Abstract
Here we tell the story of ivermectin, describing its anthelmintic and insecticidal actions and recent studies that have sought to reposition ivermectin for the treatment of other infectious diseases. The standard theory of its anthelmintic and insecticidal mode of action is that it is a selective positive allosteric modulator of glutamate-gated chloride channels found in nematodes and insects. At higher concentrations, ivermectin also acts as an allosteric modulator of ion channels found in host central nervous systems. In addition, in tissue culture, at concentrations higher than anthelmintic concentrations, ivermectin shows antiviral, antimalarial, antimetabolic, and anticancer effects. Caution is required before extrapolating from these preliminary repositioning experiments to clinical use, particularly for Covid-19 treatment, because of the high concentrations of ivermectin used in tissue-culture experiments.
Ivermectin, the Anthelmintic and Insecticide
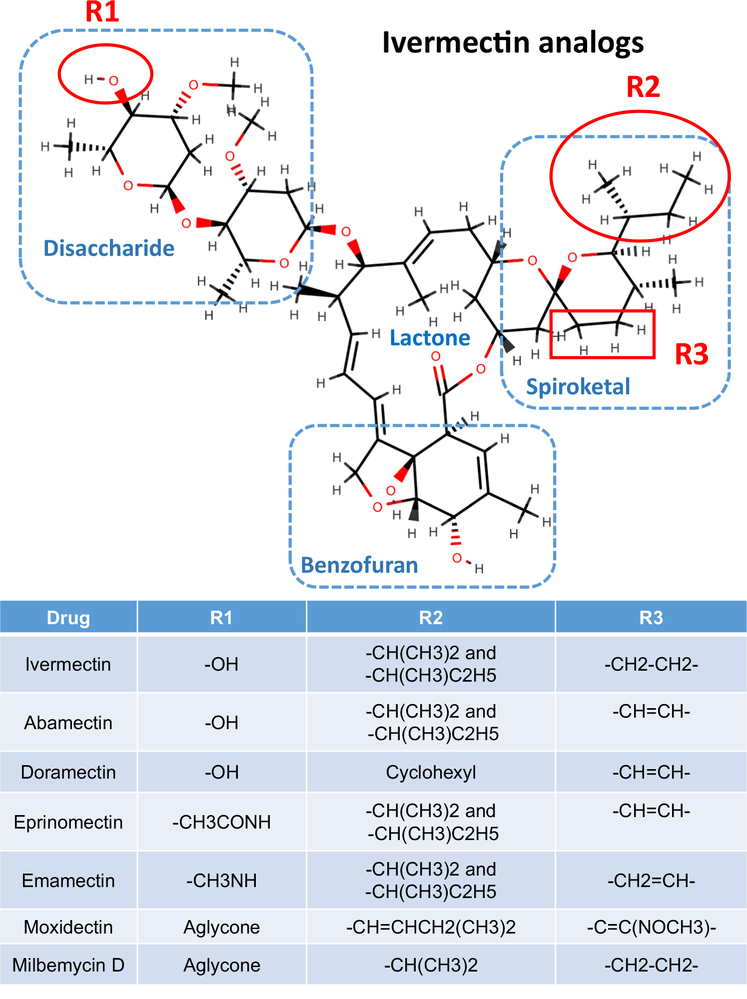
Ivermectin is a mixture of more than 80% 22,23-dihydroavermectin B1a and B1b (Figure 1). It is a remarkably potent anthelmintic and insecticide when given orally at therapeutic doses of 150 or 200 μg/kg to ruminants, pigs, horses, or humans where it yields Cmax plasma concentrations of 11–54 ng/ml or 13–63 nM [1,2]. It is generally safe, with acute LD50 (see Glossary) toxicities seen at 24 000 μg/kg in monkeys [3] and 80 000 μg/kg in beagles [4]; it has a wide spectrum of action against gastrointestinal (GI) parasitic nematodes, lungworms, lice, and mange, but it is not effective against cestodes or trematodes. It is, however, very effective at low plasma concentrations against microfilaria, and it impairs adult filaria fertility for long periods without killing most adult filaria. Here we review ivermectin’s mechanism of action as an anthelmintic, as an insecticide, and recent studies that have sought to reposition ivermectin as an antiviral, antimalarial, and antidiabetic drug (Table 1).
Figure 1. Structure of Ivermectin and Analogs.
The molecule is divided into four regions: the disaccharide, the lactone, the spiroketal, and the benzofuran. Different modifications to this structure at the positions R1, R2, and R3 give rise to ivermectin, abamectin, doramectin, eprinomectin, emamectin, moxidectin, and milbemycin.
Table 1.
Experimental Settings for Studying the Effects of Ivermectin on Ion Channels, FXR, NHR-8, Viruses, Malaria, Cancer, and Asthma
| Target | Experimental preparation | Concentration: ng/ml (nM) | Refs and comment |
|---|---|---|---|
| AVR-15b Haemonchus contortus channel opening | HEK293 expressed | EC50 19 (22 nM) | [28] |
| Abamectin on GluCls of Ascaris suum pharynx inhibition | Dissected whole worm pharynx | EC50 350 (400 nM) | [37] |
| Brugia malayi mf protein release, ES inhibition by Bma-AVR-14A GluCl | Whole Brugia and microfilaria | <88 (<100 nM) | [31] |
| Downregulation of B. malayi meiosis genes | Whole Brugia | <88 (<100 nM) | [8] |
| A. suum GABA ion-channel inhibition | Dissected preparation | <175 (<200 nM) | [27] |
| Histamine-gated Cl channel activation | Expressed channel from Drosophila melanogaster | 875 (1000 nM) used | [33] |
| pH-gated Cl channel activation | Sarcoptes scabiei oocyte expressed receptor | Single conc 10 000 (11 429 nM) | [34] |
| Nematode nAChRs | Expressed channels and A. suum preparation | EC50 368 (420 nM) | [35,37] |
| FXR activation and transcription | FXR and ivermectin Crystal structure and mice, serum glucose and cholesterol decrease | 1300 μg/kg per day for 14 days | [69–72] Repurpose or find more potent analog |
| NHR-8 transcription factor | NHR-8 of Caenorhabditis elegans and H. contortus | Knockout in C. elegansreduced ∼2 LDA (from 1.63 to 0.96 nM) | [104] Low level of resistance |
| Dengue; West Nile virus; Venezuelan equine encephalitis virus inhibition | Tissue culture inhibition transport into by nuclear importin α/β1 | IC50s 875–3500 (1000–4000 nM) | [55–61] More potent analog required to be effective |
| SARS-Covid-2 inhibition | Tissue culture | 4375 (5000 nM), ∼ 100× | [107] |
| Plasmodium inhibition | Culture: inhibition of Plasmodium in liver inhibits nuclear import of signal recognition particles of P. falciparum | IC50 ∼438 (∼500 nM) | [65–67] More potent analog required to be effective. Ivermectin has effects on mosquitoes |
| Cancer | Tissue culture and mice experiments by different mechanisms | <8000 nM | [76,77,79–83,105] High concentrations required for effects |
| Asthma | Mice: reduction of cellular and humoral responses to antigen in asthma model | 2000 μg/kg | [89] High μg/kg dose |
Standard Theory of the Ivermectin Mode of Action
Ivermectin and Analogs Are Allosteric Modulators of GluCl Ion Channels
Ivermectin and its analogs are positive allosteric modulators (PAMs) that selectively open inhibitory glutamate-gated chloride ion channels in the membranes of pharyngeal muscles, motor nerves, female reproductive tracts, and the excretory/secretory (ES) pores of nematodes and of muscle and nerves of insects and crustaceans [5,6]. The effect is: (i) inhibition of pharyngeal pumping (Figure 2) when the pharyngeal muscle is the target; (ii) inhibition of motility when motor nerves are the main target; (iii) inhibition of egg or microfilaria release when the female reproductive tract is the target site; and (iv) loss of host immunosuppression when the ES pore cannot open to release host immunosuppressants.
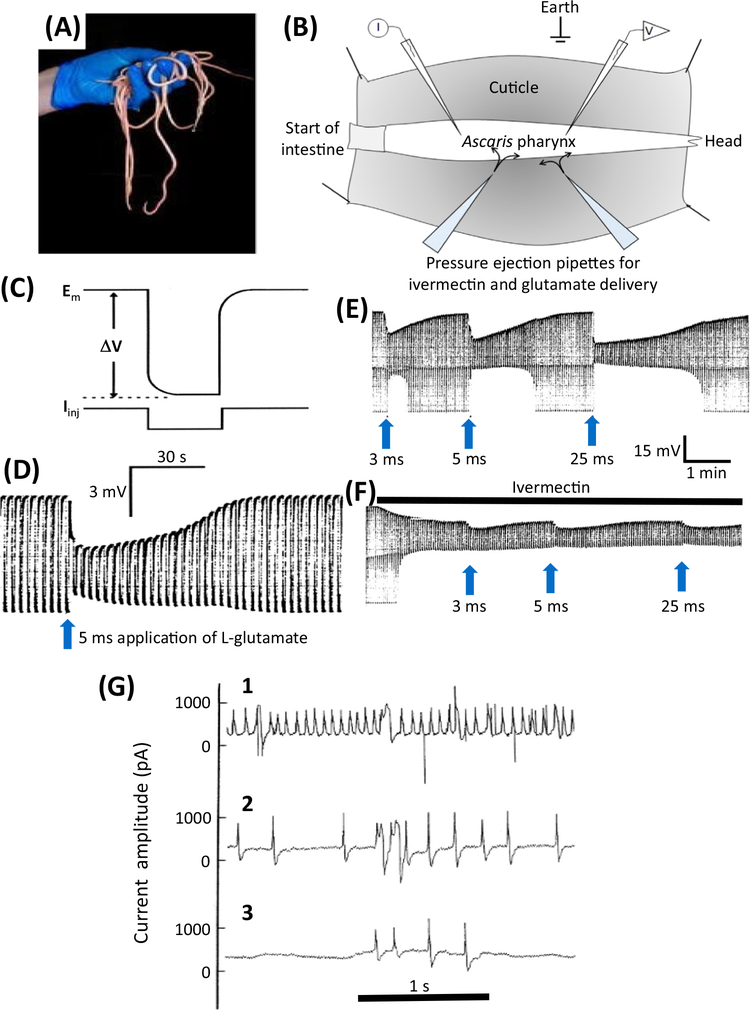
Figure 2. Electrophysiologic Effects of Ivermectin in Parasitic Nematodes.
