Abstract
In nature, bacteria form biofilms by producing exopolymeric matrix that encases its entire community. While it is widely known that biofilm matrix can prevent bacterivore predation and contain virulence factors for killing predators, it is unclear if they can alter predator motility. Here, we report a novel “quagmire” phenotype, where Pseudomonas aeruginosa biofilms could retard the motility of bacterivorous nematode Caenorhabditis elegans via the production of a specific exopolysaccharide, Psl. Psl could reduce the roaming ability of C. elegans by impeding the slithering velocity of C. elegans. Furthermore, the presence of Psl in biofilms could entrap C. elegans within the matrix, with dire consequences to the nematode. After being trapped in biofilms, C. elegans could neither escape effectively from aversive stimuli (noxious blue light), nor leave easily to graze on susceptible biofilm areas. Hence, this reduced the ability of C. elegans to roam and predate on biofilms. Taken together, our work reveals a new function of motility interference by specific biofilm matrix components, and emphasizes its importance in predator–prey interactions.
Subject terms: Biofilms, Bacterial host response
Introduction
Bacteria colonize most natural surfaces and their hosts as biofilms. The encased community of bacterial cells produces its extracellular matrix that serves as a barrier from physicochemical factors [1], and allows bacterial differentiation and specialization [2, 3]. Living in biofilms can offer strong competitive advantages in the presence of various environmental stresses, such as predation, immune attack, and antimicrobials. Depending on the stimuli and gene regulatory networks, the biofilm matrix is highly complex and dynamic. For instance, the opportunistic pathogen Pseudomonas aeruginosa secretes varying compositions and levels of exopolysaccharides (Pel, Psl, and alginate), adhesion proteins (CdrA), extracellular DNA (eDNA), allowing it colonize both abiotic and biotic surfaces [4].
The production of biofilm matrix is mainly mediated by the c-di-GMP secondary messenger system, which is common in most gram-negative bacterial species [5]. Synthesis of c-di-GMP by diguanylate cyclases (DGCs) will lead to biofilm formation via loss of bacterial motility and induced production of exopolymeric matrix, whereas phosphodiesterase (PDE)-mediated degradation of c-di-GMP causes biofilm dispersal and production of specific virulence factors, such as exotoxin A from type III secretion system (T3SS) and rhamnolipids [6–9]. The redundancy of DGCs and PDEs in P. aeruginosa leads to fine-tuning the expression of various biofilm matrix components. For instance, production of the Pel and Psl exopolysaccharides is controlled by the wsp operon, with WspR as the DGC [10], whereas MucR controls alginate production [11]. PDEs, such as DipA, are involved in biofilm dispersal [12], while RocR mediates swarming motility [13].
In the natural environment, Caenorhabditis elegans are bacterivores that roam and feed on microbial biofilms growing on rotten fruit or plant biomass. As a model organism applicable to a multitude of research fields encompassing developmental biology, behavioral studies to infections, C. elegans are experimentally grown on bacterial lawns in media plates. While Escherichia coli OP50 are common food choices for C. elegans, Yersinia biofilms were previously shown to block the mouth and prevent bacterial uptake by C. elegans, resulting in the nematodes’ death by starvation [14]. Furthermore, biofilms formed by P. aeruginosa [15] and Salmonella [16] could produce specific virulence factors, such as pyoverdine [17], that killed C. elegans after being internalized into the intestine. While these effects mainly revolved around C. elegans feeding and intestinal infection, it is unclear if biofilms can alter motility which is a key feature of C. elegans.
Here, we report for the first time that the biofilm matrix can alter C. elegans locomotion and its resulting behavior, herein termed as the “quagmire” phenotype. Using the P. aeruginosa mutant library of known components and regulators for biofilm matrix, we showed that Psl, a key exopolysaccharide present in the P. aeruginosa biofilm matrix, could impede nematode locomotion, which was adequately reflected in reduced velocity and roaming of C. elegans. Trapping of nematodes in the P. aeruginosa biofilm matrix significantly reduced the ability of C. elegans to either escape from a noxious blue-light repellent, or move toward the susceptible OP50 biofilms.
Taken altogether, our study suggests a novel mechanism by which biofilms employ to impede C. elegans movement, possibly to delay predation and boost survival. In the context of bacterial infections, our findings also suggest a plausible similarity in specific biofilm matrix components impeding immune cell migration.
Materials and methods
Bacterial strains and growth conditions
The bacterial strains and plasmids used in this study are listed in Supplementary Table S1. E. coli DH5a strain was used for standard DNA manipulations. Luria–Bertani (LB) medium was used to cultivate E. coli and P. aeruginosa strains. For plasmid maintenance in E. coli, the medium was supplemented with 100 μg ml−1 ampicillin and 15 μg ml−1 gentamycin. For marker selection in P. aeruginosa, 30 μg ml−1 gentamycin, 30 μg ml−1 tetracycline, or 100 μg ml−1 carbenicillin was used, as appropriate.
Growth and maintenance of C. elegans
The bacterial lawn, such as E. coli OP50 and P. aeruginosa strains, was cultivated on nematode growth media (NGM) agar plates at 37 °C for 16 h. The laboratory C. elegans N2 nematode was transferred to the bacterial lawn on the NGM agar plates and cultivated at room temperature for 72 h to allow the population expansion.
