Abstract
Fyn kinase in the dorsomedial striatum (DMS) of rodents plays a central role in mechanisms underlying excessive alcohol intake. The DMS is comprised of medium spiny neurons (MSNs) that project directly (dMSNs) or indirectly (iMSNs) to the substantia nigra. Here, we examined the cell-type specificity of Fyn’s actions in alcohol use. First, we knocked down Fyn selectively in DMS dMSNs or iMSNs of mice and measured the level of alcohol consumption. We found that downregulation of Fyn in dMSNs, but not in iMSNs, reduces excessive alcohol but not saccharin intake. D1Rs are coupled to Gαs/olf, which activate cAMP signaling. To examine whether Fyn’s actions are mediated through cAMP signaling, DMS dMSNs were infected with GαsDREADD, and the activation of Fyn signaling was measured following CNO treatment. We found that remote stimulation of cAMP signaling in DMS dMSNs activates Fyn and promotes the phosphorylation of the Fyn substrate, GluN2B. In contract, remote activation of GαsDREADD in DLS dMSNs did not alter Fyn signaling. We then tested whether activation of GαsDREADD in DMS dMSNs or iMSNs alters alcohol intake and observed that CNO-dependent activation of GαsDREADD in DMS dMSNs but not iMSNs increases alcohol but not saccharin intake. Finally, we examined the contribution of Fyn to GαsDREADD-dependent increase in alcohol intake, and found that systemic administration of the Fyn inhibitor, AZD0503 blocks GαsDREADD-dependent increase in alcohol consumption. Our results suggest that the cAMP-Fyn axis in the DMS dMSNs is a molecular transducer of mechanisms underlying the development of excessive alcohol consumption.
Subject terms: Reward, Motivation
Introduction
The dorsomedial striatum (DMS) is critically involved in processes such as locomotion [1], and goal-directed behaviors [2, 3]. The DMS is comprised primarily of GABAergic medium spiny projection neurons (MSNs) that receive dopaminergic input from the midbrain [4]. MSNs can be divided into two populations of neurons that take part in opposing activities [5]; MSNs that project directly to the substantia nigra pars reticula (SNr) facilitate actions and are defined as direct MSNs (dMSNs) [5], and MSNs that project indirectly to the SNr gate actions and are defined as indirect MSNs (iMSNs) [5]. dMSNs selectively express the dopamine D1 receptors (D1Rs) whereas iMSNs express the dopamine D2 receptors (D2Rs) [5]. In the striatum, D1Rs are coupled to Gαolf, a homolog of Gαs [6]. Stimulation of Gαs/olf-coupled receptors results in the production of the second messenger cyclic adenosine monophosphate (cAMP) [6, 7], which binds to, and activates, protein kinase A (PKA) [8], a kinase that plays an important role in the adult brain [9–11]. In contrast, D2Rs are coupled to Gαi, which inhibits cAMP signaling [7]. dMSNs and iMSNs exert balanced influence on locomotion and goal-directed behaviors [5], and an imbalance of dMSNs and iMSNs function has been implicated in neurodegenerative disorders such as Parkinson’s disease [5, 12], as well as psychiatric disorders such as obsessive-compulsive disorder, anxiety, and addiction [13–15].
We previously observed that Fyn kinase is activated in DMS dMSNs upon stimulation of D1Rs [16]. Fyn belongs to the Src family of non-receptor protein tyrosine kinases (PTKs) [17, 18], and is highly expressed in the developing and adult brain in regions such as cortex, hippocampus, and cerebellum as well as in the striatum [19, 20]. Fyn plays an important role in the CNS [21], as it modulates excitatory and inhibitory synaptic transmission and participates in learning and memory processes [21–28]. Dysfunction of Fyn signaling has been associated with Alzheimer’s disease [29] and pain [23].
Accumulating data in humans and rodents also suggest that Fyn plays a central role in cellular neuroadaptations that underlie alcohol use disorder (AUD) [30, 31]. Specifically, genetic mutations within the Fyn gene have been associated with increased susceptibility for the development and severity of AUD in humans [32–34], and gene network association studies identified a link between Fyn and alcohol dependence [35]. Animal data suggest that Fyn plays a role in the acute tolerance to the hypnotic sedative effect of alcohol [36, 37], as well as in alcohol drinking behavior [38–40]. Molecularly, excessive consumption of alcohol activates Fyn specifically in the DMS of mice and rats [39, 41, 42]. Once activated by alcohol, Fyn phosphorylates its substrate, GluN2B [39, 41, 42]. Alcohol-dependent Fyn-mediated phosphorylation of GluN2B produces a forward trafficking of the channel and long-lasting enhancement of GluN2B activity in the DMS [39]. Inhibition of Fyn in the DMS of rats attenuates operant self-administration of alcohol [39], and systemic administration of the Fyn inhibitor, AZD0530, attenuates goal-directed alcohol seeking and facilitates extinction in mice [40]. Together, these data suggest that Fyn in the DMS plays a central role in neuroadaptations that underlie alcohol use.
This study was aimed to explore the cellular specificity of Fyn-dependent molecular and behavioral neuroadaptations that drive AUD.
Methods
The description of purchased reagents, collection of brain samples, western blot analysis, immunoprecipitation, preparation of solutions, and the preparation of FLEX-shRNA-Fyn and FLEX-SCR is detailed in the Supplementary Information section.
Animals
C57BL/6 mice were obtained from Jackson Laboratories. Drd1a-Cre (D1-Cre) and AdoraA2-Cre (A2A-Cre) mice both of which are on C57BL/6 background, were obtained from Mutant Mice Resource and Research Centers (MMRRC) UC Davis (David, CA). Ai14 mice were purchased from Jackson Laboratory (Bar Harbor, Maine). The generation of D1-Cre/Ai14 mouse line is described in [43]. The same breeding strategy was used to generate the A2A-Cre/Ai14 mouse line. Mice were genotyped by polymerase chain reaction (PCR) analysis of products derived from tail DNA. Male mice were 8–9 weeks old at the beginning of the experiments and were individually housed in temperature and humidity-controlled rooms under a reversed 12-h light/dark cycle. Food and water were available ad libitum. All animal procedures were approved by the University of California San Francisco (UCSF) Institutional Animal Care and Use Committee and were conducted in agreement with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC, UCSF).
Infection of the DMS with FLEX-shFyn and FLEX-SCR
D1-Cre/Ai14 and A2A-Cre/Ai14 mice were anesthetized using a mixture of ketamine (120 mg/kg) and xylazine (8 mg/kg). Bilateral microinfusions were made using stainless steel injectors (33 gauge, Small Parts) into the DMS (the stereotaxic coordinates were anterioposterior +1.1 mm from bregma; mediolateral ±1.2 mm from bregma and dorsoventral −2.8 and from bregma for the first injection site and anterioposterior +1.3 mm from bregma; mediolateral ±1.2 mm from bregma and dorsoventral −3 mm from bregma for the second injection site). Animals were infused with lentivirus-expressing FLEX-shFyn or its scramble control (FLEX-SCR) (1.2 μl/site with 2 sites of injection per hemisphere) at a concentration of 107 pg/ml at an injection rate of 0.1 μl/min [16]. After each infusion, the injectors were left in place for an additional 10 min to allow the virus to diffuse.
