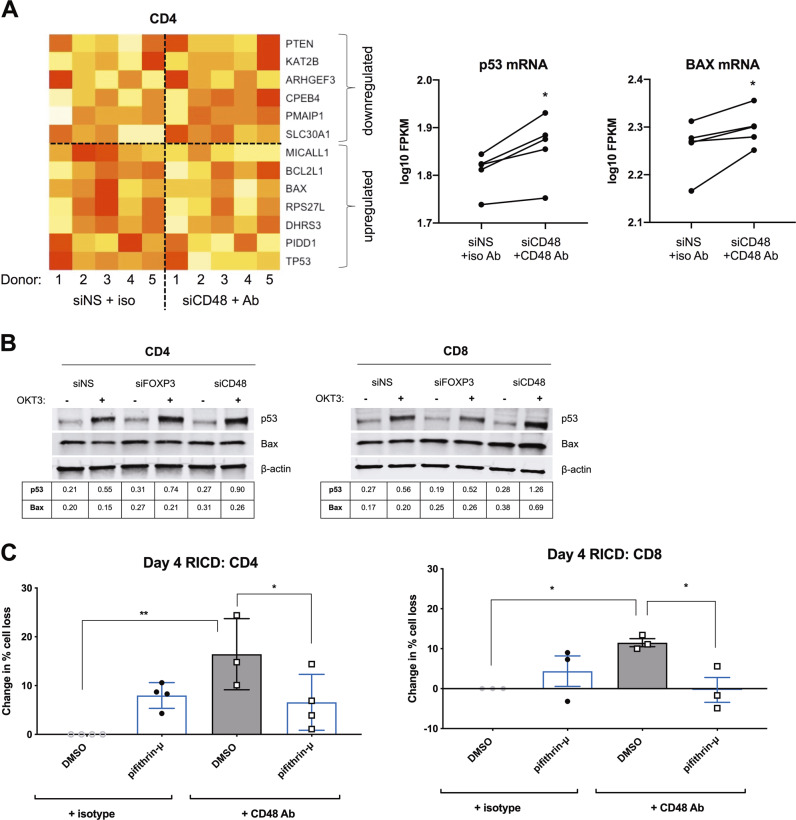
Given the effects of CD48 KD and antibody blockade on RICD, we further probed the downstream apoptosis effectors that were involved. Subsequent transcriptomic profiling of donor CD4 T cells uncovered 376 differentially expressed genes affected by CD48 KD/blockade. Gene ontology analysis using the Protein ANalysis THrough Evolutionary Relationships (PANTHER) Classification System revealed a signature of p53 pathway genes that were overrepresented in our data set (4.59-fold enrichment, p = 5.36 × 10−4). Indeed, several p53-dependent gene targets defined in the literature44 were differentially expressed as a result of CD48 KD plus blockade, including TP53 itself and the pro-apoptotic molecule BAX (Fig. 5a). We therefore asked whether p53 signaling might influence RICD sensitivity in expanding Tcons. Surprisingly, both FOXP3 and CD48-KD cells exhibited slightly elevated basal BAX expression and increased p53 accumulation upon restimulation, particularly in CD4 T cells (Fig. 5b). To test whether enhanced p53 induction may potentiate RICD, we tested whether p53 inhibition using pifithrin-μ could reverse the increase in RICD noted during CD48 blockade. Indeed, concomitant p53 inhibition reduced RICD on day 4 in CD48 Ab-treated cells to control levels (Fig. 5c). These data suggest that RICD resistance induced by FOXP3-dependent CD48 expression is mediated in part through the suppression of p53 signaling upon restimulation.
Fig. 5.
FOXP3 and CD48 expression protect T cells from p53-mediated RICD sensitization. a Activated CD4-KD T cells on day 4 post activation (NS vs. CD48) −/+CD48-blocking Ab from five anonymous healthy blood donors were subjected to RNA-seq. Normalized mean-centered log base 10 FPKM values are used for heatmap visualization of p53 target gene expression levels (yellow to red color scale represents higher to lower expression levels, respectively). The p53 and BAX transcript levels (FPKM) in each donor are displayed (right panels). Statistical significance was determined by paired t tests. p53, *p = 0.01; BAX, *p = 0.03). b Purified CD4 and CD8 T cells were electroporated with siRNA against FOXP3, CD48, or a nonspecific (NS) control. Activated T cells were restimulated with 100 ng/mL OKT3 on day 4 for 12 h. Whole-cell lysates were subjected to immunoblotting for p53 and BAX. β-actin was used for normalization, and the normalized protein levels are indicated in the tables below each blot. c Activated CD4 and CD8 T cells on day 4 post activation were incubated with 10 μg/mL anti-CD48-blocking Ab or an isotype control Ab for 1 h, −/+0.5 μM pifithrin-μ or a DMSO solvent control. Cells were then restimulated with 100 ng/mL OKT3 to induce RICD. The change in the percentage cell loss was averaged for 3–4 independent donors, and the percentage cell loss in isotype control cells + DMSO was set to 0. Statistical significance was assessed by one-way ANOVA. CD4: *p = 0.0281, **p = 0.0005. CD8: *p = 0.0486 DMSO, *p = 0.042 pifithrin-μ
FOXP3 and CD48 expression protect cells from RICD via the promotion of autophagy
In tumor cells, autophagy can repress p53 accumulation and pro-apoptotic function.45 Recent reports suggest that elevated autophagy in Tregs maintains their survival and functional integrity46, and that autophagy must be subverted to allow late-stage effector T cells to die by RICD.47 Furthermore, autophagy fosters mitochondrial health via mitophagy in late-stage effector T cells.47 Given that FOXP3 KD and CD48 blockade both resulted in decreased MOMP, we wondered whether autophagy was also altered in these conditions.