C. elegans motility assay
All P. aeruginosa strains were first inoculated and grew in 2 ml of LB medium at 37 °C with shaking at 200 rpm for 16 h. After washing the overnight culture with 0.9% NaCl, the 30 µl of bacterial culture was transferred to NGM petri dish (3 cm diameter) for spreading with a bacteriological spreader. The culture was incubated overnight to allow biofilm growth. At least 50 individuals of C. elegans from 3 independent trials were transferred from the feeding plate to the center of the biofilm with titanium wire picker. Unless stated otherwise (such as L1 larvae and adults), L3 C. elegans were used for all experiments. The nematodes were given 5 mins for them to adapt to the fresh environment. Before recording the video, the plate was tapped for three to four times to stimulate the C. elegans roaming in the bacterial biofilm lawn.
Track, displacement, and velocity analysis
The 30-sec video clip of the locomotion of each single individual was recorded by a stereomicroscope (Nikon SMZ1270i, Japan) at ×10 magnification. The video clips were processed and analyzed with image processing software “ImageJ Fiji”. The locomotion of C. elegans each frame was tracked using the manual tracking plugin. The track images were captured and the frame velocity was calculated by the software in µm per second. The frame displacement was calculated in pixels by the following formula:
x1 = the initial x-coordinate, x1 = the x-coordinate in the next frame of x1, y1 = the initial y-coordinate, y2 = the y-coordinate in the next frame of y1.
Nematode escape assay
The overnight cultures of P. aeruginosa strains were inoculated and grown in 2 ml LB medium at 37 °C overnight with shaking at 200 rpm. The bacterial culture was centrifuged to collect the cell pellet. The cell pellet was resuspended with 50 µl 0.9% NaCl. The entire resuspended culture was then transferred to the center of the NGM plate to make a concentrated spot (diameter ~3 mm) for the growth of biofilm trap at 37 °C for 16 h. The Stage L3 C. elegans were placed directly onto the biofilm trap. A noxious blue light source from the epifluorescence microscope (Nikon, Japan) was then used to illuminate the biofilm spot and stimulate the repulsion of C. elegans. The duration of C. elegans escaping from the biofilm spot was recorded using a timer.
Food choice assay
As previously described [18], P. aeruginosa PAO1 and E. coli OP50 lawns (circular shape with diameter of 1.2 cm) were grown 2.5 cm apart from each other at 37 °C for 16 h. After being washed by PBS buffer twice, 60 nematodes at L3 stage were transferred in the middle between the lawns of P. aeruginosa PAO1 and E. coli OP50. The nematodes were incubated at room temperature, allowing the nematodes to choose their preference between both species. The number of nematodes on either bacterial lawn was enumerated at 2, 4, 6, 10, and 24 h. The choice index was tabulated as follows: (worm number in PAO1 colonies − worm number in OP50 colonies)/total worm number used.
Biofilm protection assay
The overnight cultures of P. aeruginosa strains and E. coli OP50 were inoculated and grown in 2 ml LB medium at 37 °C for 16 h with shaking at 200 rpm. The bacterial culture was centrifuged to collect the cell pellet. The cell pellet was resuspended with 50 µl 0.9% NaCl. For P. aeruginosa strains, their cultures were then transferred to the center of the NGM plate to make a concentrated spot (diameter ~3 mm). On the same plate, OP50 E. coli was transferred and spread with a spreader over the remaining parts of the agar, leaving a 2 mm gap between the P. aeruginosa spot and the OP50 lawn. The NGM plate was incubated for 16 h at 37 °C to allow the growth of P. aeruginosa biofilm, which represented the “tougher” biofilm trap and OP50 lawn the susceptible biofilm.
On each plate, five individual Stage L3 C. elegans were transferred and placed onto the P. aeruginosa biofilm spot. Alternatively, as controls, E. coli OP50 treated with exogenously added 0 or 1 µg ml−1 Pel, Psl were used as biofilm spots. The extent of roaming by the C. elegans on the OP50 lawn after leaving the biofilm spot was quantified by counting the 5 × 5 mm grid squares being covered by C. elegans tracks every 10 min till the 60th minute. At least five replicate plates from three independent trials were conducted for the experiment.
Reproduction assay of C. elegans on bacterial lawns
To observe if the five nematodes could reproduce and expand their population after leaving the P. aeruginosa biofilm trap and reaching the OP50 lawn, the plates from the biofilm protection assay were further incubated at room temperature for 24 h to allow the emergence of L1 C. elegans progenies. Only the L1 C. elegans progenies on the OP50 lawns were enumerated with a tally counter.
Alternatively, when E. coli OP50 treated with 1 µg ml−1 Pel or Psl were used as biofilm spots, the plates from the E. coli biofilm protection assay were further incubated at room temperature for 120 h to allow the emergence of L1 C. elegans progenies. Only the L1 C. elegans progenies on the OP50 lawns were enumerated every 24 h with a tally counter. At least five replicate plates from three independent trials were conducted for the experiment.
Exopolysaccharide extraction
As previously described [19], Pel, Psl, and alginate were extracted by growing ΔpslBCD/plac-YedQ, ΔpelA/plac-YedQ, and ΔmucAΔpelAΔpslBCD static biofilms respectively on standard Petri dishes containing 15 ml LB supplemented with appropriate antibiotics at 37 °C for 16 h. The biofilms were collected and separated from the supernatant by centrifugation at 10,000 g for 5 min. The cell pellet was resuspended in 0.9% NaCl and treated with mild water-bath sonication (Elmasonic P120H, power = 50%, frequency = 37 KHz, 5 min) to separate the cells from the surface-associated matrix. The cells were then separated from the matrix by centrifugation, leaving behind the crude matrix extract.
The crude extract was then further treated by removal of eDNA by precipitation with 25% ethanol and 0.1 M CaCl2. Extracellular proteins were then removed from the extract with 0.5 mg ml−1 proteinase K at 60 °C for 1 h and inactivation at 80 °C for 30 min. The extract was then filtered with centrifugal filter (<3 kDa) to remove the metabolites. The extract was then lyophilized and resuspended in sterile ddH2O.