Infection of the DMS and the dorsolateral striatum (DLS) with AAV-DIO-rM3D(Gs)-mCherry
D1-Cre or A2A-Cre mice were anesthetized using a mixture of ketamine (120 mg/kg) and xylazine (8 mg/kg). Bilateral microinfusions were made using stainless steel injectors (33 gauge, small parts) into the DMS (the stereotaxic coordinates were anterioposterior +1.1 mm from bregma; mediolateral ±1.25 mm from bregma and dorsoventral −2.8 mm from bregma) or the dorsolateral striatum (DLS) (the stereotaxic coordinates were anterioposterior +1.1, medialateral ±2.3 from bregma, and dorsoventral −2.8 from bregma). Mice were infused with AAV-DIO-rM3D(Gs)-mCherry (AAV-DIO-Gs-DREADD) (1 μl per hemisphere) at a concentration of 1013 vg/ml and at an injection rate of 0.1 μl/min. After each infusion, the injectors were left in place for an additional 10 min to allow the virus to diffuse.
Drinking paradigm
Two bottle choice—20% alcohol
D1-Cre/Ai14 and A2A-Cre/Ai14 mice underwent 1 week of two bottle choice 20% (v/v) alcohol drinking paradigm (IA20%2BC) as described in [44, 45]. Specifically, 1 month after stereotaxic surgery and infection of the DMS with FLEX-shFyn or FLEX-SCR, mice had 24 h access to one bottle of 20% alcohol and one bottle of water on Monday, Wednesday, and Friday with alcohol drinking sessions starting 2 h into the dark cycle. During the 24 or 48 h (weekend) of alcohol deprivation periods, mice had access to two bottles of water.
Two bottle choice—10% alcohol
One month after stereotaxic surgery and the infection of the DMS or the DLS of D1-Cre or A2A-Cre with AAV-DIO-Gs-DREADD, mice were habituated by intraperitoneal (IP) injection of saline for three days. On test day, mice received a systemic administration of vehicle (0.5% DMSO) or Clozapine N-Oxide (CNO, 3 mg/kg). Fifteen minutes later, mice had access to one bottle of 10% (v/v) alcohol and one bottle of water. Alcohol and water intake were measured 4 h later. Mice were then given water only for one week and were tested again using a counterbalanced, within subject design.
A separate cohort of D1-Cre mice were infected with AAV-DIO-Gs-DREADD in the DMS. One month later, mice were systemically administered with vehicle (20% HPBCD) or AZD0530 (10 mg/kg) 3 h prior to the beginning of the drinking session. Subsequently, animals received a systemic administration of vehicle (0.5% DMSO) or CNO, (3 mg/kg) 15 min before the beginning of the drinking session, and alcohol and water intake were measured 4 h later. Mice were tested in a counterbalanced, within-subjects design.
Two bottle choice—0.03% saccharin
D1-Cre/Ai14 mice underwent an intermittent saccharin intake procedure [46]. Specifically, 2 weeks after the end of the alcohol drinking paradigm during which mice consumed only water, mice had access to one bottle of water and one bottle of saccharin (0.03%) for 1 week (2 session) and saccharin and water intake were evaluated.
The DMS of D1-Cre mice were infected with AAV-DIO-Gs-DREADD. On test day, mice received a systemic administration of vehicle (0.5% DMSO) or CNO (3 mg/kg). Fifteen minutes later, mice had access to a bottle of saccharin (0.03%) and one bottle of water. Saccharin and water intake were measured 4 h later. Mice were then given water only for one week and were tested again using a counterbalanced, within subject design.
To measure the level of Fyn signaling activation by saccharin, C57BL/6 were subjected to one week of home cage 0.03% intermittent saccharin intake and the DMS was dissected at the last 4 h drinking session.
Fluid consumption measurements
Alcohol, saccharin, and water bottles were presented in 50 ml graduated polypropylene cylinders with stainless steel drinking spouts inserted through 2 grommets in front of the cage. Bottles were weighted before and 4 h after the drinking session in order to determine the volume of the consumed fluid. The weight of each mouse was measured the day before the drinking session to calculate the grams of alcohol intake per kilogram of body weight. The placement (right or left) of the bottles was alternated in each session to control for side preference. Two bottles containing water and alcohol in a cage without mice were used to evaluate the spillage due to the experimental manipulations during the test sessions. The spillage was always ≤0.2 ml. Alcohol (g/kg), saccharin (ml/kg), and water (ml/kg) intake were recorded at the end of each 4-h drinking session.
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to plot and analyze the data. D’Agostino–Pearson normality test and F-test/Levene tests were used to verify the normal distribution of variables and the homogeneity of variance, respectively. Data were analyzed using the appropriate statistical test, including two-tailed unpaired or paired t-test, two-way analysis of variance (ANOVA) followed by post hoc tests as detailed in the figure legends. Experiments designed to evaluate the consequence of Fyn knockdown using FLEX-shFyn were analyzed with unpaired t-test. Experiments designed to test the contribution of cAMP signaling on alcohol intake using AAV-DIO-Gs-DREADD were analyzed with paired t-test since within subject design was used. All data are expressed as mean ± SEM, and statistical significance was set at p < 0.05.
