Abstract
Acetaminophen (APAP) overdose is the most common cause of acute liver failure in the United States and formation of APAP-protein adducts, mitochondrial oxidant stress and activation of the mitogen activated protein (MAP) kinase c-jun N-terminal kinase (JNK) are critical for APAP-induced cell death. However, direct evidence linking these mechanistic features are lacking and were investigated by examining the early temporal course of these changes in mice after 300 mg/kg APAP. Protein adducts were detectable in the liver (0.05–0.1 nmol/mg protein) by 15 and 30 min after APAP, which increased (>500%) selectively in mitochondria by 60 min. Cytosolic JNK activation was only evident at 60 min, and was significantly attenuated by scavenging superoxide specifically in the cytosol by TEMPO treatment. Treatment of mouse hepatocytes with APAP revealed mitochondrial superoxide generation within 15 minutes, accompanied by hydrogen peroxide production without change in mitochondrial respiratory function. The oxidant stress preceded JNK activation and its mitochondrial translocation. Inhibitor studies identified the putative source of mitochondrial superoxide as complex III, which released superoxide towards the intermembrane space after APAP resulting in activation of JNK in the cytosol. Our studies provide direct evidence of mechanisms involved in mitochondrial superoxide generation after NAPQI-adduct formation and its activation of the MAP kinase cascade in the cytosol, which are critical features of APAP hepatotoxicity.
Keywords: Acetaminophen, NAPQI, oxidative stress, protein adducts, mitochondria, electron transport chain
1. INTRODUCTION
Acetaminophen (APAP) overdose can cause severe liver injury and acute liver failure, which accounts for about 50% of all cases of ALF in the United States (Rubin et al., 2018). The metabolic activation of APAP generates the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which is detoxified by cellular glutathione (GSH) at therapeutic doses. However, the excessive amount of NAPQI generated after an APAP overdose causes GSH depletion, leading to binding of NAPQI to cellular proteins, especially mitochondrial proteins including ATP synthase, aldehyde dehydrogenase and glutathione peroxidase (Qiu et al., 1998; Jaeschke et al., 2019). APAP overdose also causes early activation of a MAP kinase cascade within the cytosol, ultimately culminating in activation and mitochondrial translocation of c-jun N-terminal kinase (JNK) to the mitochondria (Ramachandran and Jaeschke, 2020). Sustained JNK activation in the cytosol and its mitochondrial translocation causes excessive formation of mitochondrial superoxide, which can react with nitric oxide to produce peroxynitrite, causing nitration of protein tyrosine residues (Knight et al., 2001; Cover et al., 2005). The importance of superoxide in the process is also re-iterated by the aggravation of APAP-induced liver injury in partial manganese superoxide dismutase (MnSOD)-deficient mice (Ramachandran et al., 2011) and by the protection seen against APAP hepatotoxicity with superoxide dismutase mimetics such as Mito-TEMPO (Du et al., 2017; Du et al., 2019). These mitochondrial changes, including severe sustained oxidative and nitrosative stress ultimately cause mitochondrial permeability transition pore opening, leading to a breakdown of the proton gradient across the membrane, and consequently cell death (Ramachandran and Jaeschke 2019a, b).
Thus, while formation of mitochondrial NAPQI-protein adducts, JNK activation and mitochondrial dysfunction have been well studied, they have predominantly been investigated at the time of peak APAP-induced injury. While NAPQI-protein adducts on mitochondria have been identified to be critical to hepatocyte injury (Tirmenstein and Nelson 1989; Xie et al., 2015a), mechanistic insight into how NAPQI protein adduct formation in mitochondria causes MAP kinase cascade activation in the cytosol is not well understood. Early investigation of reactive metabolite targets on mitochondria identified several proteins, including components of the electron transport chain (Qiu et al., 1998), but were again examined at the peak of APAP injury by which time JNK translocation to mitochondria has already taken place. Hence it would be difficult to determine if these alterations occurred prior to JNK translocation or were a consequence of that event. This is important, since mechanisms of NAPQI-adduct induced mitochondrial superoxide production and activation of cytosolic JNK could be distinct from those mediating enhanced reactive oxygen species production and mitochondrial dysfunction after mitochondrial JNK translocation. Mitochondrial superoxide production can have a variety of physiological roles (Sena and Chandel 2012) and do not necessarily need to cause mitochondrial dysfunction directly. In addition, mitochondrial antioxidant enzymes such as MnSOD would typically scavenge superoxide generated within mitochondria and convert it to non-radicals such as hydrogen peroxide, which has signaling roles of its own (Bao et al., 2009). Thus, the consequences of superoxide formation -whether it results in generation of hydrogen peroxide formation through MnSOD, for example; or generates peroxynitrite through reaction with nitric oxide- would depend on the mitochondrial milieu when the radical is generated. This environment is likely to be different immediately after APAP overdose when NAPQI-adducts are just forming on the mitochondria compared to later time points when activated JNK translocation induces additional signaling cascades. Thus, our study focuses on the very early temporal course of NAPQI-adduct formation and mitochondrial superoxide formation in comparison to JNK activation to gain insight into these very early mechanistic events which initiate APAP hepatotoxicity.
2. MATERIALS AND METHODS
2.1. Animals and experimental design.
Male C57BL/6J mice (8–12 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and kept in an environmentally controlled room with 12 hours dark/light cycle. Experiments were conducted with male mice because female mice, despite similar mechanisms of APAP-induced liver injury, are less susceptible due to an enhanced response to re-synthesize GSH (Du et al., 2014; Masubuchi et al., 2011). C57BL/6 is the most frequently used mouse strain for drug hepatotoxicity research; both sub-strains (6J and 6N) show a similar injury mechanism but 6N mice are more susceptible for a given dose of APAP (Duan et al., 2016). Since most of our and others’ research into the mechanisms of APAP hepatotoxicity has been done in male C57BL/6J mice, we continue to use this substrain. The animals were provided ad libitum access to food and water. Prior to APAP treatment, food was removed, and animals fasted overnight (16 hours) before APAP administration. APAP was dissolved in warm saline and mice were intraperitoneally (i.p) injected at a dose of 300 mg/kg. TEMPO (100 mg/kg) or Mito-TEMPO (20 mg/kg) (Sigma-Aldrich, St. Louis, MO) were dissolved in saline and administered 1 hour before APAP treatment. The animals were provided water ad libitum during the experiments.
