Abstract
BACKGROUND
Low-grade endometrial stromal sarcoma (LGESS) is a rare indolent tumor with a favorable prognosis. With the importance of improving quality of life recognized, fertility-sparing surgery may be an option for those young women. However, most of the reports suggested that stage IA patients might be candidates for fertility-sparing surgery, and adjuvant hormonal treatment was considered a feasible adjuvant therapy for reducing the recurrence risk of patients with LGESS and hysterectomy was recommended after the completion of pregnancy and delivery.
CASE SUMMARY
A 28-year-old pregnant woman diagnosed with stage IB LGESS was treated by fertility-sparing surgery when term cesarean section delivery was performed. Without any adjuvant treatment, she had the other successful term pregnancy and cesarean section 45 mo after first fertility-sparing surgery. Moreover, only hysteroscopic resection was performed to retain fertility again even when the tumor recurred after 6 years. So far the patient’s fertility and disease-free status have remained for more than 8 years without any adjuvant therapy despite local resection of the sarcoma. And the two babies were in good health.
CONCLUSION
For young patients with stage I LGESS, it seems that repeated fertility-sparing surgeries could be performed even after two term deliveries and the tumor recurrence, and it might be attempted without adjuvant therapy but the counseling should be considered as mandatory.
Keywords: Endometrial stromal sarcoma, Fertility-sparing, Term pregnancy, Adjuvant therapy, Case report, Endometrial
Core Tip: Low-grade endometrial stromal sarcoma (LGESS) is an indolent tumor and fertility-sparing surgery may be an option for young women. However, hysterectomy and adjuvant therapy were recommended after the delivery completion. We present a pregnant LGESS woman who was treated by fertility-sparing surgery at term delivery. She had the other successful term pregnancy after 45 mo. Moreover, only hysteroscopic resection was performed to retain fertility again when the tumor recurred after 6 years. Her fertility and disease-free status have remained for more than 8 years without adjuvant therapy. This case represents the first attempt of fertility-sparing surgery even after two term deliveries and the tumor recurrence and an attempt of no adjuvant therapy for young patients with LGESS.
INTRODUCTION
Endometrial stromal sarcoma (ESS) is a rare tumor, constituting approximately 10% of uterine sarcomas and only 0.2% of malignancies of the uterus[1]. Based on morphology, mitotic activity, cellularity, and the presence of necrosis, the latest WHO classification scheme[2] divided ESS into endometrial stromal nodule (ESN), low-grade endometrial stromal sarcoma (LGESS), high-grade endometrial stromal sarcoma (HGESS), and undifferentiated uterine sarcoma (UUS). LGESS, the most common type, is an indolent tumor with a favorable prognosis, with 5- year and 10-year survival rates of 98% and 89%, respectively, for stage I[3]. However, a high recurrence rate up to 50% has been reported even in early stages. It occurs most frequent in women of perimenopausal age of 46 (range 18-83) years who usually present with abnormal vaginal bleeding[1], uterine enlargement , abdominal pain, and a pelvic mass, but these clinical manifestations may not appear and it is always misdiagnosed especially during pregnancy.
Surgery remains the cornerstone of treatment and consists of an abdominal hysterectomy with bilateral salpingo-oopherectomy. Over these years, with the importance of improving quality of life recognized, and given the relatively good outcomes associated with LGESS, fertility-sparing surgery has been considered an option for those young women. Despite limited experience and some controversy, some clinicians suggested that fertility-sparing surgery may be a viable option for young women, particularly nulliparous women with early stage LGESS. However, most of the reports suggested that stage IA patients may be candidates for fertility-sparing surgery and it should only be conserved for young patients with a strong desire for future fertility with fully informed consent[4]. Adjuvant hormonal treatment was considered a feasible adjuvant therapy for reducing the recurrence risk of patients with LGESS and hysterectomy was recommended after the completion of pregnancy and delivery.
So far, there has been no consensus on LGESS recurrence rates after fertility-sparing management due to the rarity of the tumor. Is adjuvant drug therapy necessary after fertility-sparing surgery? Is it feasible to perserve fertility for women who have completed one single birth? In the present case, a pregnant woman diagnosed with LGESS was treated by fertility-sparing surgery when term cesarean section delivery was performed. Without any adjuvant treatment she had the other successful term pregnancy and cesarean section 45 mo after first fertility-sparing surgery. Moreover, the patient’s fertility has remained for more than 8 years without adjuvant therapy, despite local resection of the sarcoma and the second fertility-sparing surgery for recurrence.