Results
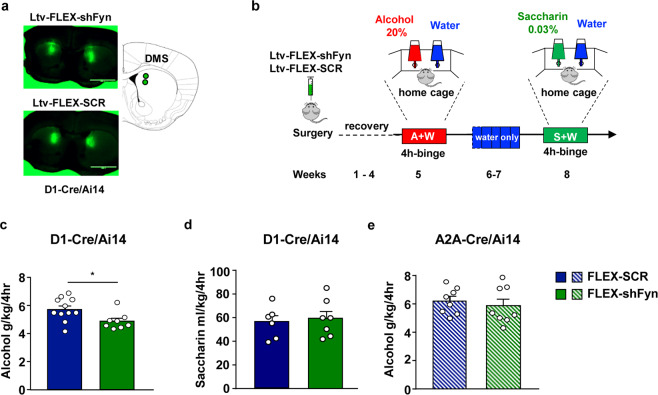
To determine whether Fyn’s actions are localized to DMS dMSNs and/or iMSNs, we used transgenic mice that express Cre recombinase and tdTomato specifically in dMSNs (D1-Cre/Ai14), or that express Cre recombinase and tdTomato specifically in iMSNs (A2A-Cre/Ai14), in combination with a Cre-dependent Flip Excision (FLEX) approach to downregulate Fyn mRNA in dMSNs or iMSNs, respectively, as described in [16]. First, we examined the consequence of Fyn knockdown in DMS dMSNs on alcohol drinking. To do so, the DMS of D1-Cre/Ai14 was infected bilaterally with a lentivirus expressing a short hairpin mRNA sequence targeting Fyn inserted in a FLEX cassette (FLEX-shFyn) (107 pg/ml, 1.2 μl per site, two sites per hemisphere) or with a FLEX virus expressing a scramble sequence, which was used as a control (FLEX-SCR, 107 pg/ml, 1.2 μl per site, two sites per hemisphere) (Fig. 1a). After 4 weeks, which enabled maximal viral infection and knockdown of the gene [16], mice were subjected to week of IA20%2BC and alcohol intake was measured at the last 4 h binge drinking session, a period in which mice drink the majority of alcohol, and in which mice reach a blood alcohol concentration (BAC) of over 80 mg% [44, 47] (Timeline, Fig. 1b). Alcohol intake was significantly reduced in D1-Cre/Ai14 mice infected with FLEX-shFyn in dMSNs as compared with the FLEX-SCR infected mice (Fig. 1c, Supplementary Table 1).
Fig. 1. Knockdown of Fyn in DMS dMSNs but not in iMSNs attenuates the development of alcohol but not saccharin intake.
a The DMS of D1-Cre/Ai14 mice was infected bilaterally with a lentivirus-expressing FLEX-shFyn or FLEX-SCR (107pg/ml, 1.2 μl per site, 2 injection per side), and viral infection was evaluated 4 weeks later. Infection was visualized on EVOS FL microscope (Life Technologies, Carlsbad, CA), scale: 2×. b Timeline of experiments. Four weeks after surgery, D1-Cre/Ai14 (c) or A2A-Cre/Ai14 mice (e) underwent 20%2BC 4 h binge drinking session, and alcohol and water intake were recorded. d At the completion of the alcohol drinking regimen, D1-Cre/Ai14 mice had access to water only for 2 weeks, followed by 1 week of home cage intermittent access to 0.03% saccharin. Saccharin and water intake were measured at the 4-h time point. c Knockdown of Fyn in the DMS of D1-Cre/Ai14 mice attenuates alcohol intake (two-tailed unpaired t-test, t = 2.491, p = 0.0234). d Knockdown of Fyn in the DMS of D1-Cre/Ai14 mice does not alter saccharin intake (two-tailed unpaired t-test, t = 0.2163, p = 0.8327). e Knockdown of Fyn in the DMS of A2A-Cre/Ai14 does not affect alcohol intake (two-tailed unpaired t-test, t = 0.583, p = 0.5692). Data are presented as individual data points and mean ± SEM. *p < 0.05. c FLEX-shFyn n = 8, FLEX-SCR n = 11. d FLEX-shFyn n = 7, FLEX-SCR n = 6, e FLEX-shFyn n = 8, FLEX-SCR n = 8.
We then assessed whether Fyn’s action in DMS dMSNs is specific for alcohol or is shared with other rewarding substances. To examine this question, we tested the consequences of Fyn knockdown in DMS dMSNs on the consumption of 0.03% saccharin (Timeline Fig. 1b). Knockdown of Fyn in DMS dMSNs did not alter saccharin intake (Fig. 1d, Supplementary Table 1).
As detailed in the introduction alcohol activates Fyn in the DMS of rodents [39, 41, 42] and that the activation of Fyn results in GluN2B phosphorylation [39, 41, 42]. To solidify the conclusion that Fyn in the DMS does not contribute to saccharin intake, we measured Fyn activation and GluN2B phosphorylation in the DMS of C57BL/6 mice consuming saccharin (Timeline, Supplementary Fig. 1a). As shown in Supplementary Fig. 1b, c, saccharin consumption (Supplementary Table 1), does not alter Fyn/GluN2B signaling in the DMS. Together, these results suggest that Fyn’s regulation of consummatory behavior in DMS dMSNs is specific for alcohol and is not generalized to other reinforcing agents.
Next, to determine whether downregulation of Fyn in iMSNs also affects alcohol intake, the DMS of A2A-Cre/Ai14 mice was infected with FLEX-shFyn or FLEX-SCR (Timeline, Fig. 1b). After 4 weeks of recovery allowing maximal virus infection, mice were subjected to 1 week of IA20%2BC period, and alcohol intake was measured at the last 4 h binge drinking session. We found that knockdown of Fyn in iMSNs does not alter alcohol intake (Fig. 1e, Supplementary Table 1) suggesting that Fyn participates in mechanisms underlying alcohol consumption through its actions in dMSNs but not in iMSNs.
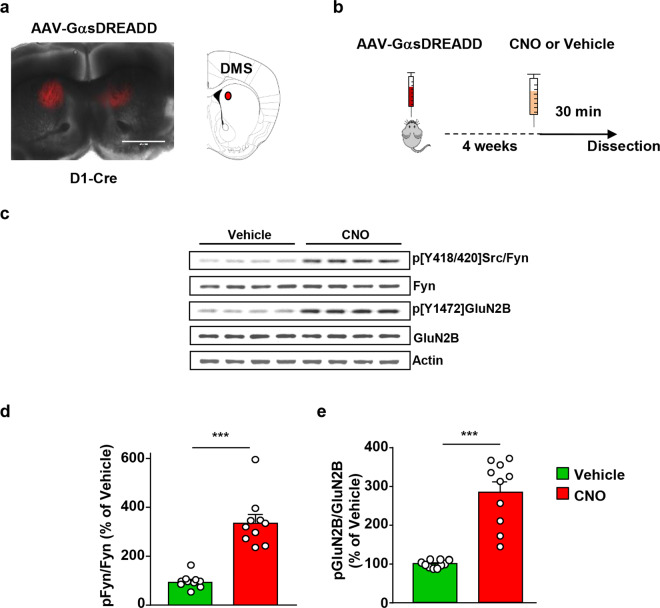
As stated above, D1Rs are selectively expressed in dMSNs [5]. D1Rs are coupled to Gαs/olf, and stimulation of Gαs/olf-coupled receptors activates cAMP/PKA signaling [6, 7]. Ex vivo data suggest that Fyn is activated in the hippocampal neurons through the cAMP/PKA pathway [24, 48–50]. We, therefore, postulated that alcohol activates Fyn through the stimulation of cAMP/PKA signaling in DMS dMSNs. To test this possibility, we utilized the Designer Receptor Exclusively Activated by Designer Drug (DREADD) system to remotely activate Gαs in DMS dMSNs or iMSNs [51]. First, the DMS of D1-Cre mice was infected bilaterally with AAV8-hSyn-DIO-rM3D(Gs)-mCherry (1013 vg/ml, 1 μl per hemisphere) (Fig. 2a). Four weeks after surgery, vehicle or CNO (3 mg/kg) was administered systemically, and the DMS was harvested 30 min later (Timeline, Fig. 2b). As shown in Fig. 2c, d, CNO administration produced a robust increase in the phosphorylation and thus activation of Fyn in the DMS. To measure Fyn activation, we utilized anti-phosphoTyrosine418/420Src/Fyn antibodies, which recognize the autophosphorylated active form of Fyn and Src [17, 18]. To confirm that the kinase that was activated in response to CNO administration was indeed Fyn and not Src, the DMS of D1-Cre mice of another cohort of animals was infected bilaterally with AAV8-hSyn-DIO-rM3D(Gs)-mCherry and treated with vehicle or CNO (3 mg/kg). Src was then immunoprecipitated using specific anti-Src antibodies (Supplementary Fig. 2a, b), and the level of Src activation in response to CNO treatment was measured using the anti-phosphoTyrosine418/420Src/Fyn antibodies. As shown in Supplementary Fig. 2c, Src was not activated upon stimulation of GαsDREADD in DMS dMSNs.