2.2. Subcellular fractionation and Western blot analysis.
Mitochondria and cytosolic fractions were isolated as described (Cover et al., 2005). Briefly, aliquots of liver tissue were homogenized in ice cold isolation buffer (220 mM mannitol, 70 mM sucrose, 2.5 mM HEPES, 10 mM EDTA, 1 mM EGTA, 0.1% bovine serum albumin; pH 7.4). Mitochondria were isolated by differential centrifugation (20,000 ×g) and washed with 2 ml of isolation buffer. The 20,000 ×g supernatant was used to measure JNK activation in the cytosol. Western blotting was carried out in mitochondria and cytosolic samples with the following primary antibodies: rabbit anti-JNK (Cat #9252S), rabbit anti-p-JNK (Cat #4668S), rabbit anti-β-actin (Cat #4970L) and rabbit anti-VDAC (Cat #4661S) from Cell Signaling Technology (Danvers, Massachusetts, USA) at 1:1000 dilution. Secondary antibodies were anti-rabbit horseradish peroxidase-coupled IgG (Santa Cruz Biotechnology) at 1:5,000 dilution. Protein bands were detected by chemiluminescence (ECL reagent, GE Bioscience, Pittsburgh, PA, USA) on the LICOR Odyssey imaging system. Densitometry was carried out using Image J software to assess differences.
2.3. Nitro-tyrosine staining.
Liver tissue was fixed and embedded in paraffin, following which 5μm sections were subject to antigen retrieval using heat in citrate buffer. Tissue sections were incubated with anti-nitrotyrosine primary antibody (Invitrogen, cat # A-21185), followed by development with an avidin/biotin-peroxidase system (Vector lab, cat# PK-6101) and detection with DAB substrate (Cell Signaling, cat# 8059).
2.4. Protein adduct and GSH measurement.
Liver and isolated mitochondrial samples were homogenized in 10 mM sodium acetate (pH 6.5) and the supernatants collected after centrifugation at 16,000 ×g for 5 min. To remove low molecular weight compounds including APAP-GSH conjugates that might interfere with detection, the liver homogenates were filtered through Bio-Spin 6 columns (Bio-Rad, Hercules, CA) that were pre-washed with 10 mM sodium acetate. The filtered samples were digested with proteases to free APAP-CYS overnight and then precipitated using 40% trichloroacetic acid (TCA) for liver tissue or cold acetonitrile for mitochondrial samples. The supernatant of liver tissues was pelleted by centrifugation using filtered tubes. The supernatant of mitochondria samples was evaporated at 55 °C and 16 psi and the protein-derived APAP-CYS containing residues were re-suspended in small volume of 10 mM sodium acetate buffer with 20% TCA. APAP-CYS was then measured using HPLC with electrochemical detection (Muldrew et al., 2002). Total GSH in samples was measured using a modification of the Tietze assay as described in detail (Jaeschke and Mitchell 1990; McGill and Jaeschke 2015).
2.5. Primary mouse hepatocyte isolation.
Cells were isolated from mice by means of the 2-step collagenase perfusion technique as described previously (Bajt et al., 2004). Cell viability was more than 80% based on trypan blue exclusion, and cell purity was more than 90% based on flow cytometry analysis. Isolated hepatocytes were plated in William E medium (Life Technologies, Grand Island, NY) containing 100 U/ml penicillin/streptomycin, 1×10−7 M insulin and 10% fetal bovine serum. After 3 hours for attachment, cells were washed with PBS and kept in William E medium without fetal bovine serum overnight before treatment with 10 mM APAP.
2.6. Detection of mitochondrial superoxide with MitoSox staining.
Assays were performed on primary mouse hepatocytes plated on glass bottom dishes as described above. After addition of 5 μM MitoSox (ThermoFisher), media was changed after 15 minutes, with addition of 10 mM APAP, 5 μM Antimycin or 5 μM Myxothiazol (Sigma-Aldrich) containing media. Images were then acquired on an inverted fluorescence microscope after 15, 30 or 60 minutes.
2.7. Measurement of hydrogen peroxide with Amplex Red.
The assay for hydrogen peroxide was performed in primary mouse hepatocytes seeded in 24-well plates as per manufacturer’s instructions (Amplex Red, Molecular Probes). Briefly, cells were treated with 5 mM APAP for 15 or 60 minutes, following which lysis buffer was added to make cell suspensions. Reaction mixture contained 50 μM Amplex Red reagent and 0.1U/mL HRP in Krebs-Ringer phosphate buffer, which was warmed at 37 °C. 100 μL of reaction mixture was mixed with 20 μL cell suspension and the absorbance was measured at 560 nm.
2.8. Measurement of mitochondrial respiratory function.
Mitochondrial respiration was measured by oxygen consumption rates on a Seahorse XF243 extracellular flux analyzer (Agilent, Santa Clara, CA) according to manufacture protocol for the mitochondrial stress test. Briefly, 3×104 cells/well were seeded in William’s E medium with 10% fetal bovine serum for 3 hours to facilitate cell attachment, followed by change to William’s E medium with 1% glutamate and left overnight for equilibration. Before running the assay, the medium was change to pre-warmed mitochondrial stress test medium with 1 mM pyruvate and 10 mM glucose and the cells were incubated at 37 °C in a non-CO2 incubator for 1 hour.
2.9. Statistics.
All data are expressed as mean ± SEM and the student’s t-test was used to determine significance for two groups with normally distributed data.