(A) Ascaris suum is a large nematode that is tractable for electrophysiologic recordings. (B) A diagram of the dissected A. suum pharynx. Two glass micropipettes are placed in the pharynx, one for injecting rectangular current pulses (I) and one for recording the membrane potential (V). Two ‘puffing’ microelectrodes are used to apply, separately, glutamate or ivermectin at different time intervals. (C) Higher time resolution recording showing the injection of a hyperpolarizing rectangular current pulse (Iinj) and the membrane potential (Em) and response to the injected current (ΔV) allowing the input conductance (= 1/resistance) of the pharynx to be measured. (D) A 5 ms puff of 0.5 M glutamate produces a hyperpolarization due to the entry of Cl− (upper envelope of the trace) and a reduction in the width of the trace as the size of the membrane potential change (ΔV) is reduced as the glutamate-gated chloride (GluCl) channels open and increase the conductance of the pharyngeal muscle membrane. (E) Effects of glutamate. The trace shows the membrane potential and input conductance responses to 3 ms, 5 ms, and 25 ms ‘puffs’ of 0.5 M glutamate from a micropipette, which produces maximal changes in input conductance of 16 μS, 33 μS, and 60 μS, respectively. Longer applications of glutamate did not produce an increase in response in this experiment, showing that the 25 μs response led to a maximum response. F) The effect of a continuous ‘puff’ of 100 μM ivermectin applied also by pressure ejection from a micropipette. Once the conductance change produced by the ivermectin reached a stable position of 32.7 μS, glutamate was again applied in the same controlled way using the same application times; this produced a maximum conductance change of 28 μS with a 25 ms puff application. The maximum conductance change obtained with the coapplication of glutamate and ivermectin was 60 μS, virtually the same conductance (cf. 60 μS) change produced after a high-dose application of glutamate. The fact that coapplication did not produce an effect greater than the high dose of glutamate was interpreted as indicating that these two substances activate the same ion channel – because application of two separate ion channels by glutamate and ivermectin at high concentrations would produce an additive response. (G) Electropharyngeograms from Trichostrongylus colubriformis A before, 5 min after, and 15 min after adding 100 nM ivermectin to the bath (from [106]). A patch-pipette was used to record the currents elicited by the pumping pharynx by sucking the head of the worm into the patch-pipette. (1) Control recording before application of 100 nM ivermectin; (2) 5 min after application of 100 nM ivermectin; (3) 15 min after application of 100 nM ivermectin.
Figure 2A–E shows a two-micropipette recording of the effects of glutamate on membrane potential and the conductance of pharyngeal muscle in Ascaris suum. When glutamate is added to the preparation, glutamate-gated chloride (GluCl) ion channels open, the resistance of the pharyngeal muscle decreases, and the membrane potential hyperpolarizes as Cl− enters the pharynx (Figure 2D,E). This is reversible on washing. Ivermectin behaves similarly, but is slower in onset, and because it is lipophilic (sticky) it does not wash off from the preparation after application (Figure 2F). When ivermectin is applied to active pumping pharyngeal muscle cells of Trichostrongylus colubriformis, the electropharyngeogram shows the reduction in electrical activity (Figure 2G).
Although equivalent recordings from the nerves, reproductive tract, and the ES pore of parasitic nematodes have not been made it is expected that similar observations would be made from all their tissues that have GluCl channels. The different tissues in the different parasitic nematodes have molecularly diverse GluCl channels and therefore different sensitivities to ivermectin. The most ivermectin-sensitive tissues, and the phenotypic effects of ivermectin, vary with the parasite species.
Li et al. [7] used in situ hybridization to localize tissue expression of the Brugia malayi GluCl channel gene avr-14 (Bma-AVR-14) in adult filarial worms. Splice variant subunits of Bma-AVR-14 were expressed in female worms in the ovary, in developing embryos, in the lateral hypodermal chords, and in the uterus wall adjacent to stretched microfilariae. The genes were also expressed in adult male worms in the spermatogonia, in the vas deferens, and in the somatic muscles adjacent to the vas deferens. The tissues of the ovary and other reproductive cells are nonexcitable, so the GluCl channels may serve a paracrine function, inhibiting the growth and development of these tissues like other ligand-gated ion channels in the nonexcitable tissues of vertebrates. The activation of GluCl channels in the reproductive tracts of nematodes, by a paracrine function, could change gene expression that produces the longer-term inhibitory effects of ivermectin on the developing embryos and reproductive tissues of filaria and suppression of microfilaria production by female worms. The significance of the effect of ivermectin on the reproductive tract was also shown by RNAseq analysis that revealed downregulation of meiosis genes in female B. malayi by low (100 nM−1 μM) concentrations of ivermectin [8]. The evidence of GluCl channel expression in the nematode reproductive tract of filaria may explain the effects of ivermectin reducing their fertility but not why this effect is so slow in onset. It is pointed out that this effect is prolonged and persists long after the ivermectin has been cleared from the host and is no longer detectable.
Composition of the GluCl Ion Channels
GluCl ion channels consist of a ring of five protein subunits that may arrange in homogeneous or heterogeneous combinations (Figure 3C). In the free-living nematode Caenorhabditis elegans, there are six GluCl genes encoding channel subunits – avr-14, avr-15, glc-1, glc-2, glc-3, and glc-4 – that contribute to the ivermectin sensitivity in C. elegans [9–14]. The GluCl channel subunit gene family is divergent in parasitic nematodes and varies between the species. In the intestinal parasite of sheep, Haemonchus contortus, and in the human hookworms Ancylostoma ceylanicum and Necator americanus, there are no glc-1 nor avr-15 orthologsi. glc-1 is not present and is missingin the filarial parasites B. malayi and Onchocerca volvulus. In H. contortus the subunit genes known to be present are: avr-14, glc-2, glc-3, glc-4, glc-5, and glc-6 [15]. Thus, the GluCl channel gene homologs present varies between the species of nematode.
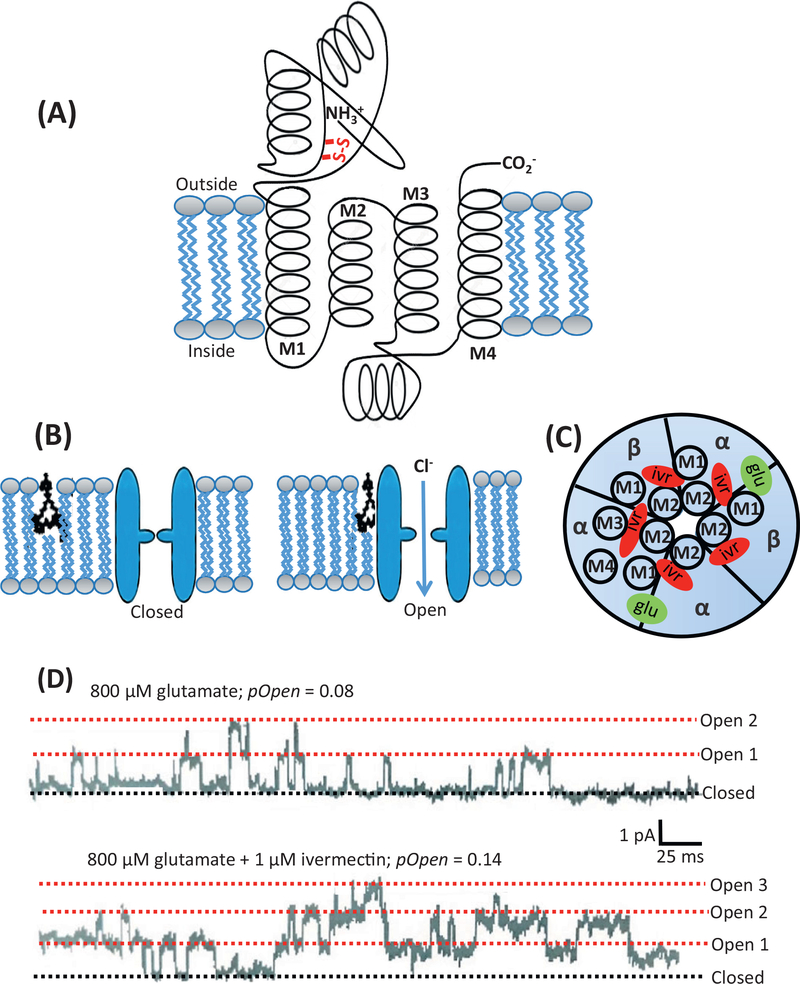
Figure 3. Glutamate-gated Chloride (GluCl) Channel Structure, Ivermectin and Glutamate Binding Sites, and Channel Currents.