Exogenous addition of exopolysaccharides to non-polysaccharide-producing strains
The ΔwspFΔpelAΔpslBCD and ΔmucAΔalgT cells from growth culture (described in the previous section on growth conditions) were washed with 0.9% NaCl and centrifuged at 13,000 × g for 3 min. Varying concentrations (0, 0.5, 1, and 2 µg ml−1) of Pel or Psl were mixed and resuspended in the ΔwspFΔpelAΔpslBCD cell pellet, while similar procedures were used for alginate and the ΔmucAΔalgT cell pellet. To test the effects of polysaccharide addition to a different bacterial species, E. coli OP50 was treated with 0 or 1 µg ml−1 Pel, Psl, or alginate.
The 30 µl of bacteria + exopolysaccharide mixture was transferred to NGM petri dish (3 cm diameter) for spreading with a spreader. The culture was incubated overnight to allow biofilm growth. At least 50 individuals of Stage L3 C. elegans were transferred from the feeding plate to the center of the biofilm with a titanium wire picker. The nematodes were given 5 min for them to adapt to the fresh environment. Before recording the video of each nematode, the plate was tapped for three to four times to stimulate the C. elegans roaming in the bacterial biofilm lawn.
Prevalence of Pel and Psl genes in microbial species
The IMG portal [20, 21] was used to search for sequenced microbial species containing Pel and Psl synthesis genes using (date accessed: 22 June 2020). The identified microbial species (P. aeruginosa, other Pseudomonads, and non-Pseudomonas species) were enumerated and tabulated as percentage of total species.
Prevalence of mutated WspF protein in sequenced P. aeruginosa strains
The WspF protein sequence of prototypic PAO1 was aligned to that from all sequenced P. aeruginosa genomes via the DIAMOND BLASTP tool [22] (date accessed: 22 June 2020). Mismatches with one to six amino acids were listed, with 750 P. aeruginosa sequenced genomes being identified.
Results
Biofilms impede locomotion and restrict roaming of C. elegans
As a proof-of-concept showing that biofilms can impede C. elegans and restrict its roaming behavior, we first need to constitutively elevate c-di-GMP signaling and promote biofilm formation via the wsp operon, which controlled both pel and psl transcription [10]. We employed the ΔwspF mutant whose mutation of wspF repressor causes the derepression of WspR DGC, resulting in increased biofilm formation via the production of Pel and Psl [10] (Supplementary Fig. S1a). It is important to note that ΔwspF was frequently isolated in in vitro and in vivo biofilm infections [23, 24]. By aligning the WspF protein sequence in prototypic PAO1 to all sequenced P. aeruginosa isolates, we also found 750 isolates with one to seven mismatches in the WspF protein (Supplementary Data S1), indicating the prevalence in wspF mutations in nature. Furthermore, to confirm that our observations was due to c-di-GMP signaling per se and not attributed to possible pleiotropic effects of the wsp operon, we also expressed the plac-YedQ plasmid containing an E. coli YedQ DGC in wild-type PAO1 to constitutively elevate intracellular c-di-GMP levels and boost Pel and Psl production [24]. While cell number in the biofilm mainly remained consistent across the wild type and mutants, both ΔwspF and PAO1/plac-YedQ produced significantly higher exopolysaccharides than PAO1 wild type (Supplementary Fig. S2).
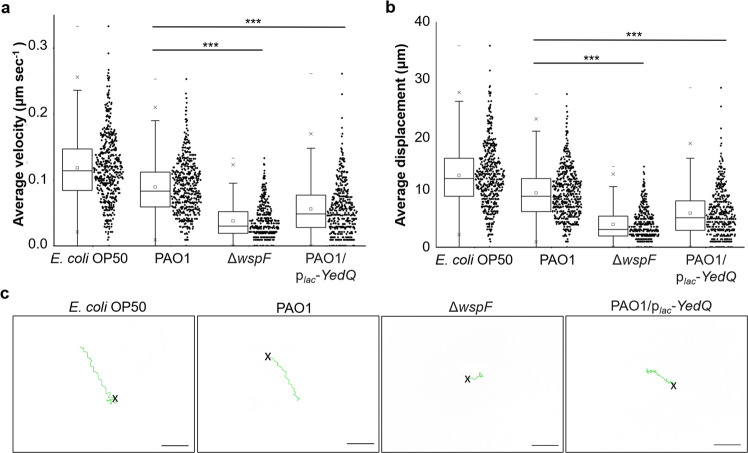
Interestingly, as compared to E. coli OP50 (Supplementary Video 1) and wild-type PAO1 (Supplementary Video 2), we observed that the nematodes moved at a slower pace and were easily trapped in the aggregates formed by ΔwspF mutant (Supplementary Video 3). By tracking the nematodes, we showed a significant reduction in velocity (Fig. 1a) and displacement (Fig. 1b) undertaken by the ΔwspF mutant as compared to wild-type PAO1. The nematodes moved constantly, albeit at lower velocity, on ΔwspF biofilms, indicating that they did not stop to rest or adopt punctuated (stop-go) movements (Supplementary Fig. S3). Higher biofilm formation by the ΔwspF mutant also significantly restricted the roaming ability of C. elegans to explore the plate, which was reflected by highly localized tracks (Fig. 1c) undertaken by C. elegans on ΔwspF lawn. Upon closer inspection of the tracks, we also observed that the nematodes switched between forward and reverse locomotion frequently on ΔwspF lawn, as compared to that of wild-type PAO1 lawn where the nematode moved in a linear direction (Fig. 1c).