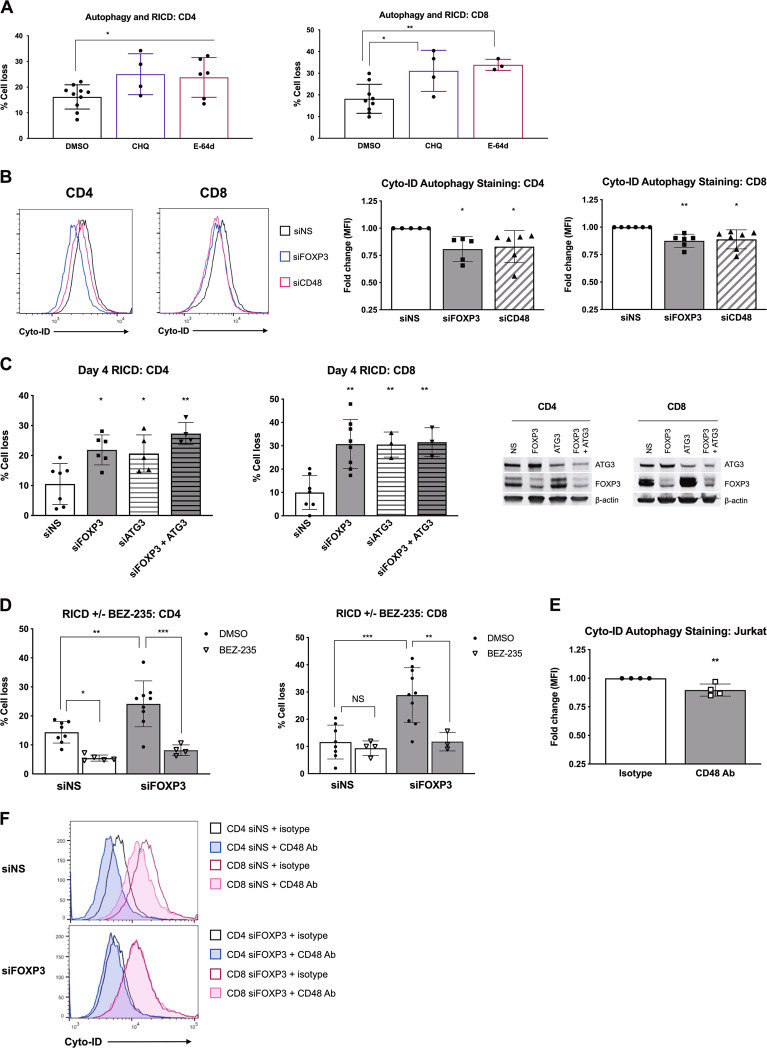
We first asked whether autophagy protects early expanding Tcons from RICD. Indeed, day 4 Tcons pretreated with chloroquine (CHQ) or E-64d to inhibit autophagy showed increased death following TCR restimulation (Fig. 6a). We then determined whether FOXP3 or CD48 KD alters basal autophagy using Cyto-ID, a highly specific, quantitative stain for autophagic vesicles.48 Remarkably, both FOXP3 and CD48 KD T cells exhibited a small but significant decrease in autophagy relative to control KD T cells (Fig. 6b).
Fig. 6.