(A) Diagram of a GluCl channel subunit. It is about 500 amino acids long and begins at the N terminal on the extracellular face of the membrane. The subunits that contain vicinal cysteines are recognized as α-subunits. Each subunit has four α-helical transmembrane regions: M1, M2, M3, and M4. The M2 region lines the pore of the transmembrane ion channel. Between the M3 and M4 regions there is an extended cytoplasmic loop. The C terminal is also found at the extracellular surface of the membrane. (B) Five subunits are arranged like the staves of a barrel around the central pore of the ion channel to form a pentomer. Ivermectin binds to the channel in the transmembrane region of the ion channel in the outer bilayer of the lipid bilayer membrane and can open the channel. (C) The five subunits of the GluCl channel viewed from above. If the five subunits of the ion channel are all the same, then the ion channel receptor is referred to as homomeric. If it is made of nonidentical subunits it is referred to as heteromeric. The orthosteric ligand-binding site, where glutamate binds, is located between two adjacent subunits (green) and in the extracellular region of the ion channel. The ivermectin binding site is deep in the ion channel and is involved in contact with the M2 region near the pore, and it contacts the M3 region as well. (D) The upper trace shows a cell-attached patch recording at −50 mV with 800 μM glutamate in the patch-pipette. The rectangular current steps representing the opening and closing of GluCl channels can be seen. Each current step is 2 pA (10−12 A). The probability of the channels being open (pOpen) is 0.08, that is, each channel was open for 8% of the time. The lower trace shows the effect of adding 1 μM ivermectin to the bath, and followed after a few minutes, allowing time for ivermectin to move into and along the membrane, the number of GluCl opening increased as did the probability of the channel being open. It increased to 0.14, or 14% of the time. The opening of many ion channels together allows more Cl− current to flow through the membranes and to increase the conductance of the membrane.
The sensitivity to ivermectin varies between the different homomeric and heteromeric GluCl channels. A few amino acid changes in the sequence of any of the subunits may alter the ivermectin sensitivity of the channel significantly. It is not surprising that there are striking differences, even between closely related species: A. ceylanicum is much more sensitive to ivermectin than the new world hookworm, N. americanus, both in vitro and in vivo [16,17]. Other explanations for the difference in sensitivity are also possible.
In many insects, a single GluCl channel gene, GluClα, is present but its transcripts are modified by mRNA splicing and editing [18–20]. The presence of gene splicing adds an additional layer of diversity to the GluCl channel and its sensitivity to ivermectin. An example of the effects of ivermectin in insects is seen with Drosophila melanogaster, where GluClα mediates sensitivity to ivermectin. The presence of GluCl channel targets in insects and arthropod parasites allows ivermectin to be used for the treatment of scabies and lice in humans [21]. Interestingly, ivermectin also has inhibitory effects on mosquitoes, and its use has been suggested to reduce the mosquito vectors in malaria [22]. In humans, it has been shown to be effective for head lice, Pediculus humanus capitis, scabies (Sarcoptes scabiei), myiasis, and mosquitoes [23]. In animals, a range of preparations of ivermectin are used and are effective against lice, mites, screw worms, bot flies, and warbles.
GluCl Channels Have Separate Orthosteric (Glutamate) and Allosteric (Ivermectin) Binding Sites
Each subunit of the pentameric GluCl ion channel is about 500 amino acids long with an N terminal and C terminal on the extracellular surface (Figure 3A). Each subunit has four transmembrane α-helices: M1, M2, M3, and M4. The five subunits of the GluCl channel may be identical (homogeneous) or nonidentical (heterogeneous). Glutamate binds to the orthosteric agonist sites (Figure 4C) which are located at the interface between two subunits [24]. There are two to five orthosteric agonist sites on each channel, depending on the subunit composition of the GluCl channel. Figures 3C and 4C show the orthosteric binding sites where glutamate binds to the channel. When glutamate binds, the channel opens, and each channel opening can be seen under single-channel patch-clamp recording of the A. suum pharynx (Figure 3D, top trace) with current pulses of about 2 pA (2 × 10−12 A) as the channel changes from the closed state to the open state.
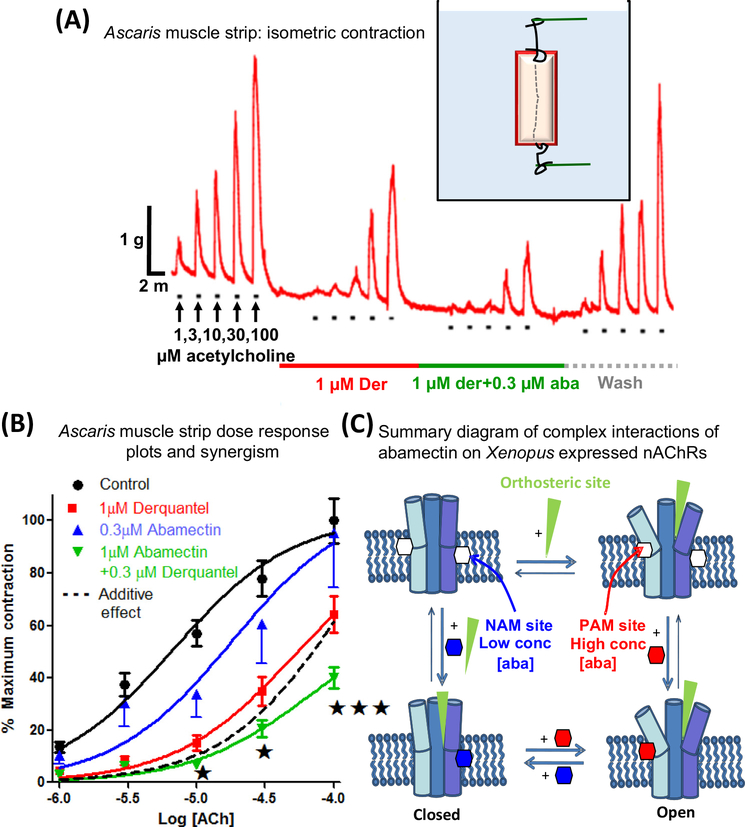
Figure 4. Interactions of Abamectin and Derquantel on Ascaris Muscle Strip Contraction and Summary Diagram of Complex Effects of Abamectin on Nicotinic Acetylcholine Receptors (nAChRs).
(A) Isometric contraction of Ascaris suum muscle strips produced by application of increasing concentrations of acetylcholine and antagonism by 1 μM derquantel (red bar), 1 μM derquantel + 0.3 μM abamectin (green bar), and wash (gray bar). Note that derquantel decreases the responses to acetylcholine and that the addition of abamectin increases the inhibition. (B) An A. suum muscle strip concentration–contraction–response plot of acetylcholine showing mean ± S.E. bars (n = 11). Control (black); in the presence of 1 μM derquantel (red); 1 μM derquantel + 0.3 μM abamectin (green); and wash (blue). Note that abamectin increases the inhibition produced by derquantel. The inhibitory effect of abamectin and derquantel is greater than an additive effect, dotted line [37]. (C) Model of ligand sites of action showing complex effects on heteromeric ion channels. The cholinergic anthelmintic agonists bind to the orthosteric sites, opening the channel. Low concentrations of abamectin (0.03 and 0.1 μM) bind to a negative allosteric site (NAM) in the lipid phase of the channel, inhibiting opening. Higher concentrations of abamectin (0.3, 1, and 10 μM) bind to a positive allosteric site (PAM), increasing opening [35].
The site of action of ivermectin is an allosteric site (Figures 3B,C and 4C), a location that is different to the physiologic glutamate ligand-binding site. Ivermectin binds to the GluCl channel within the transmembrane region of the ion channel (Figure 3B). FRAP (fluorescence recovery after photobleaching) experiments – using fluorescent bodipy (boron-dipyrromethene) ivermectin [25] quenched with trypan blue – show that ivermectin locates in the outer membrane of the phospholipid bilayer to exert its effects on the chloride channels (Figure 3B). The activation of the GluCl channels is progressive and slow, increasing over minutes following application of ivermectin to the preparation (Figure 2F), indicating diffusion into the lipid membrane and diffusion to the ion channel binding site.
Crystal structures of homopentameric GluCl channels [26] provide further details of the molecular binding sites of glutamate and ivermectin to the receptor within the outer phospholipid layer of the membrane. The pentameric GluCl channels form barrel-like staves around their central anion-selective pore. Recall that each of the pentameric subunits has four α-helix transmembrane regions, M1, M2, M3, and M4 (Figure 3A). Ivermectin can insert deeply into the subunits to make contact with the M2 pore-lining α-helix and the M2 and M3 loops because it is lipophilic (Figure 3B,C). This stabilizes the open-pore conformation of the ion channel, thereby increasing the time the ion channel stays open (Figure 3B,D). Single-channel experiments in A. suum [27] show that low concentrations of dihydroavermectin produce progressive opening of channels, giving rise to a ‘staircase’ effect. The conductance of these channels was 9–15 pS. Similar conductances were observed for expressed GluCl channels in H. contortus [28].
