Abstract
The liver is a lymphoid organ with unique immunological properties, particularly, its predominant innate immune system. The balance between immune tolerance and immune activity is critical to liver physiological functions and is responsible for the sensitivity of this organ to numerous diseases, including hepatotropic virus infection, alcoholic liver disease, nonalcoholic fatty liver disease, autoimmune liver disease, and liver cancer, which are major health problems globally. In the past decade, with the discovery of liver-resident natural killer cells, the importance of innate lymphocytes with tissue residency has gradually become the focus of research. In this review, we address the current knowledge regarding hepatic innate lymphocytes with unique characteristics, including NK cells, ILC1/2/3s, NKT cells, γδ T cells, and MAIT cells, and their potential roles in liver homeostasis maintenance and the progression of liver diseases and cancer. A better understanding of the immunopathogenesis of hepatic innate lymphocytes will be helpful for proposing effective treatments for liver diseases and cancer.
Keywords: Innate lymphocyte, Liver disease, Liver cancer, Innate lymphoid cell, Innate-like T lymphocyte
Subject terms: Innate immune cells, Mechanisms of disease
Introduction
In 2002, Ian R Mackay first proposed the concept of “hepatoimmunology”, with the recognition that the liver is a lymphoid organ with unique immunological properties, including efficient innate defenses against infection, induction of immune tolerance, apoptotic sites for the disposal of redundant lymphocytes, and susceptibility to immune-mediated disease.1 With increasing evidence, it is well known that the predominance of the innate immune system in the liver is responsible for its unique immunological properties.2–4
As the largest solid organ with digestive and metabolic functions in the body, the liver receives blood from two sources: the intestine through the portal vein (~80%) and the systemic circulation via the hepatic artery (accounting for the remaining ~20%). Enriched bacterial products, environmental toxins and food antigens constantly enter and are pressed through specific sinusoid structures in the liver by blood flow. Live sinusoids with slow blood flow and reduced pressure maximize immune cell–blood contact and interactions. To effectively ensure physiological functions while maintaining immune homeostasis and avoiding excessive immune responses leading to adverse effects, the liver harbors a unique immune system with a particularly strong innate immune component that exerts anti-inflammatory and immunotolerant functions. Furthermore, under appropriate conditions, liver immune cell populations can mount rapid and robust responses. The balance between tolerance and immune activity is critical to liver function and is tightly associated with the numerous immune cell populations in the liver.5
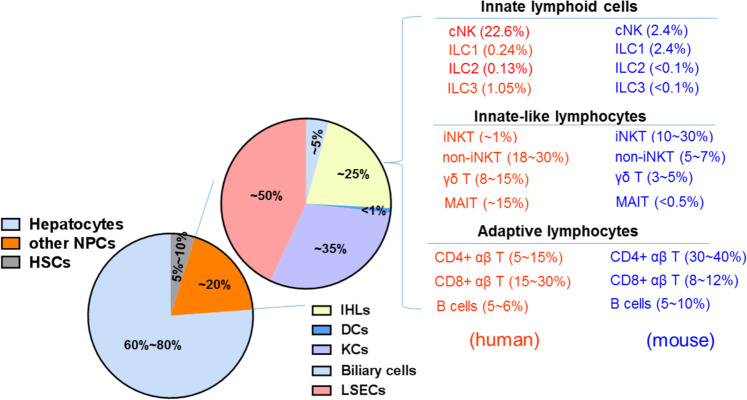
In the liver, parenchymal cells termed hepatocytes comprise ~60–80% of total cells.2 In addition to their metabolic functions, hepatocytes produce acute-phase proteins and complement factors; express innate immune receptors such as Toll-like receptors (TLRs); present antigens via major histocompatibility complex (MHC) I/MHC II and CD1d molecules; and maintain immunotolerance.6 The remaining ~20% of total cells are referred to as nonhepatocytes and include Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs), dendritic cells (DCs), biliary cells, and intrahepatic lymphocytes (IHLs). KCs are liver-resident macrophages that represent ~35% of nonparenchymal cells (NPCs) in the liver and 80–90% of tissue-resident macrophages in the body; KCs express scavenger receptors, TLRs, Fc receptor (FcRs), and complement receptors (CRs) as well as present antigens via MHC I/MHC II and CD1d molecules, producing a variety of cytokines such as interleukin (IL)-10, tumor necrosis factor-alpha (TNF-α), IL-12 and IL-18 and maintaining immunotolerance.5,7 As nonclassical immune cells, LSECs acquire immune functions by expressing a variety of pattern recognition receptors, MHC I/MHC II, CD80/CD86, adhesion molecules and scavenger receptors and are involved in maintaining liver immune homeostasis, representing ~50% of NPCs in the liver.6 HSCs, as a mesenchymal cell population, comprise ~5–10% of all liver cells, located in the space of Disse, which can secrete laminin, proteoglycans and type IV collagen, forming basement membrane-like structures. HSCs participate in the immune response to paracrine stimulation of a variety of growth factors and cytokines derived from neighboring cells in the liver, such as KCs, hepatocytes, neutrophils, LSECs, and lymphocytes.8 In addition to KCs, LSECs, and HSCs, the liver contains highly diverse IHLs, which are present at levels of ~106/g liver tissue in humans and mice (Fig. 1). The IHL cell composition in the liver is distinct from that of peripheral lymphoid organs, such as spleen, lymph nodes (LNs) and other tissues, and peripheral blood, indicating that the liver is predominantly an organ of innate lymphocytes, including natural killer (NK) cells; groups 1, 2, and 3 innate lymphoid cells (ILCs); natural killer T (NKT) cells; γδ T cells and mucosal-associated invariant T cells (MAITs), which function to maintain liver homeostasis.
Fig. 1.
Cell composition of the healthy adult liver. The liver is composed of parenchymal hepatocytes and nonparenchymal cells (NPCs), including hepatic stellate cells (HSCs) and other NPCs (KCs, LSECs, DCs, biliary cells, and IHLs). According to the features of immune responses, IHLs are divided into three groups: innate lymphoid cells, innate-like lymphocytes, and adaptive lymphocytes. The numbers indicate the estimated proportion of each population relative to the total number of IHLs, NPCs, or total liver cells. Relevant references are cited in the main text. Data on CD4+ αβT, CD8+ αβ T, and B cell frequencies are derived from ref. 41. Representative data on the frequency of ILCs in the murine liver (B6 mice) are derived from the ref. 23. Data on the frequency of ILCs among IHLs in the human liver were calculated from Fig. 1 of the ref. 48. Lymphocyte subsets within human and murine IHLs are shown in red and blue, respectively. KCs Kupffer cells, LSECs liver sinusoidal endothelial cells, DCs dendritic cells, IHLs intrahepatic lymphocytes, ILC innate lymphoid cell, NK natural killer, LTi lymphoid tissue-inducer, iNKT invariant natural killer T, non-iNKT non-invariant natural killer T, MAIT mucosal-associated invariant T cells
The liver has physiological functions, including metabolic activities, bile secretion, nutrient storage, and detoxification and is sensitive to numerous diseases, including hepatotropic virus infection, alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), autoimmune liver disease (AILD), and liver cancer, which are major health problems globally. The pathological responses of these innate lymphocytes elicited within the liver account for acute liver injury, chronic liver inflammation, liver fibrosis/cirrhosis, tumor development, and autoimmunity. In this review, we address the current knowledge regarding hepatic innate lymphocytes and their potential roles in the progression of liver diseases and cancer. In the current issue, hepatic macrophages, including KCs and liver infiltrating macrophages, are described in detail as important components of innate immune cells in the liver.9 A better understanding of hepatic innate lymphocytes will assist in the development of treatments for liver diseases.
Subsets of innate lymphocytes in the liver
As described above, innate lymphocytes are predominant in the liver and include ILCs (NK cells and ILC1/2/3s) and innate-like T lymphocytes (NKT cells, γδ T cells, and MAITs), which function to maintain liver homeostasis. Most of these innate lymphocytes exhibit the features of the liver residence, and these cells include ILCs, NKT, γδ T, and MAITs, which share many common phenotypes and functions. Furthermore, the liver tissue microenvironment reshapes liver-resident lymphocytes with unique characteristics, making them different from other tissue-resident lymphocytes, such as those in the skin, lung, or intestine. Conventional NK (cNK) cells in the liver differ from liver-resident cells, sharing some similar features with circulating NK cells in the peripheral blood and lymphoid organs. Notably, the human liver differs substantially from the mouse liver in terms of immune cell composition (Fig. 1) and phenotypes (Tables 1, 2).
Table 1.
Innate lymphocytes in the adult healthy liver (mouse)
| Cell group | Cell subset | Phenotypical identification | Other surface markers | Transcription factors | Main effector molecules | Ref. |
|---|---|---|---|---|---|---|
| ILC group 1 (CD45+ Lin− NKp46+ NK1.1+ T-bet+) | ||||||
| NK | CD3−NK1.1+CD49b+CD49a− | CD127− | Eomes+, NFIL3+, T-bet+, RORγt– | IFN-γ, Perforin, Granzyme B, TRAIL | 26,28 | |
| ILC1 | CD3−NK1.1+CD49b−CD49a+ | CD127+, CD200r1+, CXCR6+, TRAIL+, CD62L−, VLA2− | Eomes−, PLZF+, T-bet+ Hobit+, AhR+, RORγt− | TRAIL, IFN-γ, TNF-α, GM-CSF, Granzyme B | 28,34 | |
| ILC group 2 (CD45+ Lin− NK1.1− RORγt− GATA-3+) | ||||||
| ILC2 | lin− NK1.1− CD127+ ST2+Sca-1+ KLRG1+ | ICOS+, MHC II+, c-Kit+, IL-33R+, IL-25R+ | PLZF+, RORα+, GATA-3+, RORγt− | IL-4, IL-5, IL-9, IL-13 Amphiregulin (AREG) | 34,206 | |
| ILC group 3 (CD45+ Lin− RORγt+) | ||||||
| ILC3 | CD45+ Lin− RORγt+ CD4− | CD127+, NK1.1+, NKp46+/− | PLZF+, RORγt+ | IL-22, IL-17A, IL-17F, GM-CSF, IFN-γ | 34 | |
| LTia | CD45+ Lin− RORγt+ CD4+ | IL-23R+, CCR6+, arylhydrocarbon receptor+ | PLZF−, RORγt+ | IL-22, lymphotoxin | 16,34 | |
| Innate-like T lymphocytes | ||||||
| iNKT | CD3+ NK1.1+ Vα14/Jα18+ | CD4+CD8−: CD44+, CD69+, CD122+, NKG2Dlow, CD62L−; CD4−CD8−: CD44+, CD69+, CD122+, NKG2Dhi, CD62L+ | PLZF+, Hobit+, T-bet+/ GATA-3+/RORγt+ | IFN-γ, TNF-α, IL-4, IL-17, IL-2, IL-10, IL-13, IL-21, IL-22, GM-CSF, TRAIL, FasL, Perforin | 51,207–209 | |
| Non-iNKT | CD3+ NK1.1+ glycolipid-CD1d tetramers+ | CD4 + CD8−(~90%), CD122+, CD69+ | PLZF+, T-bet+, RORγt− | IFN-γ, TNF-α, IL-4, IL-13, IL-2 | 56,57,210 | |
| γδT | CD3+ TCRγδ+ (mainly Vγ1/4/6) | CD44hi, CD62L−, CD27lo, CD121lo, CD25lo, CD127lo | PLZF+, T-bet+, RORγt+ | IL-17A, IFN-γ | 59,61 | |
| MAIT | CD3+ CD4− CD161+ Vα19+ | IL-18R+ | PLZF+; T-betlo RORγthi; RORγtlo T-bet+ | Dominant IL-17; IFN-γ | 69,70 | |
ILC innate lymphoid cell, NK natural killer, LTi lymphoid tissue-inducer, iNKT invariant natural killer T, non-iNKT non-invariant natural killer T, MAIT mucosal-associated invariant T cells
aLTi: references are from intestine and lymph nodes since LTi cells are rare in the liver
Table 2.