Fig. 1. Biofilms impede locomotion and restrict roaming of C. elegans.
a Average velocity, b average displacement, and c representative tracks traveled by C. elegans (N ≥ 150) on OP50, PAO1, ΔwspF, and PAO1/plac-YedQ lawns. Means and SD are shown. ***P < 0.001, one-way ANOVA.
Similarly, the PAO1/plac-YedQ with increased biofilm formation could also dampen nematode motility and its ability to roam (Fig. 1a–d and Supplementary Video 4), thus corroborating with the results from wsp operon. This showed that the biofilms could cause the quagmire phenotype for C. elegans.
Psl is more important than Pel at impeding nematode locomotion under influence by wsp operon
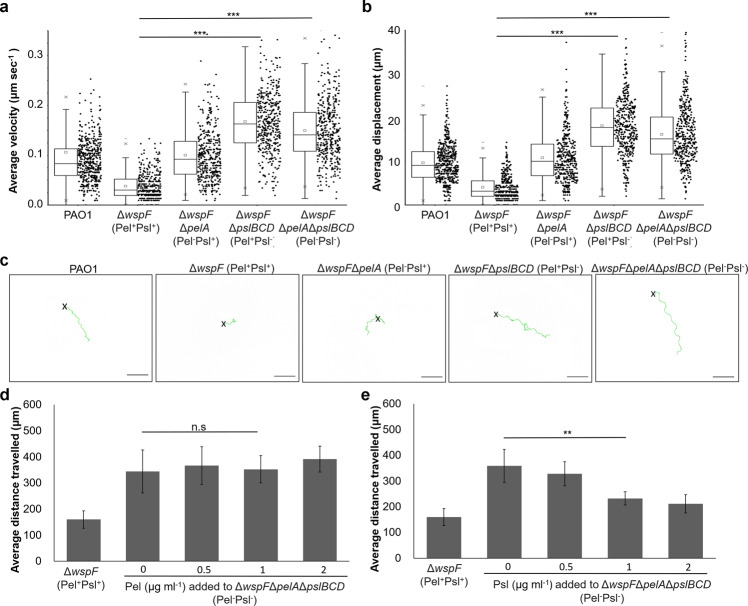
Since the wsp operon controls both Pel and Psl production [10], we next asked which exopolysaccharide played a larger role in the quagmire phenotype. To maximize the phenotypic effects of the EPS in question and ensure that our results were solely dependent on one EPS, we mutated the EPS genes in the ΔwspF mutant. The presence of wspF mutation would boost the production of the exopolysaccharide whose synthesis genes were not mutated. Hence, in this paper, unless specified otherwise, the Pel+Psl+ referred to ΔwspF; Pel+Psl− was ΔwspFΔpslBCD; Pel−Psl+ was ΔwspFΔpelA; Pel−Psl− was ΔwspFΔpelAΔpslBCD.
We found that the loss of Pel and Psl in Pel−Psl− completely abolished the quagmire phenotype, allowing the nematode to move at normal velocity and roam the bacterial lawn easily (Figs. 2a–c and S4 and Supplementary Video 5). Surprisingly, loss of Pel in Pel−Psl+ did not completely abolish the quagmire phenotype, while Psl loss in Pel+Psl− was comparable to Pel−Psl− (Figs. 2a–c and S4 and Supplementary Videos 6 and 7). While both exopolysaccharides were involved in the quagmire phenotype, Psl played a more important role in the quagmire phenotype as compared to Pel.
Fig. 2. Psl is more important than Pel at impeding nematode locomotion under influence by wsp operon.
a Average velocity, b average displacement, and c representative tracks traveled by C. elegans (N ≥ 150) on EPS mutant lawns. Changes to average distance traveled by C. elegans after exogenous addition of d Pel or e Psl to Pel−Psl− strain. Means and SD are shown. **P < 0.01, ***P < 0.001, ns not significant, one-way ANOVA.
In the similar fashion, we inserted the plac-YedQ plasmid into the EPS mutants and ultimately found that these results corroborated with our observations on the wsp operon (Supplementary Fig. S5 and Supplementary Videos 4 and 8–10). Nevertheless, we found that our results were mainly dependent on the presence of the exopolysaccharide in the biofilm matrix, as we found qualitatively identical results when we used non-wspF-mutated backgrounds on PAO1, ΔpelA, ΔpslBCD, and ΔpelAΔpslBCD mutants (Supplementary Fig. S6 and Supplementary Videos 2 and 11–13).
To confirm that the physical presence of the exopolysaccharide could contribute to the quagmire phenotype, we added Pel or Psl extracts exogenously to the Pel−Psl− strain at increasing concentrations. While increasing concentrations of Pel did not establish the quagmire phenotype in the Pel−Psl− (Fig. 2d), we found that more than 1 µg ml−1 Psl could establish the quagmire phenotype, to the point of being similar to ΔwspF (Fig. 2e). This also corroborated with the levels of exopolysaccharide extracted from the biofilms (Supplementary Fig. S2b). Hence, addition of physiologically relevant Psl concentrations to the bacterial cells could alter the biofilm matrix and impede nematode motility.
Psl immobilizes and delays C. elegans from grazing susceptible biofilms
Since C. elegans possesses chemotaxis behavior like most animals, where it moves in response either from repellents (such as noxious repellents and predators) or to attractants (such as prey, mate, and odorants) [25], we next examined the implications of Psl-mediated interference on C. elegans motility.