Ivermectin Inhibits the Nematode Parasites’ Ability to Suppress the Host Immune Response: Immune Modulation Theory
Nematode parasites can modulate the immune response of their host by releasing a complex mixture of immune modulatory compounds [29,30] and thereby survive within the host. Loss of the ability to release the immune modulators would contribute to the elimination of the parasite. Moreno et al. [31] have pointed out that ivermectin, at submicromolar concentrations, produces a rapid loss of microfilaria in host blood but fails to affect their motility in culture. An explanation for this contradictory observation is that the ivermectin-sensitive GluCl channels of microfilaria are localized on a muscle that surrounds the microfilarial ES vesicle; release of immune-modulator proteins from the ES vesicle is therefore under the control of GluCl channels. Moreno et al. [31] showed that ivermectin reduced protein release from the ES apparatus and that the loss of the ES substances could reduce the ability of the parasite to secrete proteins that allow evasion of the host immune system. Observations [32] with D. immitis microfilariae support this loss of immune modulation by the parasite and show that ivermectin produces attachment of mononuclear cells and neutrophils. These observations imply a direct effect, that is, that ivermectin prevents the parasites’ ability to release substances that inhibit the host’s immune response. The effect of ivermectin on ES substances in nonfilarial nematodes remains to be explored.
Effects of Ivermectin on Channels Other Than GluCls in Nematode Parasites and Insects: Additional Channel Theory
Although it is often assumed that ivermectin only acts therapeutically as a PAM on different GluCl channels of nematodes, insects, and arthropods, it also has PAM and inhibitory negative allosteric (NAM) effects on other ion channels. These actions may support the actions of ivermectin on GluCl channels. It has inhibitory effects on γ-aminobutyric acid (GABA) channel conductances at low concentrations of <0.2 μM in A. suum [27], but at higher (10 μM) concentrations it has potentiating effects. Ivermectin also acts as a PAM on the histamine-gated Cl− channels in D. melanogaster [33], the pH-gated Cl− channel in Sarcoptes scabiei [34], and the nematode pyrantel and levamisole nicotinic acetylcholine receptors (nAChRs) [35,36].
Derquantel and Abamectin Interact and Have Synergistic Effects on Nematode nAChRs
The regular use of anthelmintics and ivermectin has led to concerns about the development of drug resistance. Drug combinations may be more effective than single drugs and may delay the onset of resistance. A combination of the nicotinic antagonist, derquantel, and the ivermectin analog, abamectin, has been found to have synergistic anthelmintic effects against GI nematode parasites. In Ascaris muscle-strip contraction and electrophysiologic experiments [35,37], derquantel and abamectin have potent and synergistic antagonistic effects on muscle nAChRs (Figure 4A,B). The effects of abamectin on expressed nAChRs are complex [36]. At low concentrations there is an inhibition of the nicotinic responses, but this is overcome at high concentrations. The biphasic effects of abamectin suggest that it acts at two allosteric sites (Figure 4C): one, a high-affinity NAM site decreasing the agonist response, and another, lower-affinity, PAM site [35]. The observations illustrate the complex effects of macrocyclic lactones on heteromeric ion channels and that careful adjustment of concentration ratios is necessary when using combinations with other anthelmintics.
Ion Channels in the Mammalian Central Nervous System (CNS) Are Also Affected by Ivermectin
Although relatively free from toxicity, ivermectin – when large overdoses are administered – may cross the blood–brain barrier, producing depressant effects on the CNS [38–43]. Ivermectin may also enter the brain when there are mutant multiple-drug-resistance transporters in the blood–brain barrier that fail to exclude from the brain drugs that are present in the plasma. In the brain, ivermectin targets the mammalian glycine receptors (GlyRs), GABA receptors, and nAChRs [44–46]. Ivermectin acts as a PAM at a lower concentration (0.03 μM) and as an irreversible agonist at higher concentration on the mammalian GlyRs [44]. At nM concentrations, ivermectin potentiates GABA-induced Cl− currents in mouse hippocampal neurons [47].
Ivermectin also has a significant effect on other CNS ion channels [48]: (i) it modulates the adenine channel P2X4 receptor [49]; (ii) it is an inhibitor of the ether-a-go-go-related gene (hERG) K+ channels [50]; (iii) it increases Ca2+ release from sarcoplasmic reticulum (SR) vesicles by activating the ryanodine receptor (RyR) channels [51]; and (iv) it acts as an agonist of G-protein-coupled inwardly rectifying potassium (GIRK) channels. A better understanding of the modulatory effects of ivermectin and different ivermectin analogs on various ion channels could advance development of future CNS therapeutics.
The Spiroketal Groups of Ivermectin Analogs Affect Binding to the Allosteric Site of Ion Channels
Abamectin, doramectin, eprinomectin, emamectin, milbemycin, and moxidectin have a common pharmacophore: a 16-membered macrocyclic lactone ring fused with benzofuran, spiroketal, and disaccharide groups (except that there is no saccharide for moxidectin or milbemycin) (Figure 1). Binding to the ion channels in the transmembrane region involves the benzofuran group, which is inserted deeply into the transmembrane regions, reaching nearly to the pore of the channel. The spiroketal group interacts with the M1, and the disaccharide group is left on the outside of the channels [26]. The size of the spiroketal groups of the ivermectin analogs affects their ability to bind within the channels because of the limited room in the binding pocket. Abamectin is most like ivermectin, with a difference only at the C22 and C23 (R3, Figure 1). The potency of effects of abamectin is therefore similar to that of ivermectin on various ion channels [48]. Doramectin has a large spiroketal group due to the presence of the cyclohexyl moiety (R2, Figure 1) and is less potent. Moxidectin lacks the disaccharide and has a bigger R3 –C=C(NOCH3)– in the spiroketal group and has less effect on host GABA receptors, and reduced CNS toxicity [52], but is more potent on some nematode parasite GluCl channels [53].
Antiviral Effects of Ivermectin
Ivermectin Inhibits NS3 Helicase and Selected Protein Import through Nuclear Pores
The most potent antiviral effects of ivermectin reported appears to be its inhibitory effects, with an ED50 of 3–6 n M concentrations, in virus yield reduction assays with cultures of yellow fever virus. The effect is mediated by inhibition of viral NS3 helicase [54]. Ivermectin also inhibits, at higher concentrations, other flaviviruses, such as Japanese encephalitis virus and tick-borne encephalitis virus, with inhibition of replication ED50s of 200–300 nM concentrations.
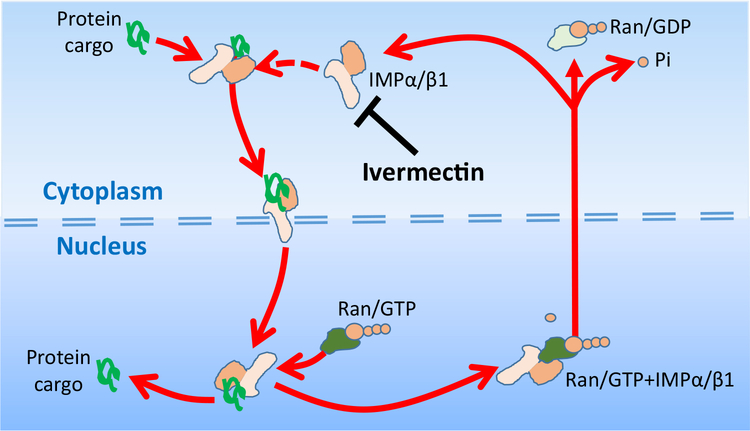
Ivermectin also inhibits the nuclear import of selected cytoplasmic proteins. Figure 5 illustrates how ivermectin inhibits by binding to the heterodimer protein importin (IMP) α/β1, and inhibits the binding of cargo proteins that are carried through the nuclear pore by IMPα/β1 into the nucleus. If IMP binding were not inhibited by ivermectin, IMPα/β1 + cargo protein would be able to pass through the nuclear pore, where the cargo protein is released into the nucleus when RanGTP binds to IMPα/β1 [55]. Viral protein cargos known to bind to IMPα/β1 include: (i) HIV-1 integrase, needed for HIV-1 propagation and incorporation into the host genome; (ii) DENV N55, a dengue virus nonstructural protein-5; and (iii) the simian virus SV40 large tumor antigen [56,57]. IC50s in the 1–4 μM range have been found to limit growth of the following RNA viruses in tissue culture: dengue virus, West Nile virus, and Venezuelan equine encephalitis virus (VEEV) [58] (Table 1). This broad-spectrum activity of ivermectin may be due to the reliance on IMPα/β1 for RNA virus protein transport during infection [56,59].
Figure 5. Ivermectin Inhibits Nuclear Import of Selected Proteins, Including Some Virus Proteins.
Illustrations of how ivermectin binds to the heterodimer protein importin, IMPα/β1, to inhibit the binding of cargo proteins that are carried through the nuclear pore by IMPα/β1 into the nucleus. The cargo protein is recognized by the IMPα/β1 protein by the α/β by a nuclear localization signal (NLS), short sequences of basic amino acids either alone or separated by a linker region of 10–12 amino acids. The import process is energy-dependent, involving GTP and its hydrolysis. Once through the nuclear pore, the protein is released when RanGTP binds to IMPα/β1 to allow release of the cargo into the nucleus. The empty IMPα/β1 is then shuttled back out through the nuclear pore to continue the cycle. GDP,; GTP,; Ran,.