CASE PRESENTATION
Chief complaints
A 28-year-old woman (gravida 2, para 0) was admitted to the Provincial Hospital Affiliated to Shandong First Medical University (China) because of 37+3 wk pregnancy complicated with serious gestational diabetes mellitus (GDM) and myoma of the uterus.
History of present illness
During this pregnancy, myoma of the uterus was found at early pregnancy and its ultrasound images were consistent with images of uterine myoma, then she was under regular antenatal examinations. The fetus was healthy except that a slight increase in the heart oval foramen was found at 26 wk pregnancy by fetal heart ultrasound. She was recommended to review ultrasound regularly.
History of past illness
The patient presented with a normal menstrual history without irregular vaginal bleeding before the pregnancy. The patient had an abortion because of an unplanned pregnancy 2 years ago.
Personal and family history
The patient had no history of smoking or alcohol abuse. Her mother had type 2 diabetes mellitus.
Physical examination
The physical examination revealed a biologically unremarkable patient in good condition and obstetric examination was normal.
Laboratory examinations
Laboratory data, including tumor markers in which the level of CA125 was 21.50 U/mL at gestational week 34, were unremarkable before delivery.
Imaging examinations
After admission, an ultrasound examination demonstrated that a low echo (6.7 cm × 3.2 cm × 5.8 cm) with a complete envelope was in the right anterior wall of the uterus, protruding to the uterine cavity, and the amniotic fluid index was 18.9 cm.
FINAL DIAGNOSIS
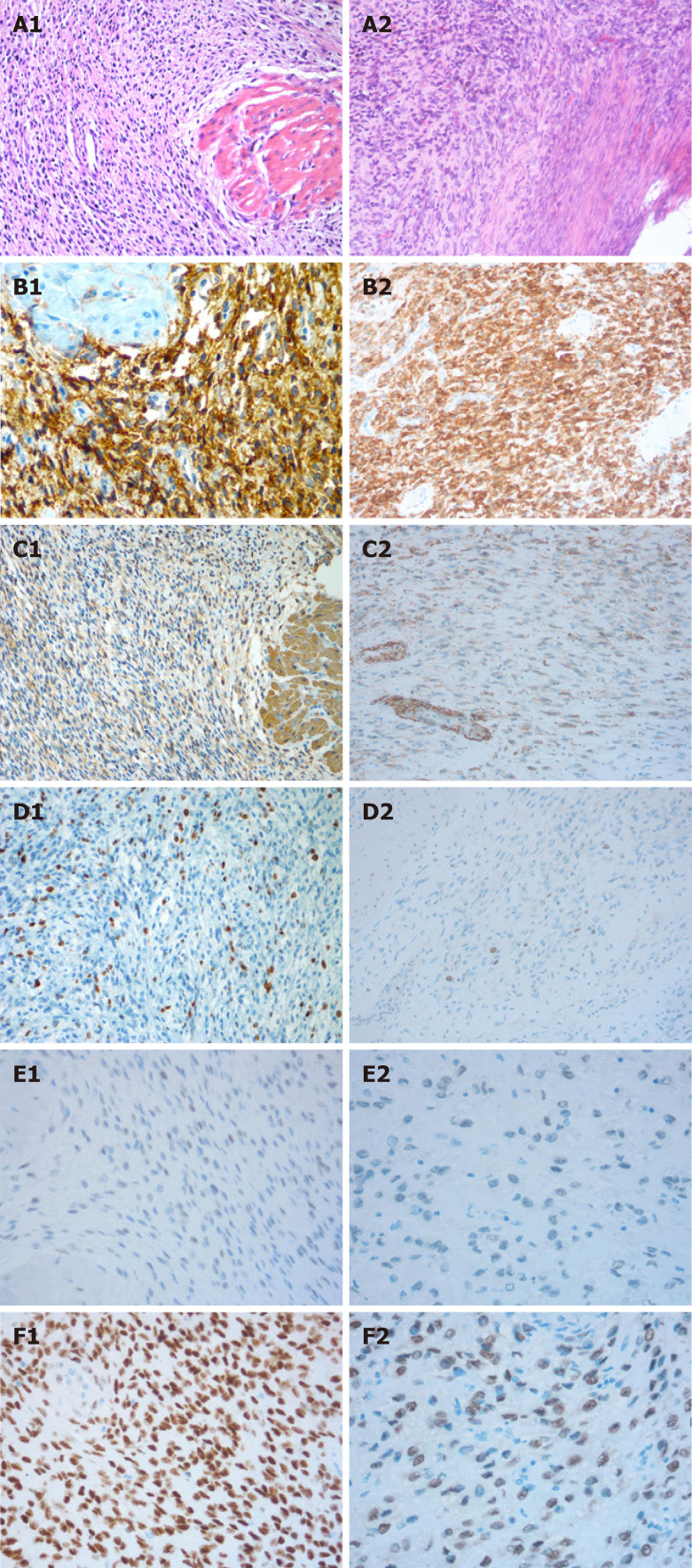
The histological examination of the mass revealed an endometrial stromal tumor with mild cell atypia and slight mitotic activity without any necrosis, and it was considered a LGESS. On immunohistochemistry, the cells were positive for CD10. Growth fraction determination with Ki-67 antibody showed that more than 10% of the tumor cells were proliferative. Immunostaining for smooth muscle actin (SMA) and Caldesmon were negative but immunohistochemical study revealed that estrogen receptor (ER) was positive in 5% of cells and progestin receptor (PR) was positive in 90% of cells (Figure 1A1-F1).
Figure 1.