FOXP3 and CD48 expression promote autophagy to protect T cells from RICD. a Activated CD4 and CD8 T cells were treated with 10 μM chloroquine (CHQ), 10 μM E-64d, or a DMSO solvent control for 1 h before restimulation with OKT3 to induce RICD. Statistical significance was determined by one-way ANOVA with Bonferroni’s multiple comparisons test. CD4: *p = 0.0222, *p = 0.0244 E-64d. CD8: *p = 0.0108, **p = 0.0051. b Activated FOXP3 and CD48 KD T cells on day 4 post activation were stained with Cyto-ID to quantify the autophagic vesicles. The Cyto-ID MFI was normalized to that of NS control KD cells. Each data point represents a value for an individual donor (right panels). Statistical significance was determined by one-way ANOVA with Dunnett’s multiple comparisons test. CD4: *p = 0.0309 siFOXP3, *p = 0.0462 siCD48. CD8: **p = 0.0064, *p = 0.0109. c Purified CD4 and CD8 T cells were electroporated with siRNA against FOXP3, ATG3, or FOXP3 + ATG3 combined or a nonspecific (NS) control siRNA. Activated T cells were restimulated with 100 ng/mL OKT3 on day 4 to conduct the RICD assays. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test. CD4: *p = 0.0115 siFOXP3, *p = 0.0349 siATG3, **p = 0.0011. CD8: ***p = 0.006, **p = 0.0082 siATG3, **p = 0.0056 siFOXP3 + ATG3. Gene knockdowns was verified by western blotting (right). d Activated CD4 and CD8 KD T cells on day 4 post activation (NS vs. FOXP3) were pretreated with 1 μM BEZ235 or DMSO for 30 min before restimulation with 100 ng/mL OKT3 to induce RICD. Statistical significance was determined by one-way ANOVA with Sidak’s multiple comparisons test. CD4: *p = 0.0213, **p = 0.0029, ***p = 0.0001. CD8: **p = 0.0083, ***p = 0.0003. e Jurkat T cells were treated with 10 μg/mL anti-CD48-blocking Ab or an isotype control Ab for 4 h and then subjected to Cyto-ID autophagy staining. The fold change in the Cyto-ID MFI (normalized to that of NS) is shown on the right for four independent experiments. Statistical significance was determined by an unpaired t test. **p = 0.0081. f Activated CD4 and CD8 KD T cells on day 4 post activation (NS vs. FOXP3) were incubated with 10 μg/mL anti-CD48-blocking Ab or an isotype control Ab for 6 h and stained with Cyto-ID. The data are representative of three independent experiments
To specifically determine the importance of autophagy in FOXP3+ Tcons, we transfected T cells with siRNA targeting FOXP3 or ATG3, an E2-like ubiquitin carrier protein that is essential for autophagosome maturation.49 Neither FOXP3 nor ATG3 KD altered TCR-induced CD25 and CD69 expression (Supplementary Fig. 5), indicating that T-cell activation remained intact under these conditions. Conssitent with the results of the pharmacological inhibition of autophagy, ATG3 KD enhanced RICD sensitivity similar to that in FOXP3 KD cells (Fig. 6c). The cosilencing of both FOXP3 and ATG3 expression resulted in a slightly greater increase in RICD for CD4, but not CD8 T cells (Fig. 6c), suggesting that the protective effect of FOXP3 could be largely dependent on autophagy. Finally, we pretreated cells with the dual PI-3K/mTOR inhibitor dactolisib (BEZ-235)50 to induce increased autophagy before TCR restimulation (Fig. 6d). BEZ-235 protected FOXP3 KD cells from death to a greater extent than the control, suggesting that the induced elevation of autophagy could rescue FOXP3-depleted Tcons from RICD.
Finally, we tested whether CD48 blockade would also decrease basal autophagy, which was consistent with the decrease in the MOMP (Fig. 4c). Cyto-ID staining was reduced in Jurkat T cells treated with a CD48-blocking Ab vs. that in cells treated with an isotype control (Fig. 6e), which was indicative of decreased autophagy. In primary Tcons, we also detected a decrease in Cyto-ID staining in control cells with CD48 blockade, which was not observed in FOXP3 KD cells that already exhibited decreased Cyto-ID fluorescence (Fig. 6f). Taken together, our results illuminate a novel pathway whereby transient FOXP3 expression protects early expanding Tcons from premature RICD by promoting a CD48-dependent autophagy program.
Discussion
Most literature concerning the function of FOXP3 in T cell-mediated immunity has focused on its role in governing Treg identity and function. Indeed, loss-of-function FOXP3 mutations cause immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome in humans due to failed Treg development, thus warranting attention. Although several groups have described transient TCR-induced FOXP3 expression in human Tcons, FOXP3 function therein has never been fully understood and may be distinct from its role in Tregs. For example, FOXP3 has been revealed as an intrinsic regulator of Th17 cells by suppressing IFN-γ production and upregulating CCR4.25 Th17 cells have higher FOXP3 expression than Th1 cells; intriguingly, Th17 cells are also more resistant to RICD.51,52 FOXP3 modulates cytokine production in equine T cells as well,28 and this may represent a conserved function of FOXP3 induction in Tcons that is more analogous to its function in humans vs. that in mice.
Tregs are highly resistant to RICD due to FOXP3-dependent repression of SAP.18,21 In this study, we determined whether transient FOXP3 upregulation protects newly activated Tcons from RICD. Indeed, our results show for the first time that FOXP3 also modulates RICD sensitivity in a proportion of both CD4 and CD8 human Tcons through a novel, SAP-independent mechanism involving CD48 upregulation and augmented autophagy. Remarkably, these findings imply that FOXP3 elicits RICD resistance in different T-cell compartments through discrete mechanisms. Furthermore, the memory T-cell pool may be distinct; additional studies are required to decipher the function of enhanced FOXP3 induction in activated human memory T cells.
