Summary
Background and purpose:
Daily intake of aspirin was shown to decrease human cerebral aneurysm rupture by 60%. The feasibility of imaging macrophages in human cerebral aneurysm walls using ferumoxytol-enhanced MRI has been demonstrated. The goal of the present study is to image aspirin effect on macrophages in the wall of human cerebral aneurysm using ferumoxytol-enhanced MRI.
Material and methods:
Five patients with known intracranial aneurysms underwent baseline imaging using T2* gradient-echo and T1 MRI sequences using ferumoxytol-enhanced MRI 72-hour post-ferumoxytol infusion. Patients then received 81 mg aspirin per os daily. After 3 months, imaging studies were repeated and analyzed by co-registration using a histogram and subtraction of follow-up images from baseline.
Results:
In all five patients, after 3 months of treatment with aspirin, the signal intensity corresponding to the uptake of ferumoxytol by macrophages in the aneurysm wall was less intense than in the baseline images. This was confirmed by co-registration of images using histogram and subtraction of follow-up images from baseline.
Conclusion:
These preliminary results suggest the feasibility of imaging aspirin effect on macrophages localized in the wall of human cerebral aneurysm using ferumoxytol-enhanced MRI. The findings provide radiographic evidence of decreased inflammation in human cerebral aneurysms with daily intake of aspirin using macrophages as a surrogate marker for inflammation.
Keywords: Aneurysm, Aspirin, Ferumoxytol, Inflammation, Macrophages, MRI
Introduction
Several experimental and human studies have demonstrated prominent inflammatory features in cerebral aneurysm walls involving macrophages and several pro-inflammatory mediators [1–11]. Depletion of macrophages in mice was shown to reduce the incidence of cerebral aneurysm formation [9]. Macrophages were also observed in the wall of experimentally induced aneurysms in rats and were found to secrete pro-inflammatory molecules including cytokines and proteolytic enzymes that degrade the extracellular matrix. The resultant inflammatory response induces fibrosis and apoptosis, ultimately leading to aneurysm formation and rupture [2,6–8,11,12].
A secondary analysis of the International Study of Unruptured Intracranial Aneurysms recently showed that daily intake of aspirin reduces the incidence of cerebral aneurysm rupture by as much as 60% [13]. Concurrently, the ability to image macrophages within human cerebral aneurysm wall using ferumoxytol-enhanced MRI has been demonstrated [14].
In the present study, we use this novel imaging technique, ferumoxytol-enhanced MRI, to image the effect of aspirin on inflammation in the wall of human cerebral aneurysms, using macrophages as a surrogate marker.
Methods
Study population
The study protocol was approved by the University Institutional Board Review. Five subjects with known unruptured, untreated intracranial aneurysms, presenting to the cerebrovascular neurosurgery department at our institution were prospectively enrolled in the study between November 2011 and April 2012. Adult patients (age ≥ 18 years) were considered eligible for the study. Exclusion criteria were as follows: children, pregnancy, history of allergy/hypersensitivity to iron, dextran, or iron-polysaccharide preparations, and history of renal insufficiency, hepatic insufficiency, or iron overload. Patients with a recent history of aspirin, non-steroidal anti-inflammatory drugs, and statin use were also excluded. All five patients received 81 mg of aspirin per os daily for 3 months.
Contrast agent
Ferumoxytol (5 mg/kg) was administered intravenously to all patients enrolled in the study immediately before images acquisition. The same imaging protocol was used in follow-up studies. The off-label use of the drug in this research protocol was approved by the Institutional Review Board at the University.
MR imaging protocol
All MR imaging was completed on a Siemens 3T TIM Trio system. Patients completed a baseline MRI consisting of T2* GE, and T1 sequences. The T2*-weighted sequence was collected using a 2D gradient-echo sequence with the following parameters: TE = 20 ms, TR = 500 ms, flip angle = 20, FOV = 220 × 220, matrix = 512 × 384, slice thickness/gap = 3.0/0.3 mm, bandwidth = 260 Hz/pixel. Imaging was performed at three time-points: preinfusion, immediately post-infusion, and 72 hours post-infusion. The T1-weighted spin-echo images were collected using these parameters: TE = 2.6 ms, TR = 317 ms, flip angle = 70°, FOV = 220 × 220, matrix = 384 × 307, bandwidth = 330 Hz/pixel, averages = 2, slice thickness/gap = 3.0/0.3 mm.
