Abstract
Atypical attention orienting has been found to be impaired in many neuropsychological disorders, but the underlying neural mechanism remains unclear. Attention can be oriented exogenously (i.e., driven by salient stimuli) or endogenously (i.e., driven by one’s goals or intentions). Genetic mouse models are useful tools to investigate the neurobiology of cognition, but a well-established assessment of attention orienting in mice is missing. This study aimed to adapt the Posner task, a widely used attention orienting task in humans, for use in mice using touchscreen technology and to test the effects of two attention-modulating drugs, methylphenidate (MPH) and atomoxetine (ATX), on the performance of mice during this task. In accordance with human performance, mice responded more quickly and more accurately to validly cued targets compared to invalidly cued targets, thus supporting mice as a valid animal model to study the neural mechanisms of attention orienting. This is the first evidence that mice can be trained to voluntarily maintain their nose-poke on a touchscreen and to complete attention orienting tasks using exogenous peripheral cues and endogenous symbolic cues. The results also showed no significant effects of MPH and ATX on attention orienting, although MPH improved overall response times in mice during the exogenous orienting task. In summary, the current study provides a critical translational task for assessing attention orienting in mice and to investigate the effects of attention-modulating drugs on attention orienting.
Subject terms: Pharmacology, Attention
Introduction
A fundamental role of attention is to direct an individual’s focus to relevant information in the environment. The ability to selectively attend to a location or modality is referred to as attention orienting [1]. Two types of attention orienting—exogenous and endogenous—have been proposed [1–3]. Exogenous orienting is a stimulus-driven process in which one’s attention is drawn automatically to salient external stimuli. Endogenous orienting represents a goal-directed process in which existing expectations and/or knowledge determine where one’s attention is given. Neuroimaging studies using spatial cueing tasks have shown that exogenous and endogenous orienting are modulated by two partially segregated brain networks [3]. Atypical attention orienting has been found in some individuals with autism spectrum disorder [4], social anxiety disorder [5], attention-deficit/hyperactivity disorder (ADHD [6]) and Parkinson’s disease [7]. Limited treatments exist for the orienting deficits in these conditions, largely due to inadequate understanding of the underlying neurobiology of atypical attention orienting.
In humans, exogenous and endogenous orienting are commonly measured by a computer-based visual spatial orienting task designed by Posner [8]. In this task, participants are instructed to respond to a left- or right-sided target after the presentation of a cue [1, 9]. The exogenous task frequently uses peripheral cues, such as a flash, that are not informative of the target location, whereas the endogenous task typically uses central informative cues, such as arrows, that indicate where the target will appear. The target appears in the cued location during valid trials and in the non-cued location during invalid trials. The outcome measures are reaction time and accuracy. The difference in performance between the valid and invalid trials is referred to as the orienting or validity effect. This effect represents the costs of disengaging and shifting attention from the incorrect to the correct location. If participants are quicker and more accurate at localising targets during valid compared with invalid trials, then their response is regarded as attention orienting that was induced by the perceived cue direction.
The Posner task has been used to investigate the neural basis of attention orienting through neuroimaging and clinical studies [3, 10, 11]. To allow the study of neural circuits through lesion and pharmacological manipulations, animal models of the Posner task have been developed in recent years. Studies in monkeys and rats showed that attention orienting was affected by lesions of the basal cholinergic nuclei [12] and administration of cholinergic medications [13–16]. Together with clinical findings showing impairments in Alzheimer’s disease, a condition associated with markedly depleted cortical cholinergic innervation [17, 18], and beneficial effects of nicotine [19, 20], these lines of evidence support acetylcholine (ACh) as a primary neurotransmitter that mediates attention orienting.
In addition to ACh, two other neurotransmitters, noradrenaline (NA) and dopamine (DA), have been associated with attention networks that interact with attention orienting. Petersen and Posner [1] have developed a model of three functionally and anatomically independent but interacting attention networks—alerting, attention orienting and executive attention. Alerting refers to the maintenance of readiness to respond to stimuli, and executive attention refers to the cognitive control of attention. Genetic and pharmacological studies in humans and animals have suggested that the alerting system modulates attention orienting via brain areas innervated by NAergic connections projecting from the midbrain [21, 22], whereas the executive attention system influences attention orienting via DAergic actions likely in the prefrontal cortex [23, 24]. NA- and DA-modulating medications, however, have shown inconsistent effects on attention orienting between humans and non-human primates [22, 25]. In contrast to cholinergic involvement, the effect of NA and DA on attention orienting is not well understood.
The mouse has emerged as a powerful animal model to investigate visuospatial attention through genetic modification or circuit manipulation [26, 27], but a well-established assessment of attention orienting in mice is missing. Recently, Wang and Krauzlis [28] provided the first adaptation of the Posner cueing task in mice. Their study demonstrated that mice exhibited shorter reaction times and higher accuracy to validly cued spatial cues, thus supporting the possibility to measure attention orienting experimentally in mice. These mice, however, were head-fixed, which might have induced high stress that affected attention processes in mice [29]. In addition, the task used peripheral cues to predict the target, which raises the question as to whether mice can endogenously orient their attention based on rule-based symbolic cues like those used in the human Posner task. Only one study has attempted to assess visuospatial attention in freely moving mice, but it did not include cues before the targets and was not designed to assess cued orienting effects [30]. Therefore, a task providing a complete assessment of exogenous and endogenous orienting in freely moving mice is required in order to explore the neural mechanisms of attention orienting.
Preclinical animal models provide a critical tool for assessing the efficacy of pharmacological treatments on attention. The beneficial effects of ADHD medications, such as methylphenidate (MPH) and atomoxetine (ATX), on attention have been documented in humans [31–33], with similar effects also described in rodents [34, 35]. Specifically, in a rodent adapted task of sustained attention, the rodent continuous performance task, MPH and ATX have been shown to improve attention performance with different effects on impulsivity [35–38]. As these compounds differ in their mechanisms of action, with MPH inhibiting both NA and DA reuptake and ATX primarily inhibiting NA reuptake [39, 40], they can be used to understand the role of these neurotransmitter systems in modulating attention. In the absence of a mouse version of the Posner task, the effect of MPH and ATX on attention orienting in mice has not been assessed.
The current study aimed to adapt the Posner cueing task for use in mice using touchscreen technology, an increasingly popular method to assess cognitive functions in rodents in a manner that is similar to cognitive tests in humans [41, 42]. A major challenge for successful task design is the requirement for mice to be trained to maintain their nose-poke at the touchscreen until the appearance of the target. This is critical to control the distance between the mice and the presentation of the stimuli, to reduce the effects of head movement on vision, and to record an accurate response time (RT). It was hypothesised that mice would respond more quickly and accurately during valid compared with invalid trials in both the exogenous and endogenous task. To further understand the role of the noradrenergic and dopaminergic neurotransmitter systems in attention orienting, the study also aimed to explore the effects of clinically effective treatments, MPH and ATX, on mice during the novel Posner-style cueing task. Given that the effects of MPH and ATX on attention orienting were inconsistent in previous research [22, 25] and that this study used a novel task, no hypothesis was proposed.