Microscopic and immunohistochemical features of the first (A1-F1) and second (A2-F2) resected tissues. A: Extensive permeation of the myometrium as irregular islands (hematoxylin-eosin, HE × 200); B: Strong CD10 positivity (brown) (B1) and moderate CD10 positivity (B2) (× 200); C: Smooth muscle actin (SMA) negativity in tumor tissue but positivity in the myometrium is positive (C1) and SMA negativity (C2) (× 200); D: Ki-67 (+;10%) (D1) and Ki-67 (+; 10-15%) (D2) (× 200); E: Estrogen receptor (ER) positivity in 5% of cells (E1 and E2) (× 400) (E2); F: Progestin receptor (PR) positivity in 90% (F1) and 50% (F2) of cells (× 400).
TREATMENT
The patient underwent a primary low transverse cesarean section for insufficient glycemic control and myomectomy at the week of 37+6, delivering a 3850 g female infant with an Apgar score of 10 at 1 min. At the time of the cesarean section, a submucosal uterine myoma (around 8 cm × 7 cm × 7 cm) protruding to the uterine cavity was explored at the uterine anterior wall near the lower part, with an irregular shape and yellowish color. The texture was soft, and the capsule was complete. Myomectomy was performed to excise the myoma completely. The uterine myoma was sent for routine pathology. And the placenta, uterus, oviduct, ovaries, and pelvis were grossly normal, thus the placenta was not sent for pathological examination. A diagnosis of LGESS was made on the specimen, although a benign lesion was presumed before. With the patient’s consent, no further treatment or surgery was performed and she was followed every 6 mo to 1 year.
After delivery, there was no clear difference for the puerperal state and subsequent menstruation when compared with the normal delivery. And the patient underwent an artificial abortion surgery because of the second unplanned pregnancy about 25 mo later. Chemotherapy and endocrine therapy were suggested as adjuvant treatments, but the patient declined chemotherapy considering its side effects and the patient's desire for the other fertility.
Forty-five months later, she was admitted to our hospital again because of the other successful pregnancy. At the 38+5 gestational weeks, she delivered the other healthy male baby whose birth weight was 3720 g by cesarean section. No sign of recurrence was discovered at the time of the operation. Because of the patient’s strong desire to keep her fertility, fertility-sparing management was performed and any adjuvant treatments were declined again. And continued postoperative follow-up included tumor markers (CEA, CA-125, and CA199) and pelvic ultrasonography was recommended.
