Abstract
The vast majority of Foxp3+ regulatory T cells (Tregs) are generated in the thymus, and several factors, such as cytokines and unique thymic antigen-presenting cells, are known to contribute to the development of these thymus-derived Tregs (tTregs). Here, we report the existence of a specific subset of Foxp3+ Tregs within the thymus that is characterized by the expression of IL-1R2, which is a decoy receptor for the inflammatory cytokine IL-1. Detailed flow cytometric analysis of the thymocytes from Foxp3hCD2xRAG1GFP reporter mice revealed that the IL-1R2+ Tregs are mainly RAG1GFP– and CCR6+CCR7–, demonstrating that these Tregs are recirculating cells entering the thymus from the periphery and that they have an activated phenotype. In the spleen, the majority of IL-1R2+ Tregs express neuropilin-1 (Nrp-1) and Helios, suggesting a thymic origin for these Tregs. Interestingly, among all tissues studied, the highest frequency of IL-1R2+ Tregs was observed in the thymus, indicating preferential recruitment of this Treg subset by the thymus. Using fetal thymic organ cultures (FTOCs), we demonstrated that increased concentrations of exogenous IL-1β blocked intrathymic Treg development, resulting in a decreased frequency of CD25+Foxp3+ tTregs and an accumulation of CD25+Foxp3− Treg precursors. Interestingly, the addition of IL-1R2+ Tregs, but not IL-1R2− Tregs, to reaggregated thymic organ cultures (RTOCs) abrogated the IL-1β-mediated blockade, demonstrating that these recirculating IL-1R2+ Tregs can quench IL-1 signaling in the thymus and thereby maintain thymic Treg development even under inflammatory conditions.
Keywords: Thymus, Treg development, Inflammation, IL-1 system
Subject terms: Immunology, T cells, Regulatory T cells
Introduction
CD4+ regulatory T cells (Tregs), which are characterized by the expression of the lineage-specific factor Foxp3, play essential roles in immune homeostasis and in self-tolerance maintenance.1 A substantial fraction of these Foxp3+ Tregs is generated in the thymus, and hence, these cells are named thymic-derived Tregs (tTregs), which are thought to comprise ~80% of the peripheral Treg population.2 In the thymus, the development of tTregs is proposed to proceed via a two-step process involving a T-cell receptor (TCR)-instructive phase and a cytokine-responsive phase.3 TCR engagement and costimulation are critical for Treg induction and are induced by unique thymic antigen-presenting cells (APCs). In this respect, the most thoroughly studied and, arguably the most important, thymic APCs for the generation of tTregs are medullary thymic epithelial cells (mTECs) and thymic dendritic cells (t-DCs).4–6 In addition, common cytokines with γ-chains, such as interleukin (IL)-2, are essential for tTreg development,7–10 and in addition to common cytokines with γ-chains, TGF-β was also shown to play a key role.11,12 However, to date, the impact of inflammatory cytokines on tTreg development has been largely ignored.
Recent studies have suggested that peripheral Tregs can recirculate to the thymus, where they constitute a large proportion of the Treg pool and display an activated and mature phenotype.13,14 The fraction of recirculating Tregs increases with cellular age and has been identified with RAG reporter mice.13,14 It has been suggested that recirculating Tregs can functionally inhibit intrathymic Treg development by competing for the limited amounts of IL-2, thereby perpetuating a negative feedback loop in which mature progeny cells return to the thymus to suppress the development of tTreg precursors.13 However, it is also possible that these recirculating Tregs can modulate the de novo generation of tTregs by other currently unidentified mechanisms.
Previously, we reported that the fraction of CD24low mature Tregs in the thymus expresses increased levels of interleukin-1 receptor 2 (Il1r2) compared to the levels expressed by the thymic CD24lowFoxp3− cells and peripheral Foxp3+ Tregs.15 IL-1R2, which is known to be expressed mainly on innate immune cells, binds to IL-1β with high affinity; however, IL-1R2 is unable to initiate signal transduction because it has only a very short intracellular domain that lacks the Toll interleukin-1 receptor (TIR) domain.16–19 Hence, IL-1R2 can be considered a decoy receptor that neutralizes the proinflammatory function of IL-1 by binding to it and thus inhibiting its interaction with the activating IL-1R1, through which its anti-inflammatory, immunoregulatory role has been mainly attributed.17,20–22 IL-1R2 is considered a novel therapeutic target, and by establishing an anti-inflammatory environment, IL-1R2 overexpression has led to reduced inflammatory responses in preclinical models such as those for collagen-induced arthritis, IL-1-induced inflammation and cardiac allograft surgery.19 However, whether IL-1 signaling plays a role in intrathymic Treg development or whether IL-1R2 expression in mature Tregs in the thymus has any functional consequence during this process remains to be elucidated.
In the present study, we analyzed the IL-1R2 expression on Tregs from different lymphoid and nonlymphoid organs. The highest frequency of IL-1R2+ Tregs was observed in the thymus, where IL-1R2 was mainly expressed on recirculating Tregs. Functional studies using fetal thymic organ cultures (FTOCs) revealed that exogenous IL-1β blocks intrathymic Treg development, resulting in a decreased frequency of CD25+Foxp3+ tTregs and an increased frequency of CD25+Foxp3− Treg precursors. Importantly, the addition of IL-1R2+ Tregs to reaggregated thymic organ cultures (RTOCs) restored normal tTreg development in the RTOCs treated with exogenous IL-1β, suggesting a role for recirculating IL-1R2+ Tregs in fine-tuning tTreg development under inflammatory conditions.
Results
In the thymus, IL-1R2 is mainly expressed on recirculating Foxp3+ Tregs
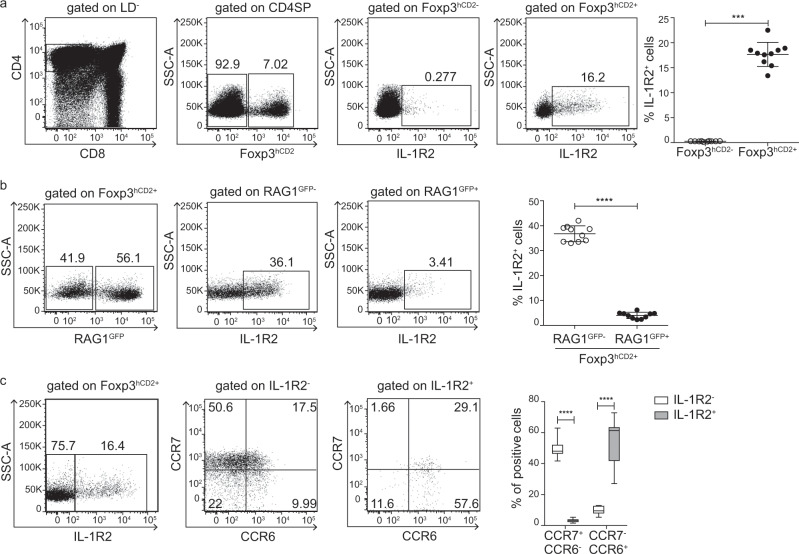
We previously reported that Il1r2 is highly expressed in mature CD24lowFoxp3+ CD4 single-positive (SP) thymocytes, while only very low levels are found in the Foxp3− CD4SP thymocyte subsets.15 Here, we performed flow cytometric analyses to validate this finding at the protein level. Analysis of single-cell suspensions from the thymi of Foxp3hCD2 reporter mice revealed a statistically significant higher frequency of Foxp3hCD2+ CD4SP thymocytes expressing IL-1R2 compared to the frequency of Foxp3hCD2− CD4SP thymocytes expressing IL-1R2 (Fig. 1a), while only a minor fraction of the double-positive (DP), double-negative (DN), and CD8SP thymocytes expressed IL-1R2 (Supplementary Fig. S1). Altogether, these data indicate that Foxp3hCD2+ CD4SP thymocytes constitute the major IL-1R2-expressing thymocyte subset.
Fig. 1. Phenotyping IL-1R2+Foxp3+ Tregs in the thymus.
Thymocytes were isolated from the Foxp3hCD2xRAG1GFP reporter mice and analyzed by flow cytometry. a Representative dot plots show the gating of the CD4SP thymocytes among the living cells (LD−, LIVE/DEAD−), Foxp3hCD2− and Foxp3hCD2+ cells among the CD4SP thymocytes, and IL-1R2+ cells among the Foxp3hCD2− and Foxp3hCD2+ CD4SP thymocytes. Numbers specify the frequencies of the cells in the indicated gates. b Representative dot plots show the gating of the RAG1GFP− and RAG1GFP+ cells among the Foxp3hCD2+ CD4SP thymocytes and the IL-1R2+ cells among the RAG1GFP− and RAG1GFP+ Foxp3hCD2+ CD4SP thymocytes. Numbers specify the frequencies of the cells in the indicated gates. a, b Scatter plots summarize the data from two independent experiments, and the bars indicate mean ± SD. Each symbol represents an individual mouse. The significance was calculated using Mann–Whitney U-test; ****p < 0.0001 and ***p < 0.001. c Representative dot plots show the gating of the IL-1R2− and IL-1R2+ cells among the Foxp3hCD2+ CD4SP thymocytes and display CCR7 and CCR6 expression levels in the IL-1R2− and IL-1R2+ Foxp3hCD2+ CD4SP thymocytes. Numbers specify frequencies of the cells in the indicated gates and quadrants. A box-and-whisker plot summarizes the data from two independent experiments (n = 16). The significance was calculated using two-way ANOVAs (Bonferroni’s multiple comparison tests); ****p < 0.0001.
