Abstract
Objective
Older emergency department (ED) patients are at high risk of mortality, and it is important to predict which patients are at highest risk. Biomarkers such as lactate, high-sensitivity cardiac troponin T (hs-cTnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), D-dimer and procalcitonin may be able to identify those at risk. We aimed to assess the discriminatory value of these biomarkers for 30-day mortality and other adverse outcomes.
Design
Prospective cohort study. On arrival of patients, five biomarkers were measured. Area under the curves (AUCs) and interval likelihood ratios (LRs) were calculated to investigate the discriminatory value of the biomarkers.
Setting
ED in the Netherlands.
Participants
Older (≥65 years) medical ED patients, referred for internal medicine or gastroenterology.
Primary and secondary outcome measures
30-day mortality was the primary outcome measure, while other adverse outcomes (intensive care unit/medium care unit admission, prolonged length of hospital stay, loss of independent living and unplanned readmission) were the composite secondary outcome measure.
Results
The median age of the 450 included patients was 79 years (IQR 73–85). In total, 51 (11.3%) patients died within 30 days. The AUCs of all biomarkers for prediction of mortality were sufficient to good, with the highest AUC of 0.73 for hs-cTnT and NT-proBNP. Only for the highest lactate values, the LR was high enough (29.0) to be applicable for clinical decision making, but this applied to a minority of patients. The AUC for the composite secondary outcome (intensive and medium care admission, length of hospital stay >7 days, loss of independent living and unplanned readmission within 30 days) was lower, ranging between 0.58 and 0.67.
Conclusions
Although all five biomarkers predict 30-day mortality in older medical ED patients, their individual discriminatory value was not high enough to contribute to clinical decision making.
Trial registration number
Keywords: accident & emergency medicine, geriatric medicine, internal medicine
Strengths and limitations of this study.
This was a prospective study in which biomarkers were measured in all older patients, irrespective of the problem they presented with.
The results of these tests were not reported back, except for lactate, and therefore did not influence the doctors.
We calculated not only the total predictive ability of the biomarkers but also interval likelihood ratios, as we hypothesised that extreme values do not add to decision making in the same way as values that are intermediate.
The limitations of the study are, besides the single-centre design, that not all consecutive patients were included because physicians prioritised in providing care at busy moments.
Introduction
Background
Biomarkers such as lactate, high-sensitivity cardiac troponin T (hs-cTnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), D-dimer and procalcitonin (PCT) are frequently used to diagnose and estimate the severity of specific diseases. They are able to detect underlying conditions or diseases that are often present in older patients (≥65 years) who visit the emergency department (ED). These include tissue hypoperfusion, myocardial injury, heart failure, thromboembolism and infections. Although several studies report that these markers are associated with adverse outcomes and predict short-term mortality,1–11 most of these were performed in relatively young ED patients,1–4 6 7 10–12 in selected ED patients with infection or sepsis6 10–12 or in patients with non-specific complaints.8 It is also noteworthy that in these studies, biomarkers were only measured when the ED physician deemed this to be indicated, because they were not routinely measured.1–4 6–8 Consequently, the true discriminatory value of these biomarkers for prediction of adverse outcomes in ED patients remains unknown.
Importance
Older patients who visit the ED are at a substantial risk of adverse outcomes including short-term mortality, intensive or medium care unit (ICU/MCU) admission, functional decline and readmissions.13–15 During the ED visit, it is crucial to establish which older patients are at highest risk, but this remains a challenging task.16 It is possible that biomarkers are helpful in establishing this risk.
Goals of this investigation
The aim of this prospective study was to assess the discriminatory value of arterial lactate, hs-cTnT, NT-proBNP, D-dimer and PCT for 30-day mortality and other adverse outcomes (ICU/MCU admission, prolonged length of hospital stay (LOS) in the hospital, loss of independent living and unplanned readmission) when measured routinely in older medical ED patients.
Methods
Study design, setting and selection of participants
This study is part of the RISE UP Study, a prospective multicentre study conducted at two EDs in the Netherlands. The study protocol of this study was published online.17 This part of the study took place in Zuyderland Medical Centre (MC), a large teaching hospital in the south of the Netherlands, because biomarkers were measured in patients included in this site only. Patients were included if they visited the ED between July 2016 and February 2017, were 65 years old or older, were examined and treated by an internist or gastroenterologist and provided written informed consent. Exclusion criteria were earlier participation in the study and inability to speak Dutch, German or English.