Materials and methods
Animals
Thirty-two male C57BL/6J mice were obtained from the Animal Resources Centre (Murdoch, Western Australia) after weaning at 4 weeks of age. Mice were housed in groups of four in individually ventilated cages (39 × 20 × 16 cm) with food and water available ad libitum, with shelter and tissue for nesting. Temperature and humidity were controlled at 22 °C and 45%, respectively. Mice were maintained on a 12 h light/dark cycle (lights on at 0700 h) and bedding changed weekly. At 7 weeks of age, mice were moved to open-top standard mouse cages (34 × 16 × 16 cm) and to a reversed light cycle (12:12 h, lights off at 0800 h). Housing groups and shelters were transferred together, though four mice were housed individually to avoid fighting. At 8 weeks of age, mice were weighed daily for 3 days to determine the baseline free feeding weight (FFW) and then food restricted to 85% FFW. Mice were fed standard chow inside their cages, at the same time of day, with a maximum 0.2 g difference in food weight between days. All procedures were approved by the Florey Institute of Neuroscience and Mental Health Animal Ethics Committee and complied with the relevant guidelines and regulations of the National Health and Medical Research Council Code of Practice for the Use of Animals for Scientific Purposes.
Drugs and treatments
MPH (Cat # M325880; Toronto Research Chemicals) and ATX (Cat # Y0001586; Sigma-Aldrich) were dissolved in 0.9% (w/v) sodium chloride, administered intraperitoneally, 30 min prior to testing in an injection volume of 10 ml/kg and a dose of 3 mg/kg. The dose of MPH and ATX was selected based on previous touchscreen studies [35–37, 43], which reported that this dose enhanced attention in mice while showing minimal changes to locomotor activity. Hence, this dose should allow for an accurate assessment of the effects of these treatments on attention.
Behavioural apparatus
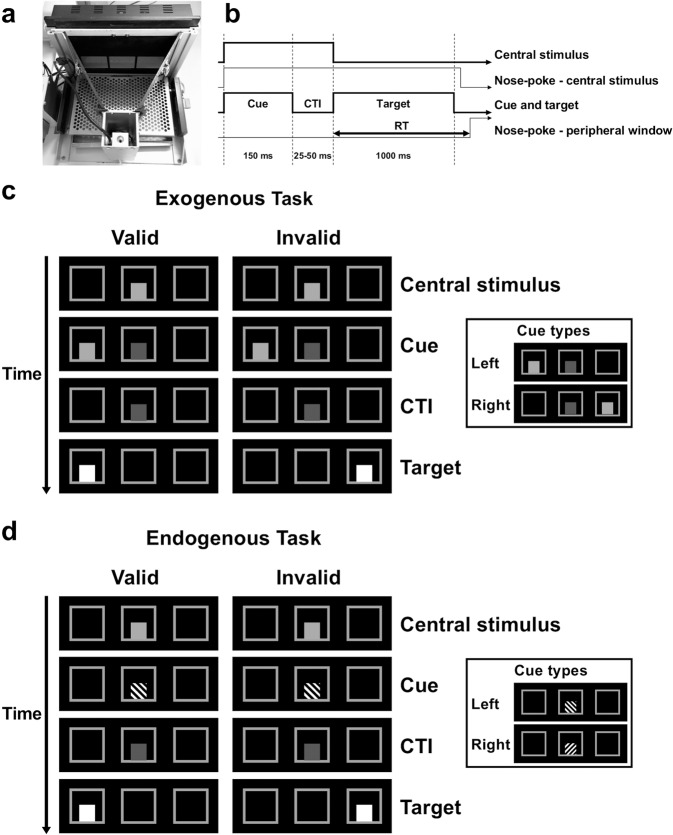
Behavioural testing was conducted in a touchscreen automated operant chamber system (Fig. 1a; Campden Instruments Ltd, UK). A black Perspex mask with three square windows (7 × 7 cm, 1.5 cm above the grid floor) was used to cover the touchscreen to reduce incidental touches. Details of the apparatus methods have been described previously [41, 44]. Whisker Server and ABET II were used to control the system and to collect data (Lafayette Instruments, Lafayette, IN, USA).
Fig. 1. Illustration of the exogenous and endogenous tasks in mice.
a Photograph of the touchscreen operant chamber. The chamber was equipped with a touchscreen on one end and a liquid-reward (milkshake) delivery magazine on the other. The screen was covered with a black Perspex mask with three response windows. b Timing of events in the probe. Trials started with illumination of the central stimulus. After a mouse nose-poked the central stimulus, a cue appeared, followed by the bright peripheral target after a random interval. CTI cue-target interval. RT response time. c Stimuli in the exogenous task. Cue validity = 50% in the probe. d Stimuli in the endogenous task. Cue validity = 80% in the probe.
Stimuli
Mice were trained to sustain their nose-poke at a stimulus in the central window during the presentation of a cue and then to respond to the target displayed in the left or right window (Fig. 1b). All stimuli measured 3.5 × 3.5 cm. The central stimulus was a square at 70% brightness and was dimmed to 20% brightness after being touched by the mouse. The cue for the exogenous task was a square at 70% brightness presented in the peripheral window. The cue for the endogenous task was a square with either 135° or 45° black grating presented in the central window (Fig. 1c, d).
Pretraining
Mice were habituated to the touchscreen chambers over two 20-min sessions. Following habituation, mice were trained to associate nose-poking at a central stimulus on the touchscreen with a food reward (Iced Strawberry Milk, Nippy’s Ltd, Australia) over two training stages (see Supplementary Materials and Methods).
Task training
After completing the pretraining stage, mice were randomly assigned to either the exogenous (n = 16) or endogenous task (n = 16). The main aim of training was for the mice to nose-poke the central stimulus for the time it took for the cue and the target to be presented. If completed correctly, mice were then rewarded with food delivery. A 5-s inter-trial-interval (ITI) would then elapse before the commencement of the next trial. If mice withdrew their nose from the central stimulus before the onset of the target (anticipation error), a 5-s ITI was initiated with no food reward. Touches to the opposite side of the target (commission error), or failure to respond to the target within a certain time (omission error), would result in no food reward and a 5-s time-out period with illumination of the house light, followed by a 5-s ITI. Omission errors occurred when mice exceeded either the maximum reaction time (i.e., time between target onset and mice leaving the central stimulus) or the maximum movement time (i.e., time between mice leaving the central stimulus and touching the peripheral window).
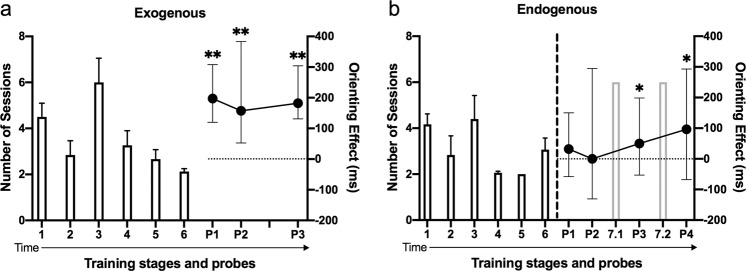
Each daily training session lasted either 60 min or 120 trials (excluding anticipation errors)—whichever came sooner. For both the exogenous and endogenous groups, training was identical except that different cues were used and that mice underwent six stages of training for the exogenous task and seven stages for the endogenous task (Fig. 2). For the exogenous task, cue validity was set at 50% in all training stages to prevent the mice from learning the association between the cue and the target. For the endogenous task, cue validity was set at 100% in training to facilitate learning that the 135° and 45° grating cue predicted the left and right target, respectively. In the final training stage for this task, cue validity was reduced to 90% to introduce the invalid endogenous cues to the mice.