Foxp3+ Tregs were reported to reenter the thymus from the periphery to inhibit the development of Treg precursors.13 To assess whether IL-1R2 is expressed on such recirculating Tregs or on newly generated Foxp3+ CD4SP thymocytes, we used mice expressing a transgene encoding green fluorescent protein (GFP) under the control of a recombination-activating gene 1 (Rag1) promoter23 to mark newly generated thymocytes (GFP+) and distinguish them from older and/or recirculating (GFP–) T cells. In line with previously published data,13 >40% of the Foxp3hCD2+ CD4SP thymocytes from 6–7-week-old Foxp3hCD2xRAG1GFP reporter mice were GFP– T cells, suggesting a substantial proportion of mature and/or recirculating Tregs is within the thymus. Interestingly, among these Foxp3hCD2+RAG1GFP– CD4SP thymocytes, IL-1R2+ cells were strongly enriched, and very few IL-1R2+ cells were found among the Foxp3hCD2+RAG1GFP+ CD4SP thymocytes (Fig. 1b), suggesting that newly generated tTregs negligibly express IL-1R2 and that the majority of the IL-1R2+ T cells in the thymus corresponds to either older tTregs or to recirculating Tregs entering the thymus from the periphery.
To obtain additional insights into the origin of thymic IL-1R2+ cells, we analyzed the expression of CCR6 and CCR7, which reportedly enable the identification of recirculating Tregs as CCR7−CCR6+ cells.14 In line with these data, CCR7−CCR6+Foxp3hCD2+ CD4SP thymocytes from the Foxp3hCD2xRAG1GFP reporter mice were mainly GFP− thymocytes, while the majority of CCR7+CCR6−Foxp3hCD2+ CD4SP thymocytes was composed of GFP+ thymocytes (Supplementary Fig. S2). Interestingly, the majority of the IL-1R2+Foxp3hCD2+ Tregs was composed of CCR7−CCR6+ Tregs, differing from the IL-1R2−Foxp3hCD2+ CD4SP thymocytes, which had the phenotype of newly generated CCR7+CCR6− tTregs (Fig. 1c). Altogether, these data support the hypothesis that most IL-1R2+ T cells in the thymus are recirculating Tregs entering the thymus from the periphery.
Splenic IL-1R2+Foxp3+ Tregs have a tTreg phenotype and characteristics of follicular regulatory T cells
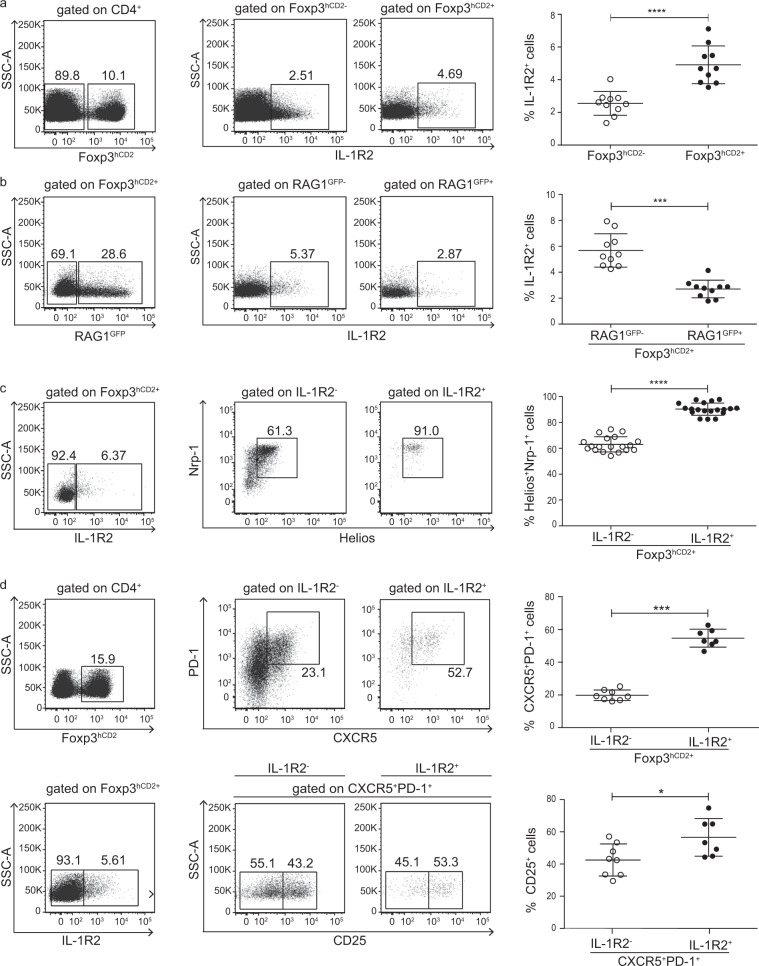
Since our data suggest that IL-1R2+ Tregs can enter the thymus from the periphery, we assessed the presence of these cells in the spleen. Flow cytometric analysis of splenocytes from the Foxp3hCD2xRAG1GFP reporter mice revealed that only small fractions of both Foxp3hCD2+ Tregs and Foxp3hCD2− conventional CD4+ T cells (Tconvs) in the spleen expressed IL-1R2, with the Foxp3hCD2+ Tregs at a slightly higher frequency than the Tconvs (Fig. 2a). Interestingly, a significantly lower frequency of the IL-1R2+ cells was found among the recent thymic emigrant Tregs, as identified by the remaining RAG1GFP expression, compared to those among the more mature RAG1GFP-Foxp3hCD2+ Tregs (Fig. 2b), suggesting that splenic Foxp3+ Tregs express IL-1R2 at a relatively more mature stage.
Fig. 2. Phenotyping IL-1R2+Foxp3+ Tregs in the spleen.
Splenocytes were isolated from the Foxp3hCD2xRAG1GFP reporter mice and analyzed by flow cytometry. a Representative dot plots show the gating of the Foxp3hCD2− and Foxp3hCD2+ cells among the CD4+ splenocytes and the IL-1R2+ cells among the Foxp3hCD2− and Foxp3hCD2+ CD4+ splenocytes. Numbers specify the frequencies of the cells in the indicated gates. b Representative dot plots show the gating of the RAG1GFP− and RAG1GFP+ cells among the Foxp3hCD2+ CD4+ splenocytes and the IL-1R2+ cells among the RAG1GFP− and RAG1GFP+ Foxp3hCD2+ CD4+ splenocytes. The numbers specify frequencies of the cells in the indicated gates. c Representative dot plots show the gating of the IL-1R2− and IL-1R2+ cells among the Foxp3hCD2+ CD4+ splenocytes and indicate the levels of Nrp-1 and Helios expression levels in the IL-1R2− and IL-1R2+ Foxp3hCD2+ CD4+ splenocytes. Numbers specify the frequencies of the cells in the indicated gates. d (Left) Representative dot plots show the gating of the Foxp3hCD2+ cells among the CD4+ splenocytes and the IL-1R2− and IL-1R2+ cells among the Foxp3hCD2+ CD4+ splenocytes. (Middle, upper row) Representative dot plots display PD-1 and CXCR5 expression levels in the IL-1R2− and IL-1R2+ Foxp3hCD2+ CD4+ splenocytes. (Middle, lower row) Representative dot plots show the gating of the CD25− and CD25+ cells among the IL-1R2− and IL-1R2+ CXCR5+PD-1+Foxp3hCD2+ CD4+ splenocytes. Numbers specify the frequencies of the cells in the indicated gates. a–d (Right) Scatter plots summarize the data from two independent experiments, with the bars indicating the means ± SD. Each symbol represents an individual mouse. The significance was calculated using Mann–Whitney U-tests; *p < 0.05, ***p < 0.001; and ****p < 0.0001.
Next, we investigated the origin of the splenic IL-1R2+Foxp3+ Tregs and assessed the expression of neuropilin-1 (Nrp-1) and Helios, two markers that define tTregs, particularly under homeostatic conditions.24–26 While only ~60% of the splenic IL-1R2−Foxp3hCD2+ Tregs expressed both Nrp-1 and Helios, the vast majority of the IL-1R2+Foxp3hCD2+ Tregs were Nrp-1+Helios+ (Fig. 2c), implying that most of the IL-1R2+Foxp3hCD2+ Tregs in the spleen had originated in the thymus.