Measurements
At the moment of routine blood sampling at the ED, an additional arterial blood gas sample and two venous blood samples were drawn. Lactate levels were measured immediately in arterial blood samples on the RAPIDPoint 500 system and were available for the attending physician. Venous blood samples were centrifuged at 1800 g for 10 min, and plasma was stored in a freezer at −20°C. D-dimer levels were measured within 4 weeks after presentation using the Sysmex CS-2100i system. Plasma was analysed for hs-cTnT, NT-proBNP and PCT levels within 3–4 months by the Cobas 8000 modular analyser. Results of all biomarkers, except those for lactate, were blinded for all healthcare providers and only available to the investigators. If one of these four biomarkers was ordered by the attending physician as part of normal clinical practice, a different blood sample was analysed, and the results were reported as usual.
All data were collected from electronic medical records. The following data were retrieved on arrival at the ED: age, sex, living situation, data on comorbidity according to the Charlson Comorbidity Index18 and triage category (using the Manchester Triage System).19 The abovementioned five biomarkers were retrieved as well.
Outcomes
Thirty-day all-cause mortality was used as primary endpoint for the discriminatory value of the biomarkers. The secondary endpoint was a composite endpoint of ICU/MCU admission, prolonged LOS (>7 days), loss of independent living and unplanned readmission within 30 days after discharge. LOS was retrieved for patients who were admitted immediately following the ED visit. Loss of independent living was defined as discharge to a nursing home/hospice or with palliative care in previously community-dwelling patients.
Data regarding the outcomes were collected by checking the electronic medical files, which are connected to the municipal administration and by contacting the general practitioner if necessary.
Patient and public involvement
No patient involved.
Analysis
The sample size available for this study depended on a prospective cohort study, the RISE UP Study, which provided data on 450 patients.17 For logistic regression analysis, at least 10 event per candidate predictor are needed, according to prediction modelling guidelines. We assumed that the mortality rate would be around 11%. Therefore, the inclusion of 450 patients provided more than sufficient observations for our primary objective.
We performed descriptive analyses of baseline characteristics, biomarker levels and outcomes on the observed data without imputation of missing values. Continuous variables are reported as means with SD or medians with IQRs and categorical variables as proportions. Comparisons between the survivor and non-survivor groups were made using unpaired t-tests for continuous variables with Gaussian distribution, Mann-Whitney tests for continuous non-Gaussian data and Pearson’s χ2 or Fisher’s exact test for categorical data.
We calculated the discriminatory value of the biomarkers for the primary and secondary outcomes by constructing the area under the curves (AUCs) of receiver operating characteristics with 95% CIs on the available data. Accuracy of the AUCs was considered excellent if between 0.9 and 1.0, very good if 0.8 and 0.9, good if 0.7 and 0.8, sufficient if 0.6 and 0.7 and bad if between 0.5 and 0.6.20
We divided the biomarkers into five groups ranging from lowest to highest values. Next, interval likelihood ratios (LRs) and mortality percentages were calculated within these groups. We considered high LRs (>10) and low LR (<0.1) as being of additional value to clinical decision making.21
We used univariable logistic regression to compute the ORs with 95% CIs for the biomarkers with respect to 30-day mortality.
As a subanalysis, we evaluated the discriminatory ability of a combination of biomarkers. For this purpose, we used logistic regression with backwards elimination using a p value of 0.10 for removal and determined the discriminatory ability of this new model by calculating the AUC with 95% CI.
Logistic regression analyses were performed on data after imputation of missing values to allow for the inclusion of all patients. Sensitivity analyses were performed to assess the impact of missing data on our results by comparing the results after imputation to complete case analysis. Missing values of biomarkers were imputed using stochastic regression imputation with predictive mean matching (online supplemental table S1). All biomarkers were tested for collinearity using Pearson’s correlation coefficient and for influential outliers using Cook’s distance. Linearity was visually checked for all biomarkers and log transformed or dichotomised depending on the relationship with the outcome. For dichotomisation, the optimum cut-off value was chosen based on the values being closest to the upper left corner of the AUC. If two values were equally distanced, Youden’s Index was used.
bmjopen-2020-042989supp001.pdf (59.4KB, pdf)
All data were analysed using IBM SPSS Statistics for Windows, V.24.0 (IBM), and p values <0.05 were considered statistically significant.
