Abstract
Background
Limited evidence exists about how to communicate breast density-informed breast cancer risk to women at elevated risk to motivate cancer prevention.
Methods
We conducted a randomized controlled trial evaluating a web-based intervention incorporating personalized breast cancer risk, information on chemoprevention, and values clarification on chemoprevention uptake vs active control. Eligible women aged 40-69 years with normal mammograms and elevated 5-year breast cancer risk were recruited from Kaiser Permanente Washington from February 2017 to May 2018. Chemoprevention uptake was measured as any prescription for raloxifene or tamoxifen within 12 months from baseline in electronic health record pharmacy data. Secondary outcomes included breast magnetic resonance imaging (MRI), mammography use, self-reported distress, and communication with providers. We calculated unadjusted odds ratios (ORs) using logistic regression models and mean differences using analysis of covariance models with 95% confidence intervals (CIs) with generalized estimating equations.
Results
We randomly assigned 995 women to the intervention arm (n = 492) or control arm (n = 503). The intervention (vs control) had no effect on chemoprevention uptake (OR = 1.04, 95% CI = 0.07 to 16.62). The intervention increased breast MRI use (OR = 5.65, 95% CI = 1.61 to 19.74) while maintaining annual mammography (OR = 0.98, 95% CI = 0.75 to 1.28). Women in the intervention (vs control) arm had 5.67-times higher odds of having discussed chemoprevention or breast MRI with provider by 6 weeks (OR = 5.67, 95% CI = 2.47 to 13.03) and 2.36-times higher odds by 12 months (OR = 2.36, 95% CI = 1.65 to 3.37). No measurable differences in distress were detected.
Conclusions
A web-based, patient-level intervention activated women at elevated 5-year breast cancer risk to engage in clinical discussions about chemoprevention, but uptake remained low.
For nearly 2 decades, the US Preventive Services Task Force has recommended use of chemoprevention agents to reduce primary breast cancer incidence among women at elevated-risk breast cancer and low risk for adverse effects (1-4). However, US chemoprevention rates remain low (5), despite compelling results in multiple placebo-controlled trials (6,7). Chemoprevention with tamoxifen, raloxifene, and aromatase inhibitors reduces 5-year breast cancer risk by 30%-55% (3). Identification of eligible women is a challenge in primary care (8), and breast cancer risk assessment tools might support this effort. Assessment tools, which incorporate both current breast cancer risk factors and breast health education, are necessary for clinical implementation and risk-informed care.
Personalized risk counseling and decision support among women at elevated breast cancer risk could be coupled with breast density notification as a potential means to increase the clinical relevance and cancer prevention impact of density notifications. Over the past decade, breast density has become more publicized as numerous states have passed legislation (and now a federal law) (9) mandating that women be notified of their density status (10-12). Density disclosure could serve as an opportunity to educate women who might otherwise be unaware of their elevated breast cancer risk and to connect them with guideline-concordant breast cancer risk management.
To fill this clinical need, we conducted a randomized controlled trial (ENGAGED-2, ClinicalTrials.gov identifier: NCT03029286) evaluating the efficacy of a web-based breast cancer risk communication and decision-making tool on uptake of chemoprevention compared with a control arm. Further, we evaluated breast magnetic resonance imaging (MRI) use, mammography maintenance, and patient-provider communication. We also measured cancer-related distress to ensure that heightened distress was not an unintended consequence of trial participation.
Methods
Trial Oversight
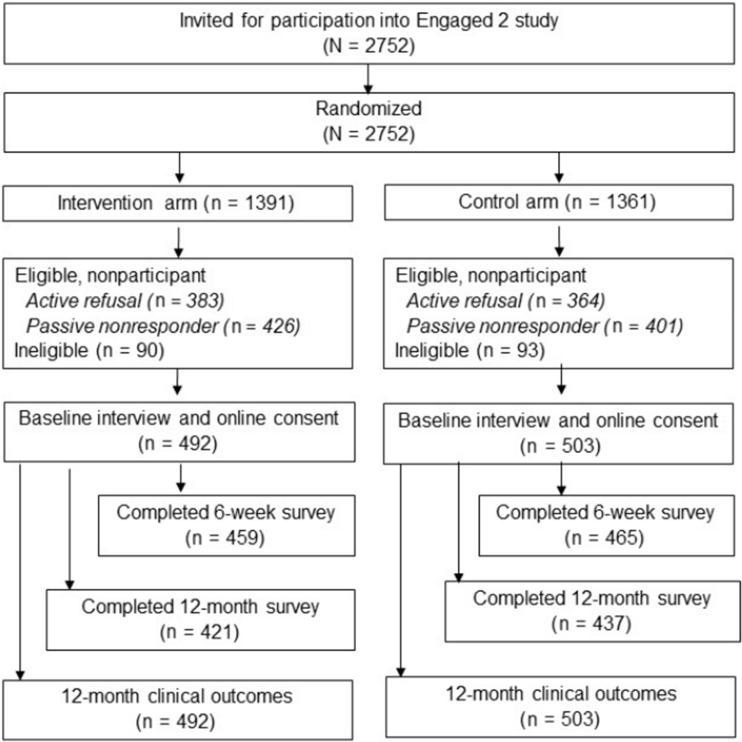
The protocol for this 2-arm randomized trial is published elsewhere (13). Women provided verbal consent prior to completing the baseline interview and electronic consent to the trial when they accessed the study website. The trial was approved by the Georgetown University institutional review board (IRB) committee as the IRB of record, with a partial waiver of informed consent for recruitment activities. The Kaiser Permanente Washington IRB ceded to the Georgetown University Institutional Review Board. This study followed the Consolidated Standards of Reporting Trials reporting guidelines (14) (see Figure 1).