The patient began to have menorrhagia with blood clot 64 mo after the first fertility-sparing surgery and myomectomy, and pelvic ultrasonography showed a submucous myoma (4.0 cm × 3.3 cm × 2.4 cm) in the first myomectomy uterine position. Three months later, an ultrasound physician reported a high echo (2.5 cm × 2.0 cm × 1.3 cm), in the uterus, which was consistent with an endometrial polyp. The boundary was clear, the internal echo was uneven, and blood flow signal from the anterior wall was detected by color Doppler flow imaging (CDFI). Then, the patient began to present irregular vaginal bleeding. Another 3 mo later, ultrasonography was performed and a submucous myoma (4.2 cm × 3.8 cm × 2.4 cm) was reported again. The myoma protruded to the uterine cavity and part of it fell into cervical canal. The tumor markers were all normal.
Hysteroscopic resection of endometrial lesions was performed almost 6 years after the first fertility-sparing surgery and myomectomy. A polyploidy necrotic vegetation with a grayish yellow color, about 3 cm × 2 cm in size, was found in the inferior segment of the right uterine wall. Based on the patient's history and clinical manifestations, recurrent LGESS was confirmed to be the final pathological examination. Immunohistochemistry showed CD10(+), smooth muscle actin(), Caldesmon(), and Ki67(+; 10-15%). Immunohistochemical study revealed strong positive staining for ER in 5% of cells and for PR in 50% of cells (Figure 1A2-F2).
OUTCOME AND FOLLOW-UP
After fully discussing with the patient and fully explaining the risk, the second fertility-preserving management was continued performed and any adjuvant treatments were declined again and again. Postoperative follow-up was continued again and there was no evidence of recurrence for more than 2 years after the second local excision of the uterine sarcoma. The patient was in still in good condition with two healthy babies more than 8 years after the first fertility-preserving surgery.
DISCUSSION
There are no reliable preoperative imaging modalities that can distinguish LGESS from uterine leiomyoma or adenomyosis[5,6]. The preoperative presumptive diagnosis was often uterine myoma or adenomyoma for many patients who were definitively diagnosed after the initial surgery[7]. The diagnosis and treatment of cancer in pregnancy are challenging, because symptoms during pregnancy may be not typical and the fetus’ interest need to be taken into account. Tumor markers are not reliable, particularly CA125 and CA153 that can be elevated during a normal pregnancy. Our patient was also misdiagnosed as having a uterine myoma before the surgery. Moreover, some of the other patients were misdiagnosed with intracavitary polyps. This case was once misdiagnosed as an endometrial polyp when the tumor recurred.
LGESS is characterized by proliferation of small uniform cells closely resembling proliferative stage endometrial stroma[5]. The tumors have less frequent mitoses (less than 5 per 10 high-power fields) and no hemorrhage or necrosis. The immuno-histochemical phenotype of ESS includes typical positive reactions for vimentin and CD10, and other markers (keratins, smooth-muscle and muscle-specific actin, estrogen and progesterone receptors) are often expressed. Of these, CD10 and smooth-muscle actin are the most useful for the diagnosis of ESS[8]. In our case, the diagnosis of ESS was partially determined by the results of immunohistochemical staining (CD10positive and SMAnegative). In addition, a histological diagnosis was established and confirmed according to the WHO classification and the tumor stage was assessed as IB using the 2009 FIGO system[9] because the size was more than 5 cm.
The majority if previous reports recommended that young patients with LGESS who require fertility retention can retain the uterus and ovary, especially for stage I. Laurelli et al[3] reported that six women with early-stage LGESS aged 18-40 years who desired childbearing and/or retaining their fertility, were submitted to hysteroscopic resection following hormonal therapy, and all patients showed no evidence of disease. Delaney et al[10] reported that a 16-year-old girl underwent local resection of the LGESS mass with uterine reconstruction. The patient remained disease-free for 8 years before achieving pregnancy spontaneously and remained disease free postpartum. Xie et al[11] reported a patient who suffered from LGESS, was treated with heavy dosage Chinese herbs by sequential therapy, and she conceived spontaneously and underwent an uncomplicated pregnancy. Therefore, it seemed that fertility-sparing surgery for LGESS is a safe procedure. Moreover, fertility-sparing surgery does not seem to affect pregnancy and the successful pregnancy rate is encouraging. Xie et al[4] reported five (62.5%) of eight patients who attempted pregnancy conceived, and they all had stage I LGESS and were treated by fertility-sparing surgery. There were four full-term pregnancies and one preterm pregnancy. The mean duration between the treatment and pregnancy was 7 mo. Jin et al[12] reported that three (60%) of five patients who received conservative surgeries for local resection of the mass underwent an uncomplicated pregnancy. Fertility-sparing management of LGESS has been demonstrated in some reports, and it seemed suitable in selected young patients because some of them reached an ideal outcome that the patient delivered a healthy baby with or without recurrence (Table 1)[3,4,7,10-19]. Our patient had two successful term pregnancies with the tumor, and this is rare in the literature.