Results
Characteristics of study subjects
For all 450 patients included during the study period, follow-up was complete (figure 1). The median age was 79 years (IQR 73–85), and 52% were men. In total, 51 (11.3%) patients died within 30 days after the ED visit, and 201 (44.7%) met the composite endpoint. The patients who died were older than those who survived (p value <0.001, table 1). Non-survivors more frequently experienced the composite endpoint (n=37, 72.5%) compared with the survivors (n=164, 41.1%; p value <0.001).
Figure 1.
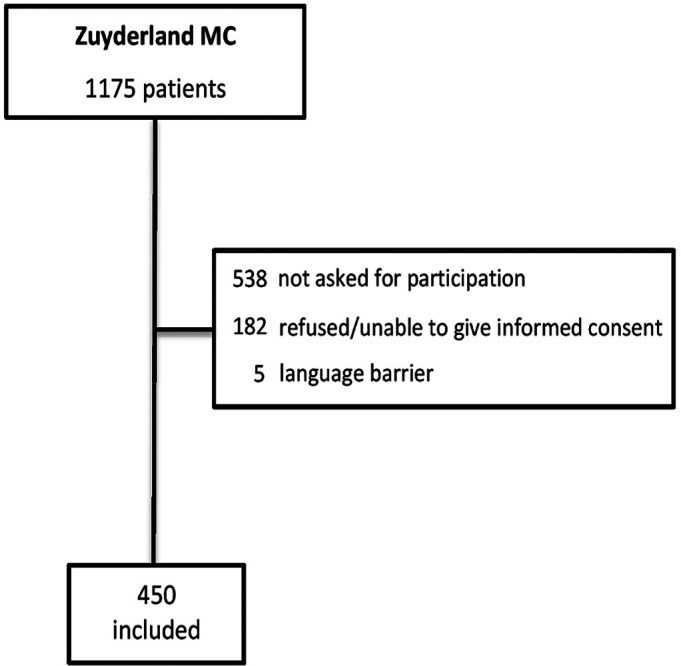
Flowchart of patient selection.
Table 1.
Baseline characteristics of study participants
| Reference values |
Missing values | Non-survivors N=51 |
Survivors N=399 |
* | |
| Age, median (IQR), years | – | 83 (77–87) | 79 (73–85) | *** | |
| Male sex, n (%) | – | 26 (51.0) | 208 (52.1) | ||
| Community-dwelling, n (%) | – | 36 (70.6) | 353 (88.5) | *** | |
| CCI score, median (IQR) | – | 3 (2–5) | 2 (1–3) | ** | |
| MTS category, n (%) | 3 (0.7) | * | |||
| Red | 2 (3.9) | 1 (0.3) | |||
| Orange | 10 (19.6) | 46 (11.6) | |||
| Yellow | 28 (54.9) | 226 (57.1) | |||
| Green | 11 (21.6) | 122 (30.8) | |||
| Blue | – | 1 (0.3) | |||
| Biomarkers | |||||
| Lactate, median (IQR), mmol/L | 0.6–1.8 | 72 (16.0) | 2.0 (1.5–2.8) | 1.4 (1.0–1.9) | *** |
| hs-cTnT, median (IQR), ng/L | <14 | 25 (5.6) | 42 (26-84) | 21 (12–39) | *** |
| NT-proBNP, median (IQR), ng/L | <125 | 26 (5.8) | 2766 (943–11597) | 759 (266–2377) | *** |
| D-dimer, median (IQR), µg/L | <500 | 43 (9.6) | 3445 (1281–6497) | 1251 (660–2804) | *** |
| PCT, median (IQR), ng/mL | <0.05 | 26 (5.8) | 0.32 (0.13–1.40) | 0.12 (0.06–0.31) | *** |
| Outcome | |||||
| Composite endpoint, n (%)† | – | 37 (72.5) | 164 (41.1) | *** |
Analysis in this table was made using non-imputed data.
*Significant difference between non-survivors and survivors with a p value of 0.01–<0.05 (*), 0.001–<0.01 (**) or <0.001 (***).