Figure 1.
CONSORT diagram
Study Population and Setting
Eligible women were aged 40-69 years with a recent normal screening mammogram in 2016-2018 and were members of Kaiser Permanente Washington, an integrated healthcare delivery system. At the time of the research study, Washington state law did not mandate reporting of breast density. However, Kaiser Permanente Washington included standard Breast Imaging-Reporting and Data System (BI-RADS) (15) breast density assessments in the mammography report via the online patient portal.
Five-year breast cancer risk was calculated to determine study eligibility using the Breast Cancer Surveillance Consortium (BCSC) risk calculator (16), the only risk calculator that incorporates breast density as a risk factor. Risk factors self-reported at the screening mammogram and in the calculator included age, race and ethnicity, first-degree family history of breast cancer, and history of breast biopsy. BI-RADS breast density assessment was determined by the reading radiologist.
The final study population was restricted to women at elevated risk of an interval breast cancer, considering both 5-year BCSC risk and breast density (17). With interval breast cancer rates of more than 1 case per 1000 screens, eligible women had either an intermediate 5-year risk (1.67%-2.49%) and extremely dense breasts or a high or very high 5-year risk (>2.50%) and either heterogeneously dense or extremely dense breasts.
We excluded women with a personal history of invasive or ductal carcinoma in situ breast cancer, lobular carcinoma in situ, any other cancer diagnosis except nonmelanoma skin cancer, and a previous referral for cancer genetic counseling and/or prior genetic testing as documented in electronic health records (EHR) (18).
No intervention components were directed at the primary care physician (PCP) or the healthcare system. However, the study team presented the trial aims and design during a routine continuing medical education module open to all PCPs and staff prior to trial initiation. During participant recruitment, PCPs received a letter stating their patient was recruited into the study because of her breast density and breast cancer risk. Kaiser Permanente Washington breast cancer screening guidelines at the time of trial recommended that women with elevated breast cancer risk consider chemoprevention and calculation of lifetime risk with a referral to oncology or clinical genetics (19).
Participant Recruitment
Women were randomly assigned 1:1 to the intervention or control arm at study sample identification and prior to recruitment by study programmer using a computer algorithm. Study recruitment materials, including letters and telephone scripts, were drafted at a sixth-grade literacy level and edited by a plain language consultant at Kaiser Permanente Washington Health Research Institute (20). All eligible women were invited to participate in a research study “because your recent mammogram showed that you had dense breast tissue. Having dense breast tissue, along with other risk factors (such as age, family history or prior breast biopsy) means your risk of developing breast cancer is higher than average for a woman of your age and race.” Eligible participants were told that if assigned to the intervention arm, they would “learn about breast density, your personal breast cancer risk, options for screening and prevention and steps you can take to manage your risk.”