Our results showing differential RICD sensitivity in early (day 4) vs. late (day 17) CD4 and CD8 T cells emphasize the importance of temporal apoptosis regulation. Induced FOXP3 expression enhanced RICD resistance during early T-cell expansion, but not during later stages when FOXP3 levels were markedly diminished. Conversely, SAP had no impact on RICD sensitivity in early Tcons, despite its critical role in boosting RICD in late-stage effectors. Our results highlight important differences in how RICD is regulated in newly activated T cells. For example, the low levels of RICD measured at day 4 post activation were completely dependent on de novo transcription. In addition, FAS ligand (FASL) and BIM expression were largely unaffected by FOXP3 KD in early Tcons and likely do not contribute substantially to residual RICD compared with late-stage effectors.35,53 Instead, FOXP3 and CD48 KD T cells showed increased p53 accumulation upon restimulation, suggesting that signals resulting from cellular stress (e.g., autophagy vs. apoptosis) govern RICD susceptibility in expanding Tcons. Clearly, RICD sensitivity is carefully calibrated throughout the life of a T cell via different mechanisms, although the RICD/AICD literature has primarily focused on thymocyte selection and the terminal stages of effector T-cell differentiation.
Our results also indicate that FOXP3 directly upregulates CD48 expression, which is consistent with genome-wide FOXP3 ChIP-Seq data from human Tregs and the presence of discernible FOXP3 binding motifs in the SLAMF2 promoter.39 CD48 is a GPI-linked protein that localizes to cholesterol-rich lipid rafts and can influence TCR signal transduction from within the immunological synapse.41,54 Importantly, our findings reveal a new function for CD48 in protecting CD4 and CD8 T cells from premature RICD. Knockdown or blockade of CD48 consistently increased RICD sensitivity, despite the variable results obtained from the blockade of its two known ligands, CD2 and 2B4. These observations underscore the complexity of SLAM receptor signaling, and it will require further investigation to determine whether (a) CD2/2B4 compete for CD48 binding and have differential signaling outcomes, (b) CD48 binds to hitherto unknown ligands on T cells, or (c) CD48 signals in a ligand-independent manner. Indeed, activating vs. inhibitory signaling through 2B4 binding to natural killer cells may depend on the abundance of CD48 on target cells.37 Regardless, the novel role we uncovered for CD48 in the promotion of apoptosis resistance may shed new light on phenotypes noted in previous murine T-cell studies. Purified CD4 T cells from CD48-deficient mice showed a profound defect in clonal expansion in vitro.55 Given that T-cell accumulation ultimately reflects the rate of proliferation minus that of cell death, the decreased number of CD48-deficient CD4 T cells noted in this study could reflect enhanced RICD sensitivity.
Our data suggest that CD48 impacts RICD sensitivity in part by promoting autophagy. A wealth of emerging evidence points to autophagy as a critical homeostatic process in T cells that regulates their proliferation, metabolism, differentiation, and survival.56 Corrado et al. recently proposed a model by which autophagy protects effector T cells from RICD via the clearance of damaged mitochondria upon TCR restimulation.47 Indeed, key autophagy proteins such as ATG12 and ATG3 have been directly implicated in regulating mitochondrial homeostasis and intrinsic cell death.57 We found that autophagy protected early activated CD4 and CD8 T cells from RICD and that FOXP3 or CD48 KD resulted in reduced basal autophagy. The blockade of CD48 also reduced autophagy in early Tcons, suggesting that some degree of stimulatory CD48 signaling at higher expression levels enhances basal autophagy. Importantly, decreased autophagy associated with FOXP3 KD or CD48 blockade was correlated with increased p53 levels and a slight but significant reduction in the MOMP, which is consistent with the results of previous T-cell studies.58 Thus, our data suggest that FOXP3-driven autophagy guards mitochondrial health in part via mitophagy in human Tcons.
By revealing the “tuning” of apoptotic sensitivity in T cells, our results highlight a potentially attractive opportunity for CD48-targeted immunotherapies, as others have suggested.41,59,60 Unlike various costimulatory and coinhibitory receptors with defined cytoplasmic signaling domains and cell-specific expression patterns, CD48 is a GPI-anchored protein ubiquitously expressed on hematopoietic cell types. Despite this, anti-CD48 antibody administration specifically ameliorated experimental autoimmune encephalomyelitis (EAE) severity in mice by limiting the number of pathogenic CD4 T cells.40 We posit that this effect may be explained in part by the suppression of the expansion of activated pathogenic cells via increased RICD sensitivity. In conclusion, our results describe a novel function for FOXP3 expression in shielding early human effector Tcons from premature RICD by upregulating CD48 and protective autophagy. Although the precise protective mechanisms linking FOXP3, CD48, and autophagy remain to be completely defined, this nascent signaling program represents a potential therapeutic target for novel immunotherapies that might attenuate or boost human T-cell responses as needed by modifying RICD susceptibility during clonal expansion.