3. RESULTS
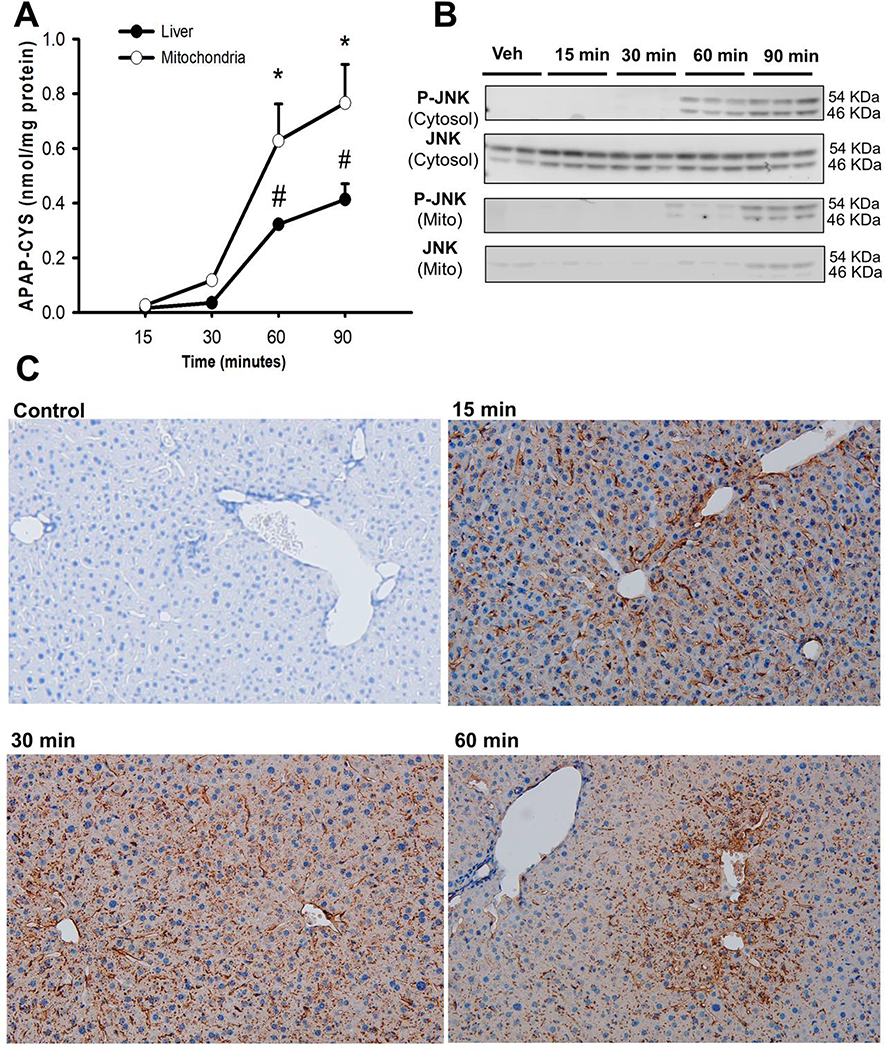
3.1. Formation of mitochondrial protein adducts after APAP overdose precedes JNK activation in the cytosol.
Initial experiments evaluated the early temporal course of protein adduct formation in liver homogenate and mitochondrial fractions after a moderate APAP overdose (300 mg/kg). Protein adducts were detectable within 15 minutes after APAP with a slight elevation especially in hepatic mitochondria by 30 minutes (Fig 1A). These levels showed significant further increase by 60 minutes, with mitochondrial adducts per mg protein being higher than those in the liver homogenate. Adducts in both mitochondria and total liver continued to rise even at 90 minutes post APAP although mitochondrial adducts were still higher than that in liver homogenate. Though mitochondrial protein adducts were elevated within 30 minutes of APAP, examination of JNK activation in the cytosol revealed phosphorylated-JNK (P-JNK) only after 60 and 90 minutes following APAP, with mitochondrial translocation of P-JNK by 90 minutes (Fig 1B). This clearly indicates that mitochondrial protein adduct formation precedes JNK activation in the cytosol. Mitochondrial NAPQI-adducts and oxidative stress have been implicated in peroxynitrite formation, which is a critical mediator of acetaminophen hepatotoxicity (Knight et al., 2002), and previous studies have identified hepatic vascular peroxynitrite within 2 hours after a 300 mg/kg dose of APAP (Knight et al., 2001). To determine whether peroxynitrite formation also occurs prior to JNK activation, liver sections were stained for the nitrotyrosine, the surrogate marker for peroxynitrite formation. As seen in Fig 1C, nitrotyrosine staining is visible in the liver within 15 minutes after APAP, but is restricted to the hepatic vasculature, a pattern which persists beyond 30 minutes; only mild scattered staining is seen in hepatocytes even at 60 minutes post APAP. Combined with the earlier findings (Knight et al., 2001), this indicates that APAP-induced hepatocyte peroxynitrite formation only occurs after JNK activation and is likely seen predominantly after JNK translocates to the mitochondria.
Figure 1:
Early adduct formation, JNK activation and peroxynitrite generation after 300 mg/kg APAP in mice. (A) Protein adduct levels in mitochondria and the liver over time. (B) Western blots evaluating temporal course of JNK activation after APAP. (C) Immunohistochemistry for nitrotyrosine as a marker of peroxynitrite in liver sections over time. Data are given as mean ± SEM, n=4; *p≤0.05 compared to 15 minutes for mitochondrial samples; # p≤0.05 compared to 15 minutes for the liver samples.
3.2. Preventing oxidative stress specifically in the cytosol delays APAP-induced JNK activation in vivo.
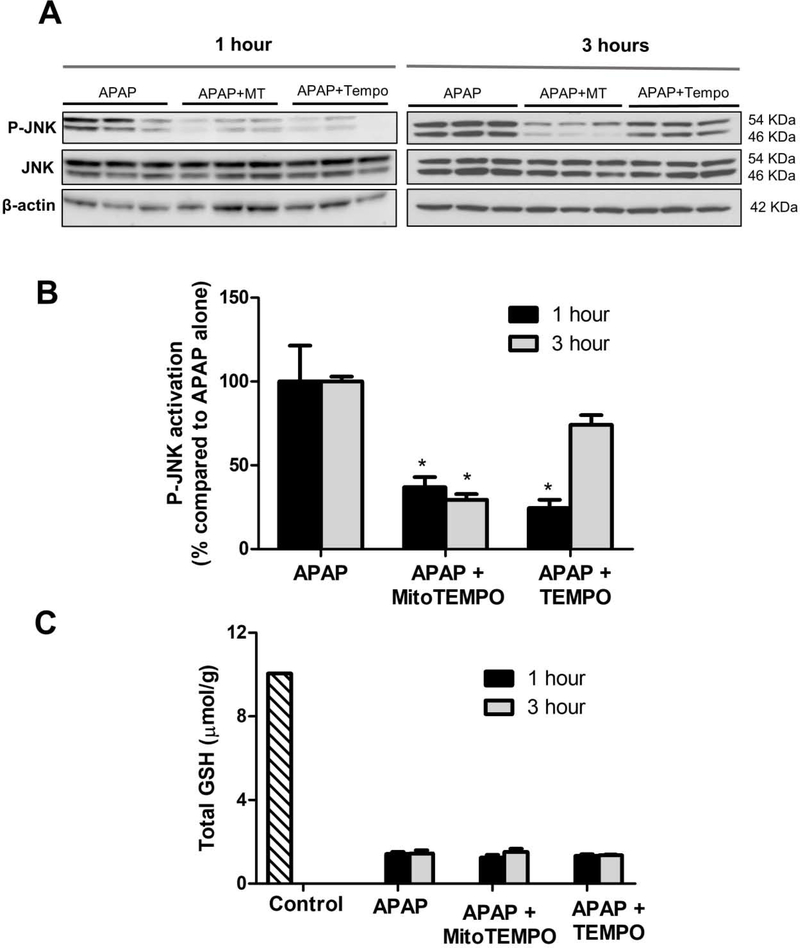
Mitochondrial oxidant stress is a well characterized outcome of NAPQI-protein adduct formation, but how does this induce JNK activation within the cytosol? We utilized compartment specific superoxide dismutase mimetics to examine this problem. Mice were pre-treated 1 hour before APAP with either TEMPO (which scavenges superoxide only in the cytosol) or mitoTEMPO (which scavenges superoxide only within mitochondria), followed by sacrifice at 1 or 3 hours after APAP. Treatment of mice with either TEMPO or MitoTEMPO attenuated JNK activation within the cytosol at 1 hour post APAP (Fig 2A), although scavenging superoxide within the cytosol with TEMPO showed better attenuation of JNK activation when compared to mitoTEMPO at this time point (Fig 2B). Interestingly, the inhibition by TEMPO was not sustained at the 3h time point, where mitoTEMPO pre-treatment still provided protection against sustained JNK activation. This suggests that mitochondrial superoxide generation is necessary for JNK activation, and the presence of the radical within the cytosolic compartment is important at the early time point for initial activation of the MAP kinase. Once mitochondrial translocation (Fig 1B) and amplification of oxidative injury has occurred, cytosolic superoxide scavenging has very limited effect (approximately 25% decrease with TEMPO compared to 80% with MitoTEMPO). Thus, this indicates that mechanisms involved in initial early activation of JNK are probably distinct from those occurring after JNK translocation to the mitochondria. These changes in JNK activation were not due to differences in APAP metabolism by these treatments since the depletion of GSH was similar among all groups (Fig 2C). The data so far indicate that mitochondrial protein adduct formation induces superoxide formation within mitochondria and its presence in the cytosol is necessary for early JNK activation. Further experiments focused on the kinetics of superoxide formation after APAP and its relationship to JNK activation and hence were carried out in primary mouse hepatocytes.
