We agree in principle with our colleagues’ endorsement in this journal of the use of the tropomyosin receptor kinase (TRK) inhibitor LOXO-101 (larotrectinib) in children.1 Our enthusiasm for the accelerated deployment of LOXO-101 has been tempered by our experience of multi-TRK inhibitor resistance though. Resistance mutations to LOXO-101 do occur but can be overcome by second- generation TRK inhibitors, such as LOXO-195 (selitrectinib).2 We, however, report the emergence of resistance mutations to both first- and second-generation TRK inhibitors in an NTRK-driven infantile fibrosarcoma (IFS). Our case questions the introduction of TRK inhibitors into clinical practice without the scrutiny of comparative clinical trials, especially in the context of NTRK-driven childhood tumours that are rarely lethal. Our cautionary words are particularly pertinent in view of the recent approval to use LOXO-101 by the UK’s National Institute for Health and Care Excellence.
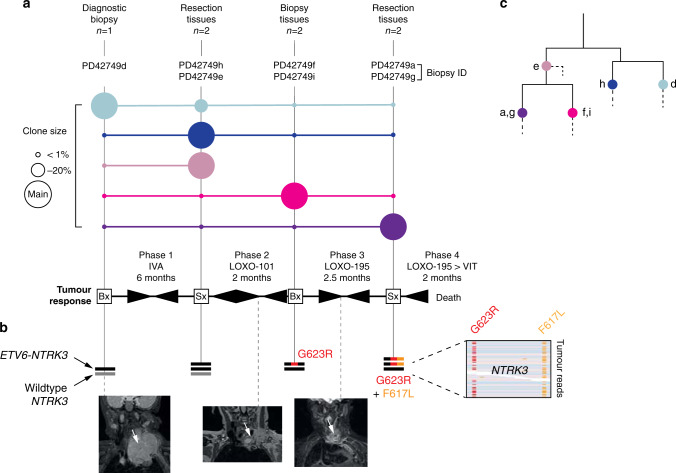
Our patient, a 10-month-old girl, suffered from an anterior mediastinal IFS extending into the neck that harboured a canonical IFS-associated ETV6-NTRK3 gene fusion. We treated her over a period of 12.5 months with cytotoxic chemotherapy, first- and second-generation TRK inhibitors and two attempts at complete surgical resection (Fig. 1a, b). The tumour repeatedly regrew and ultimately led to the child’s death. To understand the development of treatment resistance, we reconstructed the evolution of the lethal clone from somatic mutations, obtained by whole genome (fatal relapse) or exome sequences (seven tissues obtained at diagnosis and after each recurrence). At diagnosis, the tumour contained single copies of wild-type NTRK3 and the ETV6-NTRK3 fusion (Fig. 1b). Analysis of the first relapse revealed duplication of the ETV6-NTRK3 fusion. At second relapse, after 2 months of treatment with a first-generation TRK inhibitor (LOXO-101), we detected the NTRK3 resistance mutation, G623R,3 along with loss of the wild-type NTRK3 gene. Then, following 2.5 months of treatment with a second-generation TRK inhibitor (LOXO-195), multidrug resistance emerged in the shape of two mutations, NTRK3 G623R and F617L.3 Both were present in the same cells of the dominant clone, as they were captured on the same reads (i.e. the same DNA molecule) (Fig. 1b). Reconstructing the phylogeny of tumour lineages from substitutions and small indels revealed a complex clonal composition of the tumour, from which the lethal, double- mutant clone emerged (Fig. 1c). These analyses delineate the development of a seemingly “supercharged” ETV6-NTRK3 resistance fusion, forged under the selective pressure of TRK inhibitors.