Materials and methods
T-cell isolation and culture conditions
Peripheral blood mononuclear cells (PBMCs) were obtained with informed consent from anonymous healthy human donors at the National Institutes of Health (NIH) Blood Bank. Access to donors was kindly provided by Dr. Michael Lenardo. CD4 T cells were purified from PBMCs by immunomagnetic negative selection using the EasySep Human CD4 T Cell Isolation Kit (Stem Cell Technologies). CD8 T cells were purified using the Human CD8 T Cell Isolation Kit (Stem Cell Technologies), and naive CD4 T cells were purified with the Human Naive CD4 T Cell Isolation Kit II (Stem Cell Technologies). The purity of the sorted CD4 and CD8 T cells was routinely ≥90% (Supplementary Fig. 1A).
Primary T cells were activated with either a 2:1 ratio of anti-CD2/CD3/CD28 Ab-bound biotin beads (Human T Cell Activation/Expansion Kit, Miltenyi Biotec) or ImmunoCult Human CD3/CD28/CD2 T Cell Activator (Stem Cell Technologies) according to the manufacturer’s instructions and cultured in complete RPMI (RPMI 1640 (ThermoFisher Scientific) + 10% fetal calf serum (FCS) (HyClone) + 1% penicillin/streptomycin (Lonza). After 3 days, the activated cells were washed with PBS and subsequently cultured in complete RPMI plus 200 U/mL rIL-2 (PeproTech). Jurkat T cells (ATCC clone E6.1) were also maintained in complete RPMI.
siRNA-mediated knockdown in primary T cells
T cells were electroporated with siRNAs against FOXP3 (FOXP3HSS121456), SAP (SH2D1AHSS106218), ATG3 (assay ID s34733), and CD48 (assay ID s2689) (ThermoFisher) using the Amaxa Nucleofection 4D system and the P3 Primary Cell Kit (Lonza). Stealth RNAi-negative control medium GC duplex siRNA (ThermoFisher) was used as a nonspecific (NS) control, and all assays were conducted 4 days post electroporation to ensure peak knockdown efficiency. For the day 4 RICD experiments, the cells were rested for 1 h in complete RPMI at 37 °C and in 5% CO2 after isolation. The cells were then rested for an additional hour after electroporation before stimulation. The knockdown efficiencies were assessed by flow cytometry or immunoblotting for every experiment.
RICD assays
RICD assays were conducted as described previously.30 Briefly, 1 × 105 T cells were restimulated with anti-CD3 mAb (clone OKT3) in triplicate wells of a 96-well round-bottom plate for 24 h. Wells were stained with 10 μl of propidium iodide (PI, 1 μg/ml stock) (ThermoFisher) to distinguish the live and dead cells, and immediately analyzed on a BD Accuri C6 flow cytometer. Death was quantified as the percent cell loss based on quantification of viable cells collected for a constant amount of time, for which the % cell loss = (1 – [number of viable cells (treated)/number of viable cells (untreated)]) × 100. Antibody blockade experiments were conducted by incubating cells with 10 μg/mL of anti-CD48 (Biogems 10511–25), anti-CD2 (Biolegend 309212), anti-CD244 (Invitrogen 16–2449–81), or an isotype IgG1 control (Biogems 44212–25) for 1 h before restimulation with OKT3. For the small molecule inhibitor assays, T cells were pretreated for 30 min with 100 ng/ml actinomycin D (Sigma-Aldrich), 10 μM chloroquine (Sigma-Aldrich), 1 μM BEZ-235 (Cayman Chemical), 10 μM E-64d (Cayman Chemical), 0.5 μM pifithrin (Cayman Chemical), or a DMSO solvent control prior to OKT3 restimulation.
Flow cytometry
Intracellular staining of FOXP3 was conducted using the Foxp3/Transcription Factor Staining Buffer set with anti-Foxp3-APC (eBioscience 17–4777–42). For surface receptor staining, cells were washed in PBS + 1% FBS + 0.01% sodium azide, and incubated with antibodies for 30 min on ice. The surface expression of CD48 and its ligands was detected using CD48-FITC (Biolegend 336706), CD244-APC (Biolegend 188549), and CD2-PE (Biolegend 300207). T-cell activation markers were assessed with CD25-PE (BD 555432) and CD69-APC (BD 555533). Naive T cells were stained with CD45RA-FITC (Biolegend 304106), CD45RO-APC (Biolegend 304210), and CD62L-PE (Biolegend 304806) to assess purity.