Fig. 2. Timeline of training and probes.
a Exogenous task training. 1–6 on the x-axis indicate different training stages. P on the x-axis indicates the probe. Each probe comprised two sessions. Cue validity was set at 50% in all training sessions. b Endogenous task training. Cue validity was 100% in the training stages 1–6, 90% in the training stages 7.1 and 7.2 and 80% during the probes. The vertical dashed line denotes the first time that invalid trials were introduced into the endogenous task. Exogenous task: n = 16 mice in all training stages; n = 9 mice in Probe 1; n = 12 mice in Probes 2 and 3; all mice in the probes showed positive orienting effects. Endogenous task: n = 16 mice in all training stages; n = 15 mice in all probes; 60%, 53%, 67% and 80% of mice showed positive orienting effects in Probe 1, 2, 3 and 4, respectively. Numbers of sessions to complete each training stage are expressed as bars with mean + standard errors. Orienting effects in each probe are expressed as circle symbols showing the median and whiskers showing min–max values. *p < 0.05, **p < 0.001.
For the first three training stages, mice were subjected to stepwise training, in which the duration of cues and cue–target-intervals (CTIs) were adjusted in steps of 50 ms based on the performance of mice. After completing stepwise training, mice were moved to randomised training, in which the cue duration remained at 150 ms and the CTI was randomised between 25 and 50 ms. In the last training stage, the target duration was set at 1 s, maximum reaction time at 1.5 s, and the maximum movement time at 2.5 s (see Supplementary Materials and Methods).
Probes
Mice exhibiting orienting effects [(median RTs in invalid trials − valid trials) >0] were deemed to be performing the task. In the probes, cue validity remained at 50% in the exogenous task but changed to 80% in the endogenous task.
After mice showed stable orienting, the effects of attention-modulating drugs, MPH and ATX, were assessed. MPH, ATX and saline were administered in a pseudo-randomised cross-over design with a 3- to 4-day washout period between each administration. Mice were subjected to a minimum of two consecutive days of baseline training to ensure continued stable performance between each probe. Mice not reaching criteria (>70% accuracy or completion of 120 trials, excluding anticipations errors) were subjected to further baseline sessions until criteria were met.
Data analysis
All data were analysed using generalised linear, latent and mixed models (GLLAMM) with robust standard error estimation, as previously described [45]. All statistical analyses were performed in STATA (StataCorp, College Station, TX, USA). Graphs were produced using Prism (GraphPad, La Jolla, CA, USA).
Results
Mice successfully learnt the exogenous and endogenous tasks
All mice acquired stepwise and randomised training for the exogenous and endogenous tasks (Fig. 2; statistical comparisons in Supplementary Results).
Mice responded more quickly and more accurately to validly cued trials in the exogenous and endogenous tasks
Exogenous task
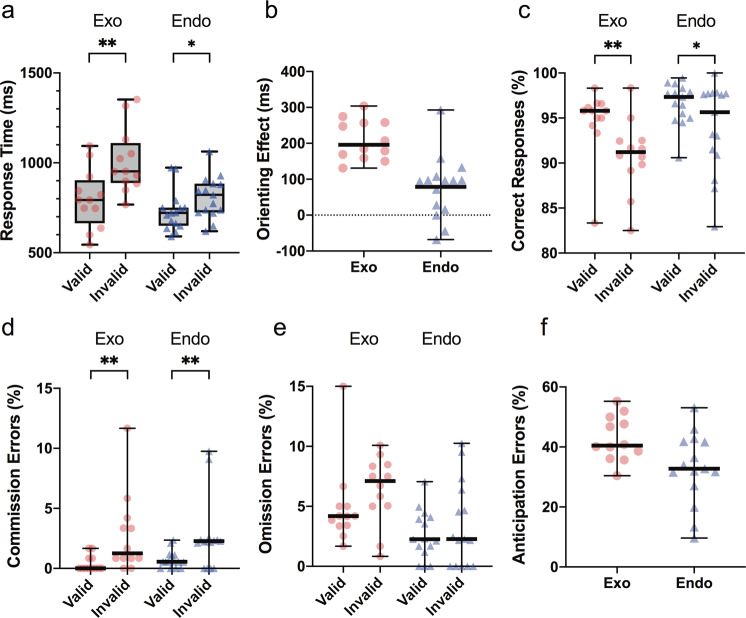
Mice showed longer RTs during invalid relative to valid trials in the last probe (Figs. 2a and 3a; Probe 3: coefficient = 198.66, 95% CI = [170.08, 227.25], p < 0.001). The mean orienting effect was 210 ms (Fig. 3b; SE = 56). Performance on invalid trials also worsened compared to valid trials, with mice showing lower odds of a correct response (Fig. 3c; OR = 0.56, 95% CI = [0.42, 0.75], p < 0.001) and higher odds of committing a commission error (Fig. 3d; OR = 6.97, 95% CI = [2.94, 16.53], p < 0.001) during invalid trials. There was no significant association between omission errors and cue validity (Fig. 3e; OR = 1.31, 95% CI = [0.95, 1.8], p = 0.1). There were no significant interactions between cue validity and nose-poking time or between cue validity and days on RT and on any types of responses, suggesting that the orienting effect was not affected by nose-poking time or days.
Fig. 3. Performance of mice following the completion of training in the exogenous task (Probe 3) and endogenous task (Probe 4).
a Response time, b orienting effect = median RT in invalid trials − median RT in valid trials, c correct responses, d commission errors, e omission errors and f anticipation errors. Data in a is expressed as box plots with the central line showing the median, box showing 25th–75th percentile, and whiskers showing min–max values. b–f are expressed as scatter plots with the bold horizontal line showing the median. *p < 0.05, **p < 0.001. Exogenous task: n = 12 mice; endogenous task: n = 15 mice.
On average, 43% of the trials initiated by mice were anticipation errors (Fig. 3f; SE = 5%) in the exogenous task. Mice showed higher odds of an anticipation error when a longer nose-poking time was required (OR = 1.03, 95% CI = [1.03, 1.04], p < 0.001). While mice with higher levels of anticipation errors responded slightly slower to the target overall (coefficient = 16.21, 95% CI = [11.85, 20.58], p < 0.001), no significant effect of anticipation error on the orienting effect (coefficient = 4.04, 95% CI = [−0.26, 8.33], p = 0.07) or any other types of responses was shown.