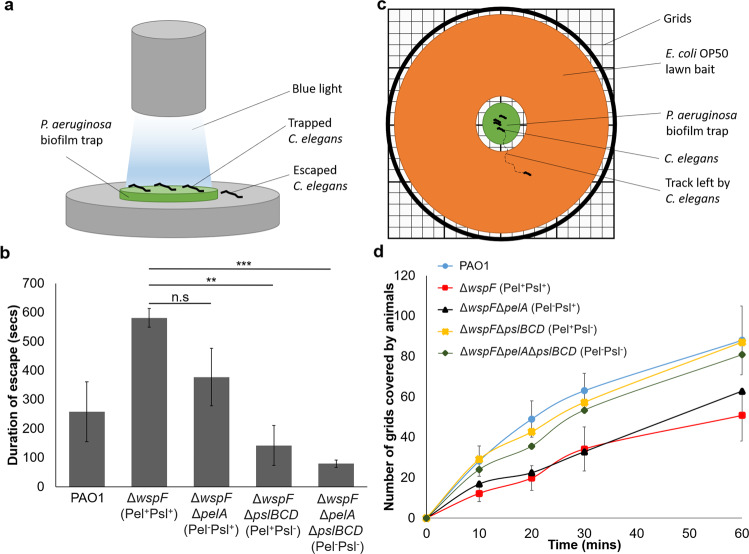
Using a modified repulsion assay [26], we designed an escape assay where we first placed C. elegans in the biofilm “trap” and shone a direct beam of blue light on the biofilms (Fig. 3a). Blue light was previously shown to be a noxious yet harmless repellent under brief exposure [26], so this would encourage C. elegans to leave the biofilm as soon as possible. By observing the average duration required by the nematodes to escape the biofilm “trap” into the safe zone, we observed that the C. elegans took significantly longer time to escape from the Pel+Psl+ and Pel−Psl+ mutants, with some of them even unable to escape from the biofilm “trap” within 10 min (Fig. 3b). In contrast, it took lesser time for the animals to escape from the Pel−Psl− and Pel+Psl− biofilms (Fig. 3b). This showed that Psl could not only impede C. elegans motility, it could effectively help the biofilm immobilize C. elegans.
Fig. 3. Psl immobilizes and delays C. elegans from escape and attacking susceptible biofilms.
a Schematic representation of trapping C. elegans by biofilms under blue light repulsion. b Duration taken by C. elegans to escape biofilm trap. c Schematic representation of trapping and baiting C. elegans. d Extent of roaming of C. elegans on bait biofilm after leaving the trap biofilm. Means and SD from triplicate experiments are shown. **P < 0.01, ***P < 0.001, ns not significant, one-way ANOVA.
We next asked what could be the possible benefit brought to the biofilms by immobilizing a predatory C. elegans within themselves. Since C. elegans is a predator which roamed around in search for food, we tested if Psl-producing biofilms could prevent or delay the roaming of C. elegans, so that the non-Psl-producing biofilms or susceptible biofilms could be spared from nematode predation. Since our food preference assay had shown that C. elegans prefer E. coli OP50 over P. aeruginosa PAO1 (Supplementary Fig. S7a), we designed an assay which set the P. aeruginosa biofilm as nematode “trap” in the center of the petri dish, which was surrounded by the susceptible E. coli OP50 biofilm as “food bait” to motivate the nematodes to leave the trap (Fig. 3c). We then compared the extent of nematode tracks on the bait after their escape from the P. aeruginosa trap. Worms notably moved across a larger area of the bait over time after escaping quickly from the poorer trap (Pel−Psl− and Pel+Psl− mutants), whereas better traps (Pel+Psl+ and Pel−Psl+ mutants) could either reduce or completely prevent C. elegans from grazing on the bait, by delaying exit or immobilizing the nematodes (Fig. 3d).
This finding had significant implications on the recolonization ability of C. elegans on the susceptible OP50 lawns, where nearly 50% fewer L1 progenies (P < 0.01) were observed growing on OP50 lawns after the adult nematodes had escaped from better traps (Pel+Psl+ and Pel−Psl+ mutants) and recolonized on susceptible OP50 lawns (Supplementary Fig. S7b). Hence, Psl was comparatively more important than Pel in entrapping predators to delay or prevent susceptible biofilms from predation, thereby improving the overall survival of the biofilms.