Innate lymphocytes in the adult healthy liver (human)
| Cell group | Cell subset | Phenotypical identification | Other surface markers | Transcription factors | Main effector molecules | Ref. |
|---|---|---|---|---|---|---|
| ILC group 1 | ||||||
| NK | CD45+ Lin− CD127− CD56+ | CD16dim/bright, NKG2D+, NKp46+, CD49b+, NKp44+, NKp80+ | T-bet+, Eomes+ | Perforin, Granzyme B, IFN-γ | 48 | |
| ILC1 | Lin− CD3− CD56bright CD16lo CD49a+ | CD69+, NKG2C+, TRAIL+, NKp46+, NKp44+ | T-bethi, Eomeslo | IFN-γ, TNF-α, GM-CSF, Perforin, Granzyme B | 30,43,48,211 | |
| CD3− CD56bright CD49e− | CD69+, CXCR6+ | T-bet+, Eomeshi | ||||
| CD3− CD56bright CXCR6+ | T-betlo, Eomeshi | |||||
| CD45+ Lin− CD127+ CD117− NKp44− CRTH2− | CD161+, CD69+, CD62L−, CD103− | T-bet+, Emoes+, RORγtint | ||||
| ILC group 2 | ||||||
| ILC2 | CD45+ Lin− CD127+CRTH2+ | CD161+, CD69+, CD62L−, CD103− | GATA-3+, RORγtint | IL-5, IL-13 | 48,49,211 | |
| ILC group 3 | ||||||
| ILC3 | CD45+ Lin− CD127+ CRTH2− CD117+ NKp44+/− | NKp46+, CD161+, CD69+, CD62L−, CD103− | RORγt+, Helios+ | IL-17A, IL-22 | 48,49,211 | |
| LTia | CD45+ Lin− CD117+ α4β7+ CD127+ | CD4−, OX40L+ | RORγt+, Id2+ | Lymphotoxin, RANKL, IL-22 | 48,212,213 | |
| Innate-like T lymphocytes | ||||||
| iNKT | CD3+ CD56+ Vα24/Vβ11+ | CD4+ CD8–, CD4– CD8– and CD4– CD8+ | PLZF+, Hobit+, T-bet+/GATA-3+/RORγt+ | TNF-α, IFN-γ, IL-4, IL-10, IL-13, IL-17A, GM-CSF, Cytotoxic | 55,56,209 | |
| Non-iNKT |
CD3+ CD56+ Vα24−; CD3+ CD56+ sulfatide-loaded CD1d tetramers+ |
CD4+ CD8–, CD4– CD8– and CD4– CD8+ | PLZF+, T-bet+, RORγt− | IFN-γ, IL-13 | 56,58,214 | |
| γδT | CD3+ Vδ1+/Vδ3+ | CD27lo/neg, CD69+, CX3CR1+, CXCR3+, CXCR6+ | PLZF+, T-bet+, RORγt+ | IFN-γ, TNF-α, IL-17A, Perforin, Granzym B | 65–67,215 | |
| MAIT | CD3+ CD4− TCR Vα7.2+ CD161+ | CD38+, PD-1+, CD69+, CD56+, NKG2D+, CD8+ (mostly) | T-bet+, Eomes+, Hobit+, RORγt+, PLZF+ | IL-17A | 77,84 | |
ILC innate lymphoid cell, NK natural killer, LTi lymphoid tissue-inducer, iNKT invariant natural killer T, non-iNKT non-invariant natural killer T, MAIT mucosal-associated invariant T cells
aLTi: references are from the fetal liver, intestine, and lymph nodes
Innate lymphoid cells (ILCs)
Research over the past decade has made a substantial contribution to understanding the cell biology in the liver, particularly through the discovery of liver-resident NK (LrNK) cells and the proposal of the nomenclature for ILCs as three groups (groups 1, 2, and 3 ILCs) in 2013.10,11 The early identification of bulk NK cells in the liver requires an update and a comprehensive description. ILCs are lymphoid immune cells that do not express antigen-specific receptors. Currently, ILCs are classified into five subsets, approved by the International Union of Immunological Societies (IUIS): NK, ILC1 (Group 1), ILC2 (Group 2), and ILC3 and LTi cells (Group 3), based on their development.12 The development of these cell lineages can be broadly described as follows: common lymphoid progenitors differentiate into common innate lymphoid progenitors, which are the progenitors of all ILC populations, and then further differentiate into either NK cell precursors (NKPs) that generate NK cells or common helper innate lymphoid progenitors (CHILPs). CHILPs generate lymphoid tissue-inducer progenitors (LTiPs) and innate lymphoid cell precursors (ILCPs) that differentiate into LTi and ILC1/2/3, respectively.12–15 ILCs play critical roles in the innate immune responses to pathogens and in the regulation of immune homeostasis and inflammation.
Group 1 ILCs
Accordingly, bulk liver NK cells should be referred to as group 1 ILCs, including cNK cells and ILC1s. Hepatic cNK cells, which emerge 2–3 weeks after birth, are defined as CD3− NK1.1+ CD49a− CD49b+ cells in mouse liver and are generated from NKPs dependent on transcription factor E4BP4 (also known as NFIL3).16 NK cells circulate in the blood; show cytotoxic function against target cells through a large panel of germline-encoded receptors; and strongly produce perforin, granzymes and cytokines, which require the transcription factor Eomes to develop and exert their functions.17–19 Thus, NK cells are also referred to as cytotoxic ILCs.20 Hepatic ILC1s are liver-resident cells that emerge before birth; they are defined as CD3− NK1.1+ CD49a+ CD49b− cells in the mouse liver and develop from ILCPs strictly dependent on transcription factor T-bet.10,16 ILC1s exhibit no/low cytotoxicity; however, they possess memory potential.21,22 IL-7Rα+ memory ILC1s generated in the lymph nodes (LNs) maintain their longevity in the liver with dependence on IL-7R signaling and CXCR6 recruitment.21 Compared with intestinal ILC1s, hepatic ILC1s exhibit more potent cytotoxicity due to the expression of granzyme B, perforin, TRAIL, and FasL.23 Additionally, hepatic ILC1s display important immune regulatory functions via high levels of inhibitory receptors, such as PD-1 and LAG-3 expression.24 In the steady state, CD49a+ ILC1s are the major subset of liver-resident ILCs, with high NKG2A expression levels and preferential localization to the perivascular spaces near DCs along the portal tracts of the liver.25
NK cells are distinct from ILC1s in development; however, NK cells, as an independent subset of ILCs, constitute group 1 ILCs together with ILC1s due to their many similarities, such as expression of NK1.1 and NKp46, production of the cytokine IFN-γ and dependence on T-bet.26 NKp46 is necessary and sufficient to regulate the expression of TRAIL posttranslationally by affecting its trafficking to the surface of group 1 ILCs, including NK cells and ILC1s.19 CD49a is a surface marker associated with ILC1s and can also be induced on NK cells after viral infection.27 Another phenotypic marker, CD200r1, is reported to be more useful for the stable and accurate identification of ILC1s during inflammation induced by MCMV infection.28 Notably, effector NK cells (CD49a− CD49b+ Eomes+) are converted into ILC1 populations, including intermediate ILC1s and ILC1s with the phenotypes of CD49a+ CD49b+ Eomes+ and CD49a+ CD49b− Eomesint, respectively, in the tumor microenvironment mediated by TGF-β signaling.29 The conversion of NK cells into ILC1-like cells (CD200r1+ CD49a+) is also observed in the obese liver, partially mediated by TGF-β signaling.30
Group 2 ILCs and Group 3 ILCs
ILC2s and ILC3s are relatively rare in the liver.31 ILC2s are the single subset included in Group 2 ILCs and are dependent on GATA-3 and ROR-α, which predominantly produce IL-4, IL-5, IL-9, and IL-13.31–34 ILC2s are mainly present in nonlymphoid tissues such as skin and lungs.31 Recent studies demonstrated that the transcription factor RelB restrained ILC2s, primarily by suppressing Bcl11b activity.35 In mice, ILC3 development is dependent on RORγt. According to the expression of NKp46, two ILC3 subsets can be distinguished: NKp46+ ILC3 and NKp46−ILC3 cells. ILC1/2/3s are called helper-like ILCs.20 LTi cells are essential for the formation of Peyer’s patches and LNs, which can produce both IL-17 and IL-22.36 In the intestine and airway, the trans-differentiation of ILC3s to ILC1s (also referred to as ex-RORγt+ ILC3s) and ILC2s toward ILC1s can be observed under certain conditions; interestingly, the shifts are reversible.37–39
Human ILCPs (Lin− CD7+ CD127+ CD117+ cells) circulate systemically and robustly generate all ILC subsets in specific tissues in response to local environmental signals.40 In humans, NK cells (CD3− CD56+ cells) are divided into CD56bright and CD56dim subsets. Compared with the mouse liver, the human liver is enriched for the CD56bright NK cell subset, most of which are considered LrNK cells.41,42 As a likely counterpart of mouse intrahepatic ILC1s, a CD56bright CD16low CD49a+ NK cell subset, namely, T-bet+ Eomes−, was identified in the human liver, expressed high levels of inflammatory cytokines, and degranulated poorly after simulation.43 Other groups reported that human CD56bright CXCR6+ NK cells or CD49e− NK cells are resident cells in the liver; however, these cells are Eomeshi T-bet+/lo and are unlike CD49a+ NK cells in the expression of transcription factors.44,45 Emoeshi T-bet+/lo CD56bright LrNK cells highly express NKG2D, NKp44, NKp46, CD107a, and perforin, indicating that they may be activated by innate signals and display cytotoxic functions.44 Furthermore, liver-resident CXCR6+ CD56bright NK cells are predominantly educated via NKG2A, since these cells generally lack the expression of self-inhibitory KIRs.46 NKp80 was reported as another phenotypic marker of human NK cells that is not expressed on ILC1s.47 The chemoattractant receptor expressed on Th2 cells (CRTH2) is selectively expressed on ILC2s, and in the human liver, the ILC population (CD45+ Lin− CD127+ CD16− NKG2A−) can be divided into distinct subsets: ILC1 (CD117− NKp44− CRTH2−), ILC2 (CRTH2+) and ILC3 (CD117+ CRTH2−) cells; and two subsets, NKp44− ILC3 and NKp44+ ILC3, defined according to NKp44 expression.48 In the healthy adult human liver, ILC2s are present at a low frequency, comprising ~0.1% of CD45+ cells, while NKp44− ILC3s account for the greatest proportion of the ILC population in the liver, unlike other organs such as the gut and tonsils, which are dominated by NKp44+ ILC3s.48,49
Innate-like T lymphocytes
Innate-like T lymphocytes comprise a diverse repertoire of unconventional T cells discovered in both mice and humans. As shown in Fig. 1, numerous innate-like T lymphocytes, including NKT cells, γδ T cells, and MAIT cells, are enriched at a high frequency in the liver. These cells are preprogrammed with limited TCR diversity and function (similar to those of innate lymphocytes) and rapidly respond to distinct molecular patterns or antigens. They recognize antigens presented via MHC class-I-like molecules and rapidly produce large quantities of cytokines, which have important roles in immunity against infection, tumors, and autoimmunity.50
NKT cells
NKT cells, known as CD1d-restricted T lymphocytes, coexpress NK cell receptors and rearrange TCRs that recognize lipid antigens or other glycolipids. Two subsets, type I or invariant NKT (iNKT) and type II or non-invariant NKT (non-iNKT), are classified according to their TCR usage and recognized antigens (Table 1, 2). CD1d tetramer staining is used to precisely identify iNKT populations with limited TCRs (Vα14-Jα18 paired with Vβ2, Vβ7, or Vβ8 in mice; Vα24-Jα18 combined with Vβ11 in humans), which are strongly reactive to the glycosphingolipid antigen α-galactosylceramide (α-GalCer).51 Type II NKT cells can respond to both glycolipids and phospholipids (such as sulfatide and lysophosphatidylcholine) that are derived from themselves or from microbes, with characteristics of both conventional T cells and type I NKT cells.52
In the liver, NKT cells are defined as tissue-resident cells with high levels of CXCR6 and LFA-1 expression.53 According to their cytokine production and expression of lineage-specific transcription factors, iNKT cells are divided into three functional subsets: iNKT1 (T-bet, IFN-γ), iNKT2 (GATA-3, IL-4), and iNKT17 (RORγt, IL-17) cells.54 Additionally, NKT cells can exert cytotoxicity induced by perforin, CD95/CD95 L, and TNF.55 In mice, NKT cells are first detected around day 5 after birth, and the frequency is highest, up to ~15–35% of IHLs, among which iNKT cells are abundant, with a frequency of 10–30% of IHLs.55–57 In humans, the CD4+ NKT cell subset is mostly present at birth, while CD4– NKT cells emerge later with age. The frequency of iNKT cells is lower in humans than in mice; however, iNKT cells are substantially enriched in the liver with a frequency of ~1% of IHLs but only 0.01–0.5% in their peripheral counterparts.51,55 Non-iNKT cells appear to be more prevalent than iNKT cells in the human liver, representing 18–30% of IHLs.56,58
γδ T cells
γδ T cells, as unconventional T lymphocytes, display immunological features of both innate and adaptive immune cells and rapidly respond to stress without clonal selection and differentiation through TCR recognition or innate recognition/stimuli.59 γδ T cells are generated during embryonic development and migrate into peripheral tissues, which are maintained for life as tissue-resident cells. Unique γδ T cell subsets were found in some tissues, but not elsewhere; for example, dendritic epidermal T cells and Vγ5 + T cells in the intestinal epithelium (γδ IEL).60 In the liver, γδ T cells are abundant with a frequency of 3–5% of all IHLs, making up 15–25% of total hepatic T cells, which are identified as liver-resident cells with predominant production of IL-17A.2,61,62 Microbiota maintain the homeostasis of liver-resident γδ T-17 cells, depending on lipid antigen/CD1d interaction, but not via TLRs or IL-1/IL-23 receptor signaling. Hepatocyte-expressed CD1d presents lipid antigens to γδ T cells and augments the number of γδ T-17 cells in the liver.61 Hepatic γδ T cells exhibit mixed Vγ chain usage, mainly including Vγ1, Vγ4, and Vγ6, and those with the CD44highCD62L− phenotype are in a more active and mature state.61 Additionally, hepatic γδ T cells rarely express CD121, CD25, and CD127.61 The unique features of hepatic γδ T-17 cells may be acquired after birth, since neonatal mice have low levels of γδ T-17 and high levels of γδ T-1 cells in the liver. Hepatocyte-derived H2-Q10, a ligand for CD8αα, also controls liver-resident γδ T cell development.63 These programmed tissue-resident γδ T cells would be shaped by and adapted to the tissue environment.