Fig. 2. Stimulation of GαsDREADD in DMS dMSNs activates Fyn/GluN2B signaling.
a The DMS of D1-Cre mice was infected bilaterally with AAV-DIO-rM3D(Gs)-mCherry (1013 vg/ml, 1 μl per side), and infection was evaluated 4 weeks later. Infection was visualized on EVOS FL microscope (Life Technologies, Carlsbad, CA), scale: 2×. b Timeline of experiment. Four weeks after surgery, vehicle (0.5% DMSO) or CNO (3 mg/kg) was systemically administered, and the DMS was dissected 30 min later. c Representative image of Fyn activation and GluN2B phosphorylation that were measure by Western blot analysis using anti-Tyr418/420[Src/Fyn] and anti-Tyr1472[GluN2B] antibodies, respectively. Total protein levels of Fyn, GluN2B, and actin, which was used as a loading control, were measured in parallel. d, e Data are presented as the individual data points and mean densitometry values of the phosphorylated protein divided by the densitometry values of the total protein ± SEM and expressed as % of vehicle. Activation of GαsDREADD in DMS dMSNs increases Fyn activation (d) (two-tailed unpaired t-test, t = 7.148, p < 0.001), and GluN2B phosphorylation (e) (two-tailed unpaired t-test, t = 6.948, p < 0.001). n = 10 per treatment. ***p < 0.001.
We also measured the phosphorylation level of the Fyn substrate, GluN2B [24], and found that Fyn activation in dMSNs was accompanied by the phosphorylation of GluN2B (Fig. 2c, e). In contrast, CNO administration did not alter Fyn’s activity or GluN2B phosphorylation in the DLS, a neighboring striatal region that was not infected with AAV8-hSyn-DIO-rM3D(Gs)-mCherry (Supplementary Fig. 3). Together, these data suggest that Fyn/GluN2B signaling is enhanced in response to remote activation of GαsDREADD in DMS dMSNs.
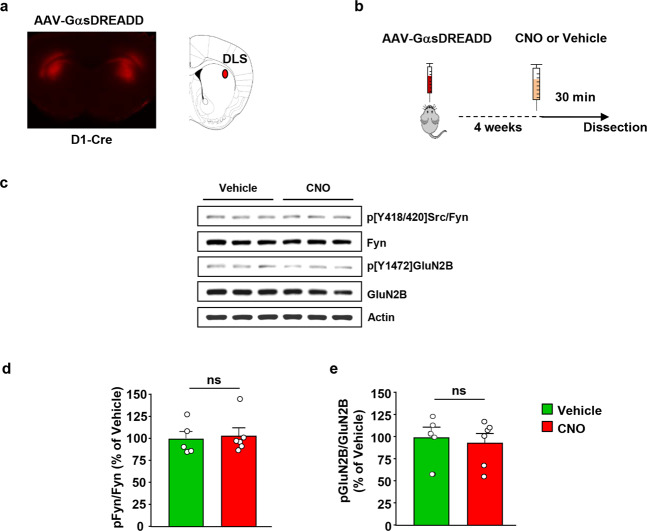
Next, we examined the level of Fyn signaling activation in the DLS dMSNs upon remote simulation of GαsDREADD. To do so, the DLS of Drd1-Cre mice was infected bilaterally with AAV8-hSyn-DIO-rM3D(Gs)-mCherry (1013 vg/ml, 1 μl per hemisphere) (Fig. 3a). Four weeks after surgery, vehicle or CNO (3 mg/kg) was administered systemically, and the DLS was harvested 30 min later (Timeline, Fig. 3b). Strikingly, as shown in Fig. 3c–e, remote activation of GαsDREADD in DLS dMSNs did not alter Fyn’s activity or GluN2B phosphorylation. These data suggest that GαsDREADD-dependent activation of Fyn signaling in dMSNs is centered in the DMS.
Fig. 3. Stimulation of GαsDREADD in DLS dMSNs does not activate Fyn/GluN2B signaling.
a The DLS of D1-Cre mice was infected bilaterally with AAV-DIO-rM3D(Gs)-mCherry (1013 vg/ml, 1 μl per side), and infection was evaluated 4 weeks later. Infection was visualized on EVOS FL microscope (Life Technologies, Carlsbad, CA), scale: 2×. b Timeline of experiment. Four weeks after surgery, vehicle (0.5% DMSO) or CNO (3 mg/kg) was systemically administered, and the DLS was dissected 30 min later. c Representative image of Fyn activation and GluN2B phosphorylation using anti-Tyr418/420[Src/Fyn] and anti-Tyr1472[GluN2B] antibodies, respectively. Total protein levels of Fyn, GluN2B and actin, which was used as a loading control, were measured in parallel. d, e Data are presented as the individual data points and mean densitometry values of the phosphorylated protein divided by the densitometry values of the total protein ± SEM and expressed as % of vehicle. Activation of GαsDREADD in DLS dMSNs does not change Fyn activation (d) (two-tailed unpaired t-test, t = 0.2957, p = 0.7742) and GluN2B phosphorylation (e) (two-tailed unpaired t-test, t = 0.4105, p = 0.6910). n = 5–6 per treatment. ns non-significant.