Fig. 1. Evolution of multi-TRK resistance.
a Clonal composition of tumour tissues over the course of illness. Coloured circles denote the clonal composition of tissues sequenced at a given time point. The scale of a clone’s contribution to these samples is relative to a circle’s size; larger circles indicate a greater contribution by that clone. Beneath this is a simplified overview of principal systemic therapies and the resultant tumour responses. IVA ifosfamide, vincristine, actinomycin D, VIT vincristine, irinotecan, temozolomide, LOXO-101 (larotrectinib) first-generation TRK inhibitor, LOXO-195 (selitrectinib) second-generation TRK inhibitor. Arrowheads tapering from left to right indicate partial/complete tumour response and vice versa for progression. b Sequence of somatic alterations of NTRK3. Black line: ETV6-NTRK3 fusion. Grey line: wild-type copy of NTRK3. Number of lines corresponds to copy number. Screen capture on the right shows the co-occurrence of both resistance mutations on the same tumour sequencing reads. At the bottom are three coronal MRI images of the tumour (white arrow): one at diagnosis (left) and two demonstrating the tumour’s best response to LOXO-101 (middle) and LOXO-195 (right). c Phylogenetic tree of tumour lineages. Solid lines: major branches where mapped mutations revealed the relationship of the clones identified. Labelled overlying coloured circles represent the clones shown in panel a. Dashed lines: private mutations that did not inform tree building.
The key evidence justifying the use of LOXO-101 in children with NTRK-driven tumours is a phase 1/2 study, which showed an impressive 93% objective response rate (ORR) amongst 15 children.4 Two of these with locally advanced disease even achieved R0 resections following treatment with TRK inhibitors. We note that one patient did develop the same NTRK3 G623R resistance mutation as our patient although responded to a second-generation inhibitor (selitrectinib). Recent work in a mixed-age cohort across histologies suggests that a 45% ORR can be achieved with LOXO-195 in patients with these NTRK kinase domain mutations.3
On this basis, it would appear that TRK inhibition is an attractive, non-cytotoxic strategy for tumour shrinkage prior to, or even in lieu of, surgery in childhood tumours of low metastatic potential, such as IFS. Our challenge has been that the same marked clinical responses to both TRK inhibitors were seen in our patient, yet were short lived and preceded the outgrowth of a lethal tumour clone. Without access to long-term efficacy data or head-to-head comparisons to best current practice, it is difficult to know how representative our experience is and so highlights the current challenge in identifying the most opportune moment to utilise TRK inhibitors in the management of IFS. Clearly, in the context of invariably lethal cancers, phase 1/2 studies may provide sufficient evidence for utilising tumour-agnostic drugs. However, in seldom lethal NTRK-driven childhood tumours, we require comparative clinical trials beyond basket studies to learn when and how to deploy these drugs. Until such evidence is available, we would advocate a cautious use of TRK inhibitors in children.
Acknowledgements
We thank Dr Grace Collord for her review of this letter. We are grateful to our patient and her family for participation in our investigation.
Author contributions
T.R.W.O. analysed sequencing data. T.J.J. and O.O. contributed to experiments. N.S. provided pathological, and O.S., M.J. and S.B. oncological expertise. M.J. and S.B. co-directed this study.
Ethics approval and consent to participate
The child’s legal guardian provided informed consent for participation in this REC-approved study (UK NHS National Research Ethics Service reference 16/EE/0394). All patient information has been anonymised for the purposes of this letter. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Express consent has been given to publish the details of this case by the child’s legal guardian.
Data availability
Raw sequencing data pertaining to this case are available in EGA.
Competing interests
Dr. Slater has advised Bayer on TRK inhibitors. The remaining authors declare no competing interests.
Funding information
S.B. and T.R.W.O. are funded by Wellcome. All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mette Jorgensen, Email: mette.jorgensen@gosh.nhs.uk.
Sam Behjati, Email: sb31@sanger.ac.uk.
References
- 1.Chisholm JC, Carceller F, Marshall LV. Tumour-agnostic drugs in paediatric cancers. Br. J. Cancer. 2020;122:1425–1427. doi: 10.1038/s41416-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drilon A, Nagasubramanian R, Blake JF, Ku N, Tuch BB, Ebata K, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017;7:963–972. doi: 10.1158/2159-8290.CD-17-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 2019;30(Suppl 8):viii23–viii30. doi: 10.1093/annonc/mdz282. [DOI] [PubMed] [Google Scholar]
- 4.Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19:705–714. doi: 10.1016/S1470-2045(18)30119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw sequencing data pertaining to this case are available in EGA.