In humans, γδ T cells are classified into two main subsets according to their TCRδ chain repertoire: Vδ1+ and Vδ2+ cells. Vδ2+ T cells are predominant in the peripheral blood, while Vδ1+ cells predominate in the mucosal tissue and skin.64,65 Vδ1+ γδ T cells are long-lived and exhibit a differentiated effector phenotype of CD27low, increased expression of granzyme B and CX3CR1 and retention of their proliferative capacity and TCR sensitivity, which are distinct from those of the Vδ2+ γδ T subset.66 In the liver, clonally expanded effector Vδ1+ T (CD27lo/neg) cells are dominant, whereas naive γδ T (CD27hi) cells are absent. Among them, a subset of Vδ1+ T cells functionally express CD69, CXCR3, and CXCR6 and clonotypically express liver-restricted TCR, indicating that they are liver-resident cells as a distinct cell population. These cells are capable of producing the polyfunctional cytokines IFN-γ and TNF-α and the cytotoxic molecules perforin and granzyme B and responding to both TCR and innate stimuli.67 Additionally, a distinct population of Vδ3+ γδ T cells is enriched in the human liver and may also be activated and expanded via CD1d recognition to release Th1, Th2, and Th17 cytokines and exert cytotoxicity to target cells.68 Furthermore, a high proportion of γδ T cells are enriched in the normal liver parenchyma, comprising 8–15% of IHLs.41,67
MAIT cells
MAIT cells are a population of innate-like T lymphocytes, defined by the coexpression of the semi-invariant TCR Vα 7.2-Jα 33/12/20 primarily paired with V β2/13 (in humans) or TCR Vα 19-Jα 33 paired with V β6/20 (in mice) and CD161.69,70 MAIT cells recognize vitamin B (riboflavin/vitamin B2; folic acid/vitamin B9) metabolites presented by the conserved MHC class-I-related molecule 1 (MR1), rapidly secrete a large amount of proinflammatory cytokines, including IFN-γ, TNF-α, IL-2, and IL-17, and exert cellular cytotoxicity against target cells.71–75 MAIT cells can be activated indirectly by IL-12, IL-18, and IL-15 produced by other immune cells in the absence of TCR signaling.76,77 Two key transcription factors, RORγt and promyelocytic leukemia zinc-finger (PLZF), appear to be responsible for the distinctive phenotypes of MAIT cells.78,79 The percentage of CD161hi Vα7.2+ MAIT cells was significantly higher in the liver than in the blood (~15% of IHLs in the liver vs. ~3% of PBLs in blood).80,81 In the human liver, highly enriched MAIT cells comprise up to 20–50% of liver-resident lymphocytes, primarily located in the biliary tract and liver sinusoids, which are constantly exposed to bacterial products from the intestine.82–84 However, in wild-type B6 mice, MAIT cells are much less abundant in the tested tissues compared with those of humans, including the liver (mean 0.6% of total αβTCR+ T cells in mice), which was speculated to be attributed to the fact that laboratory mice do not experience the microbial infections necessary for MAIT cell expansion.70 Hepatic MAIT cells are generally more activated and display tissue residency with high levels of CD69, CD56, CD38, PD-1, and NKG2D expression.77,85 MAIT cells resident in the liver adapt to exert their functions, such as dominantly producing IL-17A, which is licensed by IL-7 produced by hepatocytes in the context of inflammation.81 Furthermore, liver MAIT cells can exert TCR/MR1-independent but NKG2D-dependent cytotoxicity in response to the cytokine IL-15.77 The dominance of MAIT cells in the liver indicates that they play important roles in immune homeostasis in the steady state as well as in immune defense and inflammation in liver diseases.
Roles of innate lymphocytes in pathological conditions in the liver
The liver has various physiological functions, including metabolic activities, nutrient storage and detoxification and is sensitive to numerous diseases, including hepatotropic virus infection, ALD, NAFLD, AILD, and liver cancer, which are major health problems globally. The balance between tolerance and immunity is critical to liver functions. A variety of mechanisms in homeostasis ensure control of innate and adaptive immune responses and contribute to the activation of innate and adaptive immune cells to eliminate infected, injured, or malignant cells in the liver. As the first line of innate immune responses, enriched innate lymphocytes, including NK, ILC1s, NKT, γδ T, and MAITs, play critical roles in the resistance to viral infection and tumors and are also involved in liver damage and pathogenesis (Fig. 2).
Fig. 2.
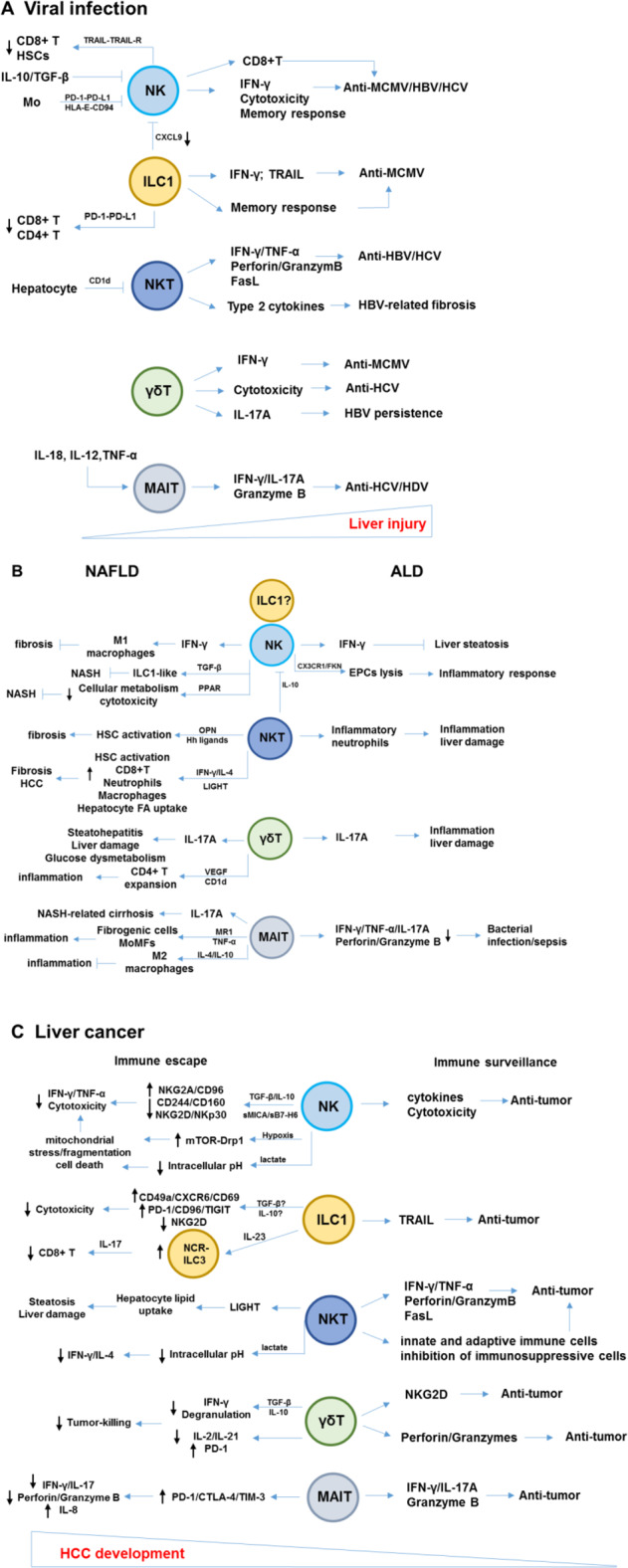
Pathological and protective roles of innate lymphocytes in liver diseases. a During viral infection of the liver, innate lymphocytes play critical antiviral roles via several mechanisms, including cytokine production, cytotoxicity, activation of adaptive immune cells, and memory responses; however, these antiviral functions may be inhibited by suppressive immune cells or cytokines in the virus-infected liver. Furthermore, innate lymphocytes, particularly NK cells and ILC1s, play an important role in the regulation of adaptive immune responses to maintain immune homeostasis. b The pathological responses of innate lymphocytes during the progression of ALD and NAFLD. NK cells exhibit protective roles in preventing liver steatosis and fibrosis. c Immunosurveillance of innate lymphocytes in liver cancer. In tumor microenvironments, a series of factors induce the dysfunction and exhaustion of innate lymphocytes, resulting in immune escape. NK natural killer, ILC innate lymphoid cell, NKT natural killer T, MAIT mucosal-associated invariant T cells. OPN osteopontin, Hh hedgehog, LIGHT LTβR ligand, PPAR peroxisome proliferator-activated receptor, MR1 MHC class-I-related molecule 1, EPCs endothelial progenitor cells, mTOR-Drp1 rapamycin-GTPase dynamin-related protein 1
Viral infection
It is well known that NK cells mediate resistance against pathogens as the first line of host defense and contribute to the activation of adaptive immune responses and their orientation during viral infections. The liver has properties of immune tolerance and is an attractive target site for pathogens, particularly HBV and HCV. Previous studies have indicated that NK cells contribute to HBV persistence by expressing the inhibitory receptor NKG2A86 and promote chronic MCMV infection by limiting virus-specific CD8+ T cell immunity.87 NK cells, featuring enhanced cytotoxicity and dysfunctional cytokine production, were demonstrated to contribute to virus persistence in patients with chronic HBV and HCV infections.88 Hepatic NK cells display more cytotoxicity than peripheral NK cells and are positively correlated with the extent of liver damage in HBV-infected immune-active patients.89 In HBV transgenic mice, NK cells were shown to mediate susceptibility to hepatocyte injury in an NKG2D-dependent manner or via enhanced production of IFN-γ.90,91 However, these studies presented the roles of bulk NK cells, including a mixture of cNK cells and LrNK/ILC1s. Here, we describe the precise roles of liver NK cells and ILC1s in detail based on data from recent studies.