T1 sequence was used mainly to validate the findings of T2* sequence (the main sequence utilized for monitoring inflammation in the wall of human cerebral aneurysms).
MR imaging analysis
Comparison of 72-hour post-infusion images was completed (Fig. 1). Post-infusion images were co-registered to the baseline images using a rigid transformation and a mutual information similarity metric. Histogram matching was then performed between the two datasets before the baseline image was subtracted from the 3-month follow-up image (i.e. Difference = Follow-up — baseline). The difference image allowed demonstration of a relative signal loss from baseline to 3-month follow-up. Two neuroradiologists independently reviewed images from all patients in a blinded fashion.
Figure 1.
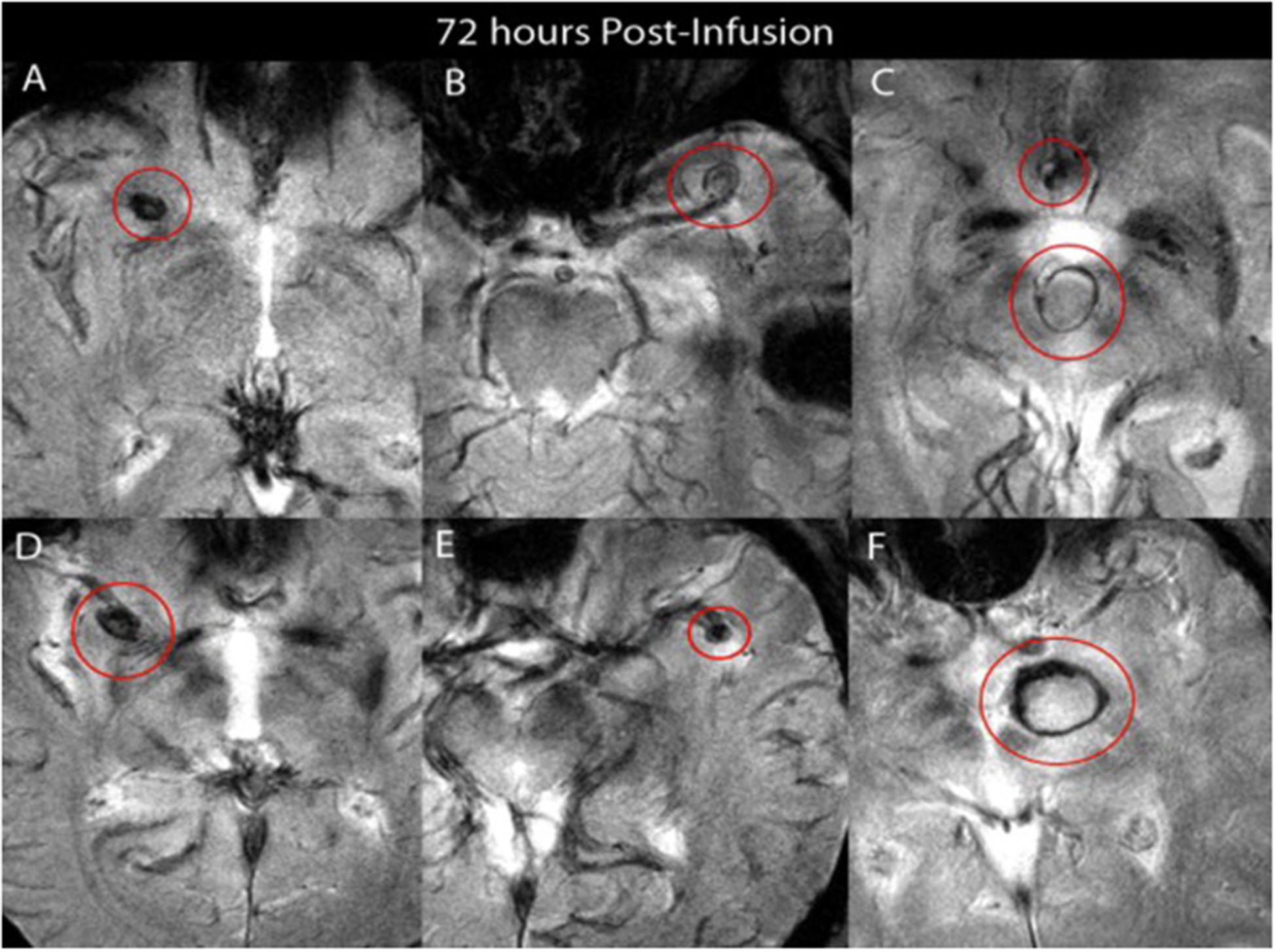
Images from five patients who received 5 mg/kg of ferumoxytol — all images are taken 72 hours post-infusion using T2* GE post-ferumoxytol infusion. A. Right middle cerebral artery aneurysm. B. Left middle cerebral artery aneurysm. C. Basilar tip and anterior communicating artery aneurysms. D. Right middle cerebral artery aneurysm. E. Left middle cerebral artery aneurysm. F. Basilar tip aneurysm.