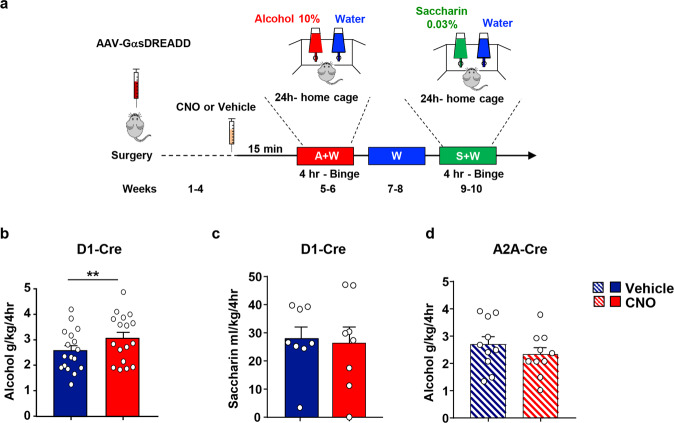
We then determined whether the cAMP-dependent activation of Fyn in dMSNs alters alcohol drinking. As Drd1-Cre mice consume large quantities of 20% alcohol ([43], Fig. 1c), we used a lower alcohol concentration (10% v/v) in order to avoid a confounding ceiling effect of alcohol intake due to GαsDREADD activation. The DMS of Drd1-Cre mice was infected bilaterally with AAV-hSyn-DIO-rM3D(Gs)-mCherry, 4 weeks later, vehicle or CNO (3 mg/kg) was administered systemically 15 min before the beginning of a 10% alcohol drinking session, and alcohol intake was measured after 4 h (Timeline, Fig. 4a). As shown in Fig. 4b, Supplementary Table 1, remote activation of GαsDREADD in DMS dMSNs significantly increased alcohol intake.
Fig. 4. Stimulation of GαsDREADD in DMS dMSNs but not in iMSNs increases alcohol but not saccharin intake.
a Timeline of experiment. The DMS of D1-Cre or A2A-Cre mice was infected bilaterally with AAV-DIO-rM3D(Gs)-mCherry (1013 vg/ml, 1 μl per side). b–d Four weeks after surgery, D1-Cre (b) or A2A-Cre (d) received a systemic administration of vehicle (0.5% DMSO) or CNO (3 mg/kg) 15 min before the beginning of the first 10% alcohol drinking session, and alcohol intake was measured 4 h later. c At the completion of the alcohol drinking regimen, D1-Cre- mice had access to water only for 2 weeks. Afterward, mice received a systemic administration of vehicle (0.5% DMSO) or CNO (3 mg/kg) 15 min before the beginning of a 0.03% saccharin drinking session, and saccharin and water intake were measured 4 h later. b Activation of GαsDREADD in DMS dMSNs increases alcohol intake (two-tailed paired t-test, t = 3.501, p = 0.003). c Activation of GαsDREADD in DMS dMSNs does not alter saccharin intake (two-tailed paired t-test, t = 0.2501, p = 0.8097). d Activation of GαsDREADD in the DMS of A2A-Cre does not affect alcohol intake (two-tailed paired t-test, t = 1.482, p = 0.1724). Data are presented as individual data points and mean ± SEM. b n = 17 per treatment, c n = 10 per treatment, d n = 8 per treatment. **p < 0.01.
Next, we examined whether remote activation of GαsDREADD in DMS dMSNs alters the consumption of saccharin. Two weeks after the end of the alcohol drinking experiment, vehicle, or CNO (3 mg/kg) was administered systemically 15 min prior to the initiation of the saccharin (0.03%) drinking session, and saccharin intake was measured after 4 h (Timeline, Fig. 4a). Activation of GαsDREADD in dMSNs did not alter saccharin intake (Fig. 4c, Supplementary Table 1) suggesting that the increase in consumption upon activation of GαsDREADD in DMS dMSNs is specific for alcohol.
We also examined whether remote activation of cAMP signaling in DMS iMSNs also alters alcohol intake. Interestingly, we found that CNO-dependent activation of GαsDREADD in DMS iMSNs does not affect alcohol intake (Fig. 4d, Supplementary Table 1) suggesting that cAMP signaling in dMSNs but not iMSNs contributes to the development of excessive alcohol consumption.
Gomez et al. reported that CNO is converted to clozapine prior to binding and activating DREADDs [52], and furthermore, CNO was reported to produce some behavioral effects on its own [53, 54]. Therefore, to ensure that the increase in alcohol intake was not due to off-target effects of the drug, we measured the level of alcohol consumption upon vehicle or CNO (3 mg/kg) treatment in D1-Cre mice that were not infected with AAV-hSyn-DIO-rM3D(Gs)-mCherry (Timeline, Supplementary Fig. 4a). CNO administration did not alter alcohol intake in uninfected D1-Cre mice (Supplementary Fig. 4b, Supplementary Table 1) suggesting that the increase in alcohol intake by CNO-dependent activation of GαsDREADD in DMS dMSNs is not due to off-target effects of the drug itself or its metabolite, clozapine.
Finally, we set out to test whether Fyn is required for GαsDREADD-dependent increase in alcohol consumption. To test this possibility, the DMS of D1-Cre mice was infected bilaterally with AAV-hSyn-DIO-rM3D(Gs)-mCherry. Four weeks later, vehicle or the Src/Fyn inhibitor AZD0530 (10 mg/kg) [29, 40, 55] was administered 3 h before the beginning of a 10% alcohol drinking session followed by the administration of vehicle or CNO (3 mg/kg) 15 min prior to the start of the session. Alcohol intake was measured at the end of a 4 h session (Timeline, Fig. 5a). In accordance with Fig. 4b, the amount of alcohol consumed by mice was elevated after the remote activation of GαsDREADD in DMS dMSNs, but the increase in alcohol intake was blocked when AZD0530 was administered prior to CNO (Fig. 5b, Supplementary Table 1).
Fig. 5. GαsDREADD-dependent increase in alcohol intake in DMS dMSNs requires Fyn.

a Timeline of experiment. The DMS of D1-Cre mice was infected bilaterally with AAV-DIO-rM3D(Gs)-mCherry (1013 vg/ml, 1 μl per side). Four weeks after surgery, animals received a systemic administration of vehicle (20% HPBCD) or AZD0530 (10 mg/kg) 3 h before the beginning of a 10% alcohol drinking session. Subsequently, animals received a systemic administration of vehicle (0.5% DMSO) or CNO (3 mg/kg) 15 min before the beginning of the drinking session, and alcohol and water intake were measured 4 h later. b AZD0530 blocks GαsDREADD-dependent enhancement of alcohol intake (Two-way RM ANOVA, effect of AZD0530: F1,10 = 9.177, p = 0.0127; effect of CNO: F1,10 = 19.43, p = 0.0013). Data are presented as individual values and mean ± SEM. n = 11. **p < 0.01. ns non-significant.
In order to ensure that the differences observed above are not due to a change in alcohol metabolism, we measured BAC after AZD0530 administration. To do so, mice received a systemic administration of vehicle or AZD0530 (10 mg/kg), 3 h later, mice received a systemic administration of alcohol (2 g/kg), and BAC was measured 30 min later (Timeline, Supplementary Fig. 5a). As shown in Supplementary Fig. 5b, BAC was similar in vehicle and AZD0530 treated mice suggesting that the drug does not affect alcohol metabolism.