For proliferation assays, day 4 activated T cells were incubated in complete RPMI + IL-2 for 3 h and then subjected to Click-It Edu Alexa Fluor 488 staining for flow-cytometric analysis according to the manufacturer’s instructions (ThermoFisher). CFSE labeling was performed using a CellTrace CFSE Proliferation Kit (ThermoFisher). Briefly, 1.5 × 106 T cells were washed two times in PBS to remove the serum and stained with 1 μM CFSE in PBS for 5 min in the dark at 37 °C. Cells were quenched/resuspended in complete RPMI, and activated as described above. Cyto-ID staining of autophagic vesicles was conducted according to the manufacturer’s instructions (Enzo Biosciences). Briefly, cells were washed with PBS and then resuspended in Cyto-ID staining solution (without serum) for 30 min at 37 °C and in 5% CO2. For the PI cell cycle analysis, 1–2 × 104 T cells were resuspended in 300 ml PBS, and 5 ml ice-cold methanol was added dropwise before a 30 min incubation at −20 °C. The fixed cells were resuspended in PBS for at least 30 min, pelleted, and resuspended in 60 μg/ml PI + 50 μg/ml RNAse A (Sigma) in PBS for 30 min at room temperature. The cells in the G1, S, and G2/M phases of the cell cycle were gated and quantified by flow cytometry (linear scale). All flow cytometry analyses were conducted using FlowJo v10 software.
Western blotting
For general immunoblotting, cells were lysed in 1% Nonidet P-40 (NP-40) lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 0.5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 1 mM Na3VO4, and 1 mM NaF) + complete protease inhibitors (Roche) for 30 min on ice. The lysates were cleared by centrifugation, boiled in 2× sample buffer (Laemmli buffer + 50 μM 2-βME) and separated with 4–20% SDS-PAGE gels (Bio-Rad). The proteins were transferred to nitrocellulose membranes for 7–10 min using the TransBlot Turbo system (Bio-Rad) and subsequently blocked with Odyssey blocking buffer (LI-COR). The blots were probed with the following antibodies: anti-FOXP3 (Novus Biologicals NB600–245), anti-SAP (Cell Signaling Technology #2778), anti-FASL (Ab3, EMD Biosciences), anti-BIM (Enzo Life Sciences), anti-ATG3 (Cell Signaling Technology #3415), anti-p53 (Cell Signaling Technology #2524), anti-Bax (BD Biosciences #610982), and anti-β-actin (Sigma-Aldrich, AC-15). The blots were incubated with IRDye anti-rabbit or anti-mouse secondary antibodies (LI-COR) and imaged using the Odyssey CLx instrument. Spot densitometric analyses were performed with the Odyssey CLx.
The nuclear and cytoplasmic fractions were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific). For each sample, 3 × 106 cells were washed in PBS and lysed in 0.1 mL of CER I lysis buffer. Lamin A/C was used as a marker of the nuclear fractions during immunoblotting (4C11 Ab, Cell Signaling Technology).
Transcriptome profiling of primary T lymphocytes
Total RNA was isolated from primary T cells using the RNeasy Plus Mini Kit with on-column DNase digestion (Qiagen). The total RNA integrity was assessed using automated capillary electrophoresis with a Fragment Analyzer (Roche). For all samples with an RQI > 8.0, a total of >75 ng RNA was used as the input for library preparation using the TruSeq Stranded mRNA Library Preparation Kit (Illumina, San Diego, CA, USA). The sequencing libraries were quantified by PCR using a KAPA Library Quantification Kit for NGS (Kapa, Wilmington, MA, USA), and the size distribution was assessed with a Fragment Analyzer. The sequencing libraries were pooled and sequenced on a NextSeq 500 Desktop Sequencer (Illumina) using a NextSeq 500 High Output Kit v2 with paired-end reads of 75 bp in length. The raw sequencing data were demuxed using bcl2fastq2 conversion software 2.17 before alignment using TopHat Alignment v1.0 software. The differential transcript expression analysis was performed using Cufflinks Assembly & DE v1.1.0 in BaseSpace Onsite (Illumina). The heatmaps visualizing the differentially expressed genes were generated using ClustVis software. The transcript abundance quantitation data were deposited in the NCBI Gene Expression Omnibus (GSE137931, GSE138272).
Statistics
The statistical analyses were conducted using GraphPad Prism 8.0.1 software. The error bars in all figures indicate +/− the standard deviation, unless otherwise specified. All statistical tests that were performed are indicated in the figure legends. For the RICD assays, each data point represents the average percentage cell loss for an individual donor (the average of triplicate samples).
Supplementary information
Acknowledgements
We thank Michael Lenardo for generously providing access to anonymous healthy donor buffy coat samples from the NIH Blood Bank. We also thank Robert Kortum, Brian Schaefer, Chou-Zen Giam, Edward Mitre, and Jason Lees for helpful discussions. We thank Kateryna Lund and Kheem Bhist for flow cytometry assistance and support. This work was funded by grants from the National Institutes of Health (1R01GM105821) and Uniformed Services University.
Author contributions
K.V. and A.L.S. conceptualized the project. K.V. designed and conducted the experiments, analyzed the results, graphed and visualized the data, and wrote the paper. N.M.L., C.L.D., A.R.S. and G.S. conducted the RNA-seq experiments and analyses. C.L., C.R.L., B.D., S.A. and B.M.B. assisted with various experiments. A.L.S. and C.L.D. reviewed and edited the paper and supervised the project.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-019-0316-z) contains supplementary material.