Results
Five patients met our eligibility criteria and were enrolled in this study. They were all females, aged 69, 70, 72, 45, and 52 years old. Their aneurysms were located respectively in the anterior communicating artery, left middle cerebral artery, basilar summit, right ophthalmic artery, and left middle cerebral artery. The sizes of the aneurysms were: 5 × 5 mm, 4 × 5 mm, 4 × 6 mm, 15 × 8 mm, and 7 × 5 respectively.
No complications were observed with daily use of aspirin. The signal intensity in the 3-month follow-up images, which corresponds to uptake of ferumoxytol by macrophages in the aneurysm wall, showed less intensity when compared to the baseline images, both at T2* GE and T1 MRI sequences (Fig. 2). This finding was confirmed by subtraction images, as described above (Fig. 2).
Figure 2.
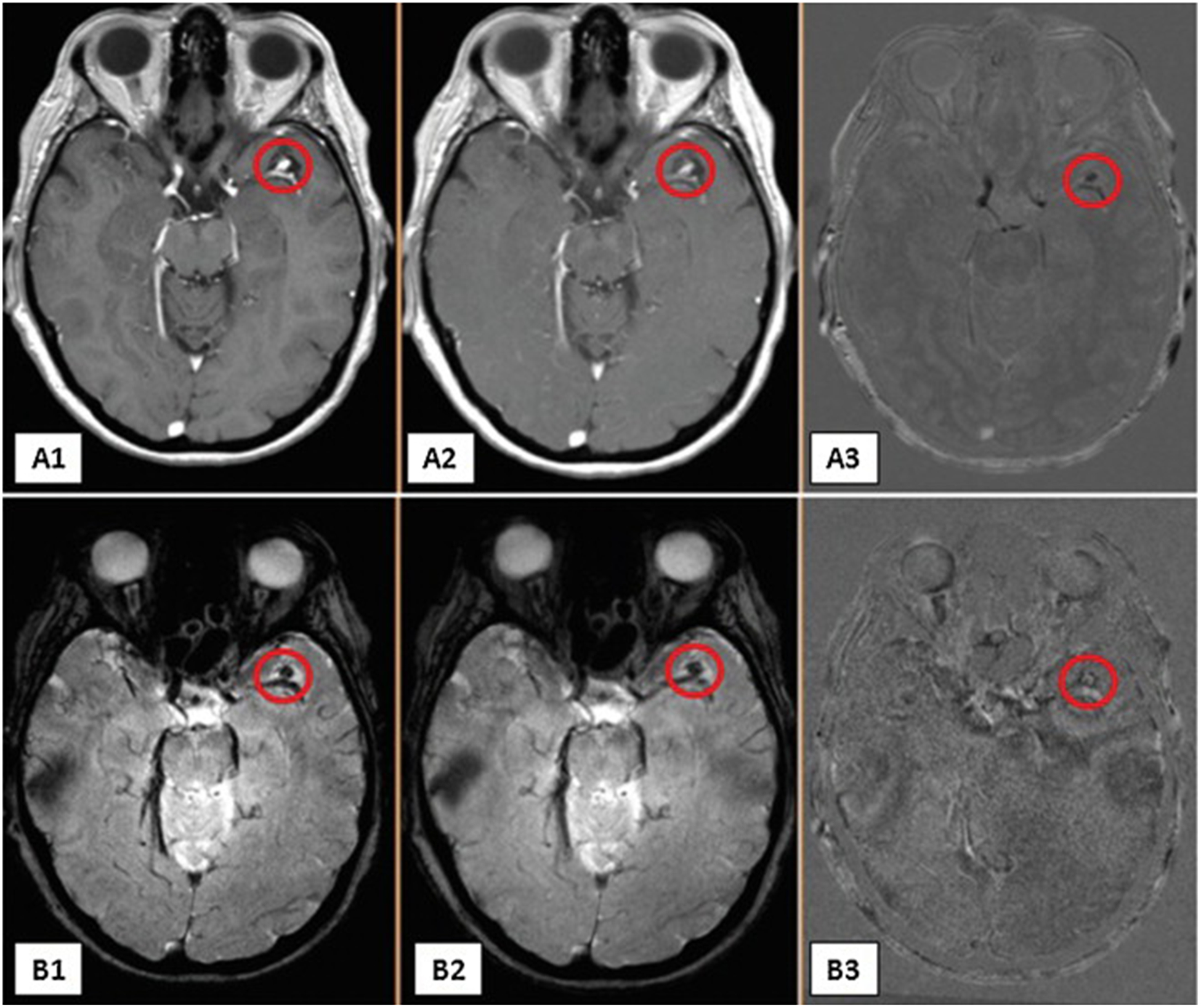
Images A1–A3 are T1 post-ferumoxytol infusion. A1 is 72 hours post-infusion of ferumoxytol at baseline of left middle cerebral artery aneurysm; A2 is 72 hours post-infusion of ferumoxytol after 3 months of aspirin; A3 is a subtraction image of the first two. Images B1–B3 are T2* GE post-ferumoxytol infusion. B1 is 72 hours post-infusion of ferumoxytol at baseline; B2 is 72 hours post-infusion of ferumoxytol after 3 months of aspirin; B3 is a subtraction image of the first two.
Discussion
Ferumoxytol-enhanced MRI allows detection of phagocytic activity of inflammatory cells, such as macrophages. Recently, the uptake of ferumoxytol by macrophages in the wall of human aneurysms and arteriovenous malformations was demonstrated histopathologically [14]. Based on these findings, we have imaged the signal intensity generated by USPIO using T2* GE MRI sequence [14]. A novel non-invasive method was therefore developed to assess the inflammatory activity in cerebral aneurysm walls, using macrophages as a surrogate marker.