At higher concentrations, ivermectin also exhibits activity against the DNA virus pseudorabies virus (PRV) in vitro and in vivo, with ivermectin treatment increasing the survival of pseudorabies virus-infected mice [60]. Ivermectin, however, has not been reported to be effective against Zika virus (ZIKV) in mice [61]. We point out that the antiviral effects of ivermectin have only been observed in tissue culture or at very high doses in mice, underlining the fact that normal therapeutic anthelmintic doses are not anticipated to show significant antiviral activity, except, perhaps, for yellow fever virus.
Covid-19
Caly et al. [107] tested the antiviral activity of 5 μM ivermectin for 48 h on SARS-CoV-2-infected Vero/hSLAM cell cultures and observed a 5000-fold reduction in viral RNA with an IC50 of 5 μM (4375 ng/ml). This concentration is ~100-fold more than the peak plasma concentration (Cmax) from a single 150 μg/kg dose that is used for normal human anthelmintic therapy [1,62]. Although 2000 μg/kg doses, which are greater than those approved by the Food and Drug Administration (FDA), have been tested on humans (producing a Cmax of 250 ng/ml, and appearing to be safe [63]), the Cmax was still well below the effective concentration of 4375 ng/ml used in the cell-culture experiments. These observations are not encouraging for ivermectin clinical trials for the treatment of Covid-19. Although a phase III trial in Thailand using ivermectin at a dose of 400 μg/kg for 3 days was found to be safe and showed a modest effect against dengue virus (DENV) [64]. These observations are scientifically interesting, but ivermectin is not yet proven to be clinically relevant for Covid-19 treatment. Further research, and the development of more potent analogs, are required for an anti-Covid-19 action. Because of concerns that some individuals have access to veterinary formulations, and that they may consider these formulations for their own use against Covid-19, on 10 April 2020 the FDA issued guidance not to use ivermectin intended for animals as a treatment for Covid-19 in humansii.
Antimalarial Effects of Ivermectin
In addition to controlling filariasis, the mass drug administration (MDA) of ivermectin has also had effects limiting the spread of malaria. Although this is likely due to an inhibitory effect on the mosquito vectors of malaria, there are reports of a direct inhibitory effect of ivermectin on the liver stages of the malaria parasite, Plasmodium sp. [65,66]. Singh et al. [67] tested a number of novel ivermectin analogs and reported that ivermectin inhibits Plasmodium falciparum erythrocyte stages in vitro, with an IC50 near 0.5 μM, and that an improved novel compound (analog-19) had a better IC50 of 0.05 μM. Mendes et al. [66] also describe the inhibitory effects of ivermectin on the development of the parasite inside liver cells in infected mice. The liver-stage inhibition reduced the subsequent blood-stage parasitemia, reduced the clinical effects, and enhanced the survival of the infected mice. The in vivo mice experiments did, however, use a very high (10 mg/kg) ivermectin dose to show the effect and used Plasmodium berghei in the liver of the mice [66].
The mechanism of this antimalarial effect involves signal recognition particles (SRPs) which are universal eukaryote ribonucleoprotein complexes that target proteins to the endoplasmic reticulum (ER). The SRPs are present in both hosts and the malaria parasite. Micromolar concentrations of ivermectin inhibit SRP nuclear import in P. falciparum by blocking the movement of the heterodimer carrier IMPα/β that carries P. falciparum SRP polypeptides into the nucleus. The effect in tissue culture is destruction of P. falciparum [65].
The clinical benefits of these antimalarial effects have not been established. Experiments by the Mendes group [68] reported that 5 mg/kg ivermectin in mice did not result in inhibition of P. berghei transmission, and ivermectin inhibition arises only in the mosquito stage of the transmission cycle. They interpreted their observations to suggest that the gamete/zygote to the ookinete stage is resistant to ivermectin analogs (eprinomectin, abamectin, ivermectin, moxidectin, doramectin, and emamectin), and the oocyst is the most vulnerable stage of the parasite’s sporogonic cycle. The published literature suggests that, at this stage, there is an in vitro effect with high concentrations of ivermectin but the repositioning of ivermectin as an effective antimalarial has not been established.
Antimetabolic Effect of Ivermectin as a Partial Agonist of the Nuclear Farnesoid Receptor (FXR)
FXR is a nuclear receptor, involved in metabolic regulation, that binds to ivermectin with an EC50 in the 200 nM range [69,70]. It is normally bound by bile acids (as the physiologic agonist) and is expressed highly in the liver, small intestine, kidney, and adrenals [71,72]. Following the binding of bile acid, the FXR translocates to the nucleus to target DNA-regulating genes that are involved in the metabolism of bile acids, lipid, and glucose. Although ivermectin has the potential to treat metabolic syndromes, such as nonalcoholic fatty liver disease [72], it is pointed out that it is not proven to do so at antiparasitic concentrations. Nonetheless, ivermectin has been found to be more selective for the FXR, with a higher affinity than bile acids, and the crystal structure of the ligand-binding domain of ivermectin to the FXR has been characterized [69,73]. Ivermectin has a potent partial agonist action on the FXR receptor and has antidiabetic and cholesterol-reducing effects in mice. Because ivermectin is a widely used clinical drug, these observations provide a useful chemical skeleton for the design of novel FXR ligands that have antimetabolic effects [69].
Anticancer Effects of Ivermectin
To overcome the cancer therapeutic bottleneck, repurposing of a number of drugs, including anthelmintics, has been suggested [74]. Juarez et al. [75] and Antoszczak et al. [76] have reviewed the anticancer effects of ivermectin at high concentrations (0.1–100 μM or doses of 3–40 mg/kg) in tissue culture and in mice. The modes of action of ivermectin on different types of cancer are proposed to involve different mechanisms: (i) the multidrug-resistance transporter protein [77]; (ii) Akt/mTOR [78] and WNT-TCF pathways [79]; (iii) purinergic receptors [80]; (iv) PAK-1 [81]; (v) the cancer-related epigenetic deregulators SIN3A and SIN3B, RNA helicase; (vi) effects on mammalian tubulin polymerization and depolymerization dynamics [82]; and (vii) preferential targeting of cancer stem-cell-like populations [83]. The potential for repurposing ivermectin for cancer treatment remains to be established, as does the mechanisms of action.
Mycobacterium
Ivermectin is not recognized as an effective antibacterial compound. Despite this, there have been some reports of very high concentrations (3–24 μg/ml) of ivermectin having effects on Mycobacterium tuberculosis [84], but these observations have been challenged because of the methods used [85]. A subsequent publication has suggested that ivermectin be considered for the treatment of Mycobacterium ulcerans, which causes Buruli ulcer [86]. No mode of action for this effect of ivermectin has been proposed, and no further publications were found to date.
Asthma
Ivermectin has been reported to exhibit anti-inflammatory properties by affecting cellular and humoral responses [87], and inhibiting lipopolysaccharide (LPS)-induced nitric oxide and prostaglandin E2 production [88]. Yan et al. [89] investigated the therapeutic potential of 2 mg/kg ivermectin for the treatment of allergic asthma in mice and found that it reduced the symptoms in mice by curtailing the recruitment of inflammatory cells and reducing the hypersecretion of mucus. These anti-inflammatory effects were likely due to decreased production of the bracheoalveolar lavage fluid cytokines, and reduced secretion of ovoalbumin-specific IgE and IgG1. Ivermectin induces immunogenic cell death by a variety of mechanisms, including the mechanisms described in the previous text associated with its anticancer action and ATP-dependent immune responses [75]. The use of ivermectin 1% cream for the inflammatory lesions of rosacea is recognized, but this may relate to its antiparasitic effect on the Demodex mite.
Resistance to Ivermectin: Candidate Genes, Pgps, and NHR-8
Resistance to ivermectin and its analogs in parasitic nematodes is of increasing concern; the mechanisms of this resistance has not been explained satisfactorily. We do have information on ivermectin resistance in the model nematode, C. elegans, where it appears that simultaneous mutations of several genes are required for very high levels (>100×) of resistance. Dent et al. [13] observed that three GluCl channel genes – glc-1, avr-14, and avr-15 – were required to produce strong ivermectin resistance, and if one of these genes were not mutated then the level of resistance was lower. The resistance phenotype was modified by mutations in the innexin genes, which code for proteins that coordinate electrical coupling between cells via gap junctions (unc-7 and unc-9), and genes of sensory head amphid neurons associated with dye uptake (osm-1, osm-5, dyf-7, dyf-11, and che-3) [90]. These high levels of resistance are less likely to be required for ivermectin resistance in nematode parasites because the maximum concentrations that the parasite in the host is exposed to during therapy is related to the maximum plasma concentrations, which are less than the concentrations applied to C. elegans in the Petri dish.
These genes of C. elegans have been pursued in parasitic nematodes as candidate genes of ivermectin resistance. Freeman et al. [91] reported that amphidial neurons were abnormal in an ivermectin-resistant isolate of H. contortus in comparison to ivermectin-sensitive isolates. However, to date, no readily identified mutant candidate gene in the parasitic nematodes has been associated with field resistance. Still, it is worth noting that a broad major quantitative trait locus (QTL) on chromosome V for ivermectin resistance has been identified in H. contortus by backcrossing experiments [92,93], so a molecular test for ivermectin resistance may emerge from these studies.