From February 2017 to May 2018, eligible participants were mailed a study recruitment letter within 6 months of their most recent normal mammogram (average = 136 days, SD = 23 days). A study team member followed up by phone within a few days to assess final eligibility and willingness to participate. All eligibility criteria were reviewed, and 6.5% of women assigned to the intervention arm and 6.8% of women assigned to the control arm were ineligible at study recruitment (see Figure 1). Eligible enrolled women completed a baseline telephone interview. Women were then emailed a link to the study website where they provided electronic informed consent. Women who completed baseline interviews but did not complete electronic consent were mailed paper consent forms. Only women who completed consent are included in the final study population (see Figure 1) (21). Participants were blinded to their condition until consented.
Study Intervention
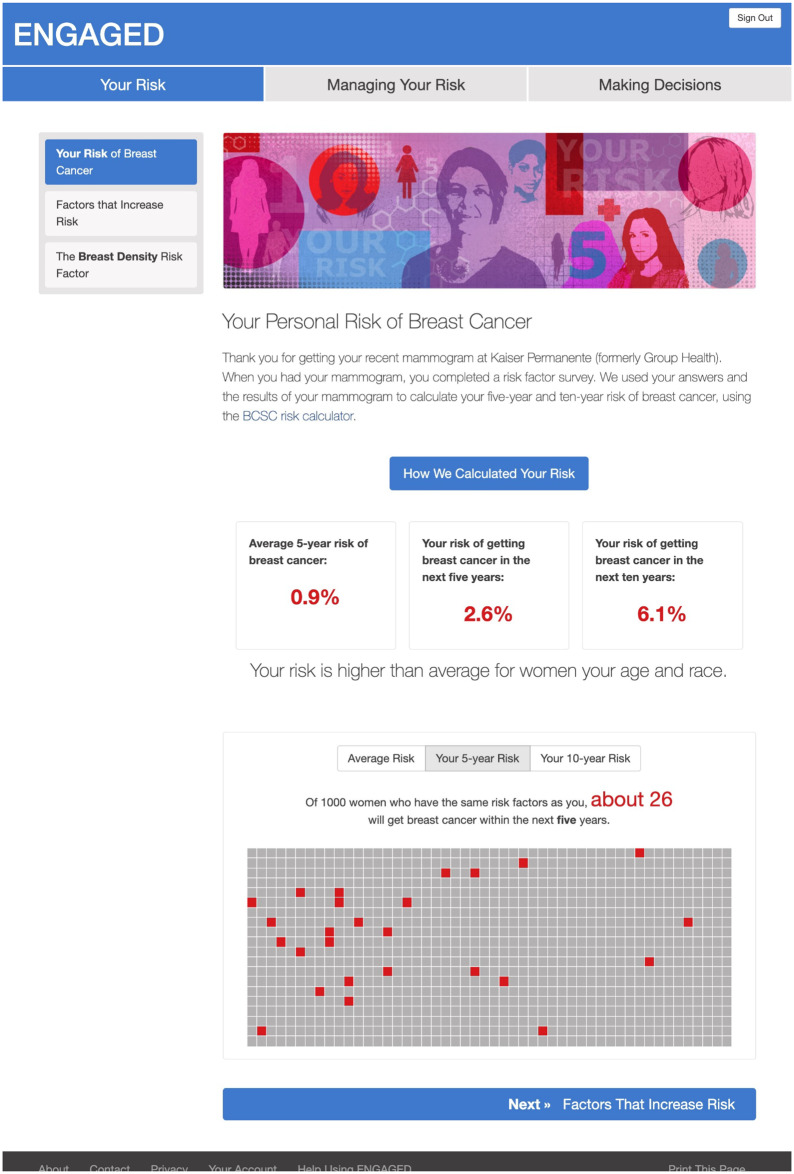
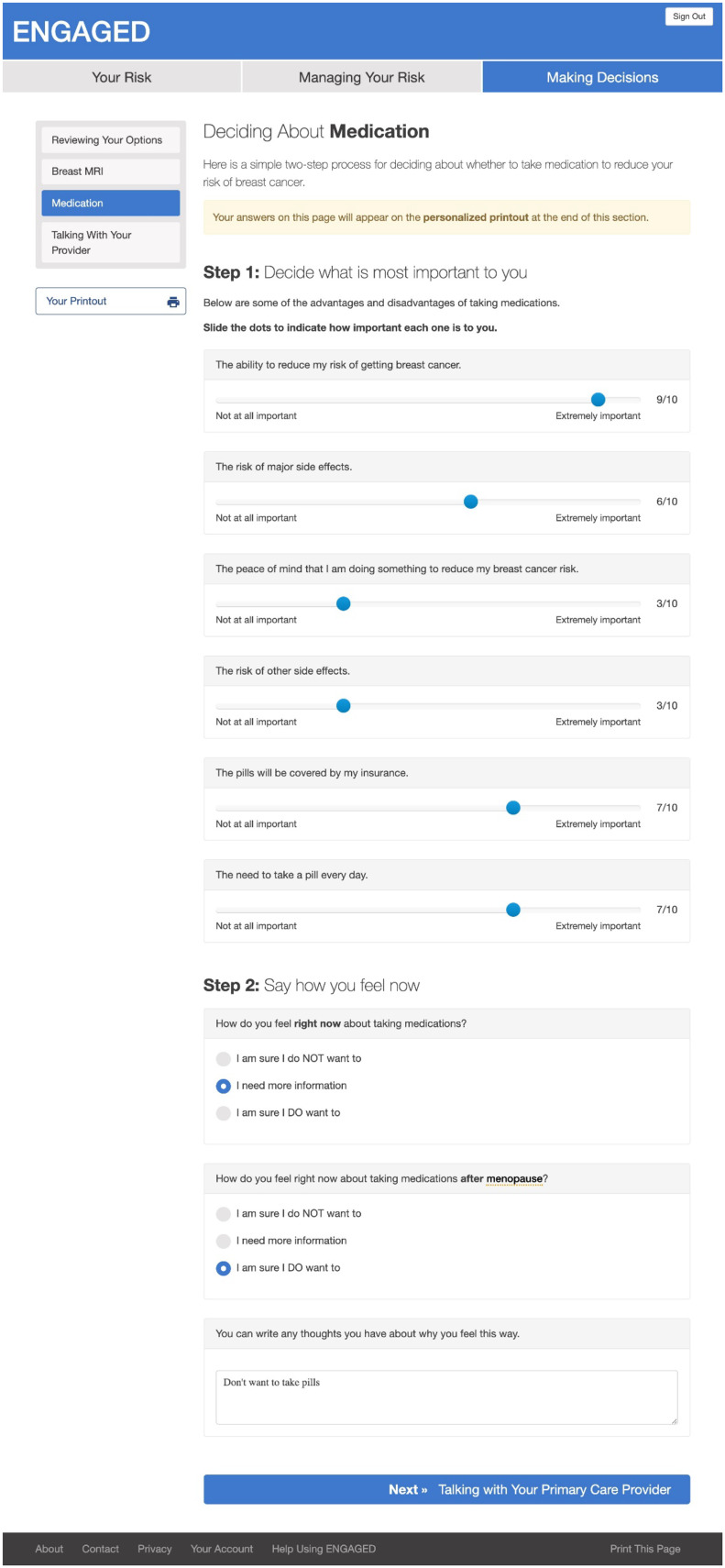
Following International Patient Decision Aid Standards (22,23), the intervention provided participants with 1) factual information about breast cancer including severity and likelihood of possible harms; 2) options for risk management, namely chemoprevention and breast MRI; 3) explanation of risks and benefits of each management option; 4) clarification of individual preferences about the options (ie, value clarification); and 5) guidance in using this information to make risk-management decisions (13). Specifically, women in the intervention arm received personalized 5- and 10-year BCSC breast cancer risk and information about chemoprevention to mitigate breast cancer risk and breast MRI. We did not have all relevant risk factors to calculate lifetime breast cancer risk; hence, all women in the intervention arm were provided with information about the use of breast MRI in women with a lifetime risk of more than 20%. Women were encouraged to speak to their PCP for a specialty referral to calculate lifetime risk to determine eligibility. Women could complete values clarification and patient activation in the form of a question prompt list to share with their PCP. A printable summary of intervention content (personalized risk estimates, values clarification, question prompt list) was available at the end of the intervention to support patient-provider communication (see Figures 2 and 3).
Figure 2.
Webpage content of ENGAGED-2 intervention displaying participant’s personalized breast cancer risk
Figure 3.