A recent study reported that follicular regulatory T (Tfr) cells lack CD25 but express IL-1R2 and suppress the IL-1-dependent activation of follicular helper T (Tfh) cells.27 In line with these published data, we observed an increased frequency of CXCR5+PD-1+ “Tfr-like” cells among the IL-1R2+ when compared to IL-1R2− Foxp3hCD2+ Tregs both in the spleen (Fig. 2d) and in the thymus (Supplementary Fig. S3). However, we could not confirm the lack of CD25 expression on the Tfr cells because both the IL-1R2+ and IL-1R2− subsets of the CXCR5+PD-1+Foxp3hCD2+ Tregs had a substantial fraction of CD25+ cells, with the frequency in the IL-1R2+ subset always being higher (Fig. 2d and Supplementary Fig. S3). Altogether, these data suggest that IL-1R2+Foxp3+ Tregs are derived from the thymus and that a large fraction of these IL-1R2+Foxp3+ Tregs has the characteristics of Tfr cells.
Tissue-resident Foxp3+ Tregs negligibly express IL-1R2
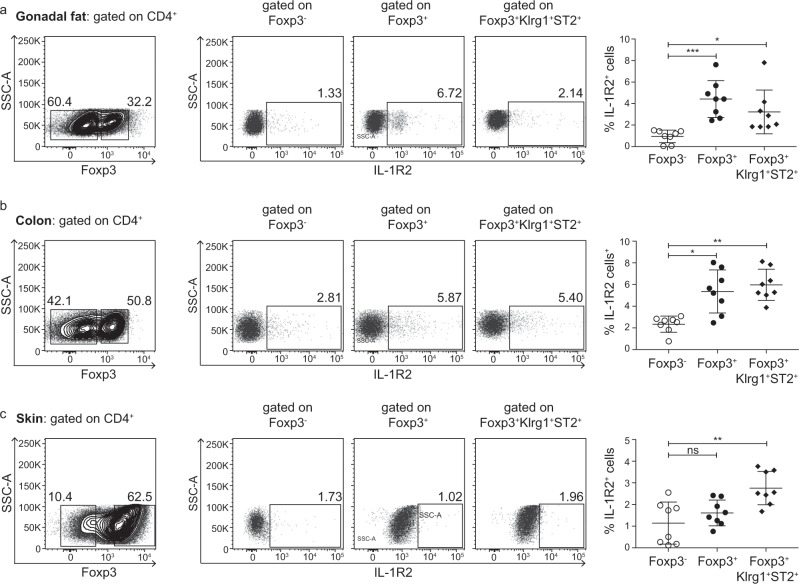
After establishing that only a small fraction of the Foxp3+ Tregs in the spleen expresses IL-1R2, we next sought to determine whether IL-1R2 expression can be detected in tissue-resident Tregs from nonlymphoid tissues.28,29 To this end, we isolated cells from gonadal fat and the colon and skin of Foxp3.IRES-DTR/GFP mice and assessed IL-1R2 expression by the Foxp3− Tconv, total Foxp3+ Tregs, and other tissue-residing Tregs, gated as Klrg1+ST2+ cells among the total Foxp3+ Tregs, as reported previously.30,31 Although tissue-resident Tregs had significantly higher IL-1R2 expression in all tissues analyzed compared to the level expressed by Foxp3− Tconv (Fig. 3), no differences were observed when tissue-resident Tregs were compared to the total Foxp3+ Tregs, and the frequency of IL-1R2+ cells was relatively low, particularly in the gonadal fat and skin samples (Fig. 3a+c). Altogether, these data suggest that tissue-resident Tregs mostly lack IL-1R2 expression, further emphasizing the high frequency of the IL-1R2+ cells among the recirculating Tregs in the thymus.
Fig. 3. Expression level of IL-1R2 on tissue-resident Tregs.
Cells were isolated from the gonadal fat (a), colon (b), and skin (c) of the Foxp3.IRES-DTR/GFP mice and analyzed by flow cytometry. a–c (Left) Representative density plots show the gating of the Foxp3− and Foxp3+ cells among the CD4+TCRβ+CD8−CD19−MHCII−LD− cells. (Middle) Representative dot plots show the gating of the IL-1R2+ cells among the Foxp3− Tconv, total Foxp3+ Tregs and Klrg1+ST2+Foxp3+ tissue-resident Tregs, all gated CD4+TCRβ+CD8−CD19−MHCII−LD− cells. Numbers specify the frequencies of the cells in the indicated gates. To increase the visibility, dot plot data from several mice were concatenated and presented. (Right) Scatter plots summarize the data from two independent experiments, and the bars indicate the mean ± SD. Each symbol represents an individual mouse. The significance was calculated using Kruskal–Wallis with Dunn’s tests (ns = not significant; *p < 0.05; **p < 0.01; and ***p < 0.001).
In the thymus, IL-1R1 is mainly expressed by thymic APCs under steady-state conditions
To obtain further insights into the IL-1-IL-1R system in the thymus, we studied which cell populations express the activating IL-1 receptor, IL-1R1. Flow cytometric analysis of the thymocytes isolated from the Foxp3hCD2xRAG1GFP reporter mice revealed that both Foxp3hCD2− and Foxp3hCD2+ CD4SP thymocytes negligibly express IL-1R1 (Fig. 4a). Slightly higher frequencies were detected for mTECs and t-DCs isolated from BALB/c mice, suggesting that, under steady-state conditions, a fraction of the thymic APCs can sense IL-1. We previously reported that Il1a and Il1b mRNA is expressed in the thymus under steady-state conditions, with t-DCs showing higher expression levels than mTECs.6 Here, we analyzed the presence of both cytokines in the thymus at the protein level using thymic lysates and demonstrated that, under steady-state conditions, both IL-1α and IL-1β were present in the thymus in the range of 5–15 pg/ml (Fig. 4b). Taken together, these data suggest that IL-1 signaling might play a role during thymic T-cell development, since both the activating IL-1R1 receptor and its ligands, IL-1α and IL-1β, are present in the thymus, even under steady-state conditions.
Fig. 4. Expression of IL-1R1 and its ligands, IL-1α and IL-1β, in the thymus.
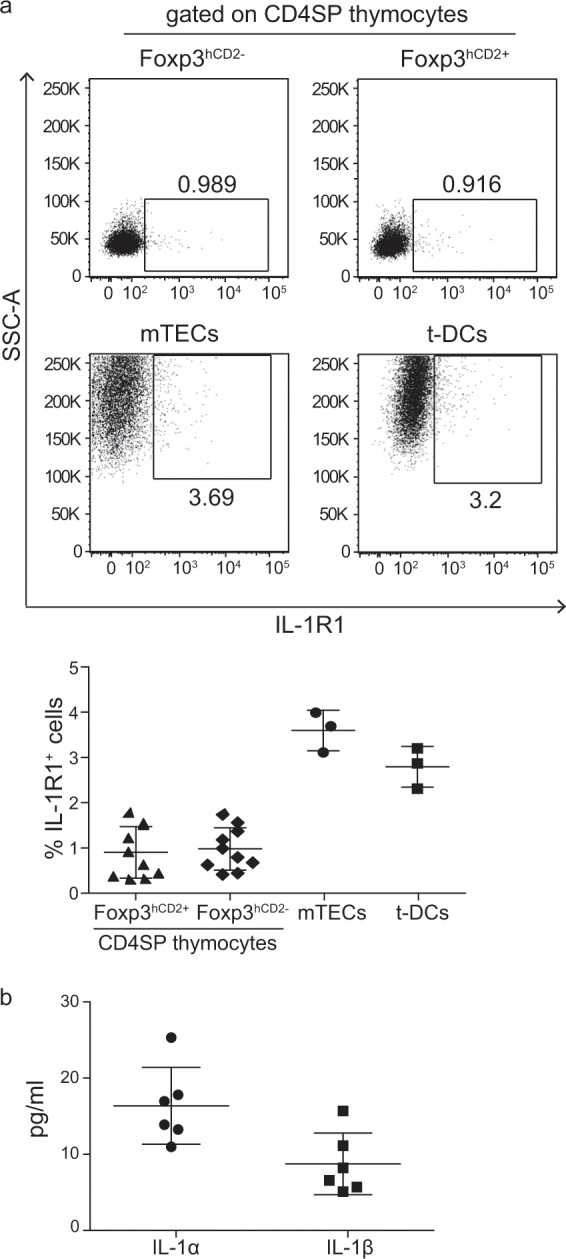
a Thymocytes isolated from Foxp3hCD2xRAG1GFP reporter mice, as well as mTECs and t-DCs isolated from the BALB/c mice were analyzed by flow cytometry. Representative dot plots show the gating of the IL-1R1+ cells among the Foxp3hCD2− and Foxp3hCD2+ CD4SP thymocytes and among the CD45−EpCAM+ mTECs and CD45+CD11chiLin− t-DCs (Lin defined as CD90, CD49b, F4/80 and CD19). Numbers specify the frequencies of the cells in the indicated gates. Scatter plot summarizes the data from two (thymocytes) and three (mTECs and t-DCs) independent experiments, and the bars indicate the mean ± SD. Each symbol represents an individual mouse. b Thymi of 6–8-week-old Foxp3hCD2xRAG1GFP reporter mice were lysed, and equal concentrations (500 µg/ml) of the lysates were used for the determination of IL-1α and IL-1β concentrations, which were normalized to that of the homogenized thymus protein content. Scatter plot summarizes the data from two independent experiments, and the bars indicate the mean ± SD. Each symbol represents an individual mouse.