Increased excretion of ivermectin analogs by P-glycoproteins (Pgps) has also been a focus of much research as a source of ivermectin resistance [94,95]. Pgps belong to the superfamily of ATP-binding cassette (ABC) transporters and form the ABCB subfamily [96]. The xenobiotic transporters are only modestly selective for their substrates, often preferring lipophilic or neutral drugs for transport [97,98].
The mechanism of anthelmintic drug resistance due to Pgps has been linked to a relatively modest increase in the expression of some specific candidate Pgps in parasitic nematodes: Pgp-2 and Pgp-9 in H. contortus [99], Pgp-9 in T. circumcincta [100], Pgp-11 in P. univalens [101] and T. circumcincta [102]. Ivermectin analogs with the disaccharide moiety are better substrates for the Pgps than the aglycones like moxidectin [103]. This difference appears to contribute to the ability of moxidectin to overcome low levels of ivermectin resistance and become ‘tolerant’. We point out, however, that the increase in Pgps in parasitic nematodes as the explanation for resistance remains relatively weak given the large number of differentially expressed genes between isolates. This difficulty is perhaps understandable given the process of gene selection under drug pressure and the polymorphisms of the genes.
If we return to recent studies on the model C. elegans and the development of ivermectin tolerance in C. elegans [104], it appears that a modest ivermectin resistance, comparable to that in parasitic nematodes, may be due to increased excretion and metabolism rather than to mutation of target genes like glc-1, avr-14, and avr-15. The increased excretion associated with increased expression of the Pgps (pgp-1, pgp-3, pgp-6, pgp-9, pgp-13, and mrp-6), metabolism by the cytochrome oxidases like CYP14, and metabolism by the glutathione S-transferases (GSTs) GST-4 and GST-10, are driven by the nuclear hormone receptor NHR-8 [104]. Thus, an effect of ivermectin activating NHR-8 would lead to a catalog of changes in expressed genes, leading to enhanced removal of ivermectin and reduced sensitivity by a polygenic mechanism and resistance.
Concluding Remarks
We have seen that ivermectin and analogs are remarkable anthelmintics and insecticides. Despite their wide spectrum of action on many species of parasite they have limited effects on adult filarial nematodes. Future research should seek to determine the explanation for this (see Outstanding Questions). If it is found that the GluCl channels of adult filaria are less sensitive to the existing macrocyclic lactones, a second generation of ivermectin analogs, or a combination with other anthelmintics, should be developed. The strongest commercial driver for the development of new ivermectin analogs may be the need to treat filarial heartworm in dogs that have become less sensitive to the current macrocyclic lactones. The development of a successful ivermectin analog for animals that is safe and effective against adult filaria could support the development of improved MDA control of onchocerciasis and lymphatic filariasis in humans.
Outstanding Questions.
Why does ivermectin have a limited effect on adult filaria? Can the limited efficacy be overcome with a second generation of ivermectin analogs?
GluCl channels are heterogeneous, varying in ivermectin sensitivity between the muscles, nerves, intestine, and reproductive tissue of individual nematodes and between the species of nematodes. What are the important locations of GluCl channels that are sensitive to ivermectin in important nematode parasites? How does activation of these GluCl channels affect the parasite?
What are the mechanisms of ivermectin resistance in nematode parasites, and can they be overcome?
Can ivermectin or its analogs be repositioned successfully for the treatment of viruses, malaria, cancer, and selected metabolic diseases such as diabetes?
Much is known about the mode of action of ivermectin as PAMs of GluCl channels. We need more research to characterize the heterogeneity of GluCl channels to determine how the sensitivity to ivermectin varies with the molecular structures of the different GluCl channels seen in the different parasite species and in their tissues. We also need more research to explain the slow and gradual effect of ivermectin on Dirofilaria immitis reproduction.
Another important area for future research is to determine the mechanisms of ivermectin resistance in parasitic nematodes. In this regard, the only substantial evidence is the large effect of the QTL on chromosome V in H. contortus. The mechanisms could include Pgps, increased metabolism, and NHR-8, but we still do not have an adequate cellular and molecular explanation for ivermectin resistance in parasitic nematodes.
Repurposing studies, using tissue culture and high concentrations of ivermectin, have revealed antiviral, antimalarial, antimetabolic, and anticancer actions, but the concentrations required to see effects in most tissue-culture studies are above the plasma concentrations seen in the usual therapeutic doses. New improved delivery systems for ivermectin that increase local concentrations without toxicity, or new analogs, should be studied for the treatment of viruses, malaria, cancer, or selected metabolic diseases such as diabetes.
Highlights.
Ivermectin and analogs are remarkable broad-spectrum anthelmintics and insecticides, but resistance is now a real concern. Resistance mechanisms have been proposed but they do not appear to account for the resistance seen in parasitic nematodes.
Ivermectin is very effective for controlling microfilaria at low doses but it has limited effects on adult filaria for unclear reasons.
Ivermectin is a positive allosteric modulator of glutamate-gated chloride channels found in both nematodes and insects, and it binds to the channels in their lipid phase.
Ivermectin and analogs also modulate other ion channels and have effects on the mammalian host brain when the blood–brain barrier is impaired.
Preliminary repositioning studies of ivermectin show antiviral, antimalarial, antimetabolic, and anticancer effects at concentrations higher than anthelmintic concentrations in tissue culture.
Acknowledgments
R.J.M. acknowledges support from NIH R01AI047194-17, R01AI155413, R21AI13967, and the E.A. Benbrook Foundation for Pathology and Parasitology. The content does not represent the official views of the National Institute of Allergy and Infectious Diseases.
Glossary
- Allosteric site
a binding site on the GluCl receptor channel that is not the same site on the receptor as the glutamate agonist. Ivermectin binds to the allosteric site to increase the opening of the channel.
- EC50
the concentration of a drug that produces 50% of the maximum response.
- Excretory/secretory (ES)
nematodes have an esophageal gland near the head region that releases ES substances; they include a range of active substances that, in animal parasitic nematodes, have immune-suppressant actions, anticoagulant effects, protease inhibitory effects, and microvesicles containing RNA.
- γ-aminobutyric acid (GABA)
an inhibitory transmitter that binds to pentameric transmembrane ion channels to open them; the channels then conduct Cl− ions to hyperpolarize the cell. These ion channels are referred to as GABA receptors; they are present on mammalian nerve cells and on nematode muscle and nerve cells.
- G-protein-coupled inwardly rectifying potassium (GIRK) channels
when potassium ion channels open they hyperpolarize the cell as K+ leaves the cell, producing inhibition. There are a number of different types of potassium ion channel. The GIRK channel is coupled to a G-protein receptor. Its current voltage relationship shows inward rectification, allowing potassium to move into the cell more easily that out of the cell.
- Glutamate-gated chloride (GluCl) ion channels
recognized target sites of ivermectin. They are pentameric Cl− ion channels that are opened by the transmitter, glutamate, and by an allosteric effect of ivermectin. They are present only in invertebrates (insects, crabs, and nematodes) – not in flukes or tapeworms or mammalian hosts. Opening of the GluCl channels by ivermectin produces inhibition of muscle, nerve, and other tissues. Eight genes, identified in nematodes, encode different subunits of the pentameric GluCl ion channels. These genes are referred to as: avr-14, avr-15, glc-1, glc-2, glc-3, glc-4, glc-5, and glc-6.
- Glycine receptors (GlyRs)
receptors present on host nerve cells in the brain and nerve ganglia, but not in the nematode parasite. They are Cl− ion-channel receptors that are opened by the transmitter glycine. Glycine, as an inhibitory transmitter, binds to the pentameric glycine transmembrane ion channels to open them; the channels then conduct Cl− ions to hyperpolarize the cells.
- hERG
the alpha subunit of the ether-a go-go potassium ion channel. This is another type of potassium channel that repolarizes the cardiac action potential. It is sensitive to some xenobiotic drugs that produce cardiac arrythmias.
- IC50
the concentration of a drug that inhibits 50% of the maximum response.
- LD50
the dose, usually μg/K or mg/Kg, that has been measured to kill half the population of the test animals. LD50 usually refers to a single oral dose but the conditions (acute, chronic, animal, and mode of administration) will affect the LD50 value.
- Mass drug administration (MDA)
a strategy used to control neglected tropical diseases by treating entire at-risk populations with a chemotherapeutic agent, like the use of ivermectin to control onchocerciasis. Usually the administration is made once or twice a year. It can result in a maintained reduction of the diseases.
- P-glycoprotein (Pgp)
a plasma membrane transporter that acts on xenobiotic drugs to export them out of the cells. There are many PgPs, each having a selective preference for the xenobiotic that they export. They are energy-dependent and require ATP.
- Positive allosteric modulators (PAMs)
pharmacological receptors can be activated by agonists binding to the normal physiological orthosteric site or they may be activated by ligands binding to another, usually nonphysiologic site known as an allosteric site. A PAM is such a ligand; it binds to the allosteric site to activate the receptor.
- Quantitative trait locus (QTL)
a section of DNA, on a chromosome, that correlates with a quantitative phenotypic trait (motility, drug-resistance, size, etc.) of a population of nematode parasites (or other organisms).