Together, these results suggest alcohol intake is driven through the cAMP/PKA/Fyn signaling in DMS dMSNs.
Discussion
Here, we present data to suggest that cAMP/PKA-dependent activation of Fyn kinase in the DMS dMSNs participates in mechanisms underlying the development of excessive alcohol intake. Specifically, we show that downregulation of Fyn levels in DMS dMSNs prior to the initiation of the IA20%2BC regimen attenuates alcohol intake. We further report that the stimulation of Gαs signaling in DMS dMSNs activates Fyn/GluN2B signaling and enhances alcohol intake in a Fyn-dependent manner. In contrast, knockdown of Fyn or activation of cAMP signaling in iMSNs does not alter alcohol intake and preference. Finally, we show that the cAMP/Fyn signaling in DMS dMSNs is specific for alcohol and does not contribute to mechanisms underlying consummatory behavior per se. Based on previous data showing that Fyn is activated in the DMS through the stimulation of D1R in DMS dMSNs [16], and since dopamine levels in the dorsal striatum are elevated by drugs of abuse including alcohol [56, 57], we propose a model in which dopamine, released in the DMS in response to alcohol exposure, stimulates D1R/cAMP/PKA signaling in dMSNs which in turn activates Fyn to initiate neuroadaptations such as GluN2B phosphorylation that promote the development of excessive alcohol use (Supplementary Fig. 6).
Our data suggest that Fyn in DMS dMSNs contributes to the development of excessive alcohol consumption. The DMS is essential for goal-directed behaviors [2, 3], and dMSNs contribute to reward learning and reinforcement [58, 59]. Thus, it is plausible that Fyn in dMSNs promotes reward learning, which in turn initiates and maintains goal-directed alcohol seeking. This possibility is in line with the finding that systemic administration of the Src/Fyn inhibitor, AZD0530, attenuates goal-directed alcohol self-administration in mice [40]. Xie et al. previously showed that administration of the Src/Fyn inhibitor PP2 into the dorsal hippocampus attenuates context-dependent cocaine seeking [60], and more recently, Belin–Rauscent reported that oral administration of the Src/Fyn s inhibitor, Masitinib, attenuates self-administration of cocaine [61]. Thus, it would be of interest to determine whether Fyn in DMS dMSNs contributes to goal-directed seeking of other drugs of abuse. Furthermore, Goto et al. previously reported that mating behavior increases PKA activity in dorsal striatal dMSNs [62]. As PKA in DMS dMSNs is upstream of Fyn, it is plausible that the Fyn in these neurons plays a role in other goal-directed behaviors such as mating. Finally, Bocarsly et al. previously showed that enhanced D1R signaling in the dorsal striatum of mice is required for the consumption of alcohol despite negative consequences, and for the enhancement of alcohol-dependent hyperlocomotion [13]. Therefore, it plausible that Fyn in DMS dMSNs also contributes to other alcohol-dependent behaviors.
We found that stimulation of cAMP/PKA signaling activates Fyn but not Src in DMS dMSNs. This observation is in line with previous data showing that Fyn but not Src is activated in the dorsal striatum by alcohol exposure [38] or D1R stimulation [16]. However, it is plausible that the cAMP/PKA-dependent activation of Src in other brain regions contribute to excessive alcohol use. For instance, Zhang et al. reported that opiate withdrawal activates Src in the locus coeruleus [63].
The data herein and previous findings [31] suggest that Fyn exerts its action on alcohol drinking in dMSNs through the phosphorylation and activation of GluN2B. However, we cannot exclude the possibility that other molecular transducers of Fyn contribute to the development of excessive alcohol use. For example, protein translation plays a critical role in mechanisms underlying AUD [64], and Fyn was shown to enhance protein translation in oligodendrocytes [65] and in neurons [66]. Fyn was also shown to promote ERK1/2 phosphorylation in oligodendrocytes [67], and NfkB signaling in microglia [68]; both signaling cascades have been linked to alcohol use [30, 69]. Finally, Fyn was reported to phosphorylate the metabotropic glutamate receptor 1 (mGluR1) [70] and the Collapsin Response Mediator Protein 2 (CRMP2) [71], which have also been implicated in alcohol’s actions in the brain [72, 73]. Exploring the contribution of these substrates and others in DMS dMSNs to the neuroadaptations underlying AUD merits further investigation.
It is highly likely that the activation of PKA in DMS dMSNs produces additional cellular consequences. For example, PKA phosphorylates the Striatal-Enriched Protein Tyrosine Phosphatase (STEP) [50] resulting in the inhibition of the activity of the phosphatase [50]. STEP is an endogenous Fyn inhibitor and is responsible for the termination of Fyn activation [50]. We previously reported that alcohol increases PKA phosphorylation of STEP in the DMS, and that knockdown of STEP in the DMS [41], or global knockout of the phosphatase increases alcohol intake [74]. It is therefore plausible that one of the consequences of PKA activation in DMS dMSNs is the phosphorylation of STEP, enabling Fyn in these neurons to stay active for a prolonged period of time.
Using the DREADD/CNO methodology, we report that the activation of GαsDREADD in dMSNs but not in iMSNs initiates the consumption of alcohol. We further show that GαsDREADD-dependent increase in alcohol intake depends, at least in part, on Fyn. To our knowledge this is the first study that provides a link between GαsDREADD activation and alcohol consummatory behavior. Cheng et al. previously showed that GαiDREADD-mediated inhibition of iMSNs enhances alcohol intake [75]. Stimulation of Gαs-coupled receptors activates adenylate cyclase that increases cAMP production, whereas the stimulation of Gαi-coupled receptors inhibits adenylate cyclase activity and cAMP production [7]. Thus, it is plausible that the development of excessive alcohol consumption depends on the inhibition of cAMP signaling in iMSNs and on the activation of cAMP signaling in dMSNs.
Our data suggest that alcohol-dependent molecular adaptations are highly specific and are segregated to a subpopulation of neurons. Future studies are necessary to determine whether this cell-type specificity is unique for the cAMP/PKA/Fyn signaling or that this is a common feature shared by some or all of the molecular targets of alcohol [30].
Although we provide a strong evidence linking PKA signaling in dMSNs to alcohol drinking behaviors, we cannot exclude the possibility that other cAMP effectors such as the guanine nucleotide exchange factor EPAC (exchange protein directly activated by cAMP) [76], and/or cyclic nucleotide-gated ion channels (CNGC) [77] also contribute to the development of excessive alcohol use. Finally, further research is required to monitor cAMP production and PKA activation in behaving animals. Recent advances in the development of reporters for cAMP [78], and PKA [79] will enable a spatial and temporal analysis of cAMP/PKA signaling in animals consuming alcohol.