†Composite endpoint consisting of intensive or medium care unit admission, prolonged length of hospital stay (>7 days), loss of independent living and unplanned readmission within 30 days after discharge.
CCI, Charlson Comorbidity Index; hs-cTnT, high-senstivity cardiac troponin T; MTS, Manchester Triage System; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin.;
Main results
Biomarkers
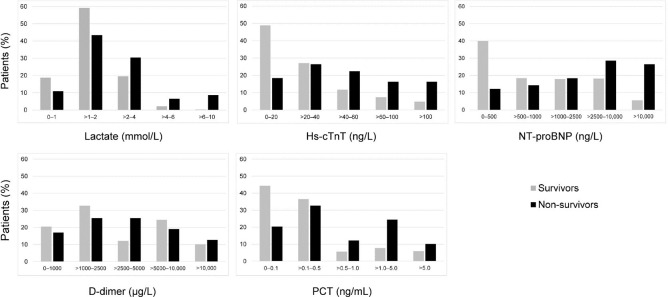
Four biomarkers, hs-cTnT, NT-proBNP, D-dimer and PCT, were above the reference range in most patients (66.4%, 86.0%, 78.0% and 79.8%, respectively), whereas for lactate, this was true in 25.6% of patients. The highest values of the biomarkers were more often present in non-survivors, whereas the lowest values were more often present in survivors, but there was a large overlap between the non-survivors and survivors (table 1 and figure 2).
Figure 2.
Distribution of the five biomarkers among survivors and non-survivors. Bars represent the proportion of patients with the according biomarker value within the survivor and non-survivor group. hs-cTnT, high-senstivity cardiac troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin.
Diagnostic accuracy of the biomarkers
The AUCs for prediction of 30-day mortality were sufficient for lactate and PCT with values of 0.68 (95% CI 0.59 to 0.77) and 0.67 (95% CI 0.60 to 0.75), respectively (table 2). The AUCs of the other biomarkers were good with the highest AUCs for hs-cTnT and NT-proBNP with a value of 0.73 (95% CI 0.66 to 0.80) for both. The AUCs of the biomarkers for the composite endpoint were mostly sufficient but lower than for mortality (ranging between 0.58 and 0.67).
Table 2.
AUCs for the biomarkers with respect to mortality and the composite endpoint
| Biomarker | n | AUC (95% CI) | |
| 30-day mortality | Composite endpoint | ||
| Lactate | 378 | 0.68 (0.59 to 0.77) | 0.62 (0.56 to 0.67) |
| hs-cTnT | 425 | 0.73 (0.66 to 0.80) | 0.67 (0.61 to 0.72) |
| NT-proBNP | 424 | 0.73 (0.66 to 0.80) | 0.65 (0.60 to 0.71) |
| D-dimer | 407 | 0.70 (0.62 to 0.77) | 0.58 (0.52 to 0.64) |
| PCT | 424 | 0.67 (0.60 to 0.75) | 0.65 (0.60 to 0.70) |
Analysis in this table was made using non-imputed data.
AUC, area under the curve; hs-cTnT, high-senstivity cardiac troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin.
LRs increased with higher biomarker values, except PCT (table 3). Most of the biomarkers had maximum LRs between 3.2 (PCT) and 4.7 (NT-proBNP), except lactate. We retrieved a maximum LR of 29.0 when lactate was between 6.0 and 10.0 mmol/L with a mortality percentage of 80.0%. The maximum LRs were, however, only applicable to a limited number of patients (n=5). The lowest LRs for all biomarkers were less variable but ranging between 0.3 (NT-proBNP) and 0.6 (lactate).
Table 3.