Figure 2:
Effect of superoxide scavenging in mitochondria or cytosol on JNK activation and GSH levels after APAP overdose. Mice were pre-treated with 100 mg/kg TEMPO or 20 mg/kg Mito-TEMPO 1 hour prior to 300 mg/kg APAP and sacrificed after 1 or 3 hours. (A) Western blots and (B) densitometry examining JNK activation after superoxide scavenging. (C) Total GSH levels in mice treated with APAP±TEMPO or Mito-TEMPO. Data are given as mean ± SEM, n=4; *p≤0.05 compared to APAP treatment alone.
3.3. APAP-induced mitochondrial superoxide and NAPQI-protein adduct formation precedes cytosolic JNK activation without compromising respiratory function.
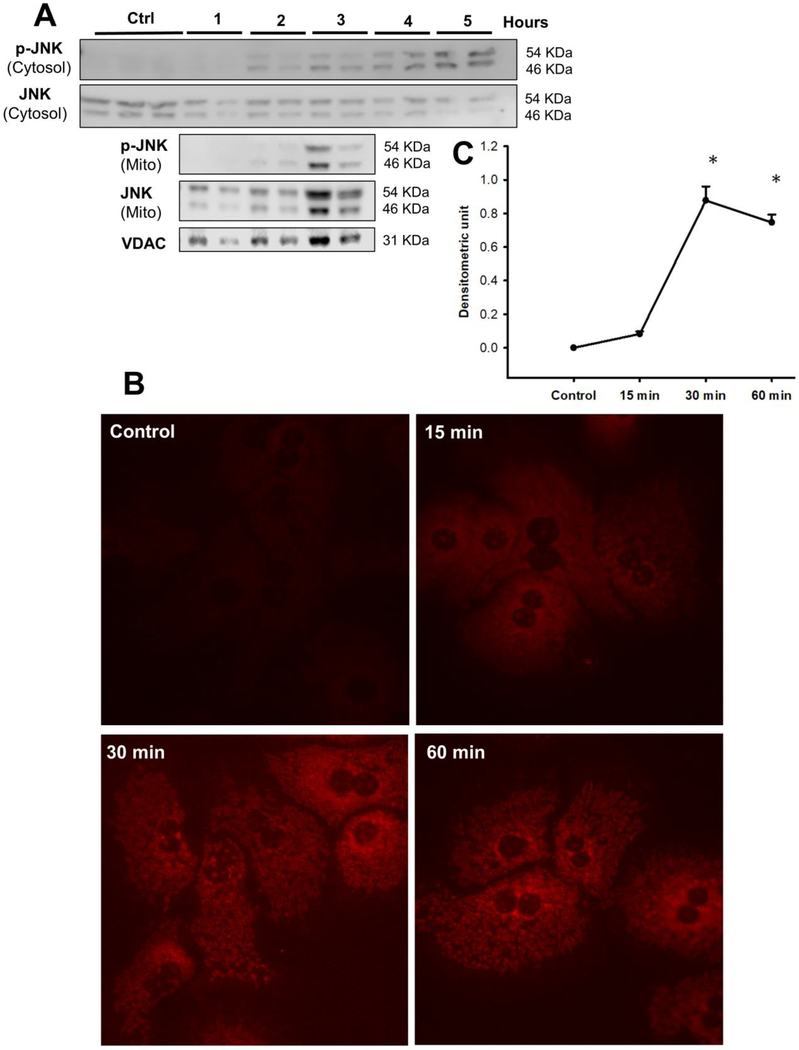
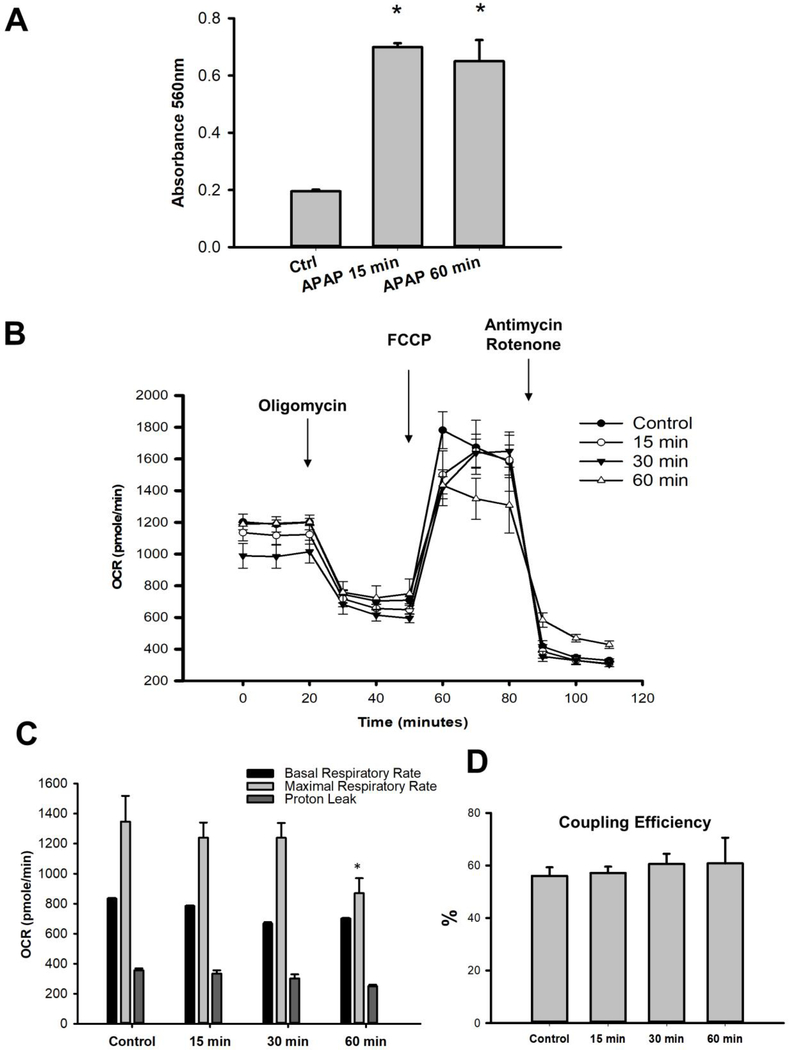
Primary mouse hepatocytes were treated with 10 mM APAP and the temporal course of JNK activation in the cytosol and subsequent mitochondrial translocation evaluated. As seen in Fig 3A, JNK activation was evident within 2 hours after APAP in the cytosol, which was then sustained up to 5 hours, with robust mitochondrial translocation of activated JNK occurring by 3 hours. How does this time course compare to mitochondrial superoxide production? This was evaluated with the fluorescent probe MitoSOX. Mitochondrial superoxide production was evident within 15 minutes after APAP treatment, which increased substantially by 30 minutes when an elevation in NAPQI-protein adducts was evident (Fig 1A) and further sustained at the 60-minute time point (Fig 3B & C). To further clarify the fate of superoxide produced from the mitochondria, we also measured cellular levels of hydrogen peroxide over time using Amplex Red. Our results show significant elevation in hydrogen peroxide within 15 minutes of APAP treatment which are sustained up to 60 minutes (Fig 4A). This closely aligns with the time course of NAPQI-adducts and mitochondrial superoxide formation suggesting that mitochondrial NAPQI-adduct induced superoxide generated within mitochondria ultimately results in elevated hydrogen peroxide levels as well. Since APAP treatment induces elevation in mitochondrial superoxide production and cellular hydrogen peroxide levels very early after exposure, does this mean that mitochondrial function is also compromised this rapidly? This does not seem to be the case, since examination of mitochondrial respiratory parameters indicate no significant difference in response over the time course of 1 hour (Fig 4B), with a decrease in maximal respiratory rate only evident by 60 minutes after APAP (Fig 4C) and no change in coupling efficiency (Fig 4D). Transient elevations in superoxide release from mitochondria, termed “superoxide flashes” have been described in a number of systems to occur without appreciable ETC dysfunction (Wang et al., 2016). Thus, the early elevation in mitochondrial superoxide could probably function in a signaling capacity (Collins et al., 2012) to induce JNK activation through the ASK1 pathway (Lim et al., 2008; Xie et al., 2015b) in the cytosol, without mitochondrial dysfunction.
Figure 3:
Mitochondrial superoxide production and JNK activation in isolated mouse hepatocytes treated with APAP (10 mM). (A) Western blots showing temporal course of JNK activation after APAP treatment in the cytosol and its translocation to mitochondria. (B) Mitochondrial superoxide generation visualized with MitoSox fluorescence and its quantitation (C) after treatment with 10 mM APAP for various times.
Figure 4:
Mitochondrial hydrogen peroxide levels and respiratory function after APAP. (A) Hydrogen peroxide released after 15 and 60 minutes of APAP treatment in primary mouse hepatocytes. (B) Representative traces of mitochondrial respiration assessed as oxygen consumption rate (OCR) in primary mouse hepatocytes after 15, 30 and 60 minutes of 10 mM APAP treatment. Temporal course of mitochondrial respiratory parameters (C) and coupling efficiency (D) after APAP treatment. Data are given as mean ± SEM from 4 experimental replicates; *p≤0.05 compared to control.