Role of other matrix components in the quagmire phenotype
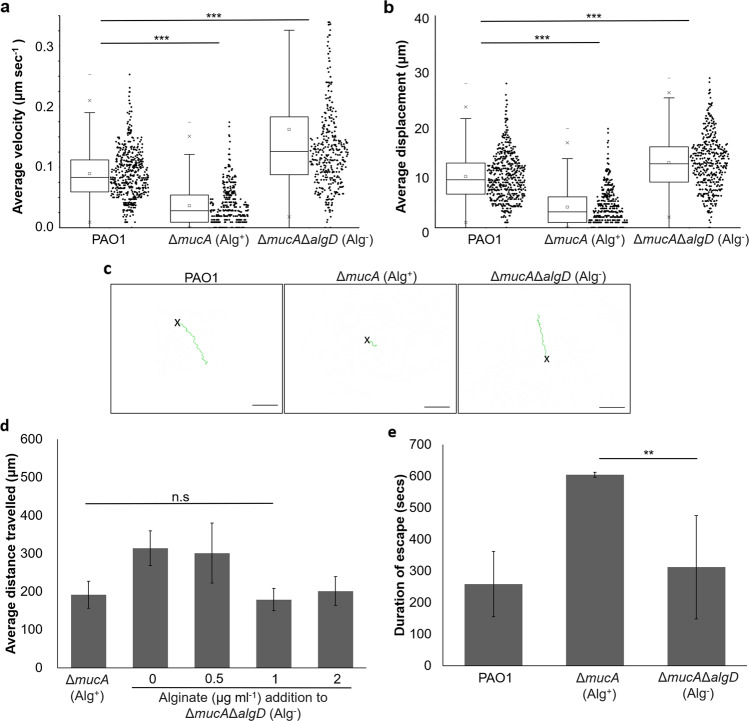
Other than Pel and Psl, the P. aeruginosa biofilm matrix comprises of a multitude of matrix components, each of which might play a role in dictating how C. elegans move across the biofilms. Since CdrA adhesion protein and eDNA were not significantly involved in the quagmire phenotype (Supplementary Fig. S8), we tested alginate, the third and final exopolysaccharide in P. aeruginosa. Alginate production was controlled by the muc c-di-GMP-signaling operon, and was responsible for mucoidy of a subset of clinical isolates in cystic fibrosis patients [27, 28]. Since its production was inversely regulated with Pel/Psl exopolysaccharides production, the presence of Pel and Psl is minimal in mucoid strains [29]. We employed the ΔmucA mutant (Alg+) whose mutation of mucA repressor causes the derepression of MucR DGC, resulting in increased mucoid biofilm formation via the production of alginate (Supplementary Fig. S1b). With higher exopolysaccharide concentration in Alg+ strain than PAO1 wild type (Supplementary Fig. S2b), we showed that increased production of alginate could also impede C. elegans motility (Fig. 4 and Supplementary Video 14). Mutagenesis of alginate synthesis gene algT in the ΔmucAΔalgT mutant (Alg−) resulted in abrogation of quagmire phenotype, allowing the C. elegans to roam the bacterial lawns freely at normal speed (Figs. 4a–c and Supplementary Fig. S9 and Supplementary Video 15). Exogenous addition of alginate to the Alg− mutant deficient in alginate production can restore the quagmire phenotype at 1 µg ml−1 (Fig. 4d). Similar to Psl, alginate could also immobilize C. elegans and prevent its escape from noxious blue light (Fig. 4e). This implied that under the influence of muc operon, alginate was solely important to impeding nematode locomotion.
Fig. 4. Role of alginate in the quagmire phenotype.
a Average velocity, b average displacement, and c representative tracks travelled by C. elegans (N ≥ 150) on PAO1, Alg+, and Alg− lawns. d Exogenous addition of alginate to Alg− strain. e Duration taken by C. elegans to escape biofilm trap. Means and SD are shown. **P < 0.01, ***P < 0.001, ns not significant, one-way ANOVA.
Discussion
Bacteria are often the target of predation by bacterivores, such as nematodes and amoebae in the environment, and phagocytes in the human body. To ensure their survival, bacteria produce a plethora of virulence factors that can effectively kill their predators, such as phenazines, hydrogen cyanide, and T3SS [30–32]. Such virulence factors typically require hours to days to kill C. elegans, which are typically demonstrated in fast paralytic (8–24 h) and slow killing assays (days to weeks) [33].
Bacteria also form biofilms whose exopolymeric matrix offers protection to bacteria by resisting predation. To our knowledge, we showed a hitherto unreported function of the biofilm matrix, which was to impede nematode locomotion and alter its grazing ability. In the case of P. aeruginosa, Psl and alginate exopolysaccharides were important in entrapping and restricting nematode movement, thereby hampering C. elegans’ ability to roam and forage for food. This observation was applicable to C. elegans of various ages and sizes tested, where young L1 larvae and adults had retarded motility on P. aeruginosa biofilms containing Psl or alginate, with increased propensity to be immobilized on the biofilm traps (Supplementary Fig. S10). Furthermore, this retarded motility was observed to be consistent for longer periods (up to 1 h), implying the long-lasting effect of biofilms on nematode motility (Supplementary Fig. S11).
While Pel and Psl genes were commonly found in most environmental and clinical isolates, alginate was only expressed in a subset of mucoid clinical isolates [34], thus Psl could be prevalently employed by P. aeruginosa biofilms in nature as compared to alginate. Nevertheless, the redundancy of exopolysaccharides involved in the quagmire phenotype conferred versatility in P. aeruginosa to respond to specific stimuli and adjust the composition of its biofilm matrix accordingly. This improved the survival of P. aeruginosa biofilms in face of varying stresses and predators.
While Pel is involved in scaffolding with eDNA-crossing-linking properties [35, 36], its viscosity allows bacterial cells to spread within the biofilm matrix, which may explain why the Pel exopolysaccharide was significantly less effective at impeding nematode locomotion than Psl [37].
Yet for Psl, it is a rigid polymer which increases effective cross-linking of cells in the biofilm matrix, thus strengthening the scaffold and promoting the formation of stiff microcolonies [37]. Without Psl, the biofilm matrix becomes less rigid [37], which may indicate why C. elegans can move across Psl-deficient biofilms easily. Nonetheless, further investigation of how these “sticky” components interact with the proteinaceous outer cuticle of C. elegans is warranted.
As for eDNA and CdrA adhesion protein, they do not have an observable effect on locomotion, which can be attributed to their lower presence in the matrix composition as compared to exopolysaccharides [38]. While biofilm matrix is directly involved in impeding nematode motility, upstream c-di-GMP-signaling proteins can indirectly influence the quagmire phenotype. Clearly, biofilms formed by DGC mutants (ΔwspR and ΔmucR) are worse off than wild-type PAO1 in retarding C. elegans locomotion (Supplementary Fig. S12), emphasizing their importance in the biofilms’ quagmire phenotype.