Webpage content of ENGAGED-2 intervention of values clarification from participants
Women in the control arm were sent from the study consent website to an American Cancer Society website with information related to breast cancer risk and prevention and cancer screening (24). Women in the control arm received their personalized risk information after study closure.
Measures
Clinical Outcomes
The primary outcome was chemoprevention uptake, measured as receipt of any prescription for tamoxifen or raloxifene, based on health plan pharmacy data. Secondary measures included receipt of breast MRI and mammography. Use of breast MRI and mammography were assessed using EHR data on receipt of breast imaging within Kaiser Permanente Washington breast imaging facilities or claims data from outside imaging facilities. All outcomes were measured within 12 months of the baseline interview.
Patient-Reported Outcomes
Study participants completed the baseline survey by telephone. Subsequent surveys at 6 weeks and 12 months were completed online, with telephone follow-up for nonresponders. Secondary outcomes included measures of conversations with a healthcare provider regarding chemoprevention and/or breast MRI and cancer-related distress, all measured at 6 weeks and 12 months. Patient-provider discussion of risk management was assessed with the following 2 questions: “Have you discussed breast MRI with your health care provider since our last interview? (Y/N)” and “Chemoprevention means you taking medication to reduce the risk of breast cancer. Have you discussed chemoprevention with your health care provider since our last interview? This would include medications such as tamoxifen (also known as Nolvadex) or raloxifene (also known as Evista) (Y/N).” These items were combined to create a composite variable. Cancer-related distress was measured using a single-item distress thermometer, adapted to cancer risk: “Please choose the number (from 0-10) that best describes how much distress you have been experiencing related to your cancer risk in the past week including today, from 0 (no distress) to 10 (extreme distress)” (25).
Statistical Analysis
The trial was designed with an effective sample size of 990 women and 90% power to detect a difference of chemoprevention uptake rates corresponding to 3.3% in the intervention arm and 0.5% in the control arm. Our trial accrued 995 participants.
We described demographics, breast cancer risk factors, and 5-year BCSC risk. We calculated frequencies for categorical variables and means (and standard deviations) for continuous variables separately by study arm. We conducted a modified intention-to-treat analysis including all randomly assigned and consented participants (26). We used logistic regression models with generalized estimating equations (GEE) to estimate odds ratios (OR) and construct corresponding 95% confidence intervals (CIs) for the associations of study arm with binary primary and secondary outcomes. Differences between mean cancer distress between study arms and corresponding 95% confidence intervals were calculated using analysis of covariance with GEE, adjusted for baseline cancer distress as a covariate. For all models, we used GEE with an exchangeable working correlation structure to account for potential correlation of outcomes within the same primary care provider (27,28). In sensitivity analyses, we constructed exact 95% confidence intervals using exact logistic regression models. Because of similarity of the results, we only report the results from the logistic regression models with GEE. In additional sensitivity analyses, we evaluated the effect of excluding women who were diagnosed with breast cancer during the 12-month follow-up. All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Of the 2752 randomly assigned women, 995 (36.1%) completed the baseline questionnaire and consented. They were used in the modified intention-to-treat analysis, with 503 randomly assigned to the control arm and 492 to the intervention arm (Figure 1). In the intervention arm, 476 (96.7%) participants reviewed all website intervention content. No data were available from control women because they were directed to a public website.
Baseline demographics (eg, age, race and ethnicity, income, and education) and breast cancer risk factors were similar across study arms (Table 1), without any noticeable differences. Nearly 99% of the women in both arms remained enrolled in Kaiser Permanente Washington by the 12-month interview. Breast cancer was diagnosed in 8 women in the intervention arm and 2 women in the control arm.
Table 1.
Baseline demographic and clinical characteristics of participants stratified by intervention and control armsa
| Characteristics | Intervention arm | Control arm |
|---|---|---|
| (n = 492) | (n = 503) | |
| Demographic | ||
| Age, y, No. (%) | ||
| 40-49 | 8 (1.6) | 11 (2.2) |
| 50-59 | 144 (29.3) | 136 (27.0) |
| 60-69 | 340 (69.1) | 356 (70.8) |
| Mean (SD) | 61.8 (5.02) | 61.9 (5.15) |
| Race and ethnicity, No. (%) | ||
| White, non-Hispanic | 465 (94.5) | 478 (95.0) |
| Other | 27 (5.5) | 25 (5.0) |
| Education level, No. (%) | ||
| High school graduate or less | 14 (4.6) | 14 (4.3) |
| Some college | 72 (23.5) | 67 (20.7) |
| College graduate/postgraduate | 221 (72.0) | 243 (75.0) |
| Missing | 185 | 179 |
| Annual household income in zip code of residence, No. (%) | ||
| $40 000 | 41 (8.9) | 45 (9.8) |
| $40 001-$80 000 | 206 (44.7) | 179 (38.8) |
| >$80 001 | 214 (46.4) | 237 (51.4) |
| Unknown/refusal | 31 | 42 |
| Breast cancer risk factors | ||
| Menopausal status, No. (%) | ||
| Premenopausal | 40 (8.1) | 35 (7.0) |
| Postmenopausal | 452 (91.9) | 468 (93.0) |
| Family history of breast cancer, No. (%) | ||
| No | 184 (45.1) | 199 (47.0) |
| Yes | 224 (54.9%) | 224 (53.0) |
| Missing | 84 | 80 |
| Breast density, No. (%) | ||
| Heterogeneously dense | 283 (57.5) | 271 (53.9) |
| Extremely dense | 209 (42.5) | 232 (46.1) |
| Prior breast biopsy, No. (%) | ||
| No | 236 (48.0) | 250 (49.7) |
| Yes | 229 (46.5) | 223 (44.3) |
| Unknown | 27 (5.5) | 30 (6.0) |
| BCSC 5-year breast cancer risk | ||
| Intermediate (1.67 to <2.50%), No. (%) | 121 (24.6) | 129 (25.6) |
| High (2.50%), No. (%) | 371 (75.4) | 374 (74.4) |
| Mean (SD) | 2.9 (0.7) | 2.9 (0.7) |
| Median (Q1, Q3) | 2.9 (2.5, 3.2) | 2.8 (2.4, 3.2) |
| Min, Max | 1.67, 5.41 | 1.67, 5.50 |
Percentages are based on nonmissing data. BCSC = Breast Cancer Surveillance Consortium; Q1 = 1st quartile; Q3 = 3rd quartile.