- Ryanodine receptor (RyR)
an intracellular calcium-permeable channel found in the membrane of the endoplasmic reticulum or sarcoplasmic reticulum of cells. It is a major cellular mediator of calcium-induced calcium release in animal cells and is activated pharmacologically by ryanodine.
- Signal recognition particles (SRPs)
conserved ribonuclear proteins that recognize and target translating proteins to the protein-conducting channel (translocon) in the endoplasmic reticulum in eukaryotes during protein synthesis.
Footnotes
References
- 1.Canga AG et al. (2008) The pharmacokinetics and interactions of ivermectin in humans – A mini-review. AAPS J. 10, 42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González Canga A et al. (2009) The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet. J. 179, 25–37 [DOI] [PubMed] [Google Scholar]
- 3.Merck (2019) Stomectol safety data sheet. p. 7 [Google Scholar]
- 4.Bauck S (1987) Ivermectin toxicity in small animals. Can. Vet. J. 28, 563–564 [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell WC et al. , eds (1989) Ivermectin and Abamectin, Springer-Verlag [Google Scholar]
- 6.Vercruysse J et al. , eds (2002) Macrocyclic Lactontes in Antiparasitic Therapy, CABI [Google Scholar]
- 7.Li BW et al. (2014) High level expression of a glutamate-gated chloride channel gene in reproductive tissues of Brugia malayi may explain the sterilizing effect of ivermectin on filarial worms. Int. J. Parasitol. Drugs Drug Resist 4, 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballesteros C et al. (2016) The effects of ivermectin on Brugia malayi females in vitro: a transcriptomic approach. PLoS Negl. Trop. Dis. 10, e0004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cully DF et al. (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371, 707–711 [DOI] [PubMed] [Google Scholar]
- 10.Cully DF et al. (1996) Molecular biology and electrophysiology of glutamate-gated chloride channels of invertebrates. Parasitology 113, S191–S200 [DOI] [PubMed] [Google Scholar]
- 11.Vassilatis DK et al. (1997) Genetic and biochemical evidence for a novel avermectin-sensitive chloride channel in Caenorhabditis elegans. Isolation and characterization. J. Biol. Chem. 272, 33167–33174 [DOI] [PubMed] [Google Scholar]
- 12.Dent JA et al. (1997) avr-15 encodes a chloride channel sub-unit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 16, 5867–5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dent JA et al. (2000) The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 97, 2674–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horoszok L et al. (2001) GLC-3: a novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. Br. J. Pharmacol. 132, 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glendinning SK et al. (2011) Glutamate-gated chloride channels of Haemonchus contortus restore drug sensitivity to ivermectin resistant Caenorhabditis elegans. PLoS One 6, e22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards JC et al. (1995) In vitro studies on the relative sensitivity to ivermectin of Necator americanus and Ancylostoma ceylanicum. Int. J. Parasitol. 25, 1185–1191 [DOI] [PubMed] [Google Scholar]
- 17.Behnke JM et al. (1993) Sensitivity to ivermectin and pyrantel of Ancylostoma ceylanicum and Necator americanus. Int. J. Parasitol. 23, 945–952 [DOI] [PubMed] [Google Scholar]
- 18.Semenov EP and Pak WL (1999) Diversification of Drosophila chloride channel gene by multiple posttranscriptional mRNA modifications. J. Neurochem. 72, 66–72 [DOI] [PubMed] [Google Scholar]
- 19.Jones AK et al. (2010) The cys-loop ligand-gated ion channel gene superfamily of the parasitoid wasp, Nasonia vitripennis. Heredity (Edinb) 104, 247–259 [DOI] [PubMed] [Google Scholar]
- 20.Jones AK and Sattelle DB (2007) The cys-loop ligand-gated ion channel gene superfamily of the red flour beetle, Tribolium castaneum. BMC Genom 8, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendel K and Rompalo A (2002) Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin. Infect. Dis. 35, S146–S151 [DOI] [PubMed] [Google Scholar]
- 22.Kobylinski KC et al. (2010) The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 116, 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashour DS (2019) Ivermectin: From theory to clinical application. Int. J. Antimicrob. Agents 54, 134–142 [DOI] [PubMed] [Google Scholar]
- 24.Degani-Katzav N et al. (2016) Subunit stoichiometry and arrangement in a heteromeric glutamate-gated chloride channel. Proc. Natl. Acad. Sci. U. S. A. 113, E644–E653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin RJ and Kusel JR (1992) On the distribution of a fluorescent ivermectin probe (4” 5,7-dimethyl-bodipy proprionylivermectin) in Ascaris membranes. Parasitology 104, 549–555 [DOI] [PubMed] [Google Scholar]
- 26.Hibbs RE and Gouaux E (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin RJ and Pennington AJ (1989) A patch-clamp study of effects of dihydroavermectin on Ascaris muscle. Br. J. Pharmacol. 98, 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atif M et al. (2017) Effects of glutamate and ivermectin on single glutamate-gated chloride channels of the parasitic nematode H. contortus. PLoS Pathog. 13, e1006663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King IL and Li Y (2018) Host–parasite interactions promote disease tolerance to intestinal helminth infection. Front. Immunol. 9, 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maizels RM (2013) Toxocara canis: molecular basis of immune recognition and evasion. Vet. Parasitol. 193, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno Y et al. (2010) Ivermectin disrupts the function of the excretory–secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. U. S. A. 107, 20120–20125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vatta AF et al. (2014) Ivermectin-dependent attachment of neutrophils and peripheral blood mononuclear cells to Dirofilaria immitis microfilariae in vitro. Vet. Parasitol. 206, 38–42 [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y et al. (2002) Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J. Biol. Chem. 277, 2000–2005 [DOI] [PubMed] [Google Scholar]
- 34.Mounsey KE et al. (2007) Molecular characterisation of a pH-gated chloride channel from Sarcoptes scabiei. Invertebr. Neurosci. 7, 149–156 [DOI] [PubMed] [Google Scholar]
- 35.Abongwa M et al. (2016) Curiouser and curiouser: the macro-cyclic lactone, abamectin, is also a potent inhibitor of pyrantel/tribendimidine nicotinic acetylcholine receptors of gastro-intestinal worms. PLoS One 11, e0146854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin R et al. (2015) The Conqueror Worm: recent advances with cholinergic anthelmintics and techniques excite research for better therapeutic drugs. J. Helminthol. 89, 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puttachary S et al. (2013) Derquantel and abamectin: effects and interactions on isolated tissues of Ascaris suum. Mol. Biochem. Parasitol. 188, 79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geyer J and Janko C (2012) Treatment of MDR1 mutant dogs with macrocyclic lactones. Curr. Pharm. Biotechnol. 13, 969–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janko C and Geyer J (2013) Moxidectin has a lower neurotoxic potential but comparable brain penetration in P-glycoprotein-deficient CF-1 mice compared to ivermectin. J. Vet. Pharmacol. Ther. 36, 275–284 [DOI] [PubMed] [Google Scholar]
- 40.Geyer J et al. (2009) Brain penetration of ivermectin and selamectin in mdr1a,b P-glycoprotein- and bcrp- deficient knockout mice. J. Vet. Pharmacol. Ther. 32, 87–96 [DOI] [PubMed] [Google Scholar]
- 41.Schinkel AH et al. (1994) Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood–brain barrier and to increased sensitivity to drugs. Cell 77, 491–502 [DOI] [PubMed] [Google Scholar]
- 42.Mealey KL et al. (2002) Frequency of the mutant MDR1 allele associated with ivermectin sensitivity in a sample population of collies from the northwestern United States. Am. J. Vet. Res. 63, 479–481 [DOI] [PubMed] [Google Scholar]
- 43.Roulet A et al. (2003) MDR1-deficient genotype in Collie dogs hypersensitive to the P-glycoprotein substrate ivermectin. Eur. J. Pharmacol. 460, 85–91 [DOI] [PubMed] [Google Scholar]
- 44.Shan Q et al. (2001) Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J. Biol. Chem. 276, 12556–12564 [DOI] [PubMed] [Google Scholar]
- 45.Adelsberger H et al. (2000) Activation of rat recombinant alpha(1)beta(2)gamma(2S) GABA(A) receptor by the insecticide ivermectin. Eur. J. Pharmacol. 394, 163–170 [DOI] [PubMed] [Google Scholar]
- 46.Krause RM et al. (1998) Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 53, 283–294 [DOI] [PubMed] [Google Scholar]
- 47.Kru°šek J and Zemková H (1994) Effect of ivermectin on γ-aminobutyric acid-induced chloride currents in mouse hippocampal embryonic neurones. Eur. J. Pharmacol. 259, 121–128 [DOI] [PubMed] [Google Scholar]
- 48.Chen IS and Kubo Y (2018) Ivermectin and its targetmolecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J. Physiol. 596, 1833–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silberberg SD et al. (2007) Ivermectin Interaction with trans- membrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron 54, 263–274 [DOI] [PubMed] [Google Scholar]
- 50.Kauthale RR et al. (2015) Assessment of temperature-induced hERG channel blockade variation by drugs. J. Appl. Toxicol. 35, 799–805 [DOI] [PubMed] [Google Scholar]
- 51.Ahern GP et al. (1999) Effects of ivermectin and midecamycin on ryanodine receptors and the Ca2+-ATPase in sarcoplasmic reticulum of rabbit and rat skeletal muscle. J. Physiol. 514, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ménez C et al. (2012) Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (−/−) mice and effects on mammalian GABA(A) channel activity. PLoS Negl. Trop. Dis. 6, e1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Njue AI et al. (2004) Mutations in the extracellular domains of glutamate-gated chloride channel alpha3 and beta subunits from ivermectin-resistant Cooperia oncophora affect agonist sensitivity. J. Neurochem. 89, 1137–1147 [DOI] [PubMed] [Google Scholar]