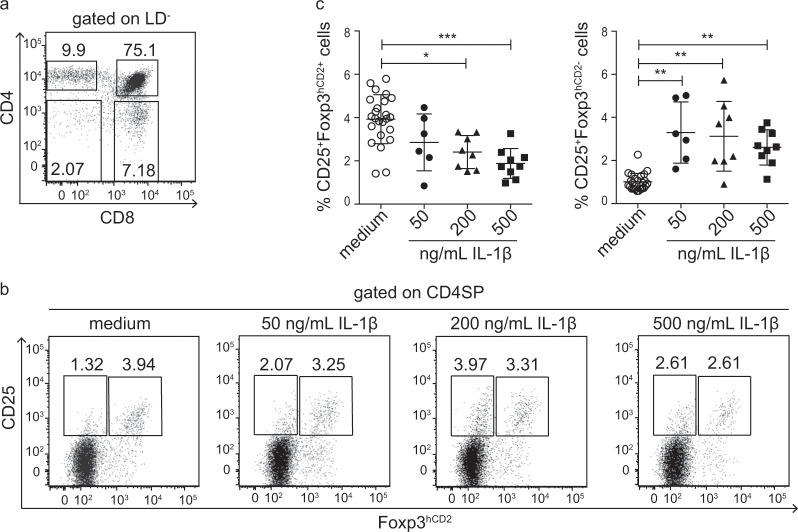
IL-1β blocks tTreg development
To investigate the functional relevance of IL-1 signaling to intrathymic Treg development, we utilized FTOCs, which enabled the assessment of the impact of exogenously added IL-1β on tTreg development and the simultaneous elimination of any influence of recirculating cells moving from the periphery to the thymus. The thymic lobes from E18.5 fetuses of Foxp3hCD2 reporter mice were cultured for 5 days in medium with increasing concentrations of exogenously added IL-1β. At the end of the culture period, the thymic lobes were harvested and frequencies of CD25+Foxp3hCD2+ tTregs and CD25+Foxp3hCD2− Treg precursors among the CD4SP thymocytes were determined by flow cytometry (Fig. 5a). Interestingly, increasing concentrations of exogenously added IL-1β resulted in a significantly decreased frequency of CD25+Foxp3hCD2+ tTregs in a dose-dependent manner when compared to control FTOCs cultured without exogenously added IL-1β (Fig. 5b). Concomitantly, the addition of IL-1β led to a significant increase in the frequency of CD25+Foxp3hCD2− Treg precursors (Fig. 5b), suggesting that inflammatory conditions, mimicked by increased concentrations of exogenous IL-1β, blocked intrathymic Treg development. To obtain further insights into the mechanism underlying the IL-1β-mediated blocking of intrathymic Treg development, we studied the IL-1R1 expression on thymocytes and APCs (mTECs and t-DCs) from the FTOCs cultured with increasing concentrations of IL-1β. In line with the abovementioned findings demonstrating negligible IL-1R1 expression on the ex vivo isolated CD4SP thymocytes (Fig. 4a), only minor fractions of both Treg precursor populations (CD25+Foxp3hCD2- and CD25−Foxp3hCD2+) and CD25+Foxp3hCD2+ tTregs from the FTOCs expressed IL-1R1, and the addition of exogenous IL-1β did not result in induction of IL-1R1 expression in the CD4SP thymocyte subsets (Supplementary Fig. S4a+b). However, larger fractions of mTECs and t-DCs from the FTOCs expressed IL-1R1 (Supplementary Fig. S4a+b), and the frequency of the IL-1R1+ cells was slightly higher compared to that of the ex vivo isolated mTECs and t-DCs (Fig. 4). However, the addition of IL-1β to the FTOCs did not result in any further induction of IL-1R1 expression in the mTECs or t-DCs; in contrast, it caused a mild reduction in IL-1R1+ cells among the mTECs (Supplementary Fig. S4b). Altogether, these data point towards the involvement of mTECs and t-DCs in the IL-1β-mediated blockade of intrathymic Treg development and suggest that CD4SP thymocytes, which mostly lack IL-1R1 expression, cannot directly sense increased IL-1β levels. The latter assumption was confirmed by sorting CD25+Foxp3hCD2- and CD25−Foxp3hCD2+ Treg precursors and culturing them in vitro for 5 days in the presence of IL-2 to generate CD25+Foxp3hCD2+ tTregs as described before.3,32,33 Here, adding increasing concentrations of IL-1β did not result in a reduced frequency of CD25+Foxp3hCD2+ tTregs (Supplementary Fig. S4c), suggesting that IL-1β has no direct effect on the Treg precursors. Interestingly, the sorted CD25+Foxp3hCD2+ tTregs were cultured under the same conditions as the controls and did not show any direct response to IL-1β treatment. Finally, when the IL-1R2− and IL-1R2+ Treg subsets sorted from spleens and lymph nodes were cultured in the presence of IL-1β, no effects on their survival or on the stability of Foxp3 expression were observed (Supplementary Fig. S5). Altogether, these data suggest that neither the CD4SP thymocyte subsets nor the peripheral Tregs can be directly modulated by IL-1β.
Fig. 5. Exogenous IL-1β affects tTreg development in the FTOCs.
Thymic lobes were isolated from E18.5 fetuses of the Foxp3hCD2 reporter mice and cultured on Transwell membranes, with medium only or medium containing the indicated concentrations of IL-1β for 5 days. At the end of the culture, the thymic lobes were analyzed by flow cytometry. a Representative dot plot shows the presence of all major thymocyte subsets (CD4SP, CD8SP, DP, and DN) among the living (LD−) thymocytes. Numbers specify the frequencies of the cells in the indicated gates. b Representative dot plots show the gating of the CD25+Foxp3hCD2− and CD25+Foxp3hCD2+ cells among CD4SP thymocytes from the FTOCs cultured under the indicated conditions. Numbers specify the frequencies of the cells in the indicated gates. c Scatter plots summarize data from four independent experiments, and the bars indicate the mean ± SD. Each symbol represents a thymic lobe. The significance was calculated using Kruskal–Wallis with Dunn’s tests (*p < 0.05; **p < 0.01; and ***p < 0.001).
IL-1R2+ Tregs fine-tune tTreg development under inflammatory conditions
After demonstrating that IL-1β blocks intrathymic Treg development in the FTOCs, we studied the impact of IL-1β on intrathymic Treg development in vivo. To this end, wild-type mice were immunized with complete Freund’s adjuvant (CFA), which is known to induce an IL-1β response.34,35 Interestingly, subcutaneous injection of CFA resulted in a significant increase in intrathymic IL-1β levels (Fig. 6a), and this increase was accompanied by reduced DP thymocyte frequency in the CFA-treated mice, compared to the levels in the phosphate-buffered saline (PBS)-treated mice (Fig. 6b). However, the increased intrathymic IL-1β levels did not affect the de novo tTreg generation, as comparable frequencies of CD25+Foxp3+ tTregs were detected among the newly generated CD73lowCD44−/int CD4SP thymocytes in both the CFA-treated mice and PBS-treated controls (Fig. 6c). These data, together with the findings from the FTOCs, suggest that in vivo the thymus is equipped with an “IL-1 quenching system” that enables normal tTreg development even in the presence of increased intrathymic IL-1β levels.
Fig. 6. Normal tTreg generation despite increased intrathymic IL-1β levels after CFA treatment.