Endogenous task
Mice exhibited longer RTs during invalid compared with valid trials in the last probe (Figs. 2a and 3a; Probe 4: coefficient = 79.42, 95% CI = [32.77, 126.08], p = 0.001). The mean orienting effect was 79 ms (Fig. 3b; SE = 23). Akin to performance in the exogenous task, mice also showed lower odds of making a correct response (Fig. 3c; OR = 0.54, 95% CI = [0.36, 0.79], p = 0.002) and higher odds of committing a commission error (Fig. 3d; OR = 3.57, 95% CI = [1.86, 6.87], p < 0.001) during invalid compared with valid trials. There was no association between omission errors and cue validity (Fig. 3e; OR = 1.32, 95% CI = [0.8, 2.16], p = 0.28). There were no significant interactions between cue validity and nose-poking time or between cue validity and days on RT and on any types of responses, suggesting that the orienting effect was not affected by nose-poking time or days.
On average, 33% of the trials initiated by mice were anticipation errors (Fig. 3f; SE = 12%) in the endogenous orienting task. Mice showed higher odds of anticipation errors on trials requiring longer nose-poking time (OR = 1.04, 95% CI = [1.03, 1.04], p < 0.001). In contrast to the exogenous task, higher anticipation errors corresponded to significantly quicker response latencies to the target (coefficient = −4.71, 95% CI = [−8.46, −0.96], p = 0.01). Apart from this, no significant effect of anticipation error on the orienting effect (coefficient = −1.33, 95% CI = [−5.62, 2.96], p = 0.54) or any other types of responses was observed.
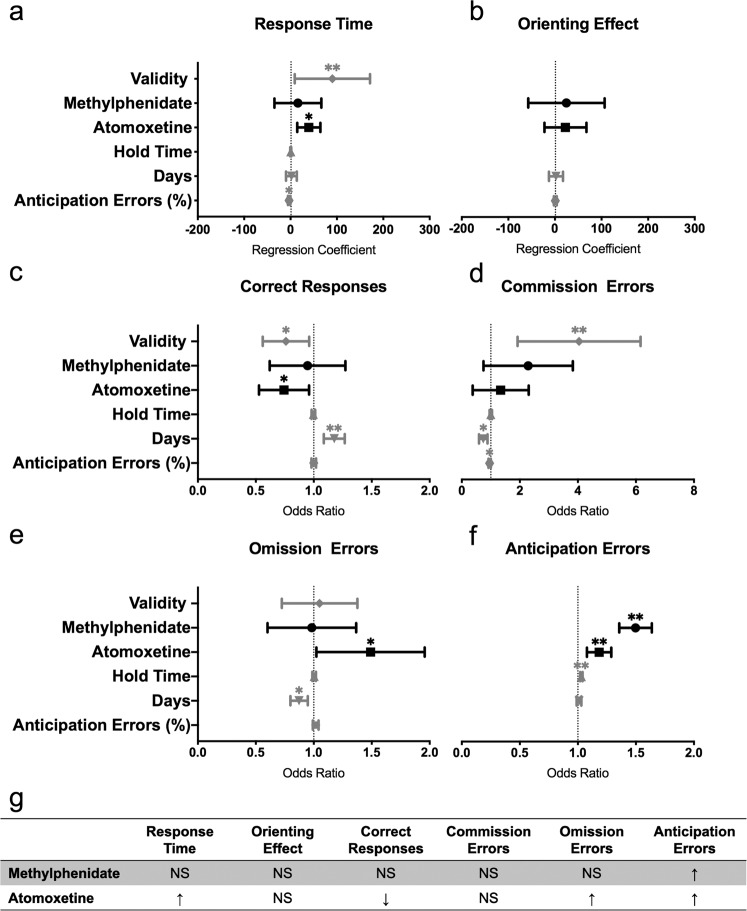
MPH quickened RT in the exogenous task, while ATX had the opposite effect
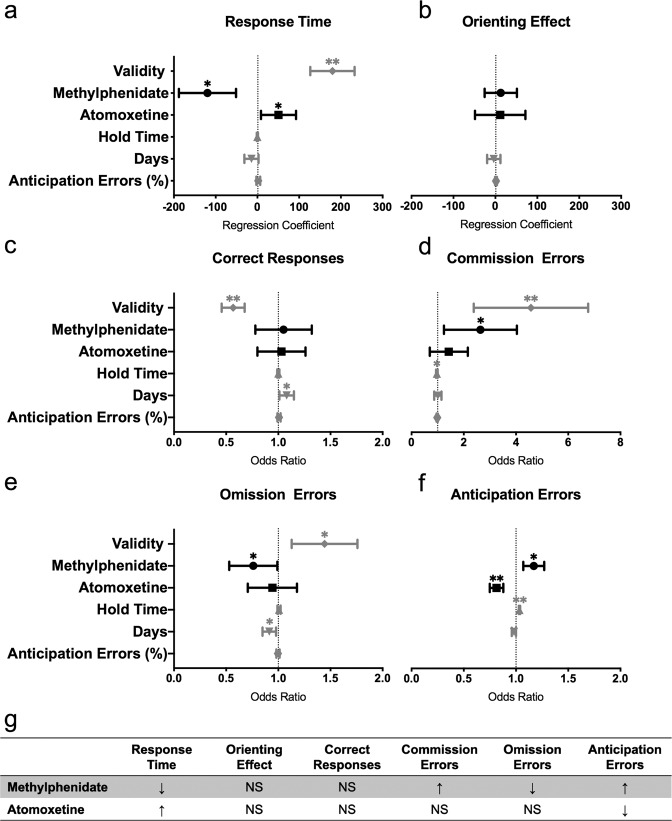
During the exogenous task, administration of MPH speeded RTs (Fig. 4a; coefficient = −119.60, 95% CI = [−118.08, −51.12], p = 0.001) and lowered the odds of omission errors in mice (Fig. 4e; OR = 0.74, 95% CI = [0.54, 1], p = 0.049), compared to saline-treated mice. MPH, however, did not alter the orienting effect (Fig. 4b; coefficient = 12.9, 95% CI = [−25.6, 51.41], p = 0.51) or the odds of correct responses (Fig. 4c; OR = 1.03, 95% CI = [0.79, 1.33], p = 0.83). There was no significant interaction between MPH and cue validity on any outcome measures. MPH appeared to influence measures related to impulsivity, with MPH-treated mice exhibiting higher odds of committing a commission error (Fig. 4d; OR = 2.39, 95% CI = [1.38, 4.14], p = 0.002) and higher odds of making an anticipation error (Fig. 4f; OR = 1.17, 95% CI = [1.07, 1.27], p = 0.001). The percentage of anticipation errors did not alter the effect of MPH on the outcome measures, except the odds of a correct response being made (OR = 1.03, 95% CI = [1.01, 1.05], p = 0.01).
Fig. 4. Effects of atomoxetine and methylphenidate on the exogenous task.
a Response time, b orienting effect (median RT in invalid trials − median RT in valid trials), c correct responses, d commission errors, e omission errors, f anticipation errors and g summary table of the effects of MPH and ATX on the outcome measures. Panels a and b depict regression coefficients ± 95% confidence intervals. Panels c–f depict odds ratios ± 95% confidence intervals. *p < 0.05, **p < 0.001. Saline: n = 13 mice; MPH: n = 11 mice; ATX: n = 13 mice.