Clinical Outcomes
At the 12-month postbaseline interview, only 1 intervention participant and 1 control participant had any prescription for tamoxifen or raloxifene (unadjusted OR = 1.04, 95% CI = 0.07 to 16.62) (Table 2). Further, 3.3% of intervention arm participants and 0.6% of control arm participants received breast MRI. The odds of receiving breast MRI in women in the intervention arm were 5.65 times the odds in the control arm (OR = 5.65, 95% CI = 1.61 to 19.74). Nearly all women who received breast MRI had a 5-year breast cancer risk of at least 2.5%. Among those with the highest 5-year breast cancer risk, the odds of receiving breast MRI were 4.54 times higher than the odds in the control arm (OR = 4.54, 95% CI = 1.26 to 16.33) (Table 2).
Table 2.
Association of the intervention with 12-month clinical outcomesa
| Clinical outcomes | Intervention arm | Control arm | Unadjusted OR (95% CI) |
|---|---|---|---|
| No. (%) | No. (%) | ||
| (n = 492) | (n = 503) | ||
| Any chemoprevention | 1 (0.2 | 1 (0.2) | 1.04 (0.07 to 16.62) |
| Tamoxifen | 1 (0.2) | 0 | — |
| Raloxifene | 0 | 1 (0.2) | — |
| Breast MRI | 16 (3.3) | 3 (0.6) | 5.65 (1.61 to 19.74) |
| BCSC risk (5-year): 1.67% to <2.50% | 3 (0.6) | 0 | — |
| BCSC risk (5-year): >2.50% | 13 (2.6) | 3 (0.6) | 4.54 (1.26 to 16.33) |
| Any mammogram | 236 (48.0) | 247 (49.1) | 0.98 (0.75 to 1.28) |
| Screening mammogram | 212 (43.1) | 211 (42.0) | 1.05 (0.80 to 1.36) |
BCSC = Breast Cancer Surveillance Consortium; CI = confidence interval; MRI = magnetic resonance image; OR = odds ratio.
In assessing mammography maintenance, 49.1% of control arm and 48.0% of intervention arm participants received a mammogram within 12 months (OR = 0.98, 95% CI = 0.75 to 1.28), with the primary indication of the mammogram for screening (89.8% of intervention arm, 85.4% of control arm).
We found no meaningful differences in magnitude of reported results for chemoprevention or breast imaging when we excluded women diagnosed with breast cancer (data not shown).
Patient-Reported Outcomes
At the 6-week postbaseline interview, 5.3% of intervention and less than 1% of control arm participants discussed chemoprevention with their provider (Table 2). Similar proportions had discussed breast MRI with their provider. The odds of having a discussion regarding chemoprevention and breast MRI at 6 weeks were 8.31 times (OR = 8.31, 95% CI = 2.50 to 27.63) and 5.22 times (OR = 5.22, 95% CI = 2.10 to 12.94) higher in the intervention arm compared with the control arm, respectively (Table 3). At 12 months, 13.9% of intervention and 3.9% of control arm participants discussed chemoprevention with providers (OR = 3.88, 95% CI = 2.33 to 6.45). At 12 months, 17.0% of intervention arm participants and 9.0% of control arm participants discussed breast MRI with their provider (OR = 2.07, 95% CI = 1.39 to 3.07). At both time points, most women who discussed breast MRI also discussed chemoprevention with their providers.
Table 3.
Association of the intervention with survey outcomes at 6 weeks and 12 monthsa
| Survey outcomes | Intervention arm | Control arm | Unadjusted OR (95% CI) |
|---|---|---|---|
| No. (%) | No. (%) | ||
| (n = 492) | (n = 503) | ||
| Discussion with a provider by 6 weeks | |||
| Chemoprevention | 24 (5.3 | 3 (0.7) | 8.31 (2.50 to 27.63) |
| Breast MRI | 29 (6.3) | 6 (1.3) | 5.22 (2.10 to 12.94) |
| Any conversation | 37 (8.1) | 7 (1.5) | 5.67 (2.47 to 13.03) |
| Discussion with a provider by 12 months | |||
| Chemoprevention | 57 (13.9) | 17 (3.9) | 3.88 (2.33 to 6.45) |
| Breast MRI | 70 (17.0) | 39 (9.0) | 2.07 (1.39 to 3.07) |
| Any conversation | 89 (21.8) | 45 (10.4) | 2.36 (1.65 to 3.37) |
CI = confidence interval; MRI = magnetic resonance image; OR = odds ratio.
Baseline measures of mean distress were similar in the intervention and control arms (0.29 and 0.21 on a 0-10 scale, respectively). Distress scores remained similar at the 6-week survey (difference = 0.08, 95% CI = -0.11 to 0.27), increasing from baseline in both arms (Table 4). At 12 months, distress measures had decreased and remained similar between study arms (difference = -0.12, 95% CI = -0.27 to 0.04) (Table 4).
Table 4.