Our findings have several implications in nature and clinical settings. In the environment, the biofilms could impede the mobility of nematode as a form of protection from grazing and act as trap to reduce further damage to susceptible biofilms, thereby improving the overall survival of the biofilms. With diverse soil microbial species that are known to interact with nematodes [39], C. elegans remain susceptible to a variety of pathogens, such as Leucobacter and Corynebacteria species, which could form robust biofilms that interact with C. elegans [40, 41]. Furthermore, certain Leucobacter strains could form aggregates, which cause swimming worms to stick together by their tails in a dead-inducing entrapment (star formation) [42]. This adds a layer of complexity in predator–prey interactions for future work on other bacterial species and bacterivores. Our findings can be also used as a gauge to test the physical parameters of various components in the biofilm matrix against predation.
Our results also raised an interesting question into the prevalence of Pel and Psl synthesis genes possessed by different microbial species. A search of sequenced bacterial genomes from the C. elegans native microbiota [43, 44] for Pel and Psl synthesis genes using the IMG portal revealed that other Pseudomonas species, such as Pseudomonas protegens and Pseudomonas lurida, and non-Pseudomonas species such as Delftia acidovorans contained Pel genes (Supplementary Data S2). One the other hand, Psl genes were exclusively found in Pseudomonas species (Supplementary Data S2). This raised the possibility that C. elegans could encounter such biofilms in the soil.
When we expanded the search to all sequenced microbial species, it was noted that more Pseudomonas species, such as Pseudomonas protegens, Pseudomonas fluorescens, and Pseudomonas chlororaphis, and non-Pseudomonas species, such as Burkholderia, Paraburkholderia, and Pseudoalteromonas, contained Pel genes (Supplementary Fig. S13a and Supplementary Data S3). Yet, Psl genes remained almost exclusive to P. aeruginosa and other Pseudomonas species (Supplementary Fig. S13b and Supplementary Data S3). Such microbial species were isolated from a variety of locations, ranging from clinics to soil, plant roots, and aquatic settings (Supplementary Data S3), indicating the prevalence of such biofilm exopolysaccharides in the environment where C. elegans could encounter. Furthermore, we observed that the addition of Psl or alginate to another species, E. coli OP50, could also impede nematode motility and even reproduction rate of its predator (Supplementary Fig. S14), raising the possibility that non-Psl-producing microbial species could utilize Psl or inhabit with Psl-producing species for similar purposes.
In clinical settings, clinical isolates such as mucoid strains with induced alginate expression and rough small colony variant strains with overexpression of Psl are frequently isolated from patients with cystic fibrosis, where phagocytosis of biofilm cells by immune cells was severely hampered [45, 46]. Our findings that the biofilm exopolysaccharides could impede predator motility raise the possibility that leukocytes have reduced migration and motility across the biofilms in human infections, thereby preventing biofilm clearance by the immune system. This warrants the need for development of anti-biofilm agents specific against biofilm matrix components [47].
Supplementary information
Acknowledgements
This research is supported by The Hong Kong Polytechnic University, Department of Applied Biology and Chemical Technology Startup Grant (BE2B) and State Key Laboratory of Chemical Biology and Drug Discovery Fund (1-BBX8).
Author contributions
SLC designed methods and experiments. SYC, SYL, and ZS carried out laboratory experiments, analyzed the data, and interpreted the results. SLC, SYL, and SYC wrote the paper. All authors have contributed to, seen, and approved the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41396-020-00779-9) contains supplementary material, which is available to authorized users.
References
- 1.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 2.Branda SS, Vik Å, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–6. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev. 2015;39:649–69. doi: 10.1093/femsre/fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Q, Ma LZ. Biofilm matrix and its regulation in Pseudomonas aeruginosa. Int J Mol Sci. 2013;14:20983–1005. doi: 10.3390/ijms141020983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tal R, Wong HC, Calhoon R, Gelfand D, Fear AL, Volman G, et al. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J Bacteriol. 1998;180:4416–25. doi: 10.1128/JB.180.17.4416-4425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua SL, Liu Y, Li Y, Ting HJ, Kohli GS, Cai Z, et al. Reduced Intracellular c-di-GMP content increases expression of quorum sensing-regulated genes in Pseudomonas aeruginosa. Front Cell Infect Microbiol. 2017;7:451. doi: 10.3389/fcimb.2017.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–73. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 9.Chua SL, Liu Y, Yam JK, Chen Y, Vejborg RM, Tan BG, et al. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat Commun. 2014;5:4462. doi: 10.1038/ncomms5462. [DOI] [PubMed] [Google Scholar]
- 10.Hickman JW, Tifrea DF, Harwood CS. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci USA. 2005;102:14422–7. [DOI] [PMC free article] [PubMed]
- 11.Hay ID, Remminghorst U, Rehm BH. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl Environ Microbiol. 2009;75:1110–20. doi: 10.1128/AEM.02416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy AB, Petrova OE, Sauer K. The phosphodiesterase DipA (PA5017) is essential for Pseudomonas aeruginosa biofilm dispersion. J Bacteriol. 2012;194:2904–15. doi: 10.1128/JB.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Tsuji G, Opoku-Temeng C, Sintim HO. Inhibition of P. aeruginosa c-di-GMP phosphodiesterase RocR and swarming motility by a benzoisothiazolinone derivative. Chem Sci. 2016;7:6238–44. doi: 10.1039/C6SC02103D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson S, Goldstone RJ, Joshua GWP, Chang C-Y, Patrick HL, Cámara M, et al. Biofilm development on Caenorhabditis elegans by Yersinia is facilitated by quorum sensing-dependent repression of type III secretion. PLOS Pathog. 2011;7:e1001250. doi: 10.1371/journal.ppat.1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan M-W, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA. 1999;96:715–20. [DOI] [PMC free article] [PubMed]