Opposite to the effects of MPH, ATX slowed RTs in mice compared to saline-treated mice (Fig. 4a; coefficient = 50.53, 95% CI = [8.55, 92.51], p = 0.02). ATX did not significantly affect the orienting effect (Fig. 4b; coefficient = 11.58, 95% CI = [−48.5, 71.65], p = 0.71), the odds of correct responses (Fig. 4c; OR = 1.01, 95% CI = [0.81, 1.27], p = 0.92; Fig. 4e), the odds of commission errors (Fig. 4d; OR = 1.30, 95% CI = [0.77, 2.21], p = 0.33) or the odds of omission errors (Fig. 4e; OR = 0.92, 95% CI = [0.72, 1.19], p = 0.53). There was no significant interaction between ATX and cue validity on any outcome measures. Mice administered ATX showed significantly lower odds of anticipation errors (Fig. 4f; OR = 0.81, 95% CI = [0.75, 0.88], p = 0.1) compared to mice on saline. The percentage of anticipation errors did not alter the effect of ATX on the outcome measures.
MPH had minimal effect on endogenous task performance, while ATX decreased performance
During the endogenous task, MPH had minimal effect on performance measures. MPH treatment did not affect RTs (Fig. 5a; coefficient = 15.85, 95% CI = [−34.71, 66.40], p = 0.54), orienting effects (Fig. 5b; coefficient = 24.70, 95% CI = [0.64, 1.29], p = 0.59), the odds of correct responses (Fig. 5c; OR = 0.91, 95% CI = [0.79, 1.33], p = 0.83), the odds of commission errors (Fig. 5d; OR = 1.94, 95% CI = [0.95, 3.97], p = 0.07) or the odds of omission errors (Fig. 5e; OR = 0.93, 95% CI = [0.63, 1.39], p = 0.73) in mice. There was also no significant interaction between MPH and cue validity on any outcome measure. Mice administered with MPH did show higher odds of committing an anticipation error (Fig. 5f; OR = 1.49, 95% CI = [1.36, 1.64], p < 0.001) compared to mice on saline. The percentage of anticipation errors did not alter the effect of MPH on the outcome measures, except the odds of commission errors (OR = 0.94, 95% CI = [0.89, 0.99], p = 0.003).
Fig. 5. Effects of atomoxetine and methylphenidate on the endogenous task.
a Response time, b orienting effect (median RT in invalid trials − median RT in valid trials), c correct responses, d commission errors, e omission errors, f anticipation errors and g summary table of the effects of MPH and ATX on the outcome measures. Panels a and b depict regression coefficients ± 95% confidence intervals. Panels c–f depict odds ratios ± 95% confidence intervals. *p < 0.05, **p < 0.001. Saline: n = 15 mice; MPH: n = 14 mice; ATX: n = 15 mice.
ATX impaired endogenous task performance in mice, across most of the measures. Mice administered with ATX showed significantly slower RTs (Fig. 5a; coefficient = 39.42, 95% CI = [14.56, 64.29], p = 0.002;), lower odds of correct responses (Fig. 5c; OR = 0.72, 95% CI = [0.54, 0.97], p = 0.03), higher odds of omission errors (Fig. 5e; OR = 1.44, 95% CI = [1.05, 1.98], p = 0.03) and higher odds of anticipation errors (Fig. 5f; OR = 1.18, 95% CI = [1.08, 1.29], p < 0.001). ATX did not significantly affect orienting effects (Fig. 5b; coefficient = 22.52, 95% CI = [−22.55, 67.59], p = 0.33) or the odds of commission errors (Fig. 5d; OR = 1.11, 95% CI = [0.51, 2.40], p = 0.26). There was no significant interaction between ATX and cue validity on any outcome measure. The percentage of anticipation errors did not alter the effect of ATX on the outcome measures, except the odds of correct response (OR = 1.03, 95% CI = [1.00, 1.05], p = 0.004).
Discussion
Mice can orient their attention both exogenously and endogenously, as assessed by a new touchscreen-based task adapted from the human Posner task. Like previous results in humans, mice responded more quickly and accurately to validly cued stimuli in both the exogenous and endogenous tasks. During the exogenous task, MPH administration resulted in a more impulsive and alert response style, with a speeding of RTs and a reduction in omission errors, but an increase in commission and anticipation errors. ATX administration, in contrast, resulted in a more cautious response style, with a slowing of RTs and a lower rate of anticipation errors. During the endogenous task, MPH administration had minimal effect, whereas ATX administration resulted in a decrease in overall performance—slowed RTs, increased odds of omission and anticipation errors, and decreased odds of correct responses being made. Although MPH and ATX showed differential effects on the performance of the mice during the attention orienting tasks, neither treatment altered attention orienting. Overall, the current study provides a novel protocol to investigate the neural mechanisms of attention orienting in mice. Using this new protocol, the current study showed that mice can be trained to voluntarily engage during the attention orienting tasks and, more importantly, can endogenously orient their attention based on learnt symbolic cues.
Task acquisition: mice can be trained to spontaneously complete the attention orienting tasks
An important achievement in the current study was to train the mice to voluntarily head fix at the centre of the touchscreen until the presentation of the target after a cue, which is critical in the successful adaptation of the Posner task to mice. The head-fixing behaviour helps to control the starting position of the mice in each trial to obtain reasonably accurate measurements of whole-body RTs. The head-fixing behaviour was also designed to resemble the procedure of the human Posner task in which participants are required to orient their attention to the appearance of the peripheral targets without moving their eyes or head. As mice lack fovea in their retinas, their orienting is primarily by head and body rather than eye movements [46]. By training the mice to maintain their heads centrally, their eyes were placed to see the central and peripheral stimuli. Previous studies have successfully trained rats to sustain the nose-poke at the centre in other adapted Posner tasks [16, 47]. Similar to rats, the current study showed that mice could be trained to extend their nose-poking time on the touchscreen after stepwise training and to maintain their nose-poking ability during the randomised training. This, to our knowledge, has not been reported in previous research.
Probe performance: mice showed both stimulus-driven and goal-driven orienting of attention
The results of the current study suggested that mice could orient their attention based on both exogenous and endogenous spatial cues, supporting the use of mice as a valid animal model to study the neural mechanisms of attention orienting. Mice were faster and more accurate at responding to validly cued targets, consistent with findings in humans [8, 48] and rats [47]. Similarly, a previous study showed that mice could use spatial cues to orient their attention [28]. In this previous study, however, the heads of the mice were restrained by an implanted head post, which might create stress and is not comparable with the human task. Using touchscreen technology, the current study extends this previous study by showing that mice can spontaneously orient their attention in a low stress setting that is directly compatible with human variations of the tasks.