Higher levels of IFN-γ produced by liver NK cells generate stronger resistance to MCMV.92 During MCMV infection, the conversion of cNK cells into CD49a+CD49b+ NK cells was observed in the liver, representing a transiently activated state of cNK cells with enhanced IFN-γ production and stronger cytotoxicity.93 Additionally, NK cells produced IL-10 during the early stage of MCMV infection, which regulated T cell activation and prevented liver damage for a better disease outcome.94 At the local site of primary MCMV infection, liver-resident ILC1s played an essential early antiviral role prior to the initiation of circulating responses in host protection through rapid IFN-γ production, promoted by IL-12-derived from tissue-resident XCR1+ conventional DCs (cDC1s). Ablation of liver ILC1s led to a significantly increased viral load, although in the presence of intact innate and adaptive immune cells critical for MCMV clearance.28 TRAIL is a key effector molecule utilized by ILC1s that can operate in defense against persistent infection; however, MCMV could evade TRAIL-mediated ILC1 defenses since MCMV m166 protein restricted the expression of TRAIL death receptors.95 Furthermore, the contribution of liver-resident ILC1s to subsequent adaptive immune responses during viral infections was demonstrated to inhibit the antiviral function of hepatic CD4+ T and CD8+ T cells via PD-1-PD-L1 interactions.24 Whether human liver-resident ILC1s can also negatively regulate T cell responses via the PD-1-PD-L1 axis during hepatotropic virus infection remains to be fully determined. In the mouse liver, CD49a+ ILC1s with high expression levels of NKG2A are present near DCs localized to perivascular spaces surrounding the portal triads. Upon hepatotropic adenovirus infection, NKG2A signaling in CD49a+ ILC1s inhibits the production of CXCL9, which is required for recruiting peripheral IFN-γ+ CD49b+ NK cells into the liver. Consequently, the activation of liver-resident CD103+ DCs is inhibited, which in turn dictates antiviral CD8+ T cell responses.25 ILC1s also contribute to maintaining the liver as a tolerogenic site by limiting peripheral NK cell migration into the liver at the early stage of viral infection.25 Additionally, liver ILC1s acquire adaptive features by recognition of the MCMV-encoded glycoprotein m12, which was independent of proinflammatory cytokines through bystander activation during MCMV infection, and the memory IL-18R+ ILC1 cell responses resulted in enhanced protective effects in response to secondary challenge with MCMV through enhanced IFN-γ production.22 These findings regarding liver-resident memory ILC1s are similar to those for MCMV-specific Ly49H+ NK cells.96
In the acute HBV infection, NK cells are important for viral clearance through cytokine production and the promotion of anti-HBV CD8+ T cell responses, which can largely be attributed to cNK cells but not ILC1s.97,98 NK cells are impaired in the chronic HBV infection, with defects in their antiviral functions. Immunosuppressive cytokines, such as IL-10 and TGF-β, which are elevated in the livers of patients with CHB, might account for the dysfunction of NK cells with higher levels of NKG2A expression and reduced levels of NKG2D and 2B4 expression.86,99 HBV employs hepatitis B surface antigen to generate suppressive monocytes, which educate NK cells to produce IL-10 via PD-L1/PD-1 and HLA-E/CD94 interactions, resulting in T cell inhibition.100 Furthermore, NK cells render antiviral T cell immunity in a TRAIL-dependent manner.101 Increased NK cell activity, particularly cytotoxicity, indicates a potential pathogenic role in viral hepatitis, consistent with the clinical observation that activated NK cells accelerate liver damage in patients with chronic HBV infection.102 In a novel liver and immune system dual humanized “human bone marrow mesenchymal stem cell (hBMSC)−Fah−/−Rag2−/−IL−2Rγc−/− SCID (FRGS)” mouse model (hBMSC-FRGS), human immune cells, including T cells, B cells, NK cells, macrophages, and DCs, were detectable, and further chronic hepatitis was induced by HBV infection, ultimately leading to the development of liver cirrhosis.103 Nevertheless, in patients with chronic HBV infection, enriched hepatic KLRG1+ NK cells with a mature phenotype of elevated expression of CD57 and DNAM-1 and reduced expression of NKp46 and NKG2A exerted antifibrotic functions by killing activated HSCs in a TRAIL-dependent manner and CD44-osteopontin-dependent manner.104 The level of elevation of ILC1s, but not ILC2s, was closely related to hepatic damage in patients with CHB, suggesting potential proinflammatory roles of ILC1s in the pathogenesis of CHB.105
NK cells are involved in the whole process of HCV infection, from providing innate protection to contributing to viral clearance induced by treatments. In acute HCV infection, NK cells exhibited an activated feature with enhanced cytotoxicity and IFN-γ production. Nevertheless, recent studies indicated that NK cell activation might favor progression to chronic HCV infection rather than HCV control by eliminating helper T cells.106 In chronic HCV infection, increased proportions of immature NK cells with higher NKG2A expression are observed in the liver and correlate inversely with the HCV viral load.107 The proportion of CD57+ FcRIneg adaptive NK cells was associated with increased HCV load, characterized by increased expression of PD-1 and reduced ADCC activity. Successful direct-acting antiviral (DAA) therapy restored a normal phenotype and enhanced IFN-γ production by adaptive NK cells.108
NKT cells responded to and controlled HBV/HCV infection at the early stage by producing IFN-γ and TNF-α and expressing the cytotoxic molecules perforin, granzyme B, and FasL, which also account for liver injury and chronic viral hepatitis.109 However, resident iNKT cells show aberrant activation and hyporesponsiveness to α-GalCer due to increased CD1d expression in HBV-infected livers of patients with CHB, which is associated with disease progression.110 Highly activated iNKT cells might migrate from the periphery into the liver and contribute to the progression of fibrosis to cirrhosis through the production of type 2 cytokines.111
γδ T cells control the viral load and attenuate organ damage in the liver during MCMV infection, and γδ T cells exhibit an effector-memory phenotype (CD44+ CD62L−) including both Vγ1 and Vγ4 subsets when responding to MCMV. Notably, γδ T cells are not the major producers of IFN-γ during the early acute MCMV infection, but ILC1s in the liver are.28,112 In HBV-carrier mice with plasmid-mediated HBV persistence in the liver, γδ T cells, particularly Vγ4+ T cells, drive myeloid-derived suppressor cell (MDSC) infiltration into the liver, resulting in MDSC-mediated CD8+ T cell exhaustion, which accounts for HBV-induced systemic immune tolerance.113 In humans, hepatic Vδ2+ T cells are activated and differentiated into effector cells with high CD38, CD56, and CD107a expression, acquiring a cytotoxic NK-like phenotype in HCV-infected patients. A markedly impaired ability to produce IFN-γ but enhanced cytotoxicity contributes to liver inflammation and virus persistence during chronic HCV infection.114
MAIT cells have also evolved to respond to viral infections in a TCR-independent manner. The frequency of MAIT cells was found to be reduced in the liver during chronic HCV infection but was improved within 4 weeks of therapy with DAA, with normalization of serum ALT activity.115 Activation of MAIT cells in a TCR-independent but cytokine-dependent manner, such as IL-18 induced by HCV infection or interferon (IFN)-α for HCV treatment, led to cytokine release and granzyme B production, correlating with reduced HCV replication.116 Patients with chronic HDV infection exhibited signs of microbial translocation in the liver and enhanced levels of IL-12 and IL-18, which induced activation of residual MAIT cells with a distinct phenotype (CD38hi CD28lo CD127lo PD-1hi) and promoted MAIT cell apoptosis, leading to loss of intrahepatic MAIT cells.117
Alcoholic liver disease (ALD)
ALD, ranging from hepatic steatosis (fatty liver), alcoholic hepatitis (AH), fibrosis, and cirrhosis to hepatocellular carcinoma (HCC), is caused by excessive alcohol consumption, with hepatocellular injury induced by ethanol and its metabolites occurring through their direct cytotoxic effects and oxidative stress. Heavy drinkers develop AH with an approximate rate of 10–35%, which is clinically characterized as severe and progressive acute chronic liver inflammation and a systemic inflammatory response syndrome with high morbidity and mortality.118 Alcohol exposure causes the dysregulation of innate and adaptive immune cells, which contributes to ALD pathogenesis.119,120 In particular, alcohol-induced loss of gut integrity, leading to enhanced bacterial and bacterial product translocation to the liver, triggers immune cell activation and inflammatory responses.121,122 Immune incompetence of CTLs and NK cells may be a key reason for increased mortality in patients with severe alcoholic hepatitis (SAH) (from fibrosis to cirrhosis) due to the increased infection risk.123
NK cells were found to play essential protective roles against liver steatosis through the production of IFN-γ, by which lipogenesis-associated gene expression was inhibited in hepatocytes in a chronic plus single-binge ethanol consumption mouse model. However, the protective roles of NK cells can be antagonized by NKT10 cells, which inhibit the recruitment and activation of NK cells by producing IL-10 in alcoholic hepatosteatosis.124 However, in human SAH, NK cells (CD3− CD16+ CD56+) induce the lysis of CD31+ CD34+ endothelial progenitor cells via the CX3CR1/FKN axis, which may be a key event contributing to proinflammatory responses and disease severity.125 In these studies, NK cells were identified in bulk; thus, the precise roles of cNK cells and ILC1s in the pathogenesis of ALD require further investigation. By using a mouse model, chronic alcohol consumption was shown to significantly decrease the number of cNK cells and their cytotoxicity by arresting the development of cNK cells at the CD27+ CD11b+ stage in the liver, spleen, lung, and LNs (while having no significant effect on ILC1s), as a result of a lack of IL-15 in the microenvironment. IL-15/IL-15Rα treatment not only led to the recovery of cNK cell numbers but also restored cNK cell maturation.123
iNKT cells are pathogenic in ALD induced by acute or chronic alcohol consumption. Alcohol consumption-activated proinflammatory iNKT cells accumulate in the liver and lead to the recruitment of inflammatory CD11b+ Gr-1hi Ly6G+ neutrophils, resulting in liver damage.126 During this process, NLRP3-triggered IL-1β from KCs plays a critical role in recruiting and activating iNKT cells in the liver, subsequently promoting the infiltration of neutrophils and inflammation after alcohol exposure.127 Chronic alcohol consumption increased CD1d expression levels on enterocytes, which activated iNKT cells in the intestine to migrate into the liver, where together with resident hepatic iNKT cells, they contributed to hepatocyte death and damage.128 Recent studies demonstrated that KCs were also activated by TLR3 recognition of EtOH-mediated generation of mitochondrial double-stranded RNA through exosomal delivery, and consequently, increased IL-1β from KCs triggered γδ T cells to produce IL-17A at the early stages of ALD. Furthermore, γδ T cells may accelerate the production of IL-17A in CD4+ T cells in the later stages of ALD.129
MAIT cells are markedly reduced in the peripheral blood of patients with severe AH and alcohol-related cirrhosis but not in the liver.130,131 Homing signals including β7-integrins and the chemoattractant CXCL10 hyperexpressed in the liver may facilitate intrahepatic MAIT cell relocation with preferential portal accumulation in ALD.130 MR1 expression is also much stronger in liver tissues from individuals with severe AH. The remaining blood and residual MAIT cells are phenotypically hyperactivated but functionally defective in cytokine production and cytotoxicity with compromised antibacterial potency in ALD as a consequence of the interaction with microbiota and microbial products.130,132 The impairment of MAIT cells may be responsible for increased susceptibility to bacterial infection and sepsis, which is one of the leading causes of alcohol-related death in patients with ALD.
Nonalcoholic fatty liver disease (NAFLD)
NAFLD is caused by excess lipid accumulation in the liver (steatosis) induced by factors other than viral infection, alcohol consumption, and medication. NAFLD is categorized into two forms: nonalcoholic fatty liver (NAFL) with simple hepatic steatosis and nonalcoholic steatohepatitis (NASH). NASH is a progressive form of NAFLD with an estimated prevalence of up to 30% in individuals with fatty liver, characterized by steatosis, hepatocyte injury, and inflammation. NASH is a global health problem that poses a high risk for the development of liver cirrhosis and HCC.133,134 NAFLD is strongly associated with several events, including obesity, metabolic syndrome (such as type 2 diabetes mellitus), insulin resistance, dyslipidemia, hypertension, dysfunctional adipose tissue, and altered gut microbiota.135 Almost all cells in the liver are involved in NAFLD pathogenesis, including hepatocytes, LSECs, HSCs, KCs, monocytes, neutrophils, DCs, T cells, B cells, and other innate lymphocytes.136
cNK cells were found to be recruited into the liver in a CXCL10-dependent manner, and they prevented NASH progression to fibrosis through the production of IFN-γ, which regulated macrophage polarization toward M1-like macrophages.137,138 IL-15 signaling in hepatocytes upregulated the expression of chemokines CXCL10, CCL2, and CCL5, accounting for the recruitment of immune cells, including NK cells, into the liver.139 In the obese liver, NK cells converted toward ILC1-like cells with increased CD200r1 and CD49a expression, partially mediated by enhanced TGF-β, which showed protective roles with reduced cytotoxicity in the progression of NASH.30 Lipid accumulation in NK cells, driven by obesity-induced robust peroxisome proliferator-activated receptor (PPAR) γ, impaired mammalian target of rapamycin (mTOR) signaling and disrupted their cellular metabolism and the expression of effector molecules, which may be responsible for the reduced cytotoxicity of hepatic NK cells.140 The role of hepatic ILC1s in the development of NAFLD remains unknown, although the number of CD49a+ NK1.1+ ILC1s is reduced in livers with NASH.137 In humans, the frequency and number of hepatic NK cells were unaltered by the clinical stages of NASH, although the levels of the attractive chemokines CXCL9, CXCL10, and CXCL11 were enhanced.141 Notably, intrahepatic T-bethi Eomeslo or T-betlo Eomeshi NK cells exhibited decreased degranulation and killing ability, which was inversely related to disease severity.30 The exact roles of human LrNK cells in the progression of NAFLD remain obscure due to their uncertain identification.
The roles of NKT cells in the progression of NASH have been reported but remain controversial. Some studies have demonstrated that NKT cells play a protective role in liver inflammation and damage during the progression of NASH.142,143 Due to apoptosis of NKT cells, excessive IFN-γ and TNF-α were present and promoted hepatic inflammation during the development of NAFLD.144 Conversely, some other studies demonstrated that accumulated NKT cells could drive fibrosis during NAFLD because osteopontin and hedgehog ligands from NKT cells induced HSC activation.145–147 During disease progression, Th1 cells triggered by oxidative stress-induced antigens contribute to NKT cell activation and recruitment by selectively upregulating IL-15.146 Additionally, iNKT cells are differentially skewed from predominant IL-17+ to IFN-γ+/IL-4+ subsets during NASH progression, which promoted the infiltration of CD8+ T cells, neutrophils and macrophages and the activation of HSCs to mediate progression from steatosis to fibrosis.148 Furthermore, in a NASH-driven HCC model induced by choline-deficient high-fat diet (CD-HFD) feeding, NKT cells secreted LIGHT, an LTβR ligand, to promote hepatocyte FA uptake, primarily initiating steatosis and hepatocyte damage, and further promoted liver damage and HCC development.149 In humans, similar results were observed to those found in mouse models of NASH, in which NKT cell-derived osteopontin was correlated with fibrosis severity during NASH.147 Significantly enhanced levels of LIGHT were also detected in the livers of patients with NASH, primarily produced by NKT cells.149 NKT cells increased significantly in patients with different disease severities, ranging from moderate/severe steatosis to NASH and NASH-cirrhosis.145,149,150
Liver-resident γδ T cells produce IL-17A and promote the recruitment of neutrophils, inflammation, and ROS induction in the presence of gut microbiota, contributing to steatohepatitis, liver injury, and dysmetabolism of glucose during the progression of high-fat and high-carbohydrate diet-induced NASH.61 Independent of IL-17 expression, hepatic γδ T cells, particularly IL-17hi Ly6C− CD44+ γδ T cells recruited in a CCR2-dependent manner, promote steatohepatitis by mitigating conventional CD4+ T cell expansion and regulating their inflammatory program, which depends on γδ T cell-derived endothelial growth factor (VEGF) in a CD1d-dependent manner.62,151 γδ T cells play pathologic roles by orchestrating innate and adaptive immune programming during the progression of NAFLD.
MAIT cells enriched in the liver were found to exert protection against liver inflammation, which was dependent on anti-inflammatory M2 macrophages induced by MAIT cell-derived IL-4 and IL-10, in an MCD-induced NASH model.152 However, in the livers of patients with NASH-related cirrhosis, MAIT cells dominantly accumulated in the mesenchymal space within fibrotic septa but not in the sinusoids, which displayed significantly enhanced exhaustion features with increased expression of PD-1 and TIM-3 and displayed an activated phenotype with higher expression levels of IL-17 but not granzyme B, IFN-γ and TNF-α, suggesting their involvement in the pathogenesis.80 Furthermore, MAIT cells promoted the proliferation of fibrogenic cells in an MR1-dependent manner, enhanced the proinflammatory properties of hepatic myofibroblasts via indirect TNF-α-mediated cell stimulation, and promoted the release of proinflammatory cytokines from monocyte-derived macrophages.80 These results indicated that MAIT cells were a profibrogenic cell population in the human liver.