3.4. Spatially distinct early release of mitochondrial superoxide from respiratory complex III facilitates cytosolic JNK activation after APAP.
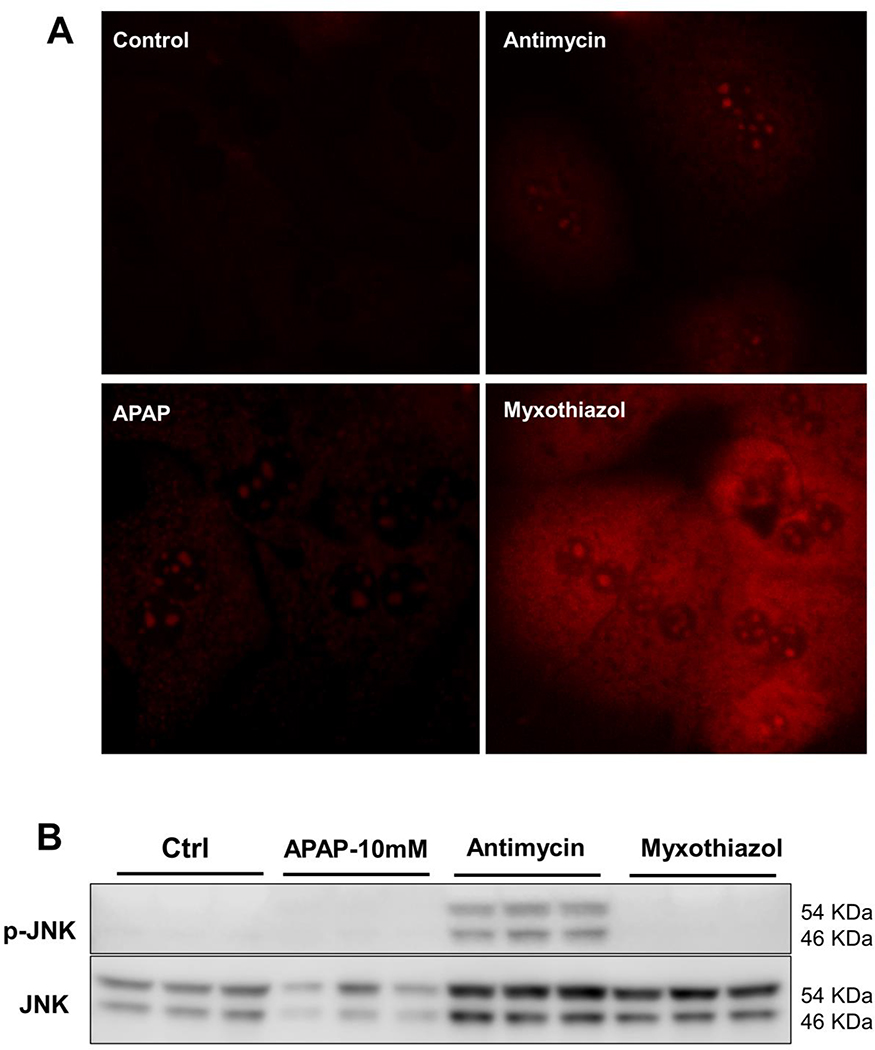
How does superoxide generation within mitochondria trigger JNK activation in the cytosol after APAP? Electron leak from the mitochondrial respiratory chain is the main source of superoxide production, and respiratory complexes I and III have been identified as important sites for superoxide production within the electron transport chain (Zhao et al., 2019). Though both complexes can generate superoxide, there are distinct spatial differences between the two. The respiratory complexes are aligned on the mitochondrial inner membrane and complex I exclusively releases superoxide into the mitochondrial matrix (St-Pierre et al., 2002). However, complex III can generate superoxide into the matrix or the intermembrane space (towards the cytosol). To further investigate the mechanisms involved in activation of cytosolic JNK by mitochondrial superoxide, we utilized the differential response of complex III to two well-known inhibitors. The first was antimycin, which inhibits complex III at the Qi site, resulting in superoxide production predominantly towards the intermembrane space, and eventually into the cytosol (Bleier and Drose 2013). The other was myxothiazol, which inhibits complex III at the Qo site, resulting in superoxide production predominantly into the mitochondrial matrix with a smaller portion into the intermembrane space matrix (Muller et al., 2004). Initial experiments used the mitochondrial matrix localization of MitoSOX to confirm the spatial orientation of superoxide production using these inhibitors. Since MitoSOX accumulates within the mitochondrial matrix, it would preferentially detect superoxide produced towards that compartment than that generated towards the intermembrane space. This seems to be the case, since hepatocytes treated with myxothiazol showed significantly more MitoSOX signal within 15 minutes of treatment than cells treated with antimycin, confirming the spatially distinct generation of superoxide from complex III by these two inhibitors (Fig 5A). In comparison, APAP treatment shows relatively negligible superoxide production within the matrix at this time (Fig 5A). Can this differential effect influence JNK activation? The answer is yes, since examination of JNK activation within the cytosol 30 minutes after APAP or either inhibitor alone shows distinct activation only in cells treated with antimycin, which evidently expediated JNK activation even when compared to 30 minutes after APAP treatment. (Fig 5B). Thus, these data confirm the directionality of superoxide production from complex III in the mitochondria and indicate that mitochondrial superoxide generation preferentially towards the intermembrane space facilitates transfer of superoxide and hydrogen peroxide to the cytosol. This also provides mechanistic insight for the in vivo observation that presence of superoxide in the cytosol is required for the early JNK activation after APAP, which is mechanistically distinct from ROS production subsequent to JNK translocation to mitochondria.
Figure 5:
Directional release of superoxide towards the cytosol is required for JNK activation. (A) Mitochondrial superoxide production after a 30-minute treatment with APAP, antimycin or myxothiazol as measured by MitoSox fluorescence. (B) Western blot showing JNK activation 30 minutes after treatment with APAP (10 mM), antimycin or myxothiazol (5 μM).
4. DISCUSSION
4.1. Early mitochondrial NAPQI-protein adduct formation after APAP overdose precedes cytosolic JNK activation, without peroxynitrite formation.