The current study provides the first demonstration of endogenous orienting in mice based on predictive symbolic cues. Previous rodent studies have typically employed predictive peripheral cues to measure endogenous orienting [47]. Predictive peripheral cues, however, have been suggested to produce both an exogenous attention capture and an endogenous shift of attention [2, 49], which may confound the measurement of endogenous orienting. The current study used symbolic cues (i.e., diagonal gratings) that predicted the location of the targets in the endogenous task, which closely resembles the endogenous cues in the human Posner task [50]. Grating cues were selected due to their neutrality [45]. With all touchscreen tasks, mice are trained over a number of sessions to acquire the required behaviour before assessment in the probe. It is possible that training led to habitual responding or automatic bias in this study. To reduce the learning effect, the probe was designed to contain a lower percentage of validly cued targets compared to the training. If mice in this study were orienting towards endogenous grating cues automatically, we would expect to see comparable orienting effects between the exogenous and endogenous orienting tasks. The orienting effect, however, was lower in the endogenous task, suggesting that the grating cues were eliciting endogenous control of attention. This finding opens up avenues for future studies to investigate endogenous orienting in mice using symbolic cues to reduce the confounding components of peripheral cues.
Mice showed a high percentage of anticipation errors (i.e., leaving the touchscreen before the onset of target) in the novel attention orienting task. This finding is different from the performance of rats in a Posner-style cueing task [47]. In that study, the rats made few anticipation errors when the interval between the cue and target was 200 ms, which is similar to the timing used in the current study. It is likely that mice are inherently more active and impulsive, as previous findings also showed that mice tended to respond before stimulus onset in the 5-choice serial reaction time task, a task used to measure visual attention and impulsive actions [51, 52]. The difference in anticipation errors between mice and rats may also be due to the different apparatuses used. In the rat study, animals were required to poke their nose into a hole rather than touching a screen, which may have prevented them from quickly withdrawing their nose-poke. Although mice showed a high percentage of anticipation errors, the current results demonstrated that the mice completed 120 trials with high accuracy of responses (>90%), other than anticipation errors, in each task session. In addition, the percentage of anticipation errors in mice did not affect the orienting effect or the odds of making correct responses or errors. Together, these findings suggest that despite the high percentage of anticipation errors, mice were able to complete the attention orienting tasks properly when they could maintain their nose-poke until the appearance of the target.
Effects of drugs: MPH and ATX exerted mixed effects on exogenous and endogenous orienting in mice
In order to investigate the neural mechanisms of attention orienting, two attention-modulating treatments, MPH and ATX, were administered to the mice in the current tasks. MPH and ATX have been observed to improve sustained and selective attention in mice in some studies [35, 53], but neither treatment exhibited significant effects on attention orienting (i.e., the orienting effects) in the current tasks. One possible explanation is that the noradrenergic (NA) and dopaminergic (DA) signalling systems, which are modulated by these compounds, have limited or indirect effects on attention orienting. Some studies suggest that NA specifically impacts alertness rather than attention orienting [54, 55], although other evidence suggests that NA might affect attention orienting through facilitating the action of ACh [56]. It has also been suggested that DA is involved in resolving conflict rather than attention orienting [56, 57]. Another possible reason is that attention-modulating drugs may only show enhancing effects when a primary attention deficit is present. Previous studies did not find consistent improvement of MPH or ATX on attention in healthy humans and animals [22, 35, 58, 59]. It is possible that healthy subjects might be able to orient their attention near their peak level, and any additional increase of noradrenergic and dopaminergic activities induced by the drugs would not necessarily improve performance. To further investigate this issue, it might be necessary to test mouse models with potential deficits in attention, or use drugs that decrease their attention functioning, such as cholinergic antagonists [15, 16].
Although MPH and ATX did not significantly affect attention orienting in mice, these treatments exerted differential effects on behavioural performance. In the exogenous task, mice administered MPH appeared to be more alert, as indicated by faster responses and reduced odds of making omission errors, and more impulsive, as indicated by increased odds of making commission and anticipation errors. In contrast, mice on ATX tended to be more cautious, as indicated by slower responses and lower odds of anticipation errors. Similarly, previous studies have suggested that ATX reduces impulsive actions in rodents [51, 60, 61] and humans [32, 62], in contrast to MPH [35, 63, 64]. While both MPH and ATX have been shown to enhance NA and DA in the prefrontal cortex, only MPH appears to directly increase DA in the basal ganglia [65–67]. It is likely that the differential effects of MPH and ATX on alertness and impulsive behaviours reflect their differential roles on subcortical DA neurotransmission.
During the endogenous task, MPH exerted minimal impact on the performance of mice, with the exception of increasing the odds of anticipation errors. The minimal effect of MPH is partially consistent with an early study in healthy people, in which MPH speeded overall RTs but did not affect the orienting effect [68]. In contrast, ATX administration in mice slowed RTs, lowered the odds of making a correct response and increased the odds of making omission and anticipation errors. Due to limited previous research in this area, it is unclear why ATX appeared to impair endogenous orienting. One possible explanation is that although the dose of ATX used in the current study was based on previous research [35], it may lead to an atypical level of arousal for the endogenous orienting task. Endogenous orienting is a goal-directed cognitive process that initially requires mice to maintain a higher level of intrinsic arousal relative to other attention processes. The equivalent dose of ATX that enhanced the performance of mice in other tasks, such as the continuous performance test [35], may have led to a state of hypo- or hyper-arousal in the endogenous orienting task, affecting the ability of the mouse to sustain attention to the task. According to the inverted U-shaped arousal-performance theory, optimal performance occurs at an intermediate level of arousal, whereas high or low levels of arousal will impair performance [21, 69]. To understand the pharmacological effects on the endogenous orienting task in mice, future studies are needed to examine the effects of different doses of drugs on task performance.
Conclusion
This study provides a novel mouse attention orienting task based on the human Posner task. In accordance with human performance, mice responded more quickly and more accurately to validly cued targets, supporting mice as a valid animal model to study the neural mechanisms of attention orienting. Our results provide the first evidence that mice can be trained to voluntarily maintain their nose-poke on the touchscreen and complete both the exogenous and endogenous orienting tasks. These findings support the use of touchscreen testing to accurately record RT in mice, which has substantial translational relevance due to the reliance on RT measurement in human studies. This study is also the first to show that mice can orient their attention based on the rule-based symbolic endogenous cues, which enables a more accurate measurement of endogenous orienting compared to the use of non-symbolic peripheral cues.
Our results did not show significant effects of MPH and ATX on attention orienting, although MPH improved overall RTs in mice during the exogenous orienting task. This is the first study to examine pharmacological effects on attention orienting in mice using our newly developed task. This paves the way for future research to investigate the effects of attention-modulating drugs and other therapeutic interventions on attention orienting and to evaluate mouse models of attention disorders.
Funding and disclosure
SL is supported by the Melbourne Research Scholarship, a graduate research scholarship established by the University of Melbourne. ELB is supported by a National Health and Medical Research Council-Australian Research Council (NHMRC-ARC) Dementia Research Development Fellowship (1111552). AJH is supported by an NHMRC Principal Research Fellowship (1117148). The authors declare no competing interests.
Supplementary information
Acknowledgements
The authors thank A/Prof. Gilberto Fernando Xavier and Mr. Mateus Torres Cruz for providing advice on the development of the training protocols. They also thank Mr. Daniel Drieberg for providing animal care, and Mr. Brett Purcell for providing the technical support.