Inflammation is increasingly thought to be a key component in the pathophysiology of intracranial aneurysms [1–11]. Several inflammatory cells and mediators appear to be involved including leukocytes, complement, immunoglobulins, cytokines, and other humoral mediators [12]. Macrophages constitute an integral part of the inflammatory response that leads to aneurysm formation and rupture [1,4,9,15]. Their presence in cerebral aneurysm walls has been consistently demonstrated, and their role in flow-induced vascular remodeling through the release of matrix metalloproteinases appears to be crucial in aneurysm pathogenesis [1,4,9,12,15].
In this study, we demonstrate, using ferumoxytol-enhanced MRI, that aspirin reduces inflammation in the wall of human cerebral aneurysms (Fig. 3). The signal intensity of T2* GE and T1 MRI sequences in the aneurysm wall represents the uptake of ferumoxytol nanoparticles by macrophages localized in the aneurysm wall. The decrease in signal intensity after 3 months of daily aspirin leads us to suggest that the inflammatory process in the aneurysm wall was attenuated by aspirin. These crucial observations bring further confirmation to previous epidemiological findings of lower risk of aneurysm rupture with daily intake of aspirin [13]. Along these lines, COX-2 and mPGES-1 were found to be upregulated in the wall of ruptured human cerebral aneurysms, and it was suggested that the protective effect of aspirin against rupture of cerebral aneurysms may be mediated in part by inhibition of COX-2/mPGES-1 [16]. This study provides the first evidence that aspirin intake may decrease inflammation in the wall of cerebral aneurysms.