- 16.Desai SK, Padmanabhan A, Harshe S, Zaidel-Bar R, Kenney LJ. Salmonella biofilms program innate immunity for persistence in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2019;116:12462–7. [DOI] [PMC free article] [PubMed]
- 17.Kirienko NV, Kirienko DR, Larkins-Ford J, Wählby C, Ruvkun G, Ausubel FM. Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host Microbe. 2013;13:406–16. doi: 10.1016/j.chom.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd MS, Sadovskaya I, Vinogradov E, Lu H, Sprinkle AB, Richardson SH, et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol. 2009;73:622–38. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen IA, Chu K, Palaniappan K, Pillay M, Ratner A, Huang J, et al. IMG/M v.5.0: an integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 2019;47:D666–77. doi: 10.1093/nar/gky901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee S, Stamatis D, Bertsch J, Ovchinnikova G, Katta HY, Mojica A, et al. Genomes OnLine database (GOLD) v.7: updates and new features. Nucleic Acids Res. 2018;47:D649–59. doi: 10.1093/nar/gky977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 23.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, et al., Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103:8487–92. [DOI] [PMC free article] [PubMed]
- 24.Chua SL, Ding Y, Liu Y, Cai Z, Zhou J, Swarup S, et al. Reactive oxygen species drive evolution of pro-biofilm variants in pathogens by modulating cyclic-di-GMP levels. Open Biol. 2016;6:160162. doi: 10.1098/rsob.160162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA. 1973;70:817–21. [DOI] [PMC free article] [PubMed]
- 26.Lee KH, Aschner M. A simple light stimulation of Caenorhabditis elegans. Curr Protoc Toxicol. 2016;67:11.21.1–5. doi: 10.1002/0471140856.tx1121s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damron FH, Yu HD. Pseudomonas aeruginosa MucD regulates the alginate pathway through activation of MucA degradation via MucP proteolytic activity. J Bacteriol. 2011;193:286–91. doi: 10.1128/JB.01132-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucher JC, Yu H, Mudd MH, Deretic V. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun. 1997;65:3838–46. doi: 10.1128/IAI.65.9.3838-3846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones CJ, Ryder CR, Mann EE, Wozniak DJ. AmrZ modulates Pseudomonas aeruginosa biofilm architecture by directly repressing transcription of the psl operon. J Bacteriol. 2013;195:1637–44. doi: 10.1128/JB.02190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cezairliyan B, Vinayavekhin N, Grenfell-Lee D, Yuen GJ, Saghatelian A, Ausubel FM. Identification of Pseudomonas aeruginosa phenazines that kill Caenorhabditis elegans. PLOS Pathog. 2013;9:e1003101. doi: 10.1371/journal.ppat.1003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallagher LA, Manoil C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol. 2001;183:6207–14. doi: 10.1128/JB.183.21.6207-6214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewenza S, Charron-Mazenod L, Giroux L, Zamponi AD. Feeding behaviour of Caenorhabditis elegans is an indicator of Pseudomonas aeruginosa PAO1 virulence. PeerJ. 2014;2:e521–1. doi: 10.7717/peerj.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan MW, Mahajan-Miklos S, Ausubel FM. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA. 1999;96:715–20. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin M, Nivens D, Weadge J, Howell P. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol. 2011;2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GCL, et al. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLOS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennings LK, Storek KM, Ledvina HE, Coulon C, Marmont LS, Sadovskaya I, et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Natl Acad Sci USA. 2015;112:11353–8. [DOI] [PMC free article] [PubMed]
- 37.Chew SC, Kundukad B, Seviour T, van der Maarel JRC, Yang L, Rice SA, et al. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. mBio. 2014;5:e01536–14. doi: 10.1128/mBio.01536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutherland IW. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol. 2001;9:222–7. doi: 10.1016/S0966-842X(01)02012-1. [DOI] [PubMed] [Google Scholar]
- 39.Samuel BS, Rowedder H, Braendle C, Félix M-A, Ruvkun G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proc Natl Acad Sci USA. 2016;113:E3941–9. [DOI] [PMC free article] [PubMed]
- 40.Muir RE, Tan MW. Virulence of Leucobacter chromiireducens subsp. solipictus to Caenorhabditis elegans: characterization of a novel host-pathogen interaction. Appl Environ Microbiol. 2008;74:4185–98. doi: 10.1128/AEM.00381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antunes CA, Clark L, Wanuske MT, Hacker E, Ott L, Simpson-Louredo L, et al. Caenorhabditis elegans star formation and negative chemotaxis induced by infection with corynebacteria. Microbiology. 2016;162:84–93. doi: 10.1099/mic.0.000201. [DOI] [PubMed] [Google Scholar]
- 42.Hodgkin J, Félix MA, Clark LC, Stroud D, Gravato-Nobre MJ. Two Leucobacter strains exert complementary virulence on Caenorhabditis including death by worm-star formation. Curr Biol. 2013;23:2157–61. doi: 10.1016/j.cub.2013.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dirksen P, Marsh SA, Braker I, Heitland N, Wagner S, Nakad R, et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC Biol. 2016;14:38. doi: 10.1186/s12915-016-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnke J, Dirksen P, Schulenburg H. Community assembly of the native C. elegans microbiome is influenced by time, substrate and individual bacterial taxa. Environ Microbiol. 2020;22:1265–79. doi: 10.1111/1462-2920.14932. [DOI] [PubMed] [Google Scholar]
- 45.Häußler S, Tümmler B, Weißbrodt H, Rohde M, Steinmetz I. Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin Infect Dis. 1999;29:621–5. doi: 10.1086/598644. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen SS, Høiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu M, Chua SL. Demolishing the great wall of biofilms in Gram-negative bacteria: to disrupt or disperse? Med Res Rev. 2020;40:1103–16. doi: 10.1002/med.21647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.