Interval likelihood ratios (LRs) for the biomarkers
| Biomarker | Mortality | N | LR | Observed mortality (%) | |
| Yes (n %) | No (n %) | ||||
| Lactate (mmol/L) | |||||
| 0–1.0 | 5 (10.9) | 62 (18.7) | 67 | 0.6 | 7.5 |
| >1.0–2.0 | 20 (43.5) | 197 (59.3) | 217 | 0.7 | 9.2 |
| >2.0–4.0 | 14 (30.4) | 65 (19.6) | 79 | 1.6 | 17.7 |
| >4.0–6.0 | 3 (6.5) | 7 (2.1) | 10 | 3.1 | 30.0 |
| >6.0–10.0 | 4 (8.7) | 1 (0.3) | 5 | 29.0 | 80.0 |
| hs-cTnT (ng/L) | |||||
| 0–20 | 9 (18.4) | 184 (48.9) | 193 | 0.4 | 4.7 |
| >20–40 | 13 (26.5) | 102 (27.1) | 115 | 1.0 | 11.3 |
| >40–60 | 11 (22.4) | 44 (11.7) | 55 | 1.9 | 20.0 |
| >60–100 | 8 (16.3) | 28 (7.4) | 36 | 2.2 | 22.2 |
| >100 | 8 (16.3) | 18 (4.8) | 26 | 3.4 | 30.8 |
| NT-proBNP (ng/L) | |||||
| 0–500 | 6 (12.2) | 150 (40.0) | 156 | 0.3 | 3.8 |
| >500–1000 | 7 (14.3) | 69 (18.4) | 76 | 0.8 | 9.2 |
| >1000–2500 | 9 (18.4) | 67 (17.9) | 76 | 1.0 | 11.8 |
| >2500–10 000 | 14 (28.6) | 68 (18.1) | 82 | 1.6 | 17.1 |
| >10 000 | 13 (26.5) | 21 (5.6) | 34 | 4.7 | 38.2 |
| D-dimer (µg/L) | |||||
| 0–1000 | 8 (17.0) | 149 (41.1) | 157 | 0.4 | 5.1 |
| >1000–2500 | 12 (25.5) | 112 (31.1) | 124 | 0.8 | 9.7 |
| >2500–5000 | 12 (25.5) | 56 (15.6) | 68 | 1.6 | 17.6 |
| >5000–10 000 | 9 (19.1) | 30 (8.3) | 39 | 2.3 | 23.1 |
| >10 000 | 6 (12.8) | 13 (3.6) | 19 | 3.6 | 31.6 |
| PCT (ng/L) | |||||
| 0–0.1 | 10 (20.4) | 166 (44.3) | 176 | 0.5 | 5.7 |
| >0.1–0.5 | 16 (32.7) | 137 (36.5) | 153 | 0.9 | 10.5 |
| >0.5–1.0 | 6 (12.2) | 21 (5.6) | 27 | 2.2 | 22.2 |
| >1.0–5.0 | 12 (24.5) | 29 (7.7) | 41 | 3.2 | 29.3 |
| >5.0 | 5 (10.2) | 22 (5.9) | 27 | 1.7 | 18.5 |
hs-cTnT, high-senstivity cardiac troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin.
Univariable logistic regression analysis
Lactate and D-dimer were dichotomised, and hs-cTnT, NT-proBNP and PCT were logarithmically transformed because they were not linearly associated with 30-day mortality. The optimum cut-off value was >1.5 mmol/L for lactate and >3000 µg/L for D-dimer. None of the biomarkers were highly correlated. In the univariable logistic regression analysis, all biomarkers were strong predictors of 30-day mortality with p values of <0.001 (table 4).
Table 4.
Univariable and multivariable logistic regression analysis for 30-day mortality
| Predictors | Univariable analysis | Multivariable analysis* | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Lactate >1.5 mmol/L | 4.29 (2.18 to 8.44) | <0.001 | 2.98 (1.46 to 6.09) | 0.003 |
| hs-cTnT—per log ng/L increase | 2.36 (1.70 to 3.27) | <0.001 | 1.53 (1.01 to 2.32) | 0.002 |
| NT-proBNP—per log ng/L increase | 1.78 (1.45 to 2.18) | <0.001 | 1.49 (1.15 to 1.92) | 0.002 |
| D-dimer >3000 µg/L | 2.91 (1.61 to 5.28) | <0.001 | 2.77 (1.44 to 5.33) | 0.045 |
| PCT—per log ng/mL increase | 1.34 (1.15 to 1.56) | <0.001 | – | – |
| AUC (95% CI) | ||||
| 0.82 (0.76 to 0.87) | ||||
Analysis in this table was made using imputed data.
*Model of biomarkers selected through backwards stepwise elimination using multivariable logistic regression analysis.
AUC, area under the curve; hs-cTnT, high-senstivity cardiac troponin T; log, logarithm; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin.