Liver injury induced by toxins
Liver ILC1s are optimally activated to produce IFN-γ, dependent on the activating receptor DNAM-1 and cytokine IL-7, after carbon tetrachloride (CCl4) injection, which promotes hepatocyte survival by upregulating the expression of Bcl-xL and suppressing CCl4-induced acute liver injury.153 During CCl4-induced acute liver injury, the elevated concentration of extracellular ATP was found to upregulate IL-12 production in the CD11b− DC subset and to subsequently accelerate IFN-γ secretion from hepatic ILC1s, particularly CD25+ ILC1s via ATP-P2RX7 signaling. Notably, extracellular ATP alone could not induce the production of IFN-γ in hepatic ILC1s.153 Unlike intestinal injury, ILC1-derived IFN-γ amplified inflammation.154,155 Other protective molecules, in addition to IFN-γ, might also be produced by activated ILC1s, as IFN-γ neutralization or deficiency in ILC1s did not completely eliminate the inhibitory effect of ILC1s on liver injury induced by CCl4.153
In the livers of mice with CCL4-induced fibrosis, IL-33-ST2 signaling was required for hepatic accumulation of ILC2s, and then activated ILC2s induced hepatic fibrosis in an IL-13-dependent mechanism, independent of adaptive immune cells.156 In humans, the proportion of intrahepatic ILC2s was also correlated with the severity of fibrosis, which potentially produced the profibrotic cytokine IL-13. Enhanced IL-33 and thymic stromal lymphopoietin from hepatocytes, HSCs and KCs might account for the activation of ILC2s.48 Additionally, in the livers of mice with CCl4-induced fibrosis, more IL-17A+ ILC3 cells and IL-22+ ILC3 cells accumulated and were demonstrated to exert profibrotic effects.157
Following CCl4 treatment, hepatic γδ T cells primarily increased the production of IL-17A and exacerbated liver fibrosis, which might be related to HSC stimulation by exosomes from damaged hepatocytes as TLR3 ligands.158 In CCl4-induced liver fibrosis, the profibrogenic functions of MAIT cells were observed in vivo, since MAIT cell-deficient mice (MR1−/− mice) were resistant to fibrosis and MAIT cell-enriched mice (Vα19 TCR Tg mice, ten-fold increase in the proportion of MAIT cells) displayed exacerbated fibrosis, which was not secondary to changes in other immune cell populations such as CD4+ and CD8+ T cells, B cells, neutrophils, macrophages, DCs, iNKT cells or γδ T cells.80
Autoimmune liver disease (AILD)
AILD is characterized by chronic immune-mediated liver damage that eventually leads to liver fibrosis and cirrhosis and includes three major forms: primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and autoimmune hepatitis (AIH), which are associated with high morbidity and mortality.159
In the livers of patients with PBC, the number of NK cells was increased, and NK cells showed strong cytotoxicity and mediated lysis of biliary epithelial cells in a TRAIL-dependent manner, during which NK cell activation required both direct TLR4 stimulation and indirect TLR3 stimulation that activated monocytes to produce IFN-α and then simulated NK cells.160,161 NK cell cytotoxicity leads to abnormal exposure of autoantigens, and NK cells may also be involved in human autoimmune diseases through their interaction with DCs, macrophages or T cells, inducing excessive inflammation and adaptive autoimmune responses.162 Using a mouse model of PBC, LrNK cells were demonstrated to limit the proliferation of CD4+ T cells and suppress autoimmune cholangitis.163 In patients with AIH, HLA-C2, the high affinity ligand for KIR2DS1 of NK cells, reportedly contributed to unwanted NK cell autoreactivity.164 In patients with PSC, the cytotoxicity of hepatic NK cells was impaired by high local production of TNF-α.165
In adults with AIH, the number of NKT cells was found to be reduced, especially during the active state, and NKT cells produced lower levels of IL-4.166 Furthermore, the proportion of sulfatide-reactive type II NKT cells (non-iNKT cells), but not iNKT cells, was selectively increased in the peripheral blood (0.11% of peripheral blood leukocytes) and liver (3.78% of intrahepatic leukocytes) in adult patients with AIH relative to healthy individuals (0.05% and 1.82%, respectively). Moreover, intrahepatic non-iNKT cells generated increased TNF-α, decreased IFN-γ and a complete lack of IL-4, indicating the involvement of non-iNKT cells with proinflammatory cytokines in patients with AIH.167 In contrast, in children with AIH, iNKT cells were increased in the liver but reduced in the peripheral blood, which was localized in the lobular area and portal spaces.168
In patients with AIH, the frequency of MAIT cells and γδ T cells in the blood and liver did not differ significantly.167 However, other studies demonstrated that MAIT cells were significantly reduced in the peripheral blood and livers of patients with AILD, including PSC, PBC, and AIH, regardless of disease subtype. In diseased livers, MAIT cells accumulated in infiltrates surrounding portal tracts and fibrotic septae, whereas in normal livers, MAIT cells were present in the parenchyma and around portal tracts. Notably, MAIT cells from patients with ALID were chronically activated and showed features of functional exhaustion with impaired production of IFN-γ and high levels of CD38, HLA-DR, and CTLA-4 expression. Additionally, MAIT cells from patients with ALID were induced to produce IL-17A by repetitive stimulation with IL-12, which upregulated RORγt expression in MAIT cells, despite their exhausted phenotype. MAIT-derived IL-17A contributed to the induction of activated proinflammatory HSCs and promoted the progression of HSC-mediated liver fibrosis.169
Liver cancer
Liver cancer was the fourth leading cause of cancer-related death globally in 2018.170 HCC, as the most common primary liver cancer, represents the largest burden of liver cancer (~85%) and often arises secondarily to chronic liver inflammation caused by HBV/HCV infection, NASH, and ALD. The liver is also a common site for tumor metastasis, which is associated with very poor prognosis, for example, in colorectal cancer (CRC).
NK cells are capable of providing immune surveillance against tumors through innate recognition, cytokine production, and natural cytotoxicity. In the liver, NK cell-mediated antitumor responses are required to prevent CRC metastatic growth independent of adaptive immune cells, and this process is mediated by NLRP3 inflammasome-mediated IL-18, which promotes the maturation of hepatic NK cells and their expression of FasL and cytotoxicity against target tumor cells.171 However, tumor cells can induce NK cell dysfunction and escape NK cell immune surveillance.172,173 In patients with HCC, the frequency of intrahepatic NK cells among tumor-infiltrating lymphocytes was found to be reduced compared with that of nontumor infiltrating lymphocytes, and intratumoral NK cells were functionally impaired, which correlated with the prognosis and survival of patients.174 Recent studies demonstrated that in HCC intratumoral tissues, NK cells significantly increased the expression levels of NKG2A and CD96 and reduced the expression levels of CD160; these cells were functionally exhausted, with impaired production of IFN-γ and TNF-α, which was strongly related to poor prognosis and high recurrence rates in HCC patients.175–177 Human CD160+ NK cells are functionally activated with high production of IFN-γ under normal conditions, while intratumoral CD160+ NK cells exhibit features of exhaustion with lower IFN-γ production than peritumoral CD160+ NK cells.177 Furthermore, the accumulation of CD49a+ tissue-resident NK cells is higher in human HCC tissue than in peritumor tissues, which is positively correlated with the frequency of NK cells expressing the inhibitory receptors PD-1, CD96, and TIGIT. These findings suggest a role for CD49a+ NK cells in the negative regulation of immune responses and the development of HCC.178 In liver tumors, up to 79% of local NK cells with a liver-resident phenotype of CXCR6+ CD69+ were reported to downregulate NKG2D with selectively impaired proliferation and reduced cytokine production and cytotoxicity against target cells.179 These dysfunctions of NK cells might have been induced by the higher levels of immunosuppressive cytokines such as IL-10 and TGF-β present in liver tumor microenvironments.175–177 In addition, high levels of HCC tumor cell-derived soluble MICA significantly obstructed activation of the NKG2D pathway of NK cells, protecting tumor cells from NK cell-mediated cytotoxicity.180 Reduced B7-H6 in HCC tissue but simultaneously increased levels in soluble form in HCC patients in the advanced stage or with a larger nodule size caused deficient NKp30-mediated function of tumor-infiltrating NKp30+ NK cells.181
In human liver cancers, tumor-infiltrating NK cells have smaller fragmented mitochondria in their cytoplasm and impaired cytotoxicity, resulting in tumor escape from NK cell-mediated immune surveillance. Hypoxia in liver cancer causes mitochondrial fragmentation due to enhanced constitutive activation of rapamycin-GTPase dynamin-related protein 1 (mTOR-Drp1) signaling in NK cells, suggesting that hypoxia is an immunosuppressive factor for intrahepatic NK cells.182 LrNK cells migrating toward colorectal liver metastatic tumors undergo apoptosis because they are unable to regulate the reduced intracellular pH, leading to mitochondrial stress and intrinsic cell death, which results from elevated lactate in the tumor microenvironment.183 Compared with nonviral-related HCC, HBV-related HCC harbors the immune landscape with more suppressive and exhausted phenotypes, including a lower frequency of CD244+ NK cells that mediate antitumor activity.184 Notably, NK cell-derived IFN-γ promotes the development of HCC through the EpCAM-EMT axis in HBV transgenic mice, revealing the importance of NK cell activation and their induction of liver injury in the pathogenesis of HBV-related HCC.185
Regarding ILCs, prototypic hepatic ILC1s with high TRAIL expression were found to exert tumoricidal activity, which was calibrated by NKp46.186 In HCC patients, elevated IL-23 serum levels were associated with poor clinical outcomes. In the tumor microenvironment, IL-23 promoted the differentiation of ILC1s to ILC3s. NCR− ILC3 cells initially responded to IL-23 and produced IL-17 in the liver during the early phase of tumor development, which directly inhibited CD8+ T cell responses by promoting cell apoptosis and limiting cell proliferation to promote HCC development.187
Type I NKT cells offer the best protection against tumors via three mechanisms: direct cytotoxicity, transactivation of innate and adaptive immune cells, and inhibition of immunosuppressive cells in the tumor microenvironment.188 Accumulated hepatic NKT cells were found to show an activated phenotype as effector-memory CD44hi CD62Llow cells with higher levels of CD69 expression and increased production of IFN-γ upon antigen stimulation, which mediated inhibition of both primary and metastatic liver tumors.189,190 CXCL16 derived from LSECs regulated CXCR6+ NKT cell accumulation, making up the majority (~75%) of hepatic CXCR6+ cells, which was controlled by primary-to-secondary bile acid conversion mediated by the gut microbiome as a messenger regulating CXCL16 levels.190 In mice, depletion of commensal bacteria with antibiotics led to primary bile acid-induced CXCL16 and reduced secondary bile acid-inhibited CXCL16, resulting in the upregulation of CXCL16 in LSECs that accumulated NKT cells into the liver. The opposing effects of bile acids on CXCL16 expression were also observed in humans; primary bile acid levels positively correlated with the levels of CXCL16, whereas the inverse was for secondary bile acid.190 However, long-term feeding of a CD-HFD diet induced the activation of CD8+ T cells and NKT cells, cooperatively promoting NASH to HCC progression through interactions with hepatocytes via NF-κB signaling. NKT cells primarily enhanced hepatocyte lipid uptake via secreted LIGHT, which interacted with hepatocellular LTβR, leading to steatosis.149 In the livers of patients with NASH-related HCC, increased LIGHT from CD8+ T cells and NKT cells was also observed.149 Thus, it remains controversial whether NKT cells ultimately have a favorable impact on the development of HCC. In tumor microenvironments, elevated lactic acid-inhibited IFN-γ and IL-4 production by NKT cells, particularly IFN-γ, which was induced by low extracellular pH by inhibiting mTOR signaling and nuclear translocation of PLZF.191 Thus, the role of NKT cells in liver cancer is closely associated with the tumor microenvironment.