References
- 1.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Snow AL, Pandiyan P, Zheng L, Krummey SM, Lenardo MJ. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol. Rev. 2010;236:68–82. doi: 10.1111/j.1600-065X.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow AL, et al. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J. Clin. Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassiri H, Janice Yeo WC, Rothman J, Koretzky GA, Nichols KE. X-linked lymphoproliferative disease (XLP): a model of impaired anti-viral, anti-tumor and humoral immune responses. Immunol. Res. 2008;42:145–159. doi: 10.1007/s12026-008-8048-7. [DOI] [PubMed] [Google Scholar]
- 5.Hislop AD, et al. Impaired Epstein-Barr virus-specific CD8+ T-cell function in X-linked lymphoproliferative disease is restricted to SLAM family-positive B-cell targets. Blood. 2010;116:3249–3257. doi: 10.1182/blood-2009-09-238832. [DOI] [PubMed] [Google Scholar]
- 6.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 7.Combadiere B, et al. Qualitative and quantitative contributions of the T cell receptor zeta chain to mature T cell apoptosis. J. Exp. Med. 1996;183:2109–2117. doi: 10.1084/jem.183.5.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.She J, Matsui K, Terhorst C, Ju ST. Activation-induced apoptosis of mature T cells is dependent upon the level of surface TCR but not on the presence of the CD3 zeta ITAM. Int. Immunol. 1998;10:1733–1740. doi: 10.1093/intimm/10.11.1733. [DOI] [PubMed] [Google Scholar]
- 9.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 10.Boehme SA, Lenardo MJ. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur. J. Immunol. 1993;23:1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz I, et al. An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death. J. Immunol. 2003;171:2930–2936. doi: 10.4049/jimmunol.171.6.2930. [DOI] [PubMed] [Google Scholar]
- 12.Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, et al. Increased proliferation of CD8+ T cells in SAP-deficient mice is associated with impaired activation-induced cell death. Eur. J. Immunol. 2007;37:663–674. doi: 10.1002/eji.200636417. [DOI] [PubMed] [Google Scholar]
- 14.Katz G, Krummey SM, Larsen SE, Stinson JR, Snow AL. SAP facilitates recruitment and activation of LCK at NTB-A receptors during restimulation-induced cell death. J. Immunol. 2014;192:4202–4209. doi: 10.4049/jimmunol.1303070. [DOI] [PubMed] [Google Scholar]
- 15.Ruffo E, et al. Inhibition of diacylglycerol kinase alpha restores restimulation-induced cell death and reduces immunopathology in XLP-1. Sci. Transl. Med. 2016;8:321ra7. doi: 10.1126/scitranslmed.aad1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinozaki K, et al. Activation-dependent T cell expression of the X-linked lymphoproliferative disease gene product SLAM-associated protein and its assessment for patient detection. Int Immunol. 2002;14:1215–1223. doi: 10.1093/intimm/dxf084. [DOI] [PubMed] [Google Scholar]
- 17.Mehrle S, Frank S, Schmidt J, Schmidt-Wolf IG, Marten A. SAP and SLAM expression in anti-CD3 activated lymphocytes correlates with cytotoxic activity. Immunol. Cell Biol. 2005;83:33–39. doi: 10.1111/j.1440-1711.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- 18.Katz, G. et al. FOXP3 renders activated human regulatory T cells resistant to restimulation-induced cell death by suppressing SAP expression. Cell Immunol.327, 54–61 (2018). [DOI] [PMC free article] [PubMed]
- 19.Fritzsching B, et al. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand-but not to TCR-mediated cell death. J. Immunol. 2005;175:32–36. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Bhaskaran N, et al. Transforming growth factor-beta1 sustains the survival of Foxp3(+) regulatory cells during late phase of oropharyngeal candidiasis infection. Mucosal Immunol. 2016;9:1015–1026. doi: 10.1038/mi.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss EM, et al. Foxp3-mediated suppression of CD95L expression confers resistance to activation-induced cell death in regulatory T cells. J. Immunol. 2011;187:1684–1691. doi: 10.4049/jimmunol.1002321. [DOI] [PubMed] [Google Scholar]
- 22.Gavin MA, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl Acad. Sci. USA. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 25.McMurchy AN, et al. A novel function for FOXP3 in humans: intrinsic regulation of conventional T cells. Blood. 2013;121:1265–1275. doi: 10.1182/blood-2012-05-431023. [DOI] [PubMed] [Google Scholar]
- 26.Torgerson TR, et al. FOXP3 inhibits activation-induced NFAT2 expression in T cells thereby limiting effector cytokine expression. J. Immunol. 2009;183:907–915. doi: 10.4049/jimmunol.0800216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan SE, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 28.Cavatorta DJ, Erb HN, Felippe MJ. Activation-induced FoxP3 expression regulates cytokine production in conventional T cells stimulated with autologous dendritic cells. Clin. Vaccin. Immunol. 2012;19:1583–1592. doi: 10.1128/CVI.00308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler SF. FOXP3: not just for regulatory T cells anymore. Eur. J. Immunol. 2007;37:21–23. doi: 10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- 30.Katz G, Snow AL. Fluorescence-activated cell sorting-based quantitation of T cell receptor restimulation-induced cell death in activated, primary human T cells. Methods Mol. Biol. 2013;979:15–23. doi: 10.1007/978-1-62703-290-2_2. [DOI] [PubMed] [Google Scholar]