Wild-type mice were immunized subcutaneously at the tail base with CFA or treated with PBS as control. Three days later, the thymi were collected and analyzed by ELISA and flow cytometry. a Scatter plot shows the levels of the IL-1β in thymic cells of the mice treated with PBS (open circles) or CFA (closed circles) and summarizes the data from three independent experiments, with the bars indicating the mean + SD. Each symbol represents an individual mouse. b (Left) Representative dot plots depict the frequencies of thymocyte subsets gated on the living thymocytes from the mice treated with PBS (left) or CFA (right). Numbers indicate the frequencies within the respective gates. (Right) Scatter plot shows the frequencies of the DP thymocytes among the living thymocytes from mice treated with PBS (open circles) or CFA (closed circles) and summarizes the data from two independent experiments, with the bars indicating the mean + SD. Each symbol represents a single mouse. c (Left) Representative dot plots depict the frequencies of the CD25+Foxp3+ Tregs among the newly generated CD73lowCD44−/int CD4SP thymocytes in the mice treated with PBS (left) or CFA (right). Numbers indicate the frequencies within the quadrants. (Right) Scatter plot summarizes the data from two independent experiments, which included eight mice per group, and depicted as the mean + SD. Each symbol represents a single mouse. The significance was calculated using Mann–Whitney U-tests; **p < 0.01 and ***p < 0.001.
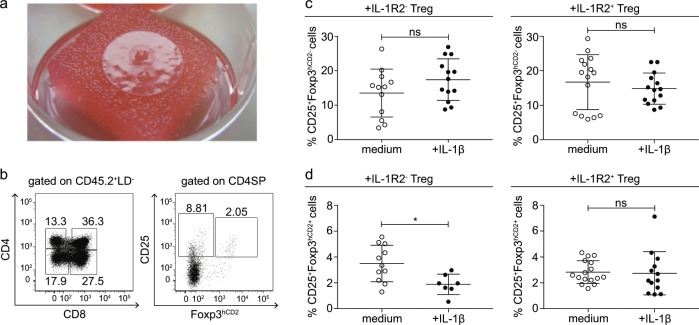
To provide experimental evidence that recirculating IL-1R2+Foxp3+ Tregs contribute to this system and fine-tune tTreg development when intrathymic IL-1 levels are increased, we utilized RTOCs (Fig. 7a). The sorted Treg subsets were added to the RTOCs to mimic the recirculation of peripheral Tregs to the thymus. For this experiment, IL-1R2+ and IL-1R2− CD4+Foxp3+ Treg subsets were isolated from pooled single-cell suspensions of spleen and lymph nodes taken from the CD45.1 congenic Foxp3hCD2 reporter mice and added in small amounts to a single-cell suspension of thymic lobes obtained from the E14.5 to E16.5 fetuses of the Foxp3hCD2 reporter mice (CD45.2), and then, these cells were reaggregated by centrifugation. The RTOCs were cultured for 8 days in the presence or absence of IL-1β. At the end of the culture period, 2–3 RTOCs were pooled to obtain a sufficient number of cells for the analysis, and the frequencies of CD25+Foxp3hCD2+ tTregs and CD25+Foxp3hCD2− Treg precursors among the CD45.2+ CD4SP thymocytes were determined by flow cytometry (Fig. 7b). Interestingly, the addition of the IL-1R2− Tregs to the RTOCs did not abrogate the IL-1β-induced blockade of intrathymic Treg development (Fig. 7c+d). Here, we identified a significant decrease in the frequency of CD25+Foxp3hCD2+ tTregs (Fig. 7d) and a slight and not statistically significant increase in the frequency of CD25+Foxp3hCD2− Treg precursors (Fig. 7c) in the IL-1β-treated RTOCs compared the frequency found in the untreated controls. Importantly, the addition of the IL-1R2+ Tregs to the RTOCs was sufficient to abrogate the IL-1β-induced blockade of intrathymic Treg development, as no differences in the frequencies of the CD25+Foxp3hCD2− Treg precursors (Fig. 7c) and the CD25+Foxp3hCD2+ tTregs (Fig. 7d) were observed between the untreated and IL-1β-treated RTOCs. In summary, the functional RTOC studies demonstrate that the IL-1R2+ Tregs prevent the IL-1β-induced blockade of intrathymic Treg development, suggesting that recirculating IL-1R2+ Tregs are critical for the fine-tuning of tTreg development under inflammatory conditions.
Fig. 7. IL-1R2+Foxp3+ Tregs fine-tune tTreg development in the RTOCs under inflammatory conditions.
RTOCs (Foxp3hCD2 reporter mice), including additional IL-1R2+Foxp3hCD2+ or IL-1R2−Foxp3hCD2+ Tregs isolated from the congenic CD45.1 Foxp3hCD2 reporter mice, were cultured in only medium or medium containing 200 ng/ml IL-1β for 8 days. At the end of the culture period, 2–3 RTOCs were pooled and analyzed by flow cytometry. Endogenous cells of the RTOCs were identified by the expression of the congenic marker CD45.2. a The photograph shows two RTOCs on a Whatman® Nuclepore™ Track-Etched Membrane at day 8 in culture. b Representative dot plots show the presence of all major thymocyte subsets (CD4SP, CD8SP, DP, and DN) among the CD45.2+LD− thymocytes (left) and the gated CD25+Foxp3hCD2− and CD25+Foxp3hCD2+ cells among the CD4SP thymocytes (right) in the control RTOCs cultured in only medium without addition of Tregs. Numbers specify the frequencies of the cells in the indicated gates. c+d Scatter plots summarize the data from four independent experiments, and the bars indicate the mean ± SD. Each symbol represents 2–3 pooled RTOCs. The significance was calculated using Mann–Whitney U-tests (ns = not significant; *p < 0.05).
Discussion
The thymus is known as the main site for the generation of Foxp3+ Tregs, and substantial progress has been made in the recent past in deciphering how tTregs develop.2 Several cellular players and molecular factors were reported to contribute to the development of tTregs; however, among these factors, common γ-chain cytokines were found to be indispensable. The majority of studies focused on IL-2 but IL-7 and IL-15 were also found to play central roles in tTreg development.3,7–10,36,37 In the present study, we identified another molecular player that fine-tunes tTreg development, the inflammatory cytokine IL-1. We demonstrated that increasing the doses of IL-1β blocked intrathymic Treg development at a point between the Treg precursor and tTreg stages and found that a large fraction of the recirculating Tregs entering the thymus from the periphery express IL-1R2, which binds IL-1β with high affinity but is unable to initiate signal transduction due to the lack of a TIR domain. Recirculation of IL-1R2+ Tregs, mimicked here by adding IL-1R2+Foxp3+ Tregs to RTOCs, enabled efficient intrathymic Treg development, even in the presence of the inflammatory cytokine IL-1β, demonstrating a connection between inflammatory immune responses in the periphery and thymic Treg development through fine-tuning of IL-1 signaling by recirculating IL-1R2+Foxp3+ Tregs.
IL-1R2+Foxp3+ Tregs had been previously found.38,39 In accordance with our own data, previous reports indicated that only activated human Tregs, but not resting human T cells, expressed IL-1R2. However, IL-1R2 expression was not upregulated on TGF-β-induced Tregs generated in vitro,39 supporting our observation that, under steady-state conditions, the majority of the IL-1R2+Foxp3+ Tregs from secondary lymphoid organs has a thymic origin, as indicated by their Helios and Nrp-1 expression. Surprisingly, we detected very small subsets of IL-1R2+ cells among the Klrg1+ST2+Foxp3+ Tregs from different tissues, although several studies had previously reported Il1r2 mRNA expression in tissue-infiltrating Tregs, such as in various tumors and healthy tissues.40–44 However, the highest frequency of IL-1R2+ Tregs was found in the thymus, and IL-1R2+ Tregs were enriched among the recirculating Tregs, suggesting that IL-1R2+ Tregs are preferentially recruited from secondary lymphoid organs or peripheral tissues and recirculated to the thymus.
Previous studies have demonstrated that mature Foxp3+ Tregs from the periphery have the capacity to recirculate to the thymus.13,14 They showed that the fraction of recirculating Tregs increases with age and efficiently inhibits intrathymic Treg development under steady-state conditions by competing for the limited amount of IL-2, thereby perpetuating a negative feedback loop. However, data from the present study suggest that, under inflammatory conditions, recirculating Tregs can also have the opposite effect and stabilize intrathymic Treg development. Our data suggest that IL-1R2 expression on recirculating Tregs plays a functional role only for intrathymic Treg development under inflammatory conditions. In line with this assumption, we found no significant differences in the frequency of CD25+Foxp3− Treg precursors or CD25+Foxp3+ tTregs comparing thymocytes from IL-1R2−/− and wild-type mice under steady-state conditions (data not shown). Together, these data suggest that the recirculating Tregs can have opposing effects on intrathymic Treg development depending on the conditions (steady-state vs. inflammatory), and we cannot formally exclude the possibility that additional molecular mechanisms have an effect in the IL-1R2− fraction of the recirculating Tregs and contribute to the neutralization of inflammatory mediators within the thymus under inflammatory conditions.
Furthermore, recirculating IL-1R2+Foxp3+ Tregs might play an additional role within the thymus, as a fraction shows characteristics of Tfr cells, in line with a recent study reporting that Tfr cells express IL-1R2 and regulate Tfh cells with respect to IL-1 signaling.27 Considering that B cells are present in the thymus and play roles in the generation of tTregs,45,46 it is tempting to speculate that the specific subpopulation of IL-1R2+Foxp3+ Tfr cells might be involved in the shaping of the Treg repertoire by interacting with and modulating the function of intrathymic B cells.