Figure 3.
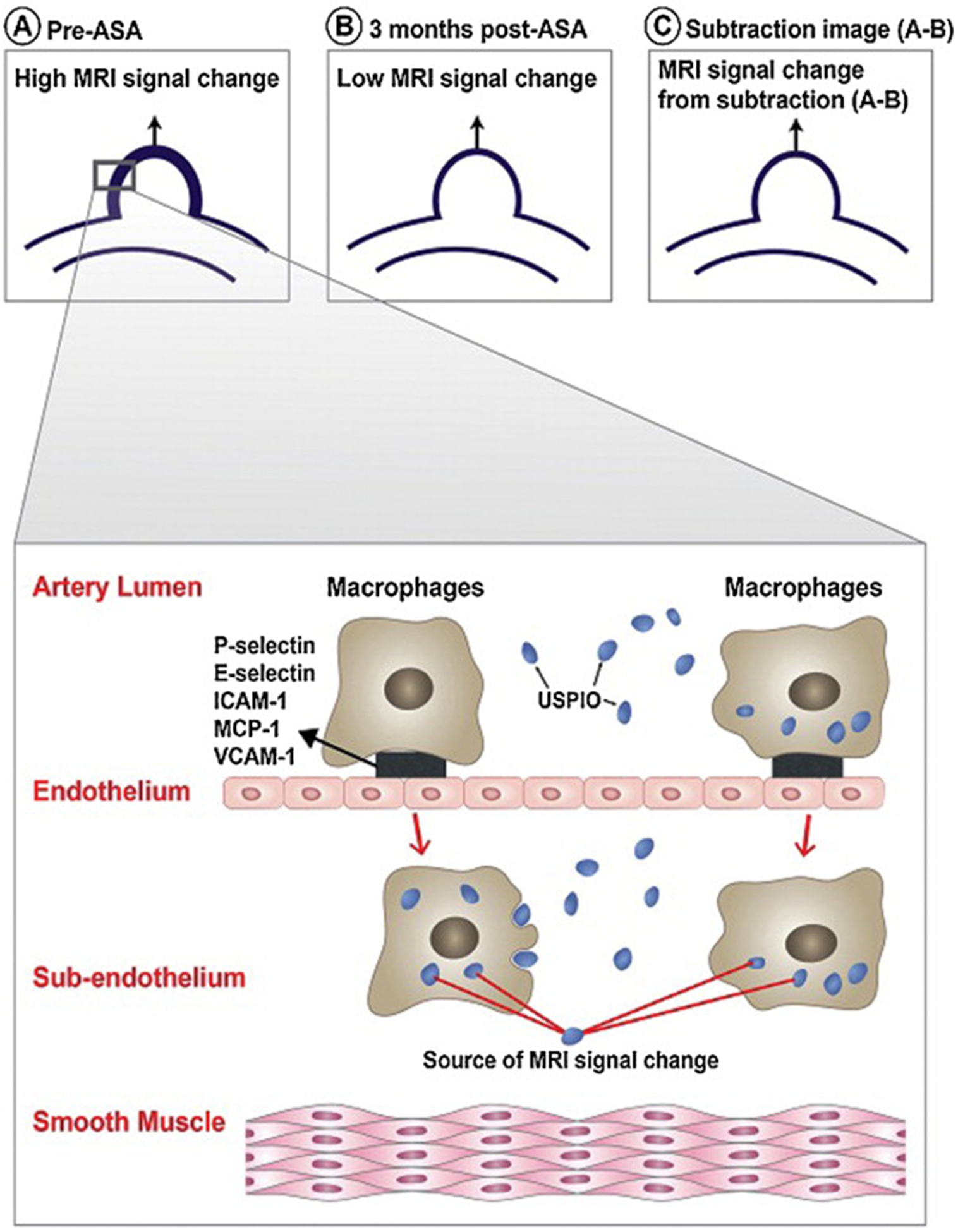
Schematic diagram illustrating (A) the high USPIO-MRI signal change in the wall of human cerebral aneurysm prior to aspirin intake, (B) decreased MRI signal change after 3 months of aspirin intake, (C) and the MRI signal change resulting from subtraction of low signal change in (B) from high signal change in (A). The insert illustrates the two mechanisms by which macrophages and USPIO result in MRI signal changes in the wall of human cerebral aneurysm.