Author contributions
SL, KAJ and ELB conceived and designed the study. SL and ELB carried out the experiments. SL analysed the data with help from CM and ELB. SL, KAJ and ELB contributed to the interpretation of the results. SL wrote the manuscript with input from KAJ, ELB and AJH. All authors read the final version and consented to publishing this manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: K. A. Johnson, E. L. Burrows.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00873-8).
References
- 1.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chica AB, Bartolomeo P, Lupiáñez J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behav Brain Res. 2013;237:107–23. doi: 10.1016/j.bbr.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 4.Keehn B, Müller R-A, Townsend J. Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev. 2013;37:164–83. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heeren A, Maurage P, Philippot P. Revisiting attentional processing of non-emotional cues in social anxiety: a specific impairment for the orienting network of attention. Psychiatry Res. 2015;228:136–42. doi: 10.1016/j.psychres.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Bellgrove MA, Johnson KA, Barry E, Mulligan A, Hawi Z, Gill M, et al. Dopaminergic haplotype as a predictor of spatial inattention in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66:1135. doi: 10.1001/archgenpsychiatry.2009.120. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S, Chen X, Wang C, Yin C, Hu P, Wang K. Selective attention deficits in early and moderate stage Parkinson’s disease. Neurosci Lett. 2012;509:50–5. doi: 10.1016/j.neulet.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 9.Posner MI. Orienting of attention: then and now. Q J Exp Psychol (Hove) 2016;69:1864–75. doi: 10.1080/17470218.2014.937446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doricchi F, Macci E, Silvetti M, Macaluso E. Neural correlates of the spatial and expectancy components of endogenous and stimulus-driven orienting of attention in the posner task. Cereb Cortex. 2009;20:1574–85. doi: 10.1093/cercor/bhp215. [DOI] [PubMed] [Google Scholar]
- 11.Proskovec AL, Heinrichs-Graham E, Wiesman AI, McDermott TJ, Wilson TW. Oscillatory dynamics in the dorsal and ventral attention networks during the reorienting of attention. Hum Brain Mapp. 2018;39:2177–90. doi: 10.1002/hbm.23997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–86. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J Neurophysiol. 2000;83:1536–49. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- 14.Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–14. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips JM, McAlonan K, Robb WGK, Brown VJ. Cholinergic neurotransmission influences covert orientation of visuospatial attention in the rat. Psychopharmacology. 2000;150:112–6. doi: 10.1007/s002130000437. [DOI] [PubMed] [Google Scholar]
- 16.Stewart C, Burke S, Marrocco R. Cholinergic modulation of covert attention in the rat. Psychopharmacology. 2001;155:210–8. doi: 10.1007/s002130100692. [DOI] [PubMed] [Google Scholar]
- 17.Ishizaki J, Meguro K, Nara N, Kasai M, Yamadori A. Impaired shifting of visuospatial attention in Alzheimer’s disease as shown by the covert orienting paradigm: implications for visual construction disability. Behav Neurol. 2013;26:121–9. doi: 10.1155/2013/147912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tales A, Snowden RJ, Haworth J, Wilcock G. Abnormal spatial and non-spatial cueing effects in mild cognitive impairment and Alzheimer’s disease. Neurocase. 2005;11:85–92. doi: 10.1080/13554790490896983. [DOI] [PubMed] [Google Scholar]
- 19.Hammersley JJ, Gilbert DG, Rzetelny A, Rabinovich NE. Moderation of nicotine effects on covert orienting of attention tasks by poor placebo performance and cue validity. Pharm Biochem Behav. 2016;149:9–16. doi: 10.1016/j.pbb.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 22.Reynaud AJ, Froesel M, Guedj C, Ben Hadj Hassen S, Cléry J, Meunier M, et al. Atomoxetine improves attentional orienting in a predictive context. Neuropharmacology. 2019;150:59–69. doi: 10.1016/j.neuropharm.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–5. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markant J, Cicchetti D, Hetzel S, Thomas KM. Relating dopaminergic and cholinergic polymorphisms to spatial attention in infancy. Dev Psychol. 2014;50:360–9. doi: 10.1037/a0033172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kratz O, Studer P, Baack J, Malcherek S, Erbe K, Moll GH, et al. Differential effects of methylphenidate and atomoxetine on attentional processes in children with ADHD: an event-related potential study using the Attention Network Test. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;37:81–9. doi: 10.1016/j.pnpbp.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62:1204–20. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Krauzlis RJ. Visual selective attention in mice. Curr Biol. 2018;28:676–85.e4. doi: 10.1016/j.cub.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pérez MÁ, Pérez-Valenzuela C, Rojas-Thomas F, Ahumada J, Fuenzalida M, Dagnino-Subiabre A. Repeated restraint stress impairs auditory attention and GABAergic synaptic efficacy in the rat auditory cortex. Neuroscience. 2013;246:94–107. doi: 10.1016/j.neuroscience.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 30.You W-K, Mysore SP. Endogenous and exogenous control of visuospatial selective attention in freely behaving mice. Nat Commun. 2020;11:1–14. doi: 10.1038/s41467-020-15909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujioka T, Takiguchi S, Yatsuga C, Hiratani M, Hong K-EM, Shin M-S, et al. Advanced test of attention in children with attention-deficit/hyperactivity disorder in Japan for evaluation of methylphenidate and atomoxetine effects. Clin Psychopharmacol Neurosci. 2016;14:79. doi: 10.9758/cpn.2016.14.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang C-Y, Pan Y-L, Lin H-Y, Huang L-W, Gau SS-F. An open-label, randomized trial of methylphenidate and atomoxetine treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2015;25:566–73. doi: 10.1089/cap.2015.0035. [DOI] [PubMed] [Google Scholar]
- 33.McGee RA, Clark S, Symons D. Does the Conners’ continuous performance test aid in ADHD diagnosis? J Abnorm Child Psychol. 2000;28:415–24. doi: 10.1023/A:1005127504982. [DOI] [PubMed] [Google Scholar]
- 34.Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, et al. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Caballero-Puntiverio M, Lerdrup LS, Grupe M, Larsen CW, Dietz AG, Andreasen JT. Effect of ADHD medication in male C57BL/6J mice performing the rodent continuous performance test. Psychopharmacology. 2019;236:1839–51. doi: 10.1007/s00213-019-5167-x. [DOI] [PubMed] [Google Scholar]
- 36.Ding Z, Brown JW, Rueter LE, Mohler EG. Profiling attention and cognition enhancing drugs in a rat touchscreen-based continuous performance test. Psychopharmacology. 2018;235:1093–105. doi: 10.1007/s00213-017-4827-y. [DOI] [PubMed] [Google Scholar]
- 37.Caballero-Puntiverio M, Lerdrup LS, Arvastson L, Aznar S, Andreasen JT. ADHD medication and the inverted U-shaped curve: a pharmacological study in female mice performing the rodent continuous performance test (rCPT) Prog Neuro-Psychopharmacol Biol Psychiatry. 2020;99:109823. doi: 10.1016/j.pnpbp.2019.109823. [DOI] [PubMed] [Google Scholar]