While mainly focusing on the phenotypic and functional characterization of IL-1R2+ Tregs from the thymus, findings in this study also suggest that thymic APCs, including mTECs and t-DCs, are the major cell types within the thymus that are capable of sensing IL-1 and mediating the blockage of intrathymic Treg development, as substantial fractions of both mTECs and t-DCs express the activating IL-1 receptor, IL-1R1, a finding in line with that of previously published studies.6,15,47 A direct sensing of IL-1 by CD4SP thymocytes is highly unlikely as they largely lack IL-1R1 expression. Indeed, addition of IL-1β to in vitro cultures of Treg precursors did not affect the generation of CD25+Foxp3hCD2+ tTregs, and similarly, culturing peripheral Treg subsets in the presence of IL-1β resulted neither in reduced survival nor impaired stability of Foxp3 expression, suggesting that neither CD4SP thymocytes nor peripheral Tregs can directly sense IL-1β. Accordingly, conditional knockout mice with Il1r1 deleted only in T cells showed normal thymic T-cell development and peripheral Treg homeostasis,48 and purified CD4+ T cells from the Il1r1−/− mice did not show any intrinsic defects upon in vitro stimulation.49 In contrast, activation of DCs with IL-1 through IL-1R1 resulted in the production of TNF-α, IFN-γ,IL-1, IL-4, IL-6, and IL-12p70,49–51 inflammatory cytokines known to inhibit the generation of Foxp3+ Tregs.52,53 Thus, we hypothesized that thymic APCs are involved in the IL-1β-mediated block of intrathymic Treg development by sensing increased levels of IL-1β and subsequently producing inflammatory cytokines that negatively affect the generation of tTregs, particularly in the absence of an IL-1 quenching system. Importantly, IL-1α and IL-1β were detected in the thymus even under steady-state conditions, and cytokine levels were rapidly increased upon subcutaneous injection of CFA, mimicking a peripheral bacterial infection. These findings are in line with data from a murine influenza virus infection model, in which elevated IL-1β levels were observed in the thymus compared to levels in the uninfected controls.54 Although mTECs and t-DCs were reported to express Il1a and Il1b mRNA already under steady-state conditions,6 it is unclear whether the elevated intrathymic IL-1β levels in response to peripheral infections result from increased local production by thymic APCs or whether they reach the thymus through the blood circulation.
Such increased levels of IL-1 can have a negative impact on intrathymic Treg development, as evidenced by the results from the FTOCs used in the present study. The results suggest IL-1β-mediated blocking of the transition from the CD25+Foxp3− Treg precursor stage to the CD25+Foxp3+ tTreg stage. However, the CFA-triggered elevation in intrathymic IL-1β levels did not affect the de novo tTreg generation, in contrast to their effects in the FTOCs. Since FTOCs lack recirculating Tregs, which exclusively express IL-1R2, our data suggest that a robust intrathymic “IL-1 quenching system” is able to mitigate the potentially harmful effects of excess IL-1β on tTreg development. Indeed, when we mimicked Treg recirculation by adding Tregs to the RTOCs, only IL-1R2+ Tregs, but not IL-1R2− Tregs, efficiently prevented the negative impact of excess IL-1β on the transition from CD25+Foxp3− Treg precursors to the CD25+Foxp3+ tTregs.
In summary, the results of the present study show that recirculating Tregs not only can inhibit intrathymic Treg development under steady-state conditions by competing for the limited amount of IL-2 but also can promote the generation of tTregs under inflammatory conditions. We identified a novel mechanism that utilizes the IL-1R2 decoy receptor expressed on recirculating IL-1R2+Foxp3+ Tregs to fine-tune IL-1 signaling in the thymus, thereby protecting intrathymic Treg development. This novel mechanism might be exploited in the future for the development of novel therapeutic strategies for the treatment of immune-mediated diseases.
Materials and methods
Mice
CByJ.SJL(B6)-Ptprca/CyJ mice (wild-type mice on a BALB/c background), B6-Foxp3tm1FlvxB6.129S6-Il10tm1Flv (Foxp3RFPxIL-10GFP mice on a C57BL/6 background), C.Foxp3tm1(CD2/CD52)Shori (Foxp3hCD2 reporter mice on a BALB/c background), C.Foxp3tm1(CD2/CD52)Shori. Thy1a mice (CD90.1 congenic Foxp3hCD2 reporter mice on a BALB/c background), B6.Foxp3tm1(CD2/CD52)Shori (Foxp3hCD2 reporter mice on a C57BL/6 background), B6.SJL-PtprcaPepcb/BoyJ.Foxp3tm1(CD2/CD52)Shori (CD45.1 congenic Foxp3hCD2 reporter mice on a C57BL/6 background) and B6.SJL-PtprcaPepcb/BoyJ.Foxp3tm1(CD2/CD52)Shori-Rag1tm1(GFP)Imku mice (CD45.1 congenic Foxp3hCD2xRAG1GFP reporter mice on a C57BL/6 background)23,55 were bred and maintained at the animal facility of the Helmholtz Center for Infection Research (Braunschweig, Germany). B6N.129(Cg)-Foxp3tm3Ayr mice (Foxp3.IRES-DTR/GFP on a C57BL/6 background)56 were bred at the animal facility of Regensburg University Clinics. C57BL/6 mice were maintained according to the guidelines of the Translational Animal Research Center (TARC, University of Mainz, Mainz, Germany). All mice were housed under specific-pathogen-free conditions in isolated, ventilated cages, and handled in accordance with good animal practice as defined by FELASA (Federation of European Laboratory Animal Science Associations) and the national animal welfare body GV-SOLAS (Society for Laboratory Animal Science). All animal experimentation was approved by the local administration and performed in accordance with federal and state policies. All mice were used at 4–12 weeks of age, and for all in vivo experiments, gender- and age-matched animals were used.
Preparation of single-cell suspensions from different organs and tissues
For the preparation of single-cell suspensions from lymphoid organs, mice were sacrificed by CO2 asphyxiation and the spleens, lymph nodes and thymi were isolated, mechanically disrupted using a plunger, and filtered through a nylon mesh with a pore size of 100 µm. Cell suspensions were washed, and splenocytes were subjected to erythrolysis buffer (0.01 M potassium bicarbonate, 0.155 M ammonium chloride, and 0.1 mM EDTA at pH 7.5). After an additional washing step, all cell suspensions were filtered through nylon meshes with a pore size of 70 µm. For the preparation of single-cell suspensions from nonlymphoid tissues, the mice were sacrificed and perfused with PBS to remove blood contaminating the tissues. In brief, the inferior vena cava was opened and the left ventricle of the heart was injected with a syringe and flushed with PBS. Subsequently, the gonadal fat pads were excised and moved into 10 ml of fat digestion buffer in a 50-ml tube (base medium Dulbecco's modified Eagle medium (DMEM); Gibco) with 1 mg/ml collagenase type II (Sigma-Aldrich,), 20 µg/ml DNase I (Roche), and 20 mg/ml bovine serum albumin (Sigma-Aldrich) and then digested for 40 min on a rotating shaker. After incubation, EDTA-PBS to a final concentration of 2 mmol/l was added and the sample was incubated for an additional 2 min and centrifuged. For the preparation of single-cell suspensions from the skin, the animals were shaved and the shaved skin was treated with hair removal creme. Hair-free skin was separated from the dorsal surface tissue and placed in 10 ml of skin digestion buffer (base medium DMEM, 4 mg/ml collagenase type IV, 10 µg/ml DNase I, and 2% fetal bovine serum). The tissue was cut into small pieces in a GentleMACS C tube (Miltenyi Biotec) and incubated under the “37_C_Multi H” program for 90 min. For the preparation of the single-cell suspensions from colon, mouse colons were excised and the feces removed. The colons were cut longitudinally and washed in PBS. The samples were preincubated according to the manual of the lamina propria dissociation kit (Miltenyi Biotec). Then, lamina propria lymphocytes were liberated using the digestion solution with the “37_C_m_LPDK_1” program of the GentleMACS dissociator.