Limitation
One limitation of this study is the small number of patients enrolled. Also at this point, quantification of USPIO uptake in the aneurysm wall is difficult. Our results are preliminary, and future studies are needed to confirm that aspirin therapy decreases macrophage count and activity in aneurysm wall.
Conclusion
These preliminary results suggest the feasibility of imaging aspirin effects on macrophages localized in human cerebral aneurysm wall using ferumoxytol-enhanced MRI. The study provides radiographic evidence of decreased inflammatory activity in human cerebral aneurysm with daily intake of aspirin.
Acknowledgments
The authors wish to acknowledge Drs. Wendy Smoker and Bruno Policeni for reading and interpreting the MRI images.
Funding: This study was supported by NIH grant No. R03NS07922 to Dr. Hasan.
Abbreviation:
- MRI
Magnetic resonance imaging
- USPIO
Ultra-supra-magnetic iron oxide nanoparticles
- COX-2
Cyclooxygenase-2
- mPGES-1
Microsomal prostaglandin E(2) Synthase-1
Footnotes
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
References
- [1].Aoki T, Kataoka H, Ishibashi R, et al. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 2009;40(3):942–51. [DOI] [PubMed] [Google Scholar]
- [2].Aoki T, Kataoka H, Shimamura M, et al. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation 2007;116(24):2830–40. [DOI] [PubMed] [Google Scholar]
- [3].Bruno G, Todor R, Lewis I, et al. Vascular extracellular matrix remodeling in cerebral aneurysms. J Neurosurg 1998;89(3):431–40. [DOI] [PubMed] [Google Scholar]
- [4].Cao Y, Zhao J, Wang S, et al. Monocyte chemoattractant protein-1 mRNA in human intracranial aneurysm walls. Zhonghua Yu Fang Yi Xue Za Zhi 2002;36(7):519–21. [PubMed] [Google Scholar]
- [5].Chyatte D, Bruno G, Desai S, et al. Inflammation and intracranial aneurysms. Neurosurgery 1999;45(5):1137–46 [discussion 1146–1137]. [DOI] [PubMed] [Google Scholar]
- [6].Frosen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke 2004;35(10):2287–93. [DOI] [PubMed] [Google Scholar]
- [7].Jayaraman T, Berenstein V, Li X, et al. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery 2005;57(3):558–64 [discussion 558–564]. [DOI] [PubMed] [Google Scholar]
- [8].Jin D, Sheng J, Yang X, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm. Surg Neurol 2007;68(Suppl. 2):S11–6 [discussion S16]. [DOI] [PubMed] [Google Scholar]
- [9].Kanematsu Y, Kanematsu M, Kurihara C, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke 2011;42(1):173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kilic T, Sohrabifar M, Kurtkaya O, et al. Expression of structural proteins and angiogenic factors in normal arterial and unruptured and ruptured aneurysm walls. Neurosurgery 2005;57(5):997–1007 [discussion 1997–1007]. [DOI] [PubMed] [Google Scholar]
- [11].Shi C, Awad IA, Jafari N, et al. Genomics of human intracranial aneurysm wall. Stroke 2009;40(4):1252–61. [DOI] [PubMed] [Google Scholar]
- [12].Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 2012;32(9):1659–76, 10.1038/jcbfm.2012.84. Epub 2012 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hasan DM, Mahaney KB, Brown RD Jr, et al. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke 2011;42(11):3156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hasan DM, Mahaney KB, Magnotta VA, et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol 2012;32(4):1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tulamo R, Frosen J, Hernesniemi J, et al. Inflammatory changes in the aneurysm wall: a review. J Neurointerv Surg 2010;2(2):120–30. [DOI] [PubMed] [Google Scholar]
- [16].Hasan D, Hashimoto T, Kung D, et al. Upregulation of cyclooxygenase-2 (COX-2) and Microsomal Prostaglandin E(2) Synthase-1 (mPGES-1) in wall of ruptured human cerebral aneurysms: preliminary results. Stroke 2012;43(7):1964–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