While mitochondrial NAPQI-protein adduct formation, mitochondrial oxidant stress and cytosolic JNK activation have been recognized to be critical early events after APAP overdose (Hanawa et al., 2008), relevant mechanisms linking these early events have not been investigated in detail. This is in contrast to the information on events subsequent to mitochondrial JNK translocation which have been extensively investigated with insight into the JNK binding partner Sab on the mitochondria (Win et al., 2011), its influence on the electron transport chain (Win et al., 2016) as well as the amplification of mitochondrial oxidant and nitrosative stress (Ramachandran and Jaeschke 2019b). Since JNK activation within the cytosol occurs very early within 1 hour of the 300 mg/kg dose of APAP (Xie et al., 2015b), we examined NAPQI-adduct formation beginning at 15 minutes post APAP up to 1.5 hour to determine the temporal relationships between adduct formation, superoxide generation and JNK activation. The preferential increase in mitochondrial protein adducts very early after APAP overdose reiterates earlier data regarding the importance of mitochondrial adducts in the pathophysiology (Tirmenstein and Nelson 1989; Xie et al., 2015a). The subsequent appearance of phosphorylated JNK in the cytosolic compartment clearly defines the time course of these changes. The data with nitro-tyrosine staining re-iterates this time course in vivo demonstrating that peroxynitrite formation within the hepatocyte is not required for the initial JNK activation and only occurs after JNK translocation to the mitochondria.
4.2. Mitochondrial-derived cytosolic superoxide/hydrogen peroxide is important for initial JNK activation, but dispensable after mitochondrial JNK translocation.
Activation of JNK within the cytosolic compartment after APAP has been well investigated with the involvement of redox-sensitive MAP kinases such as apoptosis signal inducing kinase-1 (ASK-1) (Nakagawa et al., 2008; Xie et al., 2015b) and mixed-lineage kinase 3 (MLK3) (Sharma et al., 2012) being well established (Ramachandran and Jaeschke 2018). However, a direct requirement for presence of superoxide within the cytosolic milieu for this to occur has not been demonstrated. Our data with TEMPO and mito-TEMPO now shed light on this requirement and provide novel insight elaborating the temporal differences in APAP induced signaling through the mitochondria. Mito-TEMPO has been established to specifically scavenge mitochondrial radicals without influencing the cytosolic compartment (Dikalova et al., 2010) and was shown to prevent APAP induced hepatotoxicity (Du et al., 2017; Du et al., 2019). Interestingly however, comparing its effect with TEMPO at early and later time points clearly indicates the role of cytosolic superoxide/hydrogen peroxide for initial JNK activation. This is unlike the later 3-hour time point by which time mitochondrial JNK translocation and amplification of mitochondrial oxidant stress has already occurred.
4.3. APAP-induced mitochondrial superoxide production in primary mouse hepatocytes precedes cytosolic JNK activation and its mitochondrial translocation.
Though compartment-specific scavenging of superoxide in vivo indicates the importance of early mitochondrial superoxide generation in APAP-induced JNK activation, experiments in primary mouse hepatocytes provide direct read out of mitochondrial superoxide and possible mechanisms involved. We found that the time course of mitochondrial superoxide production in response to APAP closely tracked formation of protein adducts in vivo and again preceded JNK activation within the cytosolic compartment confirming the requirement for adduct formation and mitochondrial superoxide generation to initiate JNK signaling. While the role of mitochondrial superoxide has been well established in APAP hepatotoxicity with the exacerbated injury seen in mitochondrial superoxide dismutase deficient mice (Ramachandran et al., 2011) and protection by mitochondrial targeted superoxide scavengers such as mitoTEMPO (Du et al., 2017; Du et al., 2019), our data now highlights the importance of the radical for early activation of JNK in the cytosol prior to its mitochondrial translocation.
4.4. Spatially distinct early mitochondrial superoxide release induced by APAP occurs without functional deficits and enables JNK activation in the cytosol.