- 31.Magg T, Mannert J, Ellwart JW, Schmid I, Albert MH. Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells. Eur. J. Immunol. 2012;42:1627–1638. doi: 10.1002/eji.201141838. [DOI] [PubMed] [Google Scholar]
- 32.Cencioni MT, et al. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death Dis. 2015;6:e1785. doi: 10.1038/cddis.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varadhachary AS, Perdow SN, Hu C, Ramanarayanan M, Salgame P. Differential ability of T cell subsets to undergo activation-induced cell death. Proc. Natl Acad. Sci. USA. 1997;94:5778–5783. doi: 10.1073/pnas.94.11.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz AC, et al. Fas/CD95 prevents autoimmunity independently of lipid raft localization and efficient apoptosis induction. Nat. Commun. 2016;7:13895. doi: 10.1038/ncomms13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow AL, et al. Critical role for BIM in T cell receptor restimulation-induced death. Biol. Direct. 2008;3:34. doi: 10.1186/1745-6150-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 37.Wu N, Veillette A. SLAM family receptors in normal immunity and immune pathologies. Curr. Opin. Immunol. 2016;38:45–51. doi: 10.1016/j.coi.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Kwon HK, Chen HM, Mathis D, Benoist C. Different molecular complexes that mediate transcriptional induction and repression by FoxP3. Nat. Immunol. 2017;18:1238–1248. doi: 10.1038/ni.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadlon TJ, et al. Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J. Immunol. 2010;185:1071–1081. doi: 10.4049/jimmunol.1000082. [DOI] [PubMed] [Google Scholar]
- 40.McArdel SL, Brown DR, Sobel RA, Sharpe AH. Anti-CD48 monoclonal antibody attenuates experimental autoimmune encephalomyelitis by limiting the number of pathogenic CD4+ T cells. J. Immunol. 2016;197:3038–3048. doi: 10.4049/jimmunol.1600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elishmereni M, Levi-Schaffer F. CD48: a co-stimulatory receptor of immunity. Int. J. Biochem Cell Biol. 2011;43:25–28. doi: 10.1016/j.biocel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Pahima, H., Puzzovio, P. G. & Levi-Schaffer, F. 2B4 and CD48: a powerful couple of the immune system. Clin. Immunol.204, 64–68 (2019). [DOI] [PubMed]
- 43.Ramaswamy M, et al. Specific elimination of effector memory CD4+ T cells due to enhanced Fas signaling complex formation and association with lipid raft microdomains. Cell Death Differ. 2011;18:712–720. doi: 10.1038/cdd.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer M. Census and evaluation of p53 target genes. Oncogene. 2017;36:3943–3956. doi: 10.1038/onc.2016.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White E. Autophagy andp53. Cold Spring Harb. Perspect. Med. 2016;6:a026120. doi: 10.1101/cshperspect.a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei J, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 2016;17:277–285. doi: 10.1038/ni.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corrado M, et al. Macroautophagy inhibition maintains fragmented mitochondria to foster T cell receptor-dependent apoptosis. EMBO J. 2016;35:1793–1809. doi: 10.15252/embj.201593727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo S, et al. A rapid and high content assay that measures cyto-ID-stained autophagic compartments and estimates autophagy flux with potential clinical applications. Autophagy. 2015;11:560–572. doi: 10.1080/15548627.2015.1017181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichimura Y, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 50.Maira SM, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 51.Cencioni MT, et al. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death Dis. 2015;6:e1741. doi: 10.1038/cddis.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi G, et al. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J. Immunol. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weant AE, et al. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Muhammad A, et al. Sequential cooperation of CD2 and CD48 in the buildup of the early TCR signalosome. J. Immunol. 2009;182:7672–7680. doi: 10.4049/jimmunol.0800691. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Cabrero J, et al. CD48-deficient mice have a pronounced defect in CD4(+) T cell activation. Proc. Natl Acad. Sci. USA. 1999;96:1019–1023. doi: 10.1073/pnas.96.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dowling SD, Macian F. Autophagy and T cell metabolism. Cancer Lett. 2018;419:20–26. doi: 10.1016/j.canlet.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radoshevich L, et al. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell. 2010;142:590–600. doi: 10.1016/j.cell.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J. Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 59.McArdel SL, Terhorst C, Sharpe AH. Roles of CD48 in regulating immunity and tolerance. Clin. Immunol. 2016;164:10–20. doi: 10.1016/j.clim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abadia-Molina AC, et al. CD48 controls T-cell and antigen-presenting cell functions in experimental colitis. Gastroenterology. 2006;130:424–434. doi: 10.1053/j.gastro.2005.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.