Finally, we previously showed that treatment of mice with AZD530 attenuates alcohol-dependent Fyn activation and GluN2B phosphorylation in the DMS [40], and reduces goal-directed alcohol seeking. We show herein that the enhancement of alcohol intake upon activation of GαsDREADD in DMS dMSNs is inhibited upon the administration of AZD530. AZD530 is well-tolerated in humans, as phase I and II clinical trials indicate that the drug does not produce significant side effects [80, 81], and our preclinical mouse studies show that systemic administration of the drug does not alter basal levels of locomotion [40], and does not change BAC. Together, these data give rise to the potential use of AZD530 in AUD.
Funding and disclosure
This research was supported by the National Institute of Alcohol Abuse and Alcoholism, UO1 AA023489 (DR and VAA). The authors have no conflict of interest.
Supplementary information
Acknowledgements
We thank AstraZeneca for providing us with AZD5030. The authors thank Ellanor Whiteley for her contribution.
Author contributions
YE contributed to the design of the experiments, the acquisition of data, data analysis, and to the preparation and revision of the manuscript. NM, SAS, and KP contributed to the design of the experiments, the acquisition of the data and data analysis. MFA contributed to the acquisition of data. VAA contributed to the conception of the study. DR contributed to the conception of the study, the design of the experiments, and wrote the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-0712-1).
References
- 1.Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs–roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–70. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005;32:205–16.. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- 3.Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–42. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herve D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. 2011;5:48. doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/RRS-200029981. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta. 2013;1834:1271–8. doi: 10.1016/j.bbapap.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol. 1997;7:397–403. doi: 10.1016/S0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- 11.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 12.Ryan MB, Bair-Marshall C, Nelson AB. Aberrant striatal activity in parkinsonism and levodopa-induced dyskinesia. Cell Rep. 2018;23:3438–46 e5. doi: 10.1016/j.celrep.2018.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bocarsly ME, da Silva ESD, Kolb V, Luderman KD, Shashikiran S, Rubinstein M, et al. A mechanism linking two known vulnerability factors for alcohol abuse: heightened alcohol stimulation and low striatal dopamine D2 receptors. Cell Rep. 2019;29:1147–63 e5. doi: 10.1016/j.celrep.2019.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunaydin LA, Kreitzer AC. Cortico-basal ganglia circuit function in psychiatric disease. Annu Rev Physiol. 2016;78:327–50. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- 15.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phamluong K, Darcq E, Wu S, Sakhai SA, Ron D. Fyn signaling is compartmentalized to dopamine D1 receptor expressing neurons in the dorsal medial striatum. Front Mol Neurosci. 2017;10:273. doi: 10.3389/fnmol.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resh MD. Fyn, a Src family tyrosine kinase. Int J Biochem Cell Biol. 1998;30:1159–62. doi: 10.1016/S1357-2725(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 18.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Umemori H, Wanaka A, Kato H, Takeuchi M, Tohyama M, Yamamoto T. Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system. Brain Res Mol Brain Res. 1992;16:303–10. doi: 10.1016/0169-328X(92)90239-8. [DOI] [PubMed] [Google Scholar]
- 20.Yagi T, Shigetani Y, Okado N, Tokunaga T, Ikawa Y, Aizawa S. Regional localization of Fyn in adult brain; studies with mice in which fyn gene was replaced by lacZ. Oncogene. 1993;8:3343–51. [PubMed] [Google Scholar]
- 21.Ohnishi H, Murata Y, Okazawa H, Matozaki T. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 2011;34:629–37. doi: 10.1016/j.tins.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Chattopadhyaya B, Baho E, Huang ZJ, Schachner M, Di Cristo G. Neural cell adhesion molecule-mediated Fyn activation promotes GABAergic synapse maturation in postnatal mouse cortex. J Neurosci. 2013;33:5957–68. doi: 10.1523/JNEUROSCI.1306-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildebrand ME, Xu J, Dedek A, Li Y, Sengar AS, Beggs S, et al. Potentiation of synaptic GluN2B NMDAR currents by Fyn Kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep. 2016;17:2753–65.. doi: 10.1016/j.celrep.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279:12–9. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- 25.Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–10. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 26.Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci USA. 1997;94:4761–5. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–28. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 28.Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci USA. 2002;99:5710–5. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann Neurol. 2015;77:953–71. doi: 10.1002/ana.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–91. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morisot N, Ron D. Alcohol-dependent molecular adaptations of the NMDA receptor system. Genes Brain Behav. 2017;16:139–48.. doi: 10.1111/gbb.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiguro H, Saito T, Shibuya H, Toru M, Arinami T. Mutation and association analysis of the Fyn kinase gene with alcoholism and schizophrenia. Am J Med Genet. 2000;96:716–20. doi: 10.1002/1096-8628(20001204)96:6<716::AID-AJMG3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Pastor IJ, Laso FJ, Ines S, Marcos M, Gonzalez-Sarmiento R. Genetic association between -93A/G polymorphism in the Fyn kinase gene and alcohol dependence in Spanish men. Eur Psychiatry. 2009;24:191–4. doi: 10.1016/j.eurpsy.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Schumann G, Rujescu D, Kissling C, Soyka M, Dahmen N, Preuss UW, et al. Analysis of genetic variations of protein tyrosine kinase fyn and their association with alcohol dependence in two independent cohorts. Biol Psychiatry. 2003;54:1422–6. doi: 10.1016/S0006-3223(03)00635-8. [DOI] [PubMed] [Google Scholar]
- 35.Han S, Yang BZ, Kranzler HR, Liu X, Zhao H, Farrer LA, et al. Integrating GWASs and human protein interaction networks identifies a gene subnetwork underlying alcohol dependence. Am J Hum Genet. 2013;93:1027–34. doi: 10.1016/j.ajhg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, et al. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- 37.Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–42. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, et al. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–98. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morisot N, Berger AL, Phamluong K, Cross A, Ron D. The Fyn kinase inhibitor, AZD0530, suppresses mouse alcohol self-administration and seeking. Addict Biol. 2019;24:1227–34.. doi: 10.1111/adb.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darcq E, Hamida SB, Wu S, Phamluong K, Kharazia V, Xu J, et al. Inhibition of striatal-enriched tyrosine phosphatase 61 in the dorsomedial striatum is sufficient to increased ethanol consumption. J Neurochem. 2014;129:1024–34. doi: 10.1111/jnc.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibb SL, Hamida SB, Lanfranco MF, Ron D. Ethanol-induced increase in Fyn kinase activity in the dorsomedial striatum is associated with subcellular redistribution of protein tyrosine phosphatase alpha. J Neurochem. 