The number of γδ T cells was relatively reduced in the tumor tissues of HCC patients compared to paired nontumor tissues. CCL4/5 produced by HCC tumor cells served as a chemotactic factor for γδ T cell recruitment via their CCR5 receptor, which may mainly contribute to γδ T cell migration from peritumor tissues into tumor regions. Infiltrating γδ T cells showed stronger cytotoxic activity with higher expression of NKG2D, granzymes, and perforin, indicating that γδ T cells may exhibit antitumor activities in the progression of HCC.192 However, chemotherapy accelerated immune-senescence and functional impairment of the tumor-infiltrating Vδ2+ T cell subset from liver metastases in patients with CRC, observed as a relative increase in terminally differentiated CD27neg/CD45RApos (TEMRA) cells and increased expression of the senescence marker CD57, coupled with impairments in cytotoxicity and production of TNF-α and IFN-γ.193 Additionally, significantly lower expression of IL-2 and IL-21 in γδ T cells was observed in HCC patients compared with healthy control volunteers, accounting for their reduced cytotoxic capacity.194 Degranulation and IFN-γ secretion were also substantially impaired in HCC-infiltrating γδ T cells, partially mediated directly by Tregs in a TGF-β- and IL-10-dependent manner.195 This might be strongly associated with HBV infection and the TGF-β-miR-34a-CCL22 axis for Treg recruitment into the tumor microenvironment.196
MAIT cell infiltration is significantly decreased in HCC tumor tissue compared with paired peritumor tissue or normal liver tissue, which displays typical effector-memory phenotypes (CCR7− CD45RA− CD45RO+ CD95+) but potentially impaired effector capabilities.197 Significantly downregulated expression of CCR6, CXCR6, and CCR9 on MAIT cells, particularly in the tumor center, might be responsible for the low infiltration of MAIT cells into HCC tumors. HCC-infiltrating MAIT cells were educated by malignant cells to be functionally exhausted with high expression of PD-1, CTLA-4, and TIM-3; inhibited the inherent cytokine-secreting potential of IFN-γ and IL-17, reduced the production of perforin and granzyme B, and elevated the production of the tumor-promoting cytokine IL-8.197 A large fraction of intratumoral MAIT cells were also observed in colorectal liver metastasis tissues with the failure to produce IFN-γ.198 A higher level of MAIT cell infiltration was correlated with unfavorable clinical outcomes of HCC, indicating that MAIT cells were reprogrammed in the HCC tumor microenvironment and contributed to HCC development.197
Therapeutic targeting of innate lymphocytes for the treatment of liver diseases
A better understanding of hepatic immunity and its involvement in pathogenesis can potentially help the pursuit of effective treatments for liver diseases. Innate lymphocytes play important roles in host defenses against pathogens, injured or malignant cells but also elicit inflammation to mediate tissue damage. In certain disease situations, targeting innate lymphocytes might be an effective therapeutic strategy for the treatment or control of liver diseases. In summary, dysfunctional or exhausted innate lymphocytes can be rescued by cytokine stimulation, TCR-dependent stimulation, checkpoint blockade, and metabolic regulation or can be complemented by adoptive cell transfer. In contrast, activated innate lymphocytes can be controlled by suppressive cytokine stimulation or blockade of their activating receptors. As the ultimate outcome of various liver diseases, HCC is commonly considered an inflammation-related cancer that predominantly develops under the conditions of chronic inflammation, including HBV/HCV infection, NASH, and ALD. Thus, controlling the progression of chronic viral hepatitis, chronic alcoholic hepatitis, NASH, liver fibrosis, and cirrhosis would effectively prevent HCC development.
Cytokines or immune stimulators
Cytokines, such as IL-12, IL-15, and IL-18, can selectively boost both NK cell numbers and their antiviral or antitumor efficacy. For example, IL-15 can strikingly augment the degranulation response to target cells of tumor-infiltrating NK cells to a higher percentage.179,199 NK cell defects induced by alcohol consumption are extrinsic but not intrinsic, and recovery of IL-15 in the microenvironment is critical to restore NK cell function in alcoholics.123 Systemic activation of innate immune cells, including NK cells, DCs, and macrophages, by the injection of poly I:C at the precancer stage was found to robustly suppress liver tumorigenesis induced by either the loss of PTEN or chemical carcinogens, as well as associated hepatosteatosis.200 Additionally, downregulation of activating receptors and high levels of soluble ligands were related to a poor prognosis of HCC patients.180,181 Agonists of activating receptors or neutralization of their soluble ligands is another potential therapeutic strategy to modulate the effector functions of innate lymphocytes in liver diseases.
Innate-like lymphocytes, including NKT, γδ T, and MAIT cells, can respond in a TCR-independent and MHC-unrestricted TCR-dependent manner. For example, CD1d plays a critical role in regulating γδ T cell functions in ASH or NASH; thus, targeting CD1d or γδ T cells would have therapeutic potential for the treatment of steatohepatitis.62 Injection of α-Galcer activates NKT cells and increases their removal of senescent hepatocytes, which prevents hepatocarcinogenesis.189 Targeting the LIGIT-LTβ R interaction or hedgehog pathway prevents cross-talk between NKT cells and hepatocytes, efficiently inhibiting the development of liver damage NASH and HCC.147,149 Treatment with tazarotene, a kind of RAR-γ agonist, inhibits cell proliferation and cytokine production of iNKT cells, which significantly reduces liver steatosis and fibrosis.148
Checkpoint blockade
Increasing evidence indicates the dysfunction and exhaustion of innate lymphocytes in several pathogenic situations in the liver, as described above. Targeting these inhibitory receptors, for example, by using anti-KIR and anti-NKG2A in clinical trials and anti-TIGIT and anti-CD96 in preclinical studies, fully exploits the potential of checkpoint-blockade immunotherapy in liver diseases and cancer. In the human liver, resident CXCR6+/CD56bright NK cells are predominantly educated through NKG2A and are hyporesponsive to stimulation by target cells with reduced cytokine production, indicating that novel management aimed at activating LrNK cells could be beneficial in the control of infections or cancer.46 NKG2A expression was influenced by factors from cancer nests, such as a high level of IL-10 in HCC patients, and contributed to NK cell exhaustion, suggesting that blockade of NKG2A has the potential to restore immune surveillance on liver tumors by reversing NK cell exhaustion.175 Additionally, inhibition of NKG2A signaling on NK/ILC1s can induce robust immune responses of CD8+ T cells against persistent pathogens in the liver as a novel vaccine strategy.25 IL-1R8 is a checkpoint for NK cells, inhibiting their maturation and effector function, and blockade of IL-1R8 unleashes NK cell-mediated resistance to hepatic carcinogenesis, hematogenous liver metastasis, and MCMV infection.201 High levels of TGF-β1 were found to upregulate CD96 expression in NK cells and to inhibit CD160+ NK cells in HCC patients. Blocking TGF-β1 specifically restored the expression of CD96 and CD160 on NK cells to normal levels and reversed NK cell dysfunction.176,177 Blocking the CD96–CD155 interaction restored NK cell responses against tumor cells by reversing NK cell exhaustion, indicating a potential therapeutic role for CD96 in fighting HCC. Additionally, restoring CD160 expression appears to be another promising management strategy against liver cancer.177
Metabolic modulation
As described above, the activation and function of these hepatic innate lymphocytes are regulated by lipid accumulation, oxidative stress/ER stress, glucose dysmetabolism, hypoxia, and elevated lactate in tissue microenvironments or gut microbiota via the gut–liver axis, suggesting that modulation of the metabolic profile may be an effective strategy to control their activation and effector functions. For example, inhibition of mitochondrial fragmentation by treatment with a fission inhibitor, mdivi-1, selectively targeting Dyn and attenuating Drp1 self-assembly, or Drp1 expression knockdown, improves mitochondrial metabolism, cell survival and antitumor activity of NK cells, indicating that targeting hypoxia-induced mTOR signaling could rescue NK cell exhaustion and invigorate NK cell antitumor activity for HCC.182 Additionally, metabolic regulation through targeting NK and ILC1s could prevent hepatocyte injury and inflammation during the process of NASH.30,202
Adoptive cell transfer
NK cell adoptive transfer has shown promising prospects in tumor immunotherapy. Allogeneic haploidentical NK cells have been demonstrated to be good sources of various adoptively transferred NK cells, which have marked antitumor activities with a low rate of rejection and weak side effects after short- or long-term activation or expansion in vitro. CAR-NK cells, generated using genetic modification, show enhanced cytotoxicity, specificity, and selectivity. In HCC, glypican-3- (GPC3-) specific CAR-NK92 cells were reported to show potent antitumor activities in multiple HCC xenografts with either high or low GPC3 expression, indicating that GPC3-specific NK cell adoptive transfer could be a novel treatment option for GPC3+ HCC patients.203 Furthermore, allogenic γδ T cell adoptive transfer therapy was used for HCC patients, which positively regulated peripheral immune functions, inhibited tumor activity, improved the quality of life and prolonged the life span.204
Concluding remarks
At present, the understanding of the innate immune system in the liver is incomplete. Although some potential treatments have been proposed based on the innate immune pathogenesis of liver disease, there remains some progress to be made until these approaches can be translated into clinical treatments for patients. In addition, the specific features of the liver require attention in the study of innate immune pathogenesis and therapeutic targets of liver diseases. Liver immune homeostasis is regulated by the gut–liver axis, involving the signals generated by dietary, genetic, and environmental factors. The gut microbiota is associated with various liver diseases, including NAFLD, ALD, liver fibrosis, and HCC.205 In both health and disease, gut microbiota regulate the activation and function of hepatic NK, ILCs, NKT, γδ T, and MAIT cells. Furthermore, innate lymphocytes function via interaction with other cells in the environment, such as hepatocytes, KCs, LSECs, and HSCs. In addition, interactions among these innate lymphocytes and between innate and adaptive lymphocytes participate in liver homeostasis and pathogenesis.
Accumulating evidence suggests that these innate lymphocytes not only have beneficial effects on the defense against viral infection, inhibit liver inflammation and fibrosis, and prevent carcinogenesis in the liver but also contribute to hepatocellular damage resulting in chronic inflammation and autoimmune diseases. Further investigations are required to clarify the diverse functions of these innate lymphocytes and to enhance our understanding of the immunopathogenesis of liver diseases. It is urgent that effective treatments be translated into clinical practice.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81788101, 91542000, and 81671554), the Ministry of Science & Technology of China (2017ZX10202203-002-001, 2017ZX10202203-009-002), and the National Key R&D Program of China (2019YFA0508503).
Author contributions
Y.C. prepared the manuscript, tables, and figures. Z.T. designed and provided guidance on the outline of this review and revised the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Mackay IR. Hepatoimmunology: a perspective. Immunol. Cell Biol. 2002;80:36–44. doi: 10.1046/j.1440-1711.2002.01063.x. [DOI] [PubMed] [Google Scholar]
- 2.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 3.Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 4.Crispe IN. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 5.Kubes P, Jenne C. Immune responses in the liver. Annu. Rev. Immunol. 2018;36:247–277. doi: 10.1146/annurev-immunol-051116-052415. [DOI] [PubMed] [Google Scholar]
- 6.Jenne CN, Kubes P. Immune surveillance by the liver. Nat. Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 7.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 8.Khomich O, Ivanov AV, Bartosch B. Metabolic hallmarks of hepatic stellate cells in liver fibrosis. Cells. 2019;9:24. doi: 10.3390/cells9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yankai Wen, J. L., Ju, C. & Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol. Immunol. (2021). [DOI] [PMC free article] [PubMed]
- 10.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Investig. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spits H, et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Klose CSN, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, et al. Single-cell RNA-seq identifies a PD-1(hi) ILC progenitor and defines its development pathway. Nature. 2016;539:102–106. doi: 10.1038/nature20105. [DOI] [PubMed] [Google Scholar]
- 15.Ishizuka IE, et al. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat. Immunol. 2016;17:269–276. doi: 10.1038/ni.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daussy C, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheppard S, et al. The murine natural cytotoxic receptor NKp46/NCR1 controls trail protein expression in NK cells and ILC1s. Cell Rep. 2018;22:3385–3392. doi: 10.1016/j.celrep.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Zhang C. The role of innate lymphoid cells in immune-mediated liver diseases. Front Immunol. 2017;8:695. doi: 10.3389/fimmu.2017.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, et al. Memory formation and long-term maintenance of IL-7Ralpha(+) ILC1s via a lymph node-liver axis. Nat. Commun. 2018;9:4854. doi: 10.1038/s41467-018-07405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weizman OE, et al. Mouse cytomegalovirus-experienced ILC1s acquire a memory response dependent on the viral glycoprotein m12. Nat. Immunol. 2019;20:1004–1011. doi: 10.1038/s41590-019-0430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang L, et al. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J. Autoimmun. 2016;67:29–35. doi: 10.1016/j.jaut.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, et al. Liver-resident NK cells control antiviral activity of hepatic T cells via the PD-1-PD-L1 Axis. Immunity. 2019;50:403–417 e404. doi: 10.1016/j.immuni.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Krueger PD, et al. Murine liver-resident group 1 innate lymphoid cells regulate optimal priming of anti-viral CD8+ T cells. J. Leukoc. Biol. 2017;101:329–338. doi: 10.1189/jlb.3A0516-225R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat. Immunol. 2016;17:758–764. doi: 10.1038/ni.3482. [DOI] [PubMed] [Google Scholar]
- 27.Bezman NA, et al. Molecular definition of the identity and activation of natural killer cells. Nat. Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weizman OE, et al. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171:795–808 e712. doi: 10.1016/j.cell.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017;18:1004–1015. doi: 10.1038/ni.3800. [DOI] [PubMed] [Google Scholar]