Association of the intervention on measures of patient-reported distress at 6 weeks and 12 months
| Survey outcomes | Intervention arm | Control arm | Adjusted Mean Difference (95% CI)a |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| (n = 492) | (n = 503) | ||
| Distress at 6 weeks | 0.72 (1.66) | 0.61 (1.51) | 0.08 (-0.11 to 0.27) |
| Distress at 12 months | 0.36 (1.13) | 0.47 (1.34) | −0.12 (-0.27 to 0.04) |
Differences (ie, Intervention - Control) in mean distress ratings, adjusted for baseline distress ratings. CI = confidence interval.
Discussion
In this randomized controlled trial of risk management among women at elevated risk of breast cancer, our intervention led to increased rates of patient-provider communication about breast cancer risk management approaches (ie, both chemoprevention and breast MRI). Despite this increase in communication, we observed no difference in chemoprevention uptake and a modest increase in MRI uptake. These positive findings occurred without statistically significant elevations in distress or decrements in mammography maintenance in a population of insured women with recent normal mammograms.
Although our intervention increased women’s discussion of chemoprevention with their PCPs, very few women received a prescription for a chemoprevention agent. Although not directly assessed in our study, barriers to chemoprevention uptake exist across multiple levels of the healthcare system (29). Common barriers among women include fear of adverse events (including medication side effects), confusion regarding the purpose of chemoprevention, and the need for continued adherence for 5 years (29). Among physicians, a lack of knowledge regarding both risk assessment models and chemoprevention options can decrease confidence in prescribing chemoprevention agents (29). In a recent study of women who discussed chemoprevention with a PCP, a recommendation by the PCP was the most influential factor impacting uptake (30). Our intervention addressed several of these barriers but was not sufficient to fully address all barriers, nor was it designed to identify additional barriers. Further studies should evaluate multilevel interventions that address the needs of both providers and women to support optimal breast health via guideline-concordant chemoprevention use.
The intervention increased breast MRI use, which might indicate a patient and/or provider preference for additional imaging for early detection, rather than chemoprevention to reduce breast cancer incidence. Although the relative magnitude of the effect of the intervention on breast MRI was large, overall rates of use were low (<3.5%). The majority of women who received breast MRI had high to very high risk based on the BCSC 5-year risk thresholds. However, recommendations for breast MRI as an adjunct to mammography are based on lifetime not 5-year risk, so we do not know if use of breast MRI was appropriate. Incorporation of breast cancer risk assessment within the EHR might improve guideline-concordant breast cancer risk management but will only be useful if data are captured to reflect a woman’s full history (eg, family history or personal past biopsy history) (31). Previous studies have indicated overuse of breast MRI in breast cancer screening in women with a family history alone who do not meet lifetime risk thresholds (32,33). Further integration of breast cancer risk, EHR tools, and clinical discussion would help identify women both eligible for chemoprevention and guideline-recommended adjunct breast MRI to mammography to reduce potential for overuse of imaging.
We observed no differences in annual mammography maintenance between the intervention and control arms. Some women mistrust mammography in the current climate of increased breast density awareness because of concerns about mammography’s ability to find breast cancer (34). Hence, our intervention had the intended impact of providing balanced education about breast density and cancer risk that did not further alarm women about inaccuracies of mammograms while reassuring that continuing to screen with mammography was their first line of detection.
Our study population was primarily White with high education and socioeconomic status and completely insured and receiving care in an integrated healthcare system. Our study results might not be applicable in other populations, particularly in communities of color or in other healthcare systems, whether these be federally qualified healthcare centers or settings with less continuity of care. Nonetheless, adaption of our study intervention within these communities could have a great benefit in improving women’s knowledge of personal breast cancer risk, engagement with clinicians, and access to chemoprevention. We used the BCSC risk calculator for personal 5- and 10-year breast cancer risk, which has been validated in other populations of women of color (35). The calculator is available online, such that healthcare providers could use at their discretion with any patient regardless of setting. Our study generated conversations with clinicians, and these results would likely hold regardless of setting, as the intervention acted as a prompt. Ensuring that clinicians are prepared for these conversations is essential. For example, a challenge in federally qualified healthcare centers might be appropriate support for primary care physicians who might not sufficiently feel confident in prescribing and managing chemoprevention. Further, prescribing chemoprevention must take into account the balance of risks and benefits, with the risks potentially higher in some populations (36,37). Hence, developing appropriate referral patterns would need to be established. Nonetheless, continued engagement, testing, and evaluation of breast health resources for historically underrepresented communities should be a priority in breast health research.
Our study had several key strengths including a well-developed, patient-centered intervention; high response rate by participants across multiple surveys; low attrition because of disenrollment; and rigorous assessment of key outcomes through EHR data sources. However, there are some limitations. First, in the intervention, we were unable to provide lifetime risk of breast cancer, because of the unavailability of certain risk factors (ie, age at first menstrual period and first birth) in routinely collected data using tools like the breast cancer risk assessment tool (38). Our intervention messaging clearly indicated to women that their lifetime risk of breast cancer would need to be calculated to warrant imaging with breast MRI. Further, we did not specifically evaluate aromatase inhibitor prescriptions as chemoprevention outcome, because the medication was not yet FDA approved as chemoprevention and on formulary at Kaiser Permanente Washington during the study time period. Second, we previously published that our study population had a high level of awareness of breast density (39) and was demographically homogenous by race and ethnicity, education, and insurance status (40). Further, women needed to access online information to participate in the study. In general public, breast density awareness remains low (34), specifically among women of color and without college education (41). The effectiveness of our intervention in other healthcare settings and more heterogenous populations should be tested prior to widespread implementation and dissemination. Third, our intervention was delivered at the patient level, and we did not target the physician, the clinical delivery system, or known barriers. Some ongoing studies are already evaluating chemoprevention uptake using dual patient- and physician-targeted education interventions with results forthcoming (42). Finally, our study randomly assigned women prior to informed consent, such that we could expedite participant access to study website at enrollment. Because of this, our analysis was a modified intent-to-treat analysis among those randomly assigned, enrolled, and consented. Given the proportions of ineligible and active or passive refusal were similar in both the intervention and control arm, our results suggest that allocation to study arm did not affect the decision to consent and the intent to receive the intervention, such that a modified intent-to-treat analysis was appropriate (43). Further, we note the similarity in baseline characteristics of the study arms among those enrolled and consented. Given the large number of women impacted by breast density notification legislation and potentially eligible for chemoprevention based on current guidelines, further research should continue to evaluate interventions that best support women and PCPs in clinical management of breast cancer risk.