Table 1.
Term pregnancy after fertility-sparing surgery for low-grade endometrial stromal sarcoma in the literature
|
Ref.
|
Age (yr)
|
Stage
|
Adjuvant therapy
|
Recurrence, mo
|
Term pregnancy
|
Status (mo)
|
| Koskas et al[13] (2009) | 34 | IA | No | +, 10 | 1 (NTVD) | NED (23) |
| Yan et al[7] (2010) | 25 | IA | CT | - | 1 (C/S) | NED (60) |
| Delaney et al[10] (2012) | 16 | IB | MA | - | 1 (C/S) | NED (108) |
| Sánchez-Ferrer et al[14] (2012) | 32 | IB | MA | +, 31 | 1 (Twin pregnancy, C/S) | NED (60) |
| Choi et al[15] (2014) | 31 | IA | Letrozole | - | 1 (Twin pregnancy, C/S) | NED (99) |
| Zhan et al[16] (2014) | 26 | IB | CT + MPA | - | 1 (C/S) | NED (47) |
| Dong et al[17] (2014) | 25 | IB | MPA | - | 1 (C/S) | NED (31) |
| Jain et al[18] (2014) | 23 | IB | No | +, 20 | 1 (C/S) | NED (54) |
| Maeda et al[19] (2015) | 24 | NA | No | +, 10 | 1 (C/S) | AWD (> 240) |
| Jin et al[12] (2015) | 36 | IA | MA | 15 | 1 (C/S) | NED (32) |
| Jin et al[12] (2015) | 28 | IB | MA | - | 1 (C/S) | AWD (> 39) |
| Jin et al[12] (2015) | 37 | IA | MA | - | 1 (C/S) | NED (14) |
| Laurelli et al[3] (2015) | 38 | IA | No | - | 1 (NTVD) | NED (70) |
| 40 | IA | MA | - | 1 (NTVD) | NED (48) | |
| Xie et al[4] (2017) | 36 | IA | MA | - | 1 (C/S) | NED (38) |
| Xie et al[4] (2017) | 37 | IA | MA | - | 1 (C/S) | NED (24) |
| Xie et al[4] (2017) | 28 | IB | No | 15 | 1 (C/S) | NED (54) |
| Xie et al[4] (2017) | 25 | IB | No | 52 | 1 (C/S) | NED (106) |
| Xie et al[11] (2020) | 32 | IB | CM | - | 1 (C/S) | NED (35) |
ESS: Endometrial stromal sarcoma; NA: Not available; MA: Megestrol acetate; MPA: Medroxyprogesterone acetate; CT: Chemotherapy; CM: Chinese medicine; NTVD: Normal transvaginal delivery; C/S: Cesarian section; NED: No evidence of disease; AWD: Alive with disease.
Late recurrence and distant metastases may occur and severe recurrence cases have also been reported after fertility-sparing management. Koskas et al[13] reported a 34-year-old woman treated conservatively for LGESS who conceived rapidly after hysteroscopic resection of the tumor but had severe peritoneal recurrence in the postpartum period. Bai et al[20] reported that the recurrence rate among 153 cases of LGESS was 78.9% (15/19) in the myomectomy subgroup and 25.4% (34/134) in the hysterectomy subgroup. The 5-year relapse free survival among the 153 cases was 66.1%. Thus, fertility-sparing management and myomectomy could be considered for young women with a strong desire for future fertility and with fully informed consent, and long-term follow-up is mandatory.