Superoxide generated within the mitochondria are rapidly scavenged by mitochondrial superoxide dismutase (MnSOD) and converted to hydrogen peroxide, which increases in parallel to superoxide in primary mouse hepatocytes after APAP treatment. Though MnSOD has been shown to be inactivated by peroxynitrite after APAP overdose (Agarwal et al., 2011), this only occurs by 1 hour after APAP, by which time JNK activation has already been initiated. Hence, at the very early time points after APAP, mitochondrial superoxide generated is converted to membrane permeant hydrogen peroxide by MnSOD, which dissipates to the cytosol (Munro and Treberg 2017). Though mitochondrial superoxide generation was traditionally considered to be consistently detrimental and damaging, it is now recognized that these free radicals can have significant signaling roles (Sena and Chandel 2012). This seems to be the case when NAPQI protein adducts initially form on mitochondria, since no significant effect on respiratory parameters are evident at 30 minutes, for example, when superoxide generation is detectable. This would indicate that superoxide generation per se is not immediately damaging mitochondrial function after APAP and it is probably the amplification of radical production after JNK translocation with generation of highly reactive oxidants like peroxynitrite, which cause mitochondrial functional deficits. In addition, the maintenance of coupling efficiency indicates that the increase in superoxide is not secondary to excessive proton leak and uncoupling of oxidative phosphorylation but likely arising directly from subtle modulation of individual respiratory complexes. Within the electron transport chain (ETC) of the mitochondria, respiratory complex I and III are the primary sites of superoxide production, and while superoxide from complex I is exclusively released into the matrix, complex III can release superoxide into the matrix and the intermembrane space (Bleier and Drose 2013). Within the cytochrome bc1 of complex III, the ubiquinol-oxidation center (Q0 site) resides on the positive side and the ubiquinone-reduction center (Qi site) resides on the negative side of the membrane. These anatomical differences result in a unique direction of superoxide production at complex III (St-Pierre et al., 2002). Mitochondrial respiratory complexes I and III have also been implicated in APAP hepatotoxicity (Ramsay et al., 1989; Burcham and Harman 1991), and previous studies also demonstrated increased complex I activity after an overdose of APAP, with the complex I inhibitor metformin reducing oxidative stress and injury (Du et al., 2016).
Thus, clues towards the identity of the respiratory complex involved in this early superoxide release for cytosolic JNK activation come from the distinct spatial topology of radical production (Brand 2010). If NAPQI protein adducts were predominantly inducing mitochondrial superoxide production towards the matrix, this would be expected to have a profound effect on mitochondrial function at the early time points when superoxide generation was evident. However, this was not the case- suggesting that this early NAPQI-protein adduct induced superoxide production occurred away from the matrix, towards the cytosol. This would then be expected to activate the MAP kinase cascade in that compartment, resulting in JNK activation. Thus, the source of superoxide for the initial JNK activation after APAP would be complex III, which can produce radicals towards the intermembrane space and cytosol, unlike complex I, which produces superoxide towards the mitochondrial matrix (Brand 2010). This hypothesis was confirmed by the inhibitor experiments using antimycin and myxothiazol, which clearly show the distinct spatial requirement for superoxide production towards the cytosol for JNK activation. This superoxide generated from complex III towards the inter-membrane space could be transported into the cytosol through the voltage gated anion channel (Han et al., 2003).
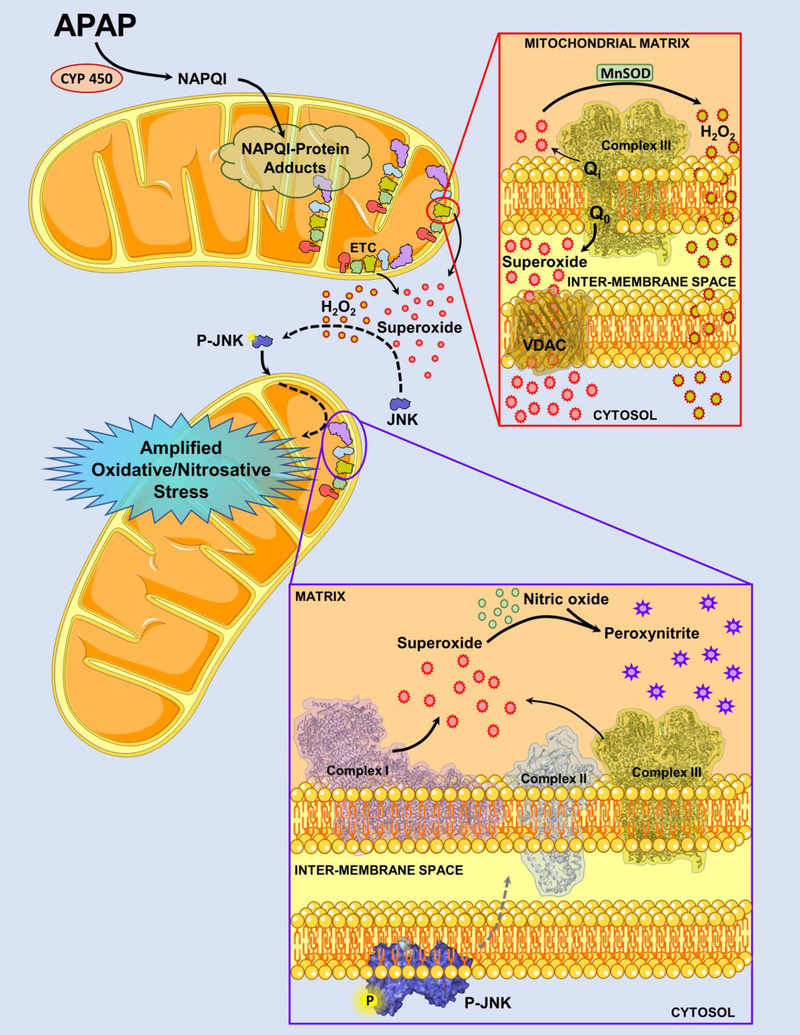
In conclusion, our data for the first time provides mechanistic insight into early activation of the MAP kinase JNK in the cytosol after an APAP overdose and provide direct evidence for the role of spatially distinct superoxide production from the mitochondria in this process. Generation of superoxide from mitochondrial respiratory complex III towards the intermembrane space spills into the cytosol and activates the signaling cascade causing cytosolic activation of the MAP kinase JNK. This early superoxide formation occurs without mitochondria dysfunction and is mechanistically distinct from the mitochondrial oxidative and nitrosative stress seen later after mitochondrial JNK translocation, which ultimately caused mitochondrial functional failure (Fig 6). Our data also emphasizes that signaling roles of mitochondrial radical production after APAP overdose are much more nuanced than appreciated earlier and highlight the variations in cell signaling with time after APAP exposure.