Subanalysis of combining biomarkers
In order to assess the discriminatory value of multiple biomarkers, we developed a model through backwards elimination in the multiple logistic regression analysis. PCT did not contribute significantly to the model (p value 0.51) and was therefore removed (table 4). This resulted in a model consisting of lactate, hs-cTnT, NT-proBNP and D-dimer. The AUC for prediction of 30-day mortality of these four biomarkers combined was 0.82 (95% CI 0.76 to 0.87).
Discussion
To the best of our knowledge, this is the first study evaluating the discriminatory value of lactate, hs-cTnT, NT-proBNP, D-dimer and PCT, when measured routinely, for predicting clinical outcome in older (≥65 years) medical ED patients. We conclude that these five biomarkers are predictive of 30-day mortality with the best discriminatory values for hs-cTnT and NT-proBNP (AUCs of 0.73). However, we observed a large overlap in biomarker values between the survivor and non-survivor group, resulting in suboptimal LRs. Overall, the predictive ability of the biomarkers for the composite endpoint turned out to be lower than for the primary endpoint.
We showed that lactate, hs-cTnT, NT-proBNP, D-dimer and PCT are sufficient to good predictors of 30-day mortality (AUCs ranging from 0.67 to 0.73) and sufficient predictors of the composite endpoint (AUCs ranging from 0.58 to 0.67) in older medical ED patients. Other studies showed the same results.6–8 10 11 22–28 However, in most of these studies, biomarkers were not measured routinely. Moreover, two studies showed that mortality was lowest in patients in whom biomarkers were not ordered during normal clinical practice.4 7 These findings show that the predictive value of biomarkers measured in all older ED patients differs from that measured only when indicated by the physician. We think that the predictive values we found for the biomarkers are more reflective of their true prognostic ability than when measured on indication.
Despite the fact that the five biomarkers were overall predictive of 30-day mortality, on an individual level, we found a large overlap in biomarker values between survivors and non-survivors. The overlap in biomarker values was most prominent in patients with non-extreme values. Especially in this group of patients, it is likely that the prognosis of the patient is less evident to the treating physician. Therefore, an estimation of prognosis provided by a biomarker is highly important. However, the discriminatory value of biomarker values in these patients was low as illustrated by the moderate LRs. In a US Study in patients with trauma, clinically meaningful contribution to decision making only occurred at lactate levels of >9 mmol/L,29 which was only present in a minority of patients. In our study, lactate had an important LR of 29 when between 6 and 10 mmol/L, which was only applicable to five patients. For the secondary composite endpoint, the discriminatory value of the biomarkers was even lower (ranging between 0.58 and 0.67). Therefore, we conclude that the five biomarkers do not contribute to clinical decision making.
Besides their discriminatory ability, the extra costs for determining the biomarkers should be taken into account. In more than 90% of patients (75% for lactate), biomarkers were not ordered by the physician (online supplemental table S2). Measuring these biomarkers on a routine basis will therefore lead to direct and indirect costs because abnormal test results (26%–86% of results were outside the reference range in our study) will undoubtedly lead to additional diagnostic tests, like CT scans.
In the multivariable analysis, stepwise elimination resulted in a new model consisting of four biomarkers, lactate, hs-cTnT, NT-proBNP and D-dimer, which yielded an AUC of 0.82. This discriminatory ability was, however, not better than that of the recently developed RISE UP Score (AUC 0.83), which consists of age, vital signs and four routine laboratory tests, albumin, blood urea nitrogen, lactate dehydrogenase and bilirubin.30 The RISE UP Score was developed in the same patient sample and has the advantage of using inexpensive variables, which are collected in routine ED care making the score feasible for use in older ED patients. In addition, we recently showed that adding these biomarkers to the RISE UP model only minimally improved the AUC of the model by 0.03.31 The limited added discriminatory ability and the expected extra costs support our conclusion that routinely determined biomarkers are not beneficial for prediction of mortality in older ED patients.
While we showed that biomarkers, measured at the ED visit, predict 30-day mortality, it is unknown whether assessment of these parameters will influence clinical decision making, outcome, well-being and medical costs. For this reason, the impact of biomarkers on clinical practice and patient-related outcome measures may be an interesting subject for future studies.