Xie et al[4] reported the clinical courses of 17 stage I LGESS patients (6 stage IA and 11 stage IB) treated by fertility-sparing surgery. The total recurrence rate was 58.8% (10/17). The six stage IA patients had no recurrence and ten (90.9%) of the eleven stage IB patients experienced a recurrence. These data suggest that stage IA patients may be more suitable candidates for fertility-sparing surgery[3]. In contrast, the recurrence rate for stage IB patients from some other studies[14,16,17,21] was relatively low. As shown in Table 1, the stage of all successful pregnancy patients with LGESS but recurrence was IB. Therefore, for stage IB LGESS patients who desire to retain fertility, complete local resection by laparotomy and surveillance to assure no growth before achieving pregnancy are recommended.
The treatment of recurrence is controversial. There are a few reports about the second fertility conservation. Xie et al[4] reported two (50%) of four stage IB patients who underwent a second fertility-sparing surgery did not have a second recurrence, and one patient conceived spontaneously and delivered a healthy baby. The recurrence limited to the uterus seems to deserve a second fertility-sparing surgery. But hysterectomy with or without salpingo-oophorectomy was always recommended once childbearing was completed. The particularity of our stage IB case is that the uterus was not removed to preserve fertility after the first cesarean section, which made another successful pregnancy and childbirth possible in the case of completed childbearing. Moreover, only hysteroscopic resection was performed to retain fertility again even when the tumor recurred.
Because ESS highly expresses estrogen and progesterone receptors, there is a theoretic potential for growth of the tumor with increasing amounts of circulating hormones in pregnancy. Amant et al[22] reported that a case of ESS succumbed to her diseases 6 d and 2 years following diagnosis during pregnancy, although aggressive treatments were performed. A possible explanation was that high levels of circulating estrogen facilitated the progression of the tumor[17]. Xie et al[4] reported that two patients among five pregnancy women who received fertility-sparing surgery for LGESS had concurrent intrauterine and extrauterine recurrences that grew quickly during pregnancy and both insisted on continuing the pregnancy. As a result, one of them delivered at 29 wk and the other had a full-term pregnancy. Our case is a patient whose LGESS was diagnosed while pregnancy. There was no recurrence in the case of high progesterone levels during the second pregnancy and delivery, until about 6 years after two pregnancies. The positive rate of progesterone receptors was about 50%-90%. It seems to indicate that progesterone and pregnancy do not promote tumor progression and recurrence, which is not in line with the previous literature and analysis. Certainly, it is too early to show that pregnancy may delay the recurrence of tumors just for this case.
Similar to adjuvant progesterone therapy for endometrial carcinoma, most LGESS are sensitive to hormones. The literatures also indicated that adjuvant hormonal therapy may reduce the risk of recurrence. Chu et al[23] reported that four (30.8%) out of thirteen LGESS patients who received adjuvant progestins experienced recurrence compared with six (66.7%) out of nine who did not. Hormonal agents include progestin, aromatase inhibitors, and GnRH analogues, which are recommended by current international guidelines[24]. Jin et al[12] reported that adjuvant endocrine therapy in young LGESS patients was recommended for about 6 mo after fertility-sparing operation. However, Cui et al[25] reported that adjuvant hormonal treatment should be considered a feasible adjuvant therapy for reducing the recurrence risk of patients with LGESS while bearing little benefit on overall survival. So far, there have been no valid data to show that adjuvant chemotherapy leads to any improvement in survival in patients with LGESS[1]. In our case, except two fertility-sparing surgeries, this patient has not received any adjuvant treatment for 8 years, but she has not recurred anymore and survived. However, the patients should have the right to make their own decisions regarding therapy. Moreover, the patients should be carefully selected and consent should be obtained.
Fertility-sparing surgery for LGESS carries a lower risk of recurrence, and it may be considered available for young patients who are eager to keep their fertility even if they have had baby already. In our case, the patient had the other successful pregnancy and delivery with first fertility-sparing surgery. Even after two successful full-term deliveries, the second fertility-sparing therapy was performed after the recurrence. This lucky patient remained disease-free and survived and no adjuvant hormone or chemotherapy therapy had been carried out since the patient was diagnosed with LGESS for more than 8 years. Further large-scale studies with long term follow-up are required to confirm our findings and to assess the safety and feasibility of this approach, because performing fertility-sparing surgery for stage I LGESS is rare.