- 38.Tomlinson A, Grayson B, Marsh S, Harte MK, Barnes SA, Marshall KM, et al. Pay attention to impulsivity: modelling low attentive and high impulsive subtypes of adult ADHD in the 5-choice continuous performance task (5C-CPT) in female rats. Eur Neuropsychopharmacol. 2014;24:1371–80. doi: 10.1016/j.euroneuro.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Arnsten AFT. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS Drugs. 2009;23:33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SRO, et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–84. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nithianantharajah J, McKechanie AG, Stewart TJ, Johnstone M, Blackwood DH, St Clair D, et al. Bridging the translational divide: identical cognitive touchscreen testing in mice and humans carrying mutations in a disease-relevant homologous gene. Sci Rep. 2015;5:14613. doi: 10.1038/srep14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–70. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- 44.Mar AC, Horner AE, Nilsson SRO, Alsiö J, Kent BA, Kim CH, et al. The touchscreen operant platform for assessing executive function in rats and mice. Nat Protoc. 2013;8:1985–2005. doi: 10.1038/nprot.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeleznikow-Johnston A, Burrows EL, Renoir T, Hannan AJ. Environmental enrichment enhances cognitive flexibility in C57BL/6 mice on a touchscreen reversal learning task. Neuropharmacology. 2017;117:219–26. doi: 10.1016/j.neuropharm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Huberman AD, Niell CM. What can mice tell us about how vision works? Trends Neurosci. 2011;34:464–73. doi: 10.1016/j.tins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marote CFO, Xavier GF. Endogenous-like orienting of visual attention in rats. Anim Cogn. 2011;14:535–44. doi: 10.1007/s10071-011-0388-3. [DOI] [PubMed] [Google Scholar]
- 48.Bonato M, Lisi M, Pegoraro S, Pourtois G. Cue-target contingencies modulate voluntary orienting of spatial attention: dissociable effects for speed and accuracy. Psychol Res. 2016;82:272–83. doi: 10.1007/s00426-016-0818-6. [DOI] [PubMed] [Google Scholar]
- 49.Warner CB, Juola JF, Koshino H. Voluntary allocation versus automatic capture of visual attention. Percept Psychophys. 1990;48:243–51. doi: 10.3758/BF03211524. [DOI] [PubMed] [Google Scholar]
- 50.Chica AB, Martín-Arévalo E, Botta F, Lupiáñez J. The spatial orienting paradigm: how to design and interpret spatial attention experiments. Neurosci Biobehav Rev. 2014;40:35–51. doi: 10.1016/j.neubiorev.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Fitzpatrick CM, Andreasen JT. Differential effects of ADHD medications on impulsive action in the mouse 5-choice serial reaction time task. Eur J Pharmacol. 2019;847:123–9. doi: 10.1016/j.ejphar.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 52.Remmelink E, Chau U, Smit AB, Verhage M, Loos M. A one-week 5-choice serial reaction time task to measure impulsivity and attention in adult and adolescent mice. Sci Rep. 2017;7:42519. doi: 10.1038/srep42519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzpatrick CM, Runegaard AH, Christiansen SH, Hansen NW, Jørgensen SH, McGirr JC, et al. Differential effects of chemogenetic inhibition of dopamine and norepinephrine neurons in the mouse 5-choice serial reaction time task. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;90:264–76. doi: 10.1016/j.pnpbp.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Posner MI, Rothbart MK. Temperament and brain networks of attention. Philos Trans R Soc Lond B Biol Sci. 2018;373:20170254. doi: 10.1098/rstb.2017.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Posner MI, Rothbart MK, Sheese BE, Voelker P. Control networks and neuromodulators of early development. Dev Psychol. 2012;48:827–35. doi: 10.1037/a0025530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beane M, Marrocco RT. Norepinephrine and acetylcholine mediation of the components of reflexive attention: implications for attention deficit disorders. Prog Neurobiol. 2004;74:167–81. doi: 10.1016/j.pneurobio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Schneider KK, Schote AB, Meyer J, Frings C. Genes of the dopaminergic system selectively modulate top-down but not bottom-up attention. Cogn Affect Behav Neurosci. 2015;15:104–16. doi: 10.3758/s13415-014-0320-9. [DOI] [PubMed] [Google Scholar]
- 58.ter Huurne N, Fallon SJ, van Schouwenburg M, van der Schaaf M, Buitelaar J, Jensen O, et al. Methylphenidate alters selective attention by amplifying salience. Psychopharmacology. 2015;232:4317–23. doi: 10.1007/s00213-015-4059-y. [DOI] [PubMed] [Google Scholar]
- 59.Repantis D, Schlattmann P, Laisney O, Heuser I. Modafinil and methylphenidate for neuroenhancement in healthy individuals: a systematic review. Pharmacol Res. 2010;62:187–206. doi: 10.1016/j.phrs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y-P, Huang T-S, Tung C-S, Lin C-C. Effects of atomoxetine on attention and impulsivity in the five-choice serial reaction time task in rats with lesions of dorsal noradrenergic ascending bundle. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;56:81–90. doi: 10.1016/j.pnpbp.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Pillidge K, Porter AJ, Vasili T, Heal DJ, Stanford SC. Atomoxetine reduces hyperactive/impulsive behaviours in neurokinin-1 receptor ‘knockout’ mice. Pharm Biochem Behav. 2014;127:56–61. doi: 10.1016/j.pbb.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kehagia AA, Housden CR, Regenthal R, Barker RA, Müller U, Rowe J, et al. Targeting impulsivity in Parkinson’s disease using atomoxetine. Brain. 2014;137:1986–97. doi: 10.1093/brain/awu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caballero-Puntiverio M, Fitzpatrick CM, Woldbye DPD, Andreasen JT. Effects of amphetamine and methylphenidate on attentional performance and impulsivity in the mouse 5-Choice Serial Reaction Time Task. J Psychopharmacol. 2017;31:272–83. doi: 10.1177/0269881116684339. [DOI] [PubMed] [Google Scholar]
- 64.Slezak JM, Ricaurte GA, Tallarida RJ, Katz JL. Methylphenidate and impulsivity: a comparison of effects of methylphenidate enantiomers on delay discounting in rats. Psychopharmacology. 2013;231:191–98. doi: 10.1007/s00213-013-3220-8. [DOI] [PubMed] [Google Scholar]
- 65.Arnsten AFT. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e145–e57. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 67.Marquand AF, De Simoni S, O’daly OG, Williams SCR, Mourao-Miranda J, Mehta MA. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology. 2011;36:1237–47. doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oken BS, Kishiyama SS, Salinsky MC. Pharmacologically induced changes in arousal: effects on behavioral and electrophysiologic measures of alertness and attention. Electroencephalogr Clin Neurophysiol. 1995;95:359–71. doi: 10.1016/0013-4694(95)00124-H. [DOI] [PubMed] [Google Scholar]
- 69.Howells FM, Stein DJ, Russell VA. Synergistic tonic and phasic activity of the locus coeruleus norepinephrine (LC-NE) arousal system is required for optimal attentional performance. Metab Brain Dis. 2012;27:267–74. doi: 10.1007/s11011-012-9287-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.