Antibodies and flow cytometry
Flow cytometric analysis was performed as described.57 In brief, single-cell suspensions were labeled directly with the following fluorochrome-conjugated anti-mouse antibodies purchased from either BioLegend, BD Biosciences, or eBioscience: CD3 (500A2), CD4 (RM4-5), CD8α (53-6.7), CD11c (N418), CD19 (6D5), CD25 (PC61.5), CD44 (IM7), CD45 (30-F11), CD45.1 (A20), CD45.2 (104), CD49b (DX5), CD73 (TY/11.8), CD90.2 (53-2.1), CD121a (35F5), CD121b (4E2), CD185 (L138D7), CD196 (129816), CD197 (4B12), CD279 (29F.1A12), CD304 (3E12), CD326 (G8.8), F4/80 (BM8), MHCII (M5/114.15.2), Klrg1 (2F1), ST-2 (DIH9), TCRbeta (H57-597), anti-human CD2 (RPA-2.10) and streptavidin. For intracellular Foxp3 (FJK-16S) and Helios (22F6) staining, a Foxp3/transcription factor staining set (eBioscience) was used. Dead cells were excluded by using 4′,6-diamidino-2′-phenylindole dihydrochloride (Sigma-Aldrich), the LIVE/DEAD™ fixable blue dead cell stain kit, fixable viability dye eFluor™ 506, or fixable viability dye eFluor™ 780 (Invitrogen). The labeled cells were acquired with BD LSRFortessa™ and BD LSRII flow cytometers (BD Biosciences), and the data were analyzed with FlowJo software (Tree Star).
Ex vivo isolation of thymic APCs
To isolate mTECs and t-DCs, thymi were collected from different mouse strains (all on a BALB/c background), and the thymic lobes were separated, finely chopped into tiny pieces, and digested in complete RPMI 1640 medium (Sigma-Aldrich) containing 2 mg/ml collagenase/dispase (Roche) and 0.2 mg/ml DNase I (Roche) and incubated at 37 °C for 80 min. The digested cells were filtered through a 100-µM nylon mesh and subjected to a Percoll gradient using 1.115 g/ml high-density Percoll and 1.06 g/ml of low-density Percoll. The gradient was centrifuged at 1350 × g for 30 min at 4 °C. The low-density interface was collected for APCs purification. The cells were sorted into CD45−EpCAM+ mTECs and CD45+CD11chi Lin− (Lin is defined as CD90, CD49b, F4/80 and CD19) t-DCs on a FACS Aria II (BD Biosciences) or MoFlo XDP (Beckman Coulter) cell sorter.
Isolation and in vitro culture of Treg precursors and tTregs
Single-cell suspensions from the thymi of the CD45.1 congenic Foxp3hCD2 reporter mice (C57BL/6 background) were labeled with anti-CD8α-APC, and CD8+ thymocytes were depleted using anti-APC MicroBeads and the autoMACS® Pro (Miltenyi Biotec). The negative fraction was labeled with respective antibodies and FACS sorted as CD4+CD8−CD25+Foxp3hCD2−, CD4+CD8−CD25−Foxp3hCD2+ or CD4+CD8−CD25+Foxp3hCD2+ cells using a FACSAria™ II (BD Biosciences) or MoFlo XDP (Beckman Coulter) cell sorter. The sorted Treg precursors (CD25+Foxp3hCD2− or CD25−Foxp3hCD2+) and CD25+Foxp3hCD2+ tTregs were cultured at 37 °C and 5% CO2 in the presence of 50 ng/ml IL-2 (R&D Systems) with or without increasing concentrations of IL-1β (50 ng/ml, 200 ng/ml or 500 ng/ml). For this purpose, as many as 5 × 104 cells/well were placed in 200 μl of RPMI 1640 medium (Gibco) supplemented with 10% FCS, 50 U/ml penicillin, 50 U/ml streptomycin, 25 mM HEPES, 1 mM sodium pyruvate (all from Biochrom AG), and 50 μM β-mercaptoethanol (Gibco) into a V-bottom 96-well plate (Sarstedt). After 5 days, the cultures were analyzed for Treg generation by flow cytometry.
Isolation and in vitro culture of IL-1R2+ and IL-1R2− Tregs
IL-1R2+ and IL-1R2− CD4+Foxp3+ Tregs were isolated from pooled single-cell suspensions of the spleens and lymph nodes taken from CD45.1 congenic Foxp3hCD2 reporter mice (C57BL/6 background). CD4+ lymphocytes were enriched using direct anti-CD4 (L3T4) microbeads followed by magnetic separation using an autoMACS® Pro Separator (Miltenyi Biotec). Subsequently, the CD4+ T cells were labeled with respective antibodies and sorted into CD4+CD25+Foxp3hCD2+IL-1R2+ and CD4+CD25+Foxp3hCD2+IL-1R2− cells using a FACSAria™ II (BD Biosciences) or MoFlo XDP (Beckman Coulter) cell sorter. The FACS-sorted Treg subsets were either added to the RTOCs (see below) or cultured in vitro. For the in vitro culture, 7 × 103–1 × 104 FACS-sorted IL-1R2+ or IL-1R2− CD4+CD25+Foxp3hCD2+ Tregs were placed into a round-bottom 96-well plate (Sarstedt) and cultured for 12 h or 5 days as described above with or without IL-1β (200 ng/ml). Anti-CD3/anti-CD28 beads (Invitrogen) were used for polyclonal stimulation at a bead-to-cell ratio of 1:1. After 12 h of culture, the cells were stained with CellEvent™ caspase-3/7 red detection reagent (Thermo Fisher Scientific) and 4′6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich), and subsequently analyzed by flow cytometry to discriminate between apoptotic and necrotic cells. After 5 days in culture, the cells were analyzed for stability of the Foxp3 expression by flow cytometry.
Fetal thymic organ cultures (FTOCs)
Thymic lobes obtained from the 18.5-embryonic-day -old (E18.5) fetuses of Foxp3hCD2 reporter mice (C57BL/6 background) were placed on a polyether sulfonate Transwell membrane with a pore size of 0.4 μm in a 24-well plate (Corning Inc.). Each well of the plate contained 300 μl of serum-free AIM V medium (Invitrogen) with or without IL-1β (BioLegend) at different concentrations (50 ng/ml, 200 ng/ml, and 500 ng/ml). Thymic lobes were cultured for 5 days at 37 °C and 5% CO2. On day 5 of culture, each thymic lobe was collected, mechanically disrupted, and pressed through a nylon filter with a pore size of 70 μm to obtain single-cell suspensions, which were analyzed by flow cytometry. For IL-1R1 staining on mTECs and t-DCs, the FTOCs were digested in complete RPMI 1640 medium (Gibco) containing 0.75 mg/ml collagenase/dispase (Roche) at 37 °C for 35 min. The digestion was stopped by addition of RPMI 1640 medium containing 10% FCS (Biochrom), and the cell suspensions were filtered through a nylon mesh with a pore size of 100 μm before analysis by flow cytometry.
Reaggregated thymic organ cultures (RTOCs)
Thymic lobes obtained from fetuses that were E14.5 to E16.5 old when taken from Foxp3hCD2 reporter mice (C57BL/6 background) were digested in complete RPMI 1640 medium (Gibco) containing 0.75 mg/ml collagenase/dispase (Roche) at 37 °C for 35 min. Digestion was stopped by the addition of RPMI 1640 medium containing 10% FCS (Biochrom), and the cell suspension was filtered through a nylon mesh with a pore size of 100 μm. A total of 0.65 × 106 cells from the obtained single-cell suspension was mixed with 7500 FACS-sorted IL-1R2+ or IL-1R2− CD4+Foxp3hCD2+ Tregs, which had been isolated from pooled single-cell suspensions of the spleens and lymph nodes taken from CD45.1 congenic Foxp3hCD2 reporter mice (C57BL/6 background), placed in a 1.5-ml Eppendorf reaction tube and centrifuged. After complete removal of the supernatant, the cell pellet was dispersed into a slurry and finally deposited as a freestanding drop on a Whatman® Nuclepore™ Track-Etched Membrane (0.8-mm pore size, 13-mm diameter) placed on a sterilized foam sponge (5 mm thick) in a 6-well plate (Sarstedt) containing 4 ml of DMEM (Gibco) in each well. The medium was supplemented with 50 µg/ml penicillin, 50 µg/ml streptomycin, 50 µM β-mercaptoethanol, 1 mM nonessential amino acids (all from Gibco), 10% FCS and 10 mM HEPES (both from Biochrom) with or without 200 ng/ml IL-1β (BioLegend). The cultures were incubated at 37 °C and in 5% CO2. For each experiment, 20–30 RTOCs were generated. After 8 days in culture, the RTOCs were collected and the single-cell suspensions consisting of 2–3 pooled RTOCs were prepared by mechanical disruption with a plunger and filtered through a nylon mesh with a pore size of 100 μm. Single-cell suspensions were analyzed by flow cytometry. Only the RTOC samples with >100 events in the final Foxp3+ gate were included in the analysis.
IL-1α and IL-1β level measurements in the thymus (steady-state)
Thymi isolated from the CD45.1 congenic Foxp3hCD2xRAG1GFP reporter mice (C57BL/6 background) were homogenized and lysed using a Bio-Plex cell lysis kit (Bio-Rad Laboratories), and the protein content of the thymic lysates was measured using a Pierce™ BCA protein assay kit (Thermo Scientific). Then, equal concentrations (500 µg/ml) of the lysates were processed using a Bio-Plex Pro Mouse Cytokine 23-Plex assay kit (Bio-Rad Laboratories) according to manufacturer’s instructions to measure the concentrations of IL-1α and IL-1β.