In summary, although the uptake of chemoprevention remained low among women at elevated breast cancer risk, intervention arm participants were activated to initiate conversations with their PCPs, without increasing their personal distress or reducing return for routine screening mammography. Given nearly 2 decades of US Preventive Services Task Force recommendations for chemoprevention, further efforts are needed to understand and address multilevel barriers to chemoprevention uptake and clinical integration.
Funding
This study is supported by the National Cancer Institute under R01CA190221, R50CA211115, P30CA05100, U01CA12958, R35CA197289, K99CA241397, and P30CA51008 and the Agency for Healthcare Research and Quality under K12HS022982. Further support was provided by Cancer Prevention Research Fellowship sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation (ASPO-17-001) and Lombardi Comprehensive Cancer Center American Cancer Society Young Investigator Award (ACS IRG 92-152-20). Collection of breast cancer risk information is supported by the National Cancer Institute-funded BCSC (P01CA154292, HHSN261201100031C, and U54CA163303).
Footnotes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to report.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Full study protocol is available on request.
Prior presentations: Results from our study were presented at the 44th Annual American Society for Preventive Oncology in March 2020.
Author contributions: Conceived and designed the analysis: Karen J. Wernli, George Luta, Jeanne S. Mandelblatt, Marc D. Schwartz, Suzanne C. O’Neill. Collected the data: Karen J. Wernli, Sarah Knerr, Kathleen Leppig, Kelly Ehrlich, David Farrell, Hongyuan Gao, Erin J. A. Bowles, Suzanne C. O’Neill. Contributed data or analysis tools: Karen J. Wernli, Tengfei Li, Kelly Ehrlich, David Farrell, Hongyuan Gao, Erin J. A. Bowles, George Luta, Suzanne C. O’Neill. Performed the analysis: Karen J. Wernli, Sarah Knerr, Tengfei Li, George Luta, Suzanne C. O’Neill. Wrote the paper: Karen J. Wernli, Sarah Knerr, Tengfei Li, Kathleen Leppig, Kelly Ehrlich, David Farrell, Hongyuan Gao, Erin J. A. Bowles, Amanda L. Graham, George Luta, Jinani Jayasekera, Jeanne S. Mandelblatt, Marc D. Schwartz, Suzanne C. O’Neill.
Data Availability
The data underlying this article cannot be shared publicly due to data privacy of the individual participants. The data will be shared on reasonable request to the corresponding author.
Contributor Information
Karen J Wernli, Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA.
Sarah Knerr, Department of Health Services, University of Washington, Seattle, WA, USA.
Tengfei Li, Department of Biostatistics, Bioinformatics, and Biomathematics, Georgetown University, Washington, DC, USA.
Kathleen Leppig, Washington Permanente Medical Group, Seattle, WA, USA.
Kelly Ehrlich, Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA.
David Farrell, PeopleDesigns, Raleigh-Durham, NC, USA.
Hongyuan Gao, Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA.
Erin J A Bowles, Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA.
Amanda L Graham, Truth Initiative, Washington, DC, USA; Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
George Luta, Department of Biostatistics, Bioinformatics, and Biomathematics, Georgetown University, Washington, DC, USA.
Jinani Jayasekera, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Jeanne S Mandelblatt, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Marc D Schwartz, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Suzanne C O’Neill, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
References
- 1. Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN.. Chemoprevention of breast cancer: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(1):59-69. [DOI] [PubMed] [Google Scholar]
- 2. Nelson HD, Fu R, Humphrey L, Smith B, Griffin JC, Nygren P. Comparative effectiveness of medications to reduce risk of primary breast cancer in women. Ann Intern Med. 2009;151(10):703-15, W-226-35. [DOI] [PubMed] [Google Scholar]
- 3. Nelson HD, Fu R, Zakher B, Pappas M, McDonagh M.. Medication use for the risk reduction of primary breast cancer in women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322(9):868-886. [DOI] [PubMed] [Google Scholar]
- 4. Owens DK, Davidson KW, Krist AH, et al. ; US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322(9):857-867. [DOI] [PubMed] [Google Scholar]
- 5. Pinsky PF, Miller E, Heckman-Stoddard B, Minasian L.. Use of raloxifene and tamoxifen by breast cancer risk level in a Medicare-eligible cohort. Am J Obstet Gynecol. 2018;218(6):606.e601-606.e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher B, Costantino JP, Wickerham DL, et al. ; other National Surgical Adjuvant Breast. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388. [DOI] [PubMed] [Google Scholar]
- 7. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727-2741. [DOI] [PubMed] [Google Scholar]
- 8. Burns RB, Schonberg MA, Tung NM, Libman H.. Should we offer medication to reduce breast cancer risk? Grand Rounds Discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2016;165(3):194-204. [DOI] [PubMed] [Google Scholar]
- 9. Federal Register. Mammography Quality Standards Act; 2019. https://www.federalregister.gov/documents/2019/03/28/2019-05803/mammography-quality-standards-act. Accessed February 15, 2020.