Figure 6:
Acetaminophen (APAP)-induced mechanisms involved in early activation of JNK within the cytosol are likely distinct from that mediating mitochondrial dysfunction after JNK translocation to the organelle. Formation of the reactive metabolite NAPQI from APAP metabolism through cytochrome P450 (CYP 450) and subsequent formation of mitochondrial protein adducts is a very early event which precedes JNK activation in the cytosol. These mitochondrial protein adducts induce changes in the mitochondrial electron transport chain (ETC), with superoxide release predominantly towards the intermembrane space from the Q0 site of mitochondrial respiratory complex III. Any superoxide produced towards the mitochondrial matrix would be converted to hydrogen peroxide by manganese superoxide dismutase (MnSOD) in the mitochondrial matrix. While this hydrogen peroxide can freely diffuse into the cytosol from the mitochondria, superoxide in the intermembrane space could be transported into the cytosol through channels such as Voltage-dependent anion channel (VDAC). This release of hydrogen peroxide and superoxide into the cytosol from the mitochondria then activates the MAP kinase cascade ultimately causing activation of JNK, the phosphorylated form of which translocates onto the mitochondria. Mitochondrial JNK translocation to the outer mitochondrial membrane then initiates a signaling cascade which amplifies mitochondrial superoxide formation from both complex I and III, which then forms peroxynitrite after reaction with nitric oxide within mitochondria. This severe oxidative/nitrosative stress then causes mitochondrial dysfunction, ultimately inducing cell necrosis. This figure includes templates from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 generic license; https://smart.servier.com.
Highlights.
Early APAP-induced mitochondrial protein adduct formation precedes JNK activation.
Mitochondrial-derived free radicals in cytosol required for initial JNK activation.
Spatially directed mitochondrial superoxide release activates cytosolic JNK.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health grants R01 DK102142 (H.J.), P20 GM103549 (H.J.) and a Pilot Grant (A.R.) from the Mechanisms of Liver Injury and Diseases COBRE (NIH P30GM118247, HJ-PI).
Footnotes
Conflict of Interest: NONE
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA, 2011. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther 337, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H, 2004. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci 80, 343–349. [DOI] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, Rice ME, 2009. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J Neurosci 29, 9002–9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleier L, Drose S, 2013. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta 1827, 1320–1331. [DOI] [PubMed] [Google Scholar]
- Brand MD, 2010. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45, 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcham PC, Harman AW, 1991. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem 266, 5049–5054. [PubMed] [Google Scholar]
- Collins Y, Chouchani ET, James AM, Menger KE, Cochemé HM, Murphy MP 2012. Mitochondrial redox signaling at a glance. J Cell Sci 125, 801–6. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H, 2005. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315, 879–887. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI, 2010. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Farhood A, Jaeschke H, 2017. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol 91, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Weemhoff JL, Chavan H, Xie Y, Krishnamurthy P, Jaeschke H, 2016. Editor’s Highlight: Metformin Protects Against Acetaminophen Hepatotoxicity by Attenuation of Mitochondrial Oxidant Stress and Dysfunction. Toxicol Sci 154, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Weemhoff JL, Woolbright BL, Jaeschke AH, Chao X, Ding WX, Jaeschke H, 2019. Mito-tempo protects against acute liver injury but induces limited secondary apoptosis during the late phase of acetaminophen hepatotoxicity. Arch Toxicol 93, 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Jaeschke H, 2014. Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol Appl Pharmacol 281, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Davis JS, Woolbright BL, Du K, Cahkraborty M, Weemhoff J, Jaeschke H, Bourdi M, 2016. Differential susceptibility to acetaminophen-induced liver injury in sub-strains of C57BL/6 mice: 6N versus 6J. Food Chem Toxicol 98, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Antunes F, Canali R, Rettori D, Cadenas E, 2003. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278, 5557–5563. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N, 2008. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283, 13565–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Duan L, Nguyen N, Ramachandran A 2019. Mitochondrial Damage and Biogenesis in Acetaminophen-induced Liver Injury. Liver Res 3, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR, 1990. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol 186, 752–759. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H, 2002. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther 303, 468–475. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H, 2001. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci 62, 212–220. [DOI] [PubMed] [Google Scholar]
- Lim PLK, Liu J, Go ML, Boelsterli UA, 2008. The mitochondrial superoxide/thioredoxin-2/Ask1 signaling pathway is critically involved in troglitazone-induced cell injury to human hepatocytes. Toxicol Sci 101, 341–9. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Nakayama J, Watanabe Y, 2011. Sex difference in susceptibility to acetaminophen hepatotoxicity is reversed by buthionine sulfoximine. Toxicology 287, 54–60. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H 2015. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol Mech Methods 25, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR, 2002. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos 30, 446–451. [DOI] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Van Remmen H, 2004. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279, 49064–49073. [DOI] [PubMed] [Google Scholar]
- Munro D, Treberg JR, 2017. A radical shift in perspective: mitochondria as regulators of reactive oxygen species. J Exp Biol 220, 1170–1180. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M, 2008. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 135, 1311–1321. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL, 1998. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem 273, 17940–17953. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2018. Acetaminophen Toxicity: Novel Insights Into Mechanisms and Future Perspectives. Gene Expr 18, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2019a. Acetaminophen Hepatotoxicity. Semin Liver Dis 39, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2019b. Acetaminophen hepatotoxicity: A mitochondrial perspective. Adv Pharmacol 85, 195–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H, 2020. A mitochondrial journey through acetaminophen hepatotoxicity. Food Chem Toxicol 140, 111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H, 2011. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 251, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RR, Rashed MS, Nelson SD, 1989. In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch Biochem Biophys 273, 449–457. [DOI] [PubMed] [Google Scholar]
- Rubin JB, Hameed B, Gottfried M, Lee WM, Sarkar M, Acute Liver Failure Study G. 2018. Acetaminophen-induced Acute Liver Failure Is More Common and More Severe in Women. Clin Gastroenterol Hepatol 16, 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LA, Chandel NS, 2012. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A, 2012. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol 82, 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD, 2002. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277, 44784–44790. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD, 1989. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J Biol Chem 264, 9814–9819. [PubMed] [Google Scholar]
- Wang W, Gong G, Wang X, Wei-LaPierre L, Cheng H, Dirksen R, Sheu SS, 2016. Mitochondrial Flash: Integrative Reactive Oxygen Species and pH Signals in Cell and Organelle Biology. Antioxid Redox Signal. 25, 534–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N, 2011. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem 286, 35071–35078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Min RW, Aghajan M, Kaplowitz N, 2016. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology 63, 1987–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, Jaeschke H, 2015. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol 289, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H, 2015a. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol 286, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Woolbright BL, Kos M, McGill MR, Dorko K, Kumer SC, Schmitt TM, Jaeschke H, 2015b. Lack of Direct Cytotoxicity of Extracellular ATP against Hepatocytes: Role in the Mechanism of Acetaminophen Hepatotoxicity. J Clin Transl Res 1, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RZ, Jiang S, Zhang L, Yu ZB, 2019. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 44, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]