In vivo CFA treatment
Wild-type mice (C57BL/6 background) aged 7–11-weeks-old were immunized subcutaneously at the tail base with 50 µl of complete Freund’s adjuvant (CFA, Difco) supplemented with 1.1 mg of heat-inactivated Mycobacterium tuberculosis (Difco) emulsified in 50 µl of Dulbecco’s phosphate-buffered saline (PBS, Sigma) or with 100 µl of PBS alone. The mice were sacrificed on day 3 postimmunization.
IL-1β level measurement in the thymus (CFA treatment)
Thymic tissue samples from the PBS- or CFA-treated mice were lysed in RIPA buffer (Roche) and mechanically dissociated. Thymic lysates were diluted 1:10 before measurements were taken of IL-1β levels using an IL-1β mouse ELISA kit (Invitrogen).
Statistical analyses
Statistical analyses were performed using GraphPad Prism software v7.0 (GraphPad). For comparison of unmatched groups, two-tailed Mann–Whitney U-tests were used, and for multiple comparisons, Bonferroni’s multiple comparison tests or Kruskal–Wallis with a Dunn’s test was used. All data are presented as the mean ± standard deviation (SD). A p-value below 0.05 was considered significant; *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001; ns = not significant.
Supplementary information
Acknowledgements
We thank Marina Wuttke and Lothar Groebe for their technical and cell sorting support. This work was supported by the CRC 738 (to J.H.), CRC/TR 128 (to A.W.) and CRC/TRR 221 (to M.F.) of the German Research Foundation and by grants from the European Research Council (ERC-CoG, #648145 REGiREG) to M.F. Y.E. was supported by Ph.D scholarship program no. 57129429 of the German Academic Exchange Service (DAAD).
Author contributions
E.N., Y.E., S.H., M.D., C.S. and I.A.M. performed the experiments and interpreted the data. A.W., C.F. and M.F. interpreted the data. E.N. and J.H. designed the research, interpreted the data, and wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Eirini Nikolouli, Yassin Elfaki
Supplementary information
The online version of this article (10.1038/s41423-019-0352-8) contains supplementary material.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein L, Robey EA, Hsieh CS. Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat. Rev. Immunol. 2019;19:7–18. doi: 10.1038/s41577-018-0083-6. [DOI] [PubMed] [Google Scholar]
- 3.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lio CW, Hsieh CS. Becoming self-aware: the thymic education of regulatory T cells. Curr. Opin. Immunol. 2011;23:213–219. doi: 10.1016/j.coi.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat. Rev. Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg G, et al. Unique properties of thymic antigen-presenting cells promote epigenetic imprinting of alloantigen-specific regulatory T cells. Oncotarget. 2017;8:35542–35557. doi: 10.18632/oncotarget.16221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 9.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur. J. Immunol. 2007;37:1817–1826. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 10.Chinen T, et al. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016;17:1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 12.Konkel JE, Jin W, Abbatiello B, Grainger JR, Chen W. Thymocyte apoptosis drives the intrathymic generation of regulatory T cells. Proc. Natl Acad. Sci. USA. 2014;111:E465–E473. doi: 10.1073/pnas.1320319111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiault N, et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 2015;16:628–634. doi: 10.1038/ni.3150. [DOI] [PubMed] [Google Scholar]
- 14.Cowan JE, McCarthy NI, Anderson G. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3+ T cells. Cell Rep. 2016;14:1041–1048. doi: 10.1016/j.celrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toker A, et al. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J. Immunol. 2013;190:3180–3188. doi: 10.4049/jimmunol.1203473. [DOI] [PubMed] [Google Scholar]
- 16.McMahan CJ, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colotta F, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 18.Garlanda C, Riva F, Bonavita E, Mantovani A. Negative regulatory receptors of the IL-1 family. Semin. Immunol. 2013;25:408–415. doi: 10.1016/j.smim.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Peters VA, Joesting JJ, Freund GG. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 2013;32:1–8. doi: 10.1016/j.bbi.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollewe C, Neumann D, Martin MU. The first two N-terminal immunoglobulin-like domains of soluble human IL-1 receptor type II are sufficient to bind and neutralize IL-1beta. FEBS Lett. 2000;487:189–193. doi: 10.1016/s0014-5793(00)02345-0. [DOI] [PubMed] [Google Scholar]
- 21.Neumann D, Kollewe C, Martin MU, Boraschi D. The membrane form of the type II IL-1 receptor accounts for inhibitory function. J. Immunol. 2000;165:3350–3357. doi: 10.4049/jimmunol.165.6.3350. [DOI] [PubMed] [Google Scholar]
- 22.Boraschi D, Italiani P, Weil S, Martin MU. The family of the interleukin-1 receptors. Immunol. Rev. 2018;281:197–232. doi: 10.1111/imr.12606. [DOI] [PubMed] [Google Scholar]
- 23.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. Cutting edge: absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J. Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- 24.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav M, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss JM, et al. Neuropilin-1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ Treg cells. J. Exp. Med. 2012;209:1723–1742. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritvo PG, et al. Tfr cells lack IL-2Ralpha but express decoy IL-1R2 and IL-1Ra and suppress the IL-1-dependent activation of Tfh cells. Sci. Immunol. 2017;2:eaan0368. doi: 10.1126/sciimmunol.aan0368. [DOI] [PubMed] [Google Scholar]
- 28.Richards DM, et al. Treg cell differentiation: from thymus to peripheral tissue. Prog. Mol. Biol. Transl. Sci. 2015;136:175–205. doi: 10.1016/bs.pmbts.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Rudra D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. 2018;9:883. doi: 10.3389/fimmu.2018.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delacher, M. et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat. Immunol. 18, 1160–1172 (2017). [DOI] [PMC free article] [PubMed]
- 31.Delacher M, et al. Rbpj expression in regulatory T cells is critical for restraining TH2 responses. Nat. Commun. 2019;10:1621. doi: 10.1038/s41467-019-09276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai X, et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38:1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen DL, et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol. 2019;20:195–205. doi: 10.1038/s41590-018-0289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shenderov K, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J. Immunol. 2013;190:5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su SB, et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 2005;175:6303–6310. doi: 10.4049/jimmunol.175.10.6303. [DOI] [PubMed] [Google Scholar]
- 36.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 37.Vang KB, et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran DQ, et al. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercer F, Kozhaya L, Unutmaz D. Expression and function of TNF and IL-1 receptors on human regulatory T cells. PLoS ONE. 2010;5:e8639. doi: 10.1371/journal.pone.0008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan TG, Mathis D, Benoist C. Singular role for T-BET+CXCR3+ regulatory T cells in protection from autoimmune diabetes. Proc. Natl Acad. Sci. USA. 2016;113:14103–14108. doi: 10.1073/pnas.1616710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Simone M, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plitas G, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molgora M, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo D, et al. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology. 2018;154:132–143. doi: 10.1111/imm.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters SN, Webster KE, Daley S, Grey ST. A role for intrathymic B cells in the generation of natural regulatory T cells. J. Immunol. 2014;193:170–176. doi: 10.4049/jimmunol.1302519. [DOI] [PubMed] [Google Scholar]
- 46.Yamano T, et al. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity. 2015;42:1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Ki S, et al. Global transcriptional profiling reveals distinct functions of thymic stromal subsets and age-related changes during thymic involution. Cell Rep. 2014;9:402–415. doi: 10.1016/j.celrep.2014.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mufazalov IA, et al. Generation of a novel T cell specific interleukin-1 receptor type 1 conditional knock out mouse reveals intrinsic defects in survival, expansion and cytokine production of CD4 T cells. PLoS ONE. 2016;11:e0161505. doi: 10.1371/journal.pone.0161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eriksson U, et al. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J. Exp. Med. 2003;197:323–331. doi: 10.1084/jem.20021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luft T, et al. IL-1 beta enhances CD40 ligand-mediated cytokine secretion by human dendritic cells (DC): a mechanism for T cell-independent DC activation. J. Immunol. 2002;168:713–722. doi: 10.4049/jimmunol.168.2.713. [DOI] [PubMed] [Google Scholar]
- 51.Wesa A, Galy A. Increased production of pro-inflammatory cytokines and enhanced T cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol. 2002;3:14. doi: 10.1186/1471-2172-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korn T, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei J, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B, et al. Severe influenza A(H1N1)pdm09 infection induces thymic atrophy through activating innate CD8+CD44hi T cells by upregulating IFN-gamma. Cell Death Dis. 2014;5:e1440. doi: 10.1038/cddis.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyao T, et al. Plasticity of Foxp3+ T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 57.Cossarizza A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur. J. Immunol. 2019;49:1457–1973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.