- 54.Mastrangelo E et al. (2012) Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J. Antimicrob. Chemother. 67, 1884–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagstaff KM et al. (2011) An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen. 16, 192–200 [DOI] [PubMed] [Google Scholar]
- 56.Caly L et al. (2012) Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antivir. Res 95, 202–206 [DOI] [PubMed] [Google Scholar]
- 57.Wagstaff KM et al. (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 443, 851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundberg L et al. (2013) Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan equine encephalitis virus replication. Antivir. Res. 100, 662–672 [DOI] [PubMed] [Google Scholar]
- 59.Jans DA et al. (2019) Inhibitors of nuclear transport. Curr. Opin. Cell Biol. 58, 50–60 [DOI] [PubMed] [Google Scholar]
- 60.Lv C et al. (2018) Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and in vivo. Antivir. Res. 159, 55–62 [DOI] [PubMed] [Google Scholar]
- 61.Ketkar H et al. (2019) Lack of efficacy of ivermectin for prevention of a lethal Zika virus infection in a murine system. Diagn. Microbiol. Infect. Dis. 95, 38–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaccour C et al. (2020) Ivermectin and COVID-19: keeping rigor in times of urgency. Am. J. Trop. Med. Hyg. 102, 1156–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzzo CA et al. (2002) Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 42, 1122–1133 [DOI] [PubMed] [Google Scholar]
- 64.Eakkawit Y et al. (2018) Efficacy and Safety of Ivermectin against Dengue Infection: A Phase III, Randomized, Double-blind, Placebo-controlled Trial. In The 34th Annual Meeting the Royal College of Physicians of Thailand: ‘Internal Medicine and One Health’ [Google Scholar]
- 65.Panchal M et al. (2014) Plasmodium falciparum signal recognition particle components and anti-parasitic effect of ivermectin in blocking nucleo-cytoplasmic shuttling of SRP. Cell Death Dis. 5, e994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendes AM et al. (2017) Inhibition of Plasmodium liver infection by ivermectin. Antimicrob. Agents Chemother. 61, e02005–e02016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh L et al. (2020) Molecular design and synthesis of iver- mectin hybrids targeting hepatic and erythrocytic stages of Plasmodium parasites. J. Med. Chem. 63, 1750–1762 [DOI] [PubMed] [Google Scholar]
- 68.Azevedo R et al. (2019) Inhibition of Plasmodium sporogonic stages by ivermectin and other avermectins. Parasit. Vectors 12, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jin L et al. (2013) The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism. Nat. Commun. 4, 1937. [DOI] [PubMed] [Google Scholar]
- 70.Jin L et al. (2015) Selective targeting of nuclear receptor FXR by avermectin analogues with therapeutic effects on nonalcoholic fatty liver disease. Sci. Rep. 5, 17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang YD et al. (2008) Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 48, 1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang YD et al. (2008) FXR: a metabolic regulator and cell protector. Cell Res. 18, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 73.Akwabi-Ameyaw A et al. (2009) FXR agonist activity of conformationally constrained analogs of GW 4064. Bioorg. Med. Chem. Lett. 19, 4733–4739 [DOI] [PubMed] [Google Scholar]
- 74.Zhang Z et al. (2020) Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target Ther. 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Juarez M et al. (2018) The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 8, 317–331 [PMC free article] [PubMed] [Google Scholar]
- 76.Antoszczak M et al. (2020) Old wine in new bottles: Drug repurposing in oncology. Eur. J. Pharmacol. 866, 172784. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X et al. (2020) Inhibition of TMEM16A Ca. Pharmacol. Res. 156, 104763. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X et al. (2020) Ivermectin augments the in vitro and in vivo efficacy of cisplatin in epithelial ovarian cancer by suppressing Akt/mTOR signaling. Am J Med Sci 359, 123–129 [DOI] [PubMed] [Google Scholar]
- 79.Melotti A et al. (2014) The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol. Med. 6, 1263–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Draganov D et al. (2015) Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci. Rep. 5, 16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L et al. (2020) Ivermectin suppresses tumour growth and metastasis through degradation of PAK1 in oesophageal squamous cell carcinoma. J. Cell. Mol. Med. 24, 5387–5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashraf S and Prichard R (2016) Ivermectin exhibits potent anti-mitotic activity. Vet. Parasitol. 226, 1–4 [DOI] [PubMed] [Google Scholar]
- 83.Dominguez-Gomez G et al. (2018) Ivermectin as an inhibitor of cancer stem-like cells. Mol. Med. Rep. 17, 3397–3403 [DOI] [PubMed] [Google Scholar]
- 84.Lim LE et al. (2013) Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob. Agents Chemother. 57, 1040–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muhammed Ameen S and Drancourt M (2014) Comment on: measurements of the in vitro anti-mycobacterial activity of ivermectin are method-dependent. J. Antimicrob. Chemother. 69, 1724–1725 [DOI] [PubMed] [Google Scholar]
- 86.Omansen TF et al. (2015) In-vitro activity of avermectins against Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 9, e0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stankiewicz M et al. (1995) Influence of ivermectin on cellular and humoral immune responses of lambs. Vet. Immunol. Immunopathol. 44, 347–358 [DOI] [PubMed] [Google Scholar]
- 88.Zhang X et al. (2009) Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 9, 354–359 [DOI] [PubMed] [Google Scholar]
- 89.Yan S et al. (2011) Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm. Res. 60, 589–596 [DOI] [PubMed] [Google Scholar]
- 90.Urdaneta-Marquez L et al. (2014) A dyf-7 haplotype causes sensory neuron defects and is associated with macrocyclic lactone resistance worldwide in the nematode parasite Haemonchus contortus. Int. J. Parasitol. 44, 1063–1071 [DOI] [PubMed] [Google Scholar]
- 91.Freeman AS et al. (2003) Amphidial structure of ivermectin- resistant and susceptible laboratory and field strains of Haemonchus contortus. Vet. Parasitol. 110, 217–226 [DOI] [PubMed] [Google Scholar]
- 92.Sallé G et al. (2019) The global diversity of Haemonchus contortus is shaped by human intervention and climate. Nat. Commun. 10, 4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doyle SR et al. (2019) Population genomic and evolutionary modelling analyses reveal a single major QTL for ivermectin drug resistance in the pathogenic nematode, Haemonchus contortus. BMC Genom. 20, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kotze A et al. (2014) Recent advances in candidate-gene and whole-genome approaches to the discovery of anthelmintic resistance markers and the description of drug/receptor inter- actions. Int. J. Parasitol. Drugs Drug Resist 4, 164–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kotze AC and Prichard RK (2016) Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Adv. Parasitol. 93, 397–428 [DOI] [PubMed] [Google Scholar]
- 96.Jones PM and George AM (2004) The ABC transporter structure and mechanism: perspectives on recent research. Cell. Mol. Life Sci. 61, 682–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hodges LM et al. (2011) Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet. Genom. 21, 152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hooiveld GJEJ et al. (2002) Stereoselective transport of hydrophilic quaternary drugs by human MDR1 and rat Mdr1b P-glycoproteins. Br. J. Pharmacol. 135, 1685–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roulet A and Prichard RK (2006) Ivermectin and moxidectin cause constitutive and induced over expression of different P-glycoproteins in resistant Haemonchus contortus. In Annual Meeting of the American Association of Veterinary Parasitologists, abstract no. 72, Honolulu, USA [Google Scholar]
- 100.Dicker AJ et al. (2011) Gene expression changes in a P-glycoprotein (Tci-pgp-9) putatively associatedwith ivermectin resistance in Teladorsagia circumcincta. Int. J. Parasitol. 41, 935–942 [DOI] [PubMed] [Google Scholar]
- 101.Janssen IJ et al. (2013) Genetic variants and increased expression of Parascaris equorum P-glycoprotein-11 in populations with decreased ivermectin susceptibility. PLoS One 8, e61635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi YJ et al. (2017) Genomic introgression mapping of field-derived multiple-anthelmintic resistance in Teladorsagia circumcincta. PLoS Genet. 13, e1006857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.David MA et al. (2016) In silico manuscript analysis of the binding of anthelmintics to Caenorhabditis elegans P-glycoprotein 1. Int. J. Parasitol. Drugs Drug Resist 6, 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ménez C et al. (2019) The transcription factor NHR-8: A new target to increase ivermectin efficacy in nematodes. PLoS Pathog. 15, e1007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nappi L et al. (2020) Ivermectin inhibits HSP27 and potentiates efficacy of oncogene targeting in tumor models. J. Clin. Invest. 130, 699–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sheriff JC et al. (2002) Effects of macrocyclic lactone anthelmintics on feeding and pharyngeal pumping in Trichostrongylus colubriformis in vitro. Parasitology 125, 477–484 [DOI] [PubMed] [Google Scholar]
- 107.Caly L et al. (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res 178. [DOI] [PMC free article] [PubMed] [Google Scholar]