Our study has some limitations. First, due to moments of crowding of the ED, it was not possible to include every possible candidate, as physicians had to give priority to providing emergency care. We detected no evidence for selection bias but cannot exclude it either.17 In addition, we only measured biomarker values immediately after arrival at the ED. It is possible that serial biomarker measurement may have yielded different information and a different predictive ability.
In conclusion, the biomarkers such as lactate, hs-cTnT, NT-proBNP, D-dimer and PCT, when measured routinely, have predictive value with regard to short-term mortality and other adverse outcomes in older medical ED patients, but given the large overlap in values between those with and without adverse outcomes, they are unlikely to individually contribute to clinical decision making. Therefore, we conclude that routine measurement of these parameters is not recommended.
Supplementary Material
Acknowledgments
We acknowledge the medical and nursing staff of the emergency department of Zuyderland Medical Centre for their contribution to our study and, most of all, all patients who participated in the study.
Footnotes
Contributors: NZ, JB, PWDL and PS are responsible for developing the research question and study design. NZ and RH collected all data. NZ and PS are responsible for study management and data collection. NZ and SvK performed the statistical analysis. Data were interpreted by all authors, and NZ drafted the first version of the manuscript. MTMR performed all laboratory tests. RH, JB, PWDL, MTMR, SvK and PS critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Funding: Zuyderland MC supported the study by funding NZ.
Disclaimer: The funding source had no involvement in the study design, analysis, interpretation, or decision to submit this work.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the medical ethics committee of Zuyderland MC (NL55867.096.15).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data are available upon reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Barfod C, Lundstrøm LH, Lauritzen MMP, et al. Peripheral venous lactate at admission is associated with in-hospital mortality, a prospective cohort study. Acta Anaesthesiol Scand 2015;59:514–23. 10.1111/aas.12503 [DOI] [PubMed] [Google Scholar]
- 2.Datta D, Walker C, Gray AJ, et al. Arterial lactate levels in an emergency department are associated with mortality: a prospective observational cohort study. Emerg Med J 2015;32:673–7. 10.1136/emermed-2013-203541 [DOI] [PubMed] [Google Scholar]
- 3.Pedersen M, Brandt VS, Holler JG, et al. Lactate level, aetiology and mortality of adult patients in an emergency department: a cohort study. Emerg Med J 2015;32:678–84. 10.1136/emermed-2014-204305 [DOI] [PubMed] [Google Scholar]
- 4.van den Nouland DPA, Brouwers MCGJ, Stassen PM. Prognostic value of plasma lactate levels in a retrospective cohort presenting at a university hospital emergency department. BMJ Open 2017;7:e011450. 10.1136/bmjopen-2016-011450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahrmann P, Christ M, Hofner B, et al. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovasc Care 2016;5:568–78. 10.1177/2048872615612455 [DOI] [PubMed] [Google Scholar]
- 6.de Groot B, Verdoorn RCW, Lameijer J, et al. High-Sensitivity cardiac troponin T is an independent predictor of inhospital mortality in emergency department patients with suspected infection: a prospective observational derivation study. Emerg Med J 2014;31:882–8. 10.1136/emermed-2013-202865 [DOI] [PubMed] [Google Scholar]
- 7.Courtney D, Conway R, Kavanagh J, et al. High-sensitivity troponin as an outcome predictor in acute medical admissions. Postgrad Med J 2014;90:311–6. 10.1136/postgradmedj-2013-132325 [DOI] [PubMed] [Google Scholar]
- 8.Nickel CH, Kuster T, Keil C, et al. Risk stratification using D-dimers in patients presenting to the emergency department with nonspecific complaints. Eur J Intern Med 2016;31:20–4. 10.1016/j.ejim.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 9.Luchner A, Möckel M, Spanuth E, et al. N-terminal pro brain natriuretic peptide in the management of patients in the medical emergency department (prompt): correlation with disease severity, utilization of hospital resources, and prognosis in a large, prospective, randomized multicentre trial. Eur J Heart Fail 2012;14:259–67. 10.1093/eurjhf/hfr171 [DOI] [PubMed] [Google Scholar]