- 30.Cuff AO, et al. The obese liver environment mediates conversion of NK cells to a less cytotoxic ILC1-like phenotype. Front. Immunol. 2019;10:2180. doi: 10.3389/fimmu.2019.02180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH, Hashimoto-Hill S, Kim M. Migration and tissue tropism of innate lymphoid cells. Trends Immunol. 2016;37:68–79. doi: 10.1016/j.it.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong SH, et al. Transcription factor RORalpha is critical for nuocyte development. Nat. Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHedlidze T, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, L. et al. The transcription factor RelB restrains group 2 innate lymphoid cells and type 2 immune pathology in vivo. Cell Mol. Immunol.10.1038/s41423-020-0404-0 (2020). [DOI] [PMC free article] [PubMed]
- 36.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernink JH, et al. Interleukin-12 and -23 control plasticity of CD127(+) Group 1 and Group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Ohne Y, et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat. Immunol. 2016;17:646–655. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- 39.Bal SM, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 2016;17:636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 40.Lim AI, et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell. 2017;168:1086–1100 e1010. doi: 10.1016/j.cell.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–594. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Heymann F, Tacke F. Immunology in the liver-from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 43.Marquardt N, et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J. Immunol. 2015;194:2467–2471. doi: 10.4049/jimmunol.1402756. [DOI] [PubMed] [Google Scholar]
- 44.Harmon C, et al. Tissue-resident Eomes(hi) T-bet(lo) CD56(bright) NK cells with reduced proinflammatory potential are enriched in the adult human liver. Eur. J. Immunol. 2016;46:2111–2120. doi: 10.1002/eji.201646559. [DOI] [PubMed] [Google Scholar]
- 45.Aw Yeang HX, et al. Cutting edge: human CD49e- NK cells are tissue resident in the liver. J. Immunol. 2017;198:1417–1422. doi: 10.4049/jimmunol.1601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lunemann S, et al. Human liver-derived CXCR6(+) NK cells are predominantly educated through NKG2A and show reduced cytokine production. J. Leukoc. Biol. 2019;105:1331–1340. doi: 10.1002/JLB.1MA1118-428R. [DOI] [PubMed] [Google Scholar]
- 47.Freud AG, et al. NKp80 defines a critical step during human natural killer cell development. Cell Rep. 2016;16:379–391. doi: 10.1016/j.celrep.2016.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forkel M, et al. Composition and functionality of the intrahepatic innate lymphoid cell-compartment in human nonfibrotic and fibrotic livers. Eur. J. Immunol. 2017;47:1280–1294. doi: 10.1002/eji.201646890. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Polo V, et al. Group 2 innate lymphoid cells exhibit progressively higher levels of activation during worsening of liver fibrosis. Ann. Hepatol. 2019;18:366–372. doi: 10.1016/j.aohep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T cell targets for cancer immunotherapy. Immunity. 2018;48:453–473. doi: 10.1016/j.immuni.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat. Protoc. 2008;3:70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- 52.Dhodapkar MV, Kumar V. Type II NKT cells and their emerging role in health and disease. J. Immunol. 2017;198:1015–1021. doi: 10.4049/jimmunol.1601399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas SY, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J. Exp. Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gapin L. Development of invariant natural killer T cells. Curr. Opin. Immunol. 2016;39:68–74. doi: 10.1016/j.coi.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat. Rev. Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 56.Rhost S, Sedimbi S, Kadri N, Cardell SL. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand. J. Immunol. 2012;76:246–255. doi: 10.1111/j.1365-3083.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- 57.Jahng A, et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Exley MA, et al. Cutting edge: compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J. Immunol. 2002;168:1519–1523. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]
- 59.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 60.Khairallah C, Chu TH, Sheridan BS. Tissue adaptations of memory and tissue-resident gamma delta T cells. Front. Immunol. 2018;9:2636. doi: 10.3389/fimmu.2018.02636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li F, et al. The microbiota maintain homeostasis of liver-resident gammadeltaT-17 cells in a lipid antigen/CD1d-dependent manner. Nat. Commun. 2017;7:13839. doi: 10.1038/ncomms13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Gao B. GammadeltaT Cells and CD1d, novel immune players in alcoholic and nonalcoholic steatohepatitis? Hepatology. 2020;71:408–410. doi: 10.1002/hep.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodall KJ, et al. Multiple receptors converge on H2-Q10 to regulate NK and gammadeltaT-cell development. Immunol. Cell Biol. 2019;97:326–339. doi: 10.1111/imcb.12222. [DOI] [PubMed] [Google Scholar]
- 64.Halary F, et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davey MS, et al. The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(-) subsets. Nat. Commun. 2018;9:1760. doi: 10.1038/s41467-018-04076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davey MS, et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat. Commun. 2017;8:14760. doi: 10.1038/ncomms14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hunter S, et al. Human liver infiltrating gammadelta T cells are composed of clonally expanded circulating and tissue-resident populations. J. Hepatol. 2018;69:654–665. doi: 10.1016/j.jhep.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mangan BA, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vdelta3 T cells. J. Immunol. 2013;191:30–34. doi: 10.4049/jimmunol.1300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin E, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahimpour A, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Treiner E, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 72.Huang S, et al. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc. Natl Acad. Sci. USA. 2009;106:8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Bourhis L, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 74.Gibbs A, et al. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017;10:35–45. doi: 10.1038/mi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel O, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat. Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- 76.Jo J, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10:e1004210. doi: 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rha MS, et al. Human liver CD8(+) MAIT cells exert TCR/MR1-independent innate-like cytotoxicity in response to IL-15. J. Hepatol. 2020;73:640–650. doi: 10.1016/j.jhep.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 78.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 79.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hegde P, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 2018;9:2146. doi: 10.1038/s41467-018-04450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang XZ, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J. Immunol. 2013;190:3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 82.Dusseaux M, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 83.Jeffery HC, et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J. Hepatol. 2016;64:1118–1127. doi: 10.1016/j.jhep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Provine NM, Klenerman P. MAIT cells in health and disease. Annu. Rev. Immunol. 2020;38:203–228. doi: 10.1146/annurev-immunol-080719-015428. [DOI] [PubMed] [Google Scholar]
- 85.Salou M, et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 2019;216:133–151. doi: 10.1084/jem.20181483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li F, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144:392–401. doi: 10.1053/j.gastro.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 87.Lang PA, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl Acad. Sci. USA. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliviero B, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1160. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, et al. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology. 2011;53:73–85. doi: 10.1002/hep.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, et al. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 91.Chen Y, Sun R, Jiang W, Wei H, Tian Z. Liver-specific HBsAg transgenic mice are over-sensitive to Poly(I:C)-induced liver injury in NK cell- and IFN-gamma-dependent manner. J. Hepatol. 2007;47:183–190. doi: 10.1016/j.jhep.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 92.Wieduwild E, et al. Beta2-adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J. Exp. Med. 2020;217:e20190554. doi: 10.1084/jem.20190554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li, W. et al. CD49a(+)CD49b(+) NK cells induced by viral infection reflect an activated state of conventional NK cells. Sci. China Life Sci. 10.1007/s11427-019-1665-1 (2020). [DOI] [PubMed]
- 94.Ali AK, Komal AK, Almutairi SM, Lee SH. Natural killer cell-derived IL-10 prevents liver damage during sustained murine cytomegalovirus infection. Front. Immunol. 2019;10:2688. doi: 10.3389/fimmu.2019.02688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Picarda G, et al. Cytomegalovirus evades TRAIL-mediated innate lymphoid cell 1 defenses. J. Virol. 2019;93:e00617–19. doi: 10.1128/JVI.00617-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Min-Oo G, Lanier LL. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J. Exp. Med. 2014;211:2669–2680. doi: 10.1084/jem.20141172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng H, Tian Z. NK cells in liver homeostasis and viral hepatitis. Sci. China Life Sci. 2018;61:1477–1485. doi: 10.1007/s11427-018-9407-2. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y, Tian Z. HBV-induced immune imbalance in the development of HCC. Front. Immunol. 2019;10:2048. doi: 10.3389/fimmu.2019.02048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun C, et al. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li H, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut. 2018;67:2035–2044. doi: 10.1136/gutjnl-2017-314098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peppa D, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J. Exp. Med. 2013;210:99–114. doi: 10.1084/jem.20121172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng Q, et al. Activated natural killer cells accelerate liver damage in patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 2015;180:499–508. doi: 10.1111/cei.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuan L, et al. HBV infection-induced liver cirrhosis development in dual-humanised mice with human bone mesenchymal stem cell transplantation. Gut. 2019;68:2044–2056. doi: 10.1136/gutjnl-2018-316091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wijaya RS, et al. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. J. Hepatol. 2019;71:252–264. doi: 10.1016/j.jhep.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Yang Z, Tang T, Wei X, Yang S, Tian Z. Type 1 innate lymphoid cells contribute to the pathogenesis of chronic hepatitis B. Innate Immun. 2015;21:665–673. doi: 10.1177/1753425915586074. [DOI] [PubMed] [Google Scholar]
- 106.Hengst J, et al. Role of soluble inflammatory mediators and different immune cell populations in early control of symptomatic acute hepatitis C virus infection. J. Viral Hepat. 2019;26:466–475. doi: 10.1111/jvh.13050. [DOI] [PubMed] [Google Scholar]
- 107.Bonorino P, et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J. Hepatol. 2009;51:458–467. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 108.Mele, D. et al. Adaptive natural killer cell functional recovery in hepatitis C virus cured patients. Hepatology. 10.1002/hep.31273 (2020). [DOI] [PubMed]
- 109.Zhu S, Zhang H, Bai L. NKT cells in liver diseases. Front. Med. 2018;12:249–261. doi: 10.1007/s11684-018-0622-3. [DOI] [PubMed] [Google Scholar]
- 110.Tan X, et al. Elevated hepatic CD1d levels coincide with invariant NKT cell defects in chronic hepatitis B virus infection. J. Immunol. 2018;200:3530–3538. doi: 10.4049/jimmunol.1701801. [DOI] [PubMed] [Google Scholar]
- 111.Wei X, et al. Hyperactivated peripheral invariant natural killer T cells correlate with the progression of HBV-relative liver cirrhosis. Scand. J. Immunol. 2019;90:e12775. doi: 10.1111/sji.12775. [DOI] [PubMed] [Google Scholar]
- 112.Khairallah C, et al. Gammadelta T cells confer protection against murine cytomegalovirus (MCMV) PLoS Pathog. 2015;11:e1004702. doi: 10.1371/journal.ppat.1004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kong X, Sun R, Chen Y, Wei H, Tian Z. GammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J. Immunol. 2014;193:1645–1653. doi: 10.4049/jimmunol.1303432. [DOI] [PubMed] [Google Scholar]
- 114.Yin W, et al. Functional dichotomy of Vdelta2 gammadelta T cells in chronic hepatitis C virus infections: role in cytotoxicity but not for IFN-gamma production. Sci. Rep. 2016;6:26296. doi: 10.1038/srep26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kefalakes H, Rehermann B. Inflammation drives an altered phenotype of mucosal-associated invariant T cells in chronic hepatitis D virus infection. J. Hepatol. 2019;71:237–239. doi: 10.1016/j.jhep.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 116.van Wilgenburg B, et al. MAIT cells are activated during human viral infections. Nat. Commun. 2016;7:11653. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dias J, et al. Chronic hepatitis delta virus infection leads to functional impairment and severe loss of MAIT cells. J. Hepatol. 2019;71:301–312. doi: 10.1016/j.jhep.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fung P, Pyrsopoulos N. Emerging concepts in alcoholic hepatitis. World J. Hepatol. 2017;9:567–585. doi: 10.4254/wjh.v9.i12.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pasala S, Barr T, Messaoudi I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res. 2015;37:185–197. [PMC free article] [PubMed] [Google Scholar]
- 120.Nagy LE. The role of innate immunity in alcoholic liver disease. Alcohol Res. 2015;37:237–250. [PMC free article] [PubMed] [Google Scholar]
- 121.Leclercq S, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl Acad. Sci. USA. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang F, Little A, Zhang H. Chronic alcohol consumption inhibits peripheral NK cell development and maturation by decreasing the availability of IL-15. J. Leukoc. Biol. 2017;101:1015–1027. doi: 10.1189/jlb.1A0716-298RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui K, et al. Suppression of natural killer cell activity by regulatory NKT10 cells aggravates alcoholic hepatosteatosis. Front. Immunol. 2017;8:1414. doi: 10.3389/fimmu.2017.01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sehgal R, et al. Natural killer cells contribute to pathogenesis of severe alcoholic hepatitis by inducing lysis of endothelial progenitor cells. Alcohol Clin. Exp. Res. 2020;44:78–86. doi: 10.1111/acer.14242. [DOI] [PubMed] [Google Scholar]