CONCLUSION
Recent reports including our case suggest that fertility-sparing surgery is possible and likely associated with a very low risk of disease relapse for young patients with stage IA or IB LGESS. It seems that second fertility-sparing surgery could be performed even after two term deliveries and the tumor recurrence. This case also suggested that fertility-sparing surgery might be attempted without adjuvant therapy, but the early reproductive counseling should be considered as mandatory. Moreover, large-scale studies with long term follow-up are required to confirm our findings.
ACKNOWLEDGEMENTS
The authors thank the patient’s family for participating in this study.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare no conflicts of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: November 10, 2020
First decision: November 23, 2020
Article in press: December 11, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsujinaka S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
Contributor Information
Yong-Zhong Gu, Department of Obstetrics and Gynecology, Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong Province, China; Department of Obstetrics and Gynecology, Provincial Hospital Affiliated to Shandong University, Jinan 250021, Shandong Province, China.
Ning-Ya Duan, Department of Obstetrics and Gynecology, Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China.
Hong-Xia Cheng, Department of Pathology, Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong Province, China.
Lian-Qiong Xu, Department of Obstetrics and Gynecology, Provincial Hospital Affiliated to Shandong University, Jinan 250021, Shandong Province, China.
Jin-Lai Meng, Department of Obstetrics and Gynecology, Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250021, Shandong Province, China; Department of Obstetrics and Gynecology, Provincial Hospital Affiliated to Shandong University, Jinan 250021, Shandong Province, China. sdslyymjl@163.com.
References
- 1.Thiel FC, Halmen S. Low-Grade Endometrial Stromal Sarcoma - a Review. Oncol Res Treat. 2018;41:687–692. doi: 10.1159/000494225. [DOI] [PubMed] [Google Scholar]
- 2.Conklin CM, Longacre TA. Endometrial stromal tumors: the new WHO classification. Adv Anat Pathol. 2014;21:383–393. doi: 10.1097/PAP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 3.Laurelli G, Falcone F, Scaffa C, Messalli EM, Del Giudice M, Losito S, Greggi S. Fertility-sparing management of low-grade endometrial stromal sarcoma: analysis of an institutional series and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2015;195:61–66. doi: 10.1016/j.ejogrb.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Xie W, Cao D, Yang J, Jiang X, Shen K, Pan L, Huang H, Lang J, You Y, Chen J. Fertility-sparing surgery for patients with low-grade endometrial stromal sarcoma. Oncotarget. 2017;8:10602–10608. doi: 10.18632/oncotarget.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188–1198. doi: 10.1016/S1470-2045(09)70226-8. [DOI] [PubMed] [Google Scholar]
- 6.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. The impact of tumor morcellation during surgery on the outcomes of patients with apparently early low-grade endometrial stromal sarcoma of the uterus. Ann Surg Oncol. 2011;18:3453–3461. doi: 10.1245/s10434-011-1751-y. [DOI] [PubMed] [Google Scholar]
- 7.Yan L, Tian Y, Fu Y, Zhao X. Successful pregnancy after fertility-preserving surgery for endometrial stromal sarcoma. Fertil Steril 2010; 93: 269.e1-269. :e3. doi: 10.1016/j.fertnstert.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Abeler VM, Nenodovic M. Diagnostic immunohistochemistry in uterine sarcomas: a study of 397 cases. Int J Gynecol Pathol. 2011;30:236–243. doi: 10.1097/PGP.0b013e318200caff. [DOI] [PubMed] [Google Scholar]
- 9.Prat J, Mbatani N. Uterine sarcomas. Int J Gynaecol Obstet. 2015;131 Suppl 2:S105–S110. doi: 10.1016/j.ijgo.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Delaney AA, Gubbels AL, Remmenga S, Tomich P, Molpus K. Successful pregnancy after fertility-sparing local resection and uterine reconstruction for low-grade endometrial stromal sarcoma. Obstet Gynecol. 2012;120:486–489. doi: 10.1097/AOG.0b013e31825a7397. [DOI] [PubMed] [Google Scholar]