- 10. Bahl M, Baker JA, Bhargavan-Chatfield M, Brandt EK, Ghate SV.. Impact of breast density notification legislation on radiologists’ practices of reporting breast density: a multi-state study. Radiology. 2016;280(3):701-706. [DOI] [PubMed] [Google Scholar]
- 11. Slanetz PJ, Freer PE, Birdwell RL.. Breast-density legislation: practical considerations. N Engl J Med. 2015;372(7):593-595. [DOI] [PubMed] [Google Scholar]
- 12. Lourenco AP, DiFlorio-Alexander RM, Slanetz PJ.. Breast density legislation in New England: a survey study of practicing radiologists. Acad Radiol. 2017;24(10):1265-1267. [DOI] [PubMed] [Google Scholar]
- 13. Knerr S, Wernli KJ, Leppig K, et al. A web-based personalized risk communication and decision-making tool for women with dense breasts: design and methods of a randomized controlled trial within an integrated health care system. Contemp Clin Trials. 2017;56:25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulz KF, Altman DG, Moher D; for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332-c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American College of Radiology. ACR BI-RADS Atlas. Quality & Safetyhttps://www.acr.org/Quality-Safety/Resources/BIRADS. Accessed January 31, 2017.
- 16. Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K.. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross TR, Ng D, Brown JS, et al. The HMO Research Network Virtual Data Warehouse: a public data model to support collaboration. eGEMs. 2014;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaiser Permanente. Breast Cancer Screening; 2020. https://cl.kp.org/nw/home/refcontainerpage.dam.html?damrefpath=/content/dam/clinicallibrary/natl/cmi/programs/cancer-breastscreen/guideline/files/Breast_Cancer_Screening_Guidelines.pdf. Accessed December 29, 2020.
- 20. KPWA Health Research Institute. Program for Readability in Science & Medicine (PRISM); 2018. https://www.kpwashingtonresearch.org/about-us/capabilities/research-communications/prism/. Accessed December 29, 2020.
- 21. Flory JH, Mushlin AI, Goodman ZI.. Proposals to conduct randomized controlled trials without informed consent: a narrative review. J Gen Intern Med. 2016;31(12):1511-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Volk RJ, Llewellyn-Thomas H, Stacey D, Elwyn G.. Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak. 2013;13(S2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Cancer Society. Breast Cancer Risk and Prevention Breast Cancer; 2020. https://www.cancer.org/cancer/breast-cancer/risk-and-prevention.html. Accessed May 8, 2020.
- 25. Cutillo A, O’Hea E, Person S, Lessard D, Harralson T, Boudreaux E.. The distress thermometer: cutoff points and clinical use. Oncol Nurs Forum. 2017;44(3):329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liang KY, Zeger SL.. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13-22. [Google Scholar]
- 28. Diggle P, Heagerty P, Liang K, Zeger S.. Analysis of Longitudinal Data. Oxford University Press; 2002. [Google Scholar]
- 29. Ball S, Arevalo M, Juarez E, Payne JD, Jones C.. Breast cancer chemoprevention: an update on current practice and opportunities for primary care physicians. Prev Med. 2019;129:105834-105834. [DOI] [PubMed] [Google Scholar]
- 30. Holmberg C, Bandos H, Fagerlin A, et al. NRG Oncology/National Surgical Adjuvant Breast and Bowel Project Decision-Making Project-1 results: decision making in breast cancer risk reduction. Cancer Prev Res. 2017;10(11):625-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang X, McGuinness JE, Sin M, Silverman T, Kukafka R, Crew KD.. Identifying women at high risk for breast cancer using data from the electronic health record compared with self-report. J Clin Oncol Clin Cancer Inform. 2019;3(3):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haas JS, Hill DA, Wellman RD, et al. Disparities in the use of screening magnetic resonance imaging of the breast in community practice by race, ethnicity, and socioeconomic status. Cancer. 2016;122(4):611-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill DA, Haas JS, Wellman R, et al. Utilization of breast cancer screening with magnetic resonance imaging in community practice. J Gen Intern Med. 2018;33(3):275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schifferdecker KE, Tosteson ANA, Kaplan C, et al. Knowledge and perception of breast density, screening mammography, and supplemental screening: in search of “informed”. J Gen Intern Med. 2020;35(6):1654-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tice JA, Bissell MCS, Miglioretti DL, et al. Validation of the breast cancer surveillance consortium model of breast cancer risk. Breast Cancer Res Treat. 2019;175(2):519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson C, Nichols HB, House M, Sandler DP.. Risk versus benefit of chemoprevention among raloxifene and tamoxifen users with a family history of breast cancer. Cancer Prev Res. 2019;12(11):801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886. [DOI] [PubMed] [Google Scholar]
- 39. Mahorter SS, Knerr S, Bowles EJA, et al. Prior breast density awareness, knowledge, and communication in a health system-embedded behavioral intervention trial. Cancer. 2020;126(8):1614-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wernli KJ, Bowles EA, Knerr S, et al. Characteristics associated with participation in ENGAGED 2–a web-based breast cancer risk communication and decision support trial. Perm J .2020; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kressin NR, Wormwood JB, Battaglia TA, Gunn CM.. Differences in breast density awareness, knowledge, and plans based on state legislation status and sociodemographic characteristics. J Gen Intern Med. 2020;35(6):1923-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crew KD, Silverman TB, Vanegas A, et al. Study protocol: randomized controlled trial of web-based decision support tools for high-risk women and healthcare providers to increase breast cancer chemoprevention. Contemp Clin Trials Commun. 2019;16:100433-100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fergusson D, Aaron SD, Guyatt G, Hébert P.. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to data privacy of the individual participants. The data will be shared on reasonable request to the corresponding author.