- 126.Maricic I, et al. Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology. 2015;61:1357–1369. doi: 10.1002/hep.27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cui K, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1beta in mice. J. Hepatol. 2015;62:1311–1318. doi: 10.1016/j.jhep.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 128.Lee KC, et al. Intestinal iNKT cells migrate to liver and contribute to hepatocyte apoptosis during alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G585–G597. doi: 10.1152/ajpgi.00269.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee JH, et al. Mitochondrial double-stranded RNA in exosome promotes interleukin-17 production through toll-like receptor 3 in alcoholic liver injury. Hepatology. 2020;72:609–625. doi: 10.1002/hep.31041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riva A, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018;67:918–930. doi: 10.1136/gutjnl-2017-314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marrero I, et al. Differential activation of unconventional T cells, including iNKT cells, in alcohol-related liver disease. Alcohol Clin. Exp. Res. 2020;44:1061–1074. doi: 10.1111/acer.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li W, et al. Alcohol abstinence does not fully reverse abnormalities of mucosal-associated invariant T cells in the blood of patients with alcoholic hepatitis. Clin. Transl. Gastroenterol. 2019;10:e00052. doi: 10.14309/ctg.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology.158, 1851–1864 (2020). [DOI] [PubMed]
- 134.Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin. Liver Dis. 2018;22:23–37. doi: 10.1016/j.cld.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 135.Liu W, Baker RD, Bhatia T, Zhu L, Baker SS. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol. Life Sci. 2016;73:1969–1987. doi: 10.1007/s00018-016-2161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cai J, Zhang XJ, Li H. The role of innate immune cells in nonalcoholic steatohepatitis. Hepatology. 2019;70:1026–1037. doi: 10.1002/hep.30506. [DOI] [PubMed] [Google Scholar]
- 137.Tosello-Trampont AC, et al. NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology. 2016;63:799–812. doi: 10.1002/hep.28389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan Y, et al. Hepatic NK cells attenuate fibrosis progression of non-alcoholic steatohepatitis in dependent of CXCL10-mediated recruitment. Liver Int. 2020;40:598–608. doi: 10.1111/liv.14307. [DOI] [PubMed] [Google Scholar]
- 139.Cepero-Donates Y, et al. Interleukin-15-mediated inflammation promotes non-alcoholic fatty liver disease. Cytokine. 2016;82:102–111. doi: 10.1016/j.cyto.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 140.Michelet X, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018;19:1330–1340. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 141.Stiglund N, et al. Retained NK cell phenotype and functionality in non-alcoholic fatty liver disease. Front. Immunol. 2019;10:1255. doi: 10.3389/fimmu.2019.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miyagi T, et al. Absence of invariant natural killer T cells deteriorates liver inflammation and fibrosis in mice fed high-fat diet. J. Gastroenterol. 2010;45:1247–1254. doi: 10.1007/s00535-010-0272-y. [DOI] [PubMed] [Google Scholar]
- 143.Martin-Murphy BV, et al. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS One. 2014;9:e80949. doi: 10.1371/journal.pone.0080949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 145.Syn WK, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sutti S, et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59:886–897. doi: 10.1002/hep.26749. [DOI] [PubMed] [Google Scholar]
- 147.Syn WK, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maricic I, et al. Differential activation of hepatic invariant NKT cell subsets plays a key role in progression of nonalcoholic steatohepatitis. J. Immunol. 2018;201:3017–3035. doi: 10.4049/jimmunol.1800614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wolf MJ, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 150.Adler M, et al. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J. Gastroenterol. 2011;17:1725–1731. doi: 10.3748/wjg.v17.i13.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Torres-Hernandez A, et al. Gammadelta T cells promote steatohepatitis by orchestrating innate and adaptive immune programming. Hepatology. 2020;71:477–494. doi: 10.1002/hep.30952. [DOI] [PubMed] [Google Scholar]
- 152.Li Y, et al. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Front. Immunol. 2018;9:1994. doi: 10.3389/fimmu.2018.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nabekura T, Riggan L, Hildreth AD, O’Sullivan TE, Shibuya A. Type 1 innate lymphoid cells protect mice from acute liver injury via interferon-gamma secretion for upregulating Bcl-xL expression in hepatocytes. Immunity. 2020;52:96–108 e109. doi: 10.1016/j.immuni.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fuchs A, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Peters CP, Mjosberg JM, Bernink JH, Spits H. Innate lymphoid cells in inflammatory bowel diseases. Immunol. Lett. 2016;172:124–131. doi: 10.1016/j.imlet.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 156.Tan Z, et al. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell Mol. Immunol. 2018;15:388–398. doi: 10.1038/cmi.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang S, et al. Type 3 innate lymphoid cell: a new player in liver fibrosis progression. Clin. Sci. 2018;132:2565–2582. doi: 10.1042/CS20180482. [DOI] [PubMed] [Google Scholar]
- 158.Seo W, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by gammadelta T cells in liver fibrosis. Hepatology. 2016;64:616–631. doi: 10.1002/hep.28644. [DOI] [PubMed] [Google Scholar]
- 159.Liaskou E, Hirschfield GM, Gershwin ME. Mechanisms of tissue injury in autoimmune liver diseases. Semin Immunopathol. 2014;36:553–568. doi: 10.1007/s00281-014-0439-3. [DOI] [PubMed] [Google Scholar]
- 160.Gao B, Bertola A. Natural killer cells take two tolls to destruct bile ducts. Hepatology. 2011;53:1076–1079. doi: 10.1002/hep.24275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shimoda S, et al. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53:1270–1281. doi: 10.1002/hep.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schleinitz N, Vely F, Harle JR, Vivier E. Natural killer cells in human autoimmune diseases. Immunology. 2010;131:451–458. doi: 10.1111/j.1365-2567.2010.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao ZB, et al. Liver-resident NK cells suppress autoimmune cholangitis and limit the proliferation of CD4(+) T cells. Cell Mol. Immunol. 2020;17:178–189. doi: 10.1038/s41423-019-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Littera R, et al. Exploring the role of killer cell immunoglobulin-like receptors and their HLA class I ligands in autoimmune hepatitis. PLoS One. 2016;11:e0146086. doi: 10.1371/journal.pone.0146086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bo X, Broome U, Remberger M, Sumitran-Holgersson S. Tumour necrosis factor alpha impairs function of liver derived T lymphocytes and natural killer cells in patients with primary sclerosing cholangitis. Gut. 2001;49:131–141. doi: 10.1136/gut.49.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ferri S, et al. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999–1007. doi: 10.1002/hep.23792. [DOI] [PubMed] [Google Scholar]
- 167.Sebode M, et al. Inflammatory phenotype of intrahepatic sulfatide-reactive type II NKT cells in humans with autoimmune hepatitis. Front. Immunol. 2019;10:1065. doi: 10.3389/fimmu.2019.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chernavsky AC, et al. Simultaneous expression of Th1 cytokines and IL-4 confers severe characteristics to type I autoimmune hepatitis in children. Hum. Immunol. 2004;65:683–691. doi: 10.1016/j.humimm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 169.Bottcher K, et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology. 2018;68:172–186. doi: 10.1002/hep.29782. [DOI] [PubMed] [Google Scholar]
- 170.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 171.Dupaul-Chicoine J, et al. The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity. 2015;43:751–763. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 172.Peng H, Wisse E, Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cell Mol. Immunol. 2016;13:328–336. doi: 10.1038/cmi.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol. Immunol. 2015;12:292–302. doi: 10.1038/cmi.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharm. Sin. 2015;36:1191–1199. doi: 10.1038/aps.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun C, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology. 2017;6:e1264562. doi: 10.1080/2162402X.2016.1264562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun H, et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology. 2019;70:168–183. doi: 10.1002/hep.30347. [DOI] [PubMed] [Google Scholar]
- 177.Sun H, et al. Reduced CD160 expression contributes to impaired NK-cell function and poor clinical outcomes in patients with HCC. Cancer Res. 2018;78:6581–6593. doi: 10.1158/0008-5472.CAN-18-1049. [DOI] [PubMed] [Google Scholar]
- 178.Sun H, et al. Accumulation of tumor-infiltrating CD49a(+) NK cells correlates with poor prognosis for human hepatocellular carcinoma. Cancer Immunol. Res. 2019;7:1535–1546. doi: 10.1158/2326-6066.CIR-18-0757. [DOI] [PubMed] [Google Scholar]
- 179.Easom NJW, et al. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction. Front. Immunol. 2018;9:1009. doi: 10.3389/fimmu.2018.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Luo Q, et al. Tumor-derived soluble MICA obstructs the NKG2D pathway to restrain NK cytotoxicity. Aging Dis. 2020;11:118–128. doi: 10.14336/AD.2019.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mantovani S, et al. Deficient natural killer cell NKp30-mediated function and altered NCR3 splice variants in hepatocellular carcinoma. Hepatology. 2019;69:1165–1179. doi: 10.1002/hep.30235. [DOI] [PubMed] [Google Scholar]
- 182.Zheng X, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 2019;20:1656–1667. doi: 10.1038/s41590-019-0511-1. [DOI] [PubMed] [Google Scholar]
- 183.Harmon C, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol. Res. 2019;7:335–346. doi: 10.1158/2326-6066.CIR-18-0481. [DOI] [PubMed] [Google Scholar]
- 184.Lim CJ, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68:916–927. doi: 10.1136/gutjnl-2018-316510. [DOI] [PubMed] [Google Scholar]
- 185.Chen Y, Hao X, Sun R, Wei H, Tian Z. Natural killer cell-derived interferon-gamma promotes hepatocellular carcinoma through the epithelial cell adhesion molecule-epithelial-to-mesenchymal transition axis in hepatitis B virus transgenic mice. Hepatology. 2019;69:1735–1750. doi: 10.1002/hep.30317. [DOI] [PubMed] [Google Scholar]
- 186.Turchinovich G, Ganter S, Barenwaldt A, Finke D. NKp46 calibrates tumoricidal potential of type 1 innate lymphocytes by regulating TRAIL expression. J. Immunol. 2018;200:3762–3768. doi: 10.4049/jimmunol.1701333. [DOI] [PubMed] [Google Scholar]
- 187.Liu Y, et al. NCR(-) group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine. 2019;41:333–344. doi: 10.1016/j.ebiom.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nair S, Dhodapkar MV. Natural killer T cells in cancer immunotherapy. Front. Immunol. 2017;8:1178. doi: 10.3389/fimmu.2017.01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mossanen JC, et al. CXCR6 inhibits hepatocarcinogenesis by promoting natural killer T- and CD4(+) T-cell-dependent control of senescence. Gastroenterology. 2019;156:1877–1889 e1874. doi: 10.1053/j.gastro.2019.01.247. [DOI] [PubMed] [Google Scholar]
- 190.Ma C, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xie D, Zhu S, Bai L. Lactic acid in tumor microenvironments causes dysfunction of NKT cells by interfering with mTOR signaling. Sci. China Life Sci. 2016;59:1290–1296. doi: 10.1007/s11427-016-0348-7. [DOI] [PubMed] [Google Scholar]
- 192.Zhao, N. et al. Intratumoral gammadelta T-cell infiltrates, CCL4/5 protein expression and survival in patients with hepatocellular carcinoma. Hepatology. 10.1002/hep.31412 (2020). [DOI] [PMC free article] [PubMed]
- 193.Bruni E, et al. Chemotherapy accelerates immune-senescence and functional impairments of Vdelta2(pos) T cells in elderly patients affected by liver metastatic colorectal cancer. J. Immunother. Cancer. 2019;7:347. doi: 10.1186/s40425-019-0825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jiang H, et al. Gammadelta T cells in hepatocellular carcinoma patients present cytotoxic activity but are reduced in potency due to IL-2 and IL-21 pathways. Int. Immunopharmacol. 2019;70:167–173. doi: 10.1016/j.intimp.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 195.Yi Y, et al. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J. Hepatol. 2013;58:977–983. doi: 10.1016/j.jhep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 196.Yang P, et al. TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Duan M, et al. Activated and exhausted MAIT cells foster disease progression and indicate poor outcome in hepatocellular carcinoma. Clin. Cancer Res. 2019;25:3304–3316. doi: 10.1158/1078-0432.CCR-18-3040. [DOI] [PubMed] [Google Scholar]
- 198.Shaler CR, et al. Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment. Cancer Immunol. Immunother. 2017;66:1563–1575. doi: 10.1007/s00262-017-2050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhuang L, et al. Activity of IL-12/15/18 primed natural killer cells against hepatocellular carcinoma. Hepatol. Int. 2019;13:75–83. doi: 10.1007/s12072-018-9909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee J, et al. Preventive inhibition of liver tumorigenesis by systemic activation of innate immune functions. Cell Rep. 2017;21:1870–1882. doi: 10.1016/j.celrep.2017.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Molgora M, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Luci C, Vieira E, Perchet T, Gual P, Golub R. Natural killer cells and type 1 innate lymphoid cells are new actors in non-alcoholic fatty liver disease. Front. Immunol. 2019;10:1192. doi: 10.3389/fimmu.2019.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu M, et al. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol. Ther. 2018;26:366–378. doi: 10.1016/j.ymthe.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alnaggar M, et al. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J. Immunother. Cancer. 2019;7:36. doi: 10.1186/s40425-019-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu X, Tian Z. Gut-liver axis: gut microbiota in shaping hepatic innate immunity. Sci. China Life Sci. 2017;60:1191–1196. doi: 10.1007/s11427-017-9128-3. [DOI] [PubMed] [Google Scholar]
- 206.Steinmann S, et al. Hepatic ILC2 activity is regulated by liver inflammation-induced cytokines and effector CD4(+) T cells. Sci. Rep. 2020;10:1071. doi: 10.1038/s41598-020-57985-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mao AP, et al. Multiple layers of transcriptional regulation by PLZF in NKT-cell development. Proc. Natl Acad. Sci. USA. 2016;113:7602–7607. doi: 10.1073/pnas.1601504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mackay LK, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 209.Bennstein SB. Unraveling Natural Killer T-Cells Development. Front. Immunol. 2017;8:1950. doi: 10.3389/fimmu.2017.01950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Singh AK, Rhost S, Lofbom L, Cardell SL. Defining a novel subset of CD1d-dependent type II natural killer T cells using natural killer cell-associated markers. Scand. J. Immunol. 2019;90:e12794. doi: 10.1111/sji.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bernink JH, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 212.Tachibana M, et al. Runx1/Cbfbeta2 complexes are required for lymphoid tissue inducer cell differentiation at two developmental stages. J. Immunol. 2011;186:1450–1457. doi: 10.4049/jimmunol.1000162. [DOI] [PubMed] [Google Scholar]
- 213.Withers DR. Lymphoid tissue inducer cells. Curr. Biol. 2011;21:R381–R382. doi: 10.1016/j.cub.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 214.Paquin-Proulx D, et al. IL13Ralpha2 expression identifies tissue-resident IL-22-producing PLZF(+) innate T cells in the human liver. Eur. J. Immunol. 2018;48:1329–1335. doi: 10.1002/eji.201747334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Eidson M, et al. Altered development of NKT cells, gammadelta T cells, CD8 T cells and NK cells in a PLZF deficient patient. PLoS One. 2011;6:e24441. doi: 10.1371/journal.pone.0024441. [DOI] [PMC free article] [PubMed] [Google Scholar]