- 11.Xie HP, Peng SH, Yi WM, Wang FL, Wu WK. Natural Pregnancy and Successful Delivery in A Woman with Endometrial Stromal Sarcoma After Sequential Therapy with Heavy Dosage of Chinese Medicine: A Case Report. Chin J Integr Med. 2020;26:783–785. doi: 10.1007/s11655-020-3251-z. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, Li Y, Deng CY, Tian QJ, Chen H, Pan LY. Fertility-sparing treatment of low-grade endometrial stromal sarcoma. Int J Clin Exp Med. 2015;8:5818–5821. [PMC free article] [PubMed] [Google Scholar]
- 13.Koskas M, Morice P, Yazbeck C, Duvillard P, Walker F, Madelenat P. Conservative management of low-grade endometrial stromal sarcoma followed by pregnancy and severe recurrence. Anticancer Res. 2009;29:4147–4150. [PubMed] [Google Scholar]
- 14.Sánchez-Ferrer ML, Machado-Linde F, Ferri-Ñíguez B, Sánchez-Ferrer M, Parrilla-Paricio JJ. Reproductive outcome after uterine-sparing surgery for endometrial stromal sarcoma. Gynecol Oncol Case Rep. 2012;3:4–6. doi: 10.1016/j.gynor.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi MC, Kim G, Hwang YY. Fertility-sparing management combined with photodynamic therapy for endometrial stromal sarcoma: a case report. Photodiagnosis Photodyn Ther. 2014;11:533–536. doi: 10.1016/j.pdpdt.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhan R, Wen H, Gao X, Yin L. Successful term pregnancy after laparoscopic surgery of low grade endometrial stromal sarcoma. Chin Med J (Engl) 2014;127:391–392. [PubMed] [Google Scholar]
- 17.Dong R, Pang Y, Mao H, Yang N, Liu P. Successful pregnancy following conservative management of low-grade endometrial stromal sarcoma: A case report. Oncol Lett. 2014;7:1039–1042. doi: 10.3892/ol.2014.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain PS, Jariwala MC. Successful Pregnancy with Endometrial Stromal Sarcoma (ESS) J Obstet Gynaecol India. 2014;64:297–298. doi: 10.1007/s13224-012-0249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda O, Moritani S, Ichihara S, Inoue T, Ishihara Y, Yamamoto S, Ito M, Matsumura Y, Sugiyama K, Horio M, Kondo I. Long-term survival in low-grade endometrial stromal sarcoma with childbirth and multidisciplinary treatment: a case report. J Med Case Rep. 2015;9:233. doi: 10.1186/s13256-015-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai H, Yang J, Cao D, Huang H, Xiang Y, Wu M, Cui Q, Chen J, Lang J, Shen K. Ovary and uterus-sparing procedures for low-grade endometrial stromal sarcoma: a retrospective study of 153 cases. Gynecol Oncol. 2014;132:654–660. doi: 10.1016/j.ygyno.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Noventa M, Gizzo S, Conte L, Dalla Toffola A, Litta P, Saccardi C. Fertility sparing surgery in young women affected by endometrial stromal sarcoma: an oncologic dilemma or a reliable option? Onco Targets Ther. 2015;8:29–35. doi: 10.2147/OTT.S69507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amant F, Van Calsteren K, Debiec-Rychter M, Heyns L, De Beeck KO, Sagaert X, Bollen B, Vergote I. High-grade endometrial stromal sarcoma presenting in a 28-year-old woman during pregnancy: a case report. J Med Case Rep. 2010;4:243. doi: 10.1186/1752-1947-4-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 2003;90:170–176. doi: 10.1016/s0090-8258(03)00258-0. [DOI] [PubMed] [Google Scholar]
- 24.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, Chu C, Cohn D, Crispens MA, Dizon DS, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, George S, Han E, Higgins S, Huh WK, Lurain JR 3rd, Mariani A, Mutch D, Fader AN, Remmenga SW, Reynolds RK, Tillmanns T, Valea FA, Yashar CM, McMillian NR, Scavone JL National Comprehensive Cancer Network. Uterine Sarcoma, Version 1.2016: Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2015;13:1321–1331. doi: 10.6004/jnccn.2015.0162. [DOI] [PubMed] [Google Scholar]
- 25.Cui R, Cao G, Bai H, Zhang Z. The clinical benefits of hormonal treatment for LG-ESS: a meta-analysis. Arch Gynecol Obstet. 2019;300:1167–1175. doi: 10.1007/s00404-019-05308-4. [DOI] [PubMed] [Google Scholar]