- 10.Rodelo JR, De la Rosa G, Valencia ML, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med 2012;30:1991–9. 10.1016/j.ajem.2012.04.033 [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm J, Hettwer S, Hammer D, et al. Outcome prediction using clinical scores and biomarkers in patients with presumed severe infection in the emergency department. Med Klin Intensivmed Notfmed 2012;107:558–63. 10.1007/s00063-012-0147-5 [DOI] [PubMed] [Google Scholar]
- 12.Freund Y, Delerme S, Goulet H, et al. Serum lactate and procalcitonin measurements in emergency room for the diagnosis and risk-stratification of patients with suspected infection. Biomarkers 2012;17:590–6. 10.3109/1354750X.2012.704645 [DOI] [PubMed] [Google Scholar]
- 13.Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med 2002;39:238–47. 10.1067/mem.2002.121523 [DOI] [PubMed] [Google Scholar]
- 14.Samaras N, Chevalley T, Samaras D, et al. Older patients in the emergency department: a review. Ann Emerg Med 2010;56:261–9. 10.1016/j.annemergmed.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 15.Salvi F, Morichi V, Grilli A, et al. The elderly in the emergency department: a critical review of problems and solutions. Intern Emerg Med 2007;2:292–301. 10.1007/s11739-007-0081-3 [DOI] [PubMed] [Google Scholar]
- 16.Carpenter CR, Shelton E, Fowler S, et al. Risk factors and screening instruments to predict adverse outcomes for undifferentiated older emergency department patients: a systematic review and meta-analysis. Acad Emerg Med 2015;22:1–21. 10.1111/acem.12569 [DOI] [PubMed] [Google Scholar]
- 17.Zelis N, Buijs J, de Leeuw PW, et al. Study protocol for a multicentre prospective cohort study to identify predictors of adverse outcome in older medical emergency department patients (the risk stratification in the emergency department in acutely ill older patients (rise up) study). BMC Geriatr 2019;19:65. 10.1186/s12877-019-1078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19.Mackway-Jones K Emergency triage. 1997 London: BMJ Publishing Group, 1997. [Google Scholar]
- 20.Šimundić A-M Measures of diagnostic accuracy: basic definitions. EJIFCC 2009;19:203–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet 2005;365:1500–5. 10.1016/S0140-6736(05)66422-7 [DOI] [PubMed] [Google Scholar]
- 22.Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med 2011;19:74. 10.1186/1757-7241-19-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park YJ, Kim DH, Kim SC, et al. Serum lactate upon emergency department arrival as a predictor of 30-day in-hospital mortality in an unselected population. PLoS One 2018;13:e0190519. 10.1371/journal.pone.0190519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puskarich MA, Illich BM, Jones AE. Prognosis of emergency department patients with suspected infection and intermediate lactate levels: a systematic review. J Crit Care 2014;29:334–9. 10.1016/j.jcrc.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 25.del Portal DA, Shofer F, Mikkelsen ME, et al. Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med 2010;17:260–8. 10.1111/j.1553-2712.2010.00681.x [DOI] [PubMed] [Google Scholar]
- 26.Marchetti M, Benedetti A, Mimoz O, et al. Predictors of 30-day mortality in patients admitted to ED for acute heart failure. Am J Emerg Med 2017;35:444–7. 10.1016/j.ajem.2016.11.050 [DOI] [PubMed] [Google Scholar]
- 27.Meyer B, Huelsmann M, Wexberg P, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of outcome in an unselected cohort of critically ill patients. Crit Care Med 2007;35:2268–73. 10.1097/01.CCM.0000284509.23439.5B [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Li C, Jia Y. Evaluation of the mortality in emergency department sepsis score combined with procalcitonin in septic patients. Am J Emerg Med 2013;31:1086–91. 10.1016/j.ajem.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 29.Baron BJ, Nguyen A, Stefanov D, et al. Clinical value of triage lactate in risk stratifying trauma patients using interval likelihood ratios. Am J Emerg Med 2018;36:784–8. 10.1016/j.ajem.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 30.Zelis N, Buijs J, de Leeuw PW, et al. A new simplified model for predicting 30-day mortality in older medical emergency department patients: the rise up score. Eur J Intern Med 2020;77:36–43. 10.1016/j.ejim.2020.02.021 [DOI] [PubMed] [Google Scholar]
- 31.Zelis N, Buijs J, de Leeuw PW, et al. Do biomarkers add anything to clinical prediction of mortality in older medical emergency department patients? Eur J Intern Med 2020;81:106–7. 10.1016/j.ejim.2020.07.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042989supp001.pdf (59.4KB, pdf)