2011;119:879–89.. doi: 10.1111/j.1471-4159.2011.07485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, et al. Alcohol elicits functional and structural plasticity selectively in dopamine D1 receptor-expressing neurons of the dorsomedial striatum. J Neurosci. 2015;35:11634–43. doi: 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warnault V, Darcq E, Levine A, Barak S, Ron D. Chromatin remodeling–a novel strategy to control excessive alcohol drinking. Transl Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laguesse S, Morisot N, Shin JH, Liu F, Adrover MF, Sakhai SA, et al. Prosapip1-dependent synaptic adaptations in the nucleus accumbens drive alcohol intake, seeking, and reward. Neuron. 2017;96:145–59 e8. doi: 10.1016/j.neuron.2017.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben Hamida S, Neasta J, Lasek AW, Kharazia V, Zou M, Carnicella S, et al. The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. J Neurosci. 2012;32:15849–58. doi: 10.1523/JNEUROSCI.2846-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D. The first alcohol drink triggers mTORC1-dependent synaptic plasticity in nucleus accumbens dopamine D1 receptor neurons. J Neurosci. 2016;36:701–13. doi: 10.1523/JNEUROSCI.2254-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–8. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- 49.Thornton C, Tang KC, Phamluong K, Luong K, Vagts A, Nikanjam D, et al. Spatial and temporal regulation of RACK1 function and N-methyl-D-aspartate receptor activity through WD40 motif-mediated dimerization. J Biol Chem. 2004;279:31357–64. doi: 10.1074/jbc.M402316200. [DOI] [PubMed] [Google Scholar]
- 50.Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, et al. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharm Rev. 2012;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ, et al. A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology. 2013;38:854–62. doi: 10.1038/npp.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–07.. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, et al. Clozapine N-oxide administration produces behavioral effects in long-evans rats: implications for designing DREADD experiments. eNeuro. 2016;3. [DOI] [PMC free article] [PubMed]
- 54.Goutaudier R, Coizet V, Carcenac C, Carnicella S. DREADDs: the power of the lock, the weakness of the Key. Favoring the pursuit of specific conditions rather than specific ligands. eNeuro. 2019;6. [DOI] [PMC free article] [PubMed]
- 55.Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. 2006;49:6465–88. J Med Chem. 2006;49:6465–88. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- 56.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lof E, Chau PP, Stomberg R, Soderpalm B. Ethanol-induced dopamine elevation in the rat–modulatory effects by subchronic treatment with nicotinic drugs. Eur J Pharm. 2007;555:139–47. doi: 10.1016/j.ejphar.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 58.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie X, Arguello AA, Wells AM, Reittinger AM, Fuchs RA. Role of a hippocampal SRC-family kinase-mediated glutamatergic mechanism in drug context-induced cocaine seeking. Neuropsychopharmacology. 2013;38:2657–65. doi: 10.1038/npp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belin-Rauscent A, Lacoste J, Hermine O, Moussy A, Everitt BJ, Belin D. Decrease of cocaine, but not heroin, self-administration and relapse by the tyrosine kinase inhibitor masitinib in male Sprague Dawley rats. Psychopharmacol (Berl) 2018;235:1545–56.. doi: 10.1007/s00213-018-4865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goto A, Nakahara I, Yamaguchi T, Kamioka Y, Sumiyama K, Matsuda M, et al. Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc Natl Acad Sci USA. 2015;112:6718–23. doi: 10.1073/pnas.1507121112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, Kibaly C, Wang YJ, Xu C, Song KY, McGarrah PW, et al. Src-dependent phosphorylation of mu-opioid receptor at Tyr(336) modulates opiate withdrawal. EMBO Mol Med. 2017;9:1521–36.. doi: 10.15252/emmm.201607324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laguesse S, Ron D. Protein translation and psychiatric disorders. Neuroscientist. 2020;26:21–42. doi: 10.1177/1073858419853236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White R, Gonsior C, Kramer-Albers EM, Stohr N, Huttelmaier S, Trotter J. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J Cell Biol. 2008;181:579–86. doi: 10.1083/jcb.200706164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C, Gotz J. Somatodendritic accumulation of Tau in Alzheimer’s disease is promoted by Fyn-mediated local protein translation. EMBO J. 2017;36:3120–38.. doi: 10.15252/embj.201797724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peckham H, Giuffrida L, Wood R, Gonsalvez D, Ferner A, Kilpatrick TJ, et al. Fyn is an intermediate kinase that BDNF utilizes to promote oligodendrocyte myelination. Glia. 2016;64:255–69. doi: 10.1002/glia.22927. [DOI] [PubMed] [Google Scholar]
- 68.Panicker N, Saminathan H, Jin H, Neal M, Harischandra DS, Gordon R, et al. Fyn kinase regulates microglial neuroinflammatory responses in cell culture and animal models of Parkinson’s disease. J Neurosci. 2015;35:10058–77. doi: 10.1523/JNEUROSCI.0302-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin DZ, Mao LM, Wang JQ. An essential role of fyn in the modulation of metabotropic glutamate receptor 1 in neurons. eNeuro. 2017;4. [DOI] [PMC free article] [PubMed]
- 71.Uchida Y, Ohshima T, Yamashita N, Ogawara M, Sasaki Y, Nakamura F, et al. Semaphorin3A signaling mediated by Fyn-dependent tyrosine phosphorylation of collapsin response mediator protein 2 at tyrosine 32. J Biol Chem. 2009;284:27393–401. doi: 10.1074/jbc.M109.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharm. 2010;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu F, Laguesse S, Legastelois R, Morisot N, Ben Hamida S, Ron D. mTORC1-dependent translation of collapsin response mediator protein-2 drives neuroadaptations underlying excessive alcohol-drinking behaviors. Mol Psychiatry. 2017;22:89–101. doi: 10.1038/mp.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Legastelois R, Darcq E, Wegner SA, Lombroso PJ, Ron D. Striatal-enriched protein tyrosine phosphatase controls responses to aversive stimuli: implication for ethanol drinking. PLoS One. 2015;10:e0127408. doi: 10.1371/journal.pone.0127408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, et al. Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption. Biol Psychiatry. 2017;81:918–29.. doi: 10.1016/j.biopsych.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharm Rev. 2013;65:670–709. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- 77.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 78.Muntean BS, Zucca S, MacMullen CM, Dao MT, Johnston C, Iwamoto H, et al. Interrogating the spatiotemporal landscape of neuromodulatory GPCR signaling by real-time imaging of cAMP in intact neurons and circuits. Cell Rep. 2018;22:255–68.. doi: 10.1016/j.celrep.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma L, Jongbloets BC, Xiong WH, Melander JB, Qin M, Lameyer TJ, et al. A highly sensitive A-kinase activity reporter for imaging neuromodulatory events in awake mice. Neuron. 2018;99:665–79 e5. doi: 10.1016/j.neuron.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res Ther. 2015;7:35. doi: 10.1186/s13195-015-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nygaard HB. Targeting Fyn kinase in alzheimer’s disease. Biol Psychiatry. 2018;83:369–76. doi: 10.1016/j.biopsych.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.