Abstract
Context
In active acromegaly, the lipolytic and insulin antagonistic effects of growth hormone (GH) excess alter adipose tissue (AT) deposition, reduce body fat, and increase insulin resistance. This pattern reverses with surgical therapy. Pegvisomant treats acromegaly by blocking GH receptor (GHR) signal transduction and lowering insulin-like growth factor 1 (IGF-1) levels. The long-term effects of GHR antagonist treatment of acromegaly on body composition have not been studied.
Methods
We prospectively studied 21 patients with active acromegaly who were starting pegvisomant. Body composition was examined by whole body magnetic resonance imaging, proton magnetic resonance spectroscopy of liver and muscle and dual-energy x-ray absorptiometry, and endocrine and metabolic markers were measured before and serially during 1.0 to 13.4 years of pegvisomant therapy. The data of patients with acromegaly were compared with predicted and to matched controls.
Results
Mass of visceral AT (VAT) increased to a peak of 187% (1.56-229%) (P < .001) and subcutaneous AT (SAT) to 109% (–17% to 57%) (P = .04) of baseline. These remained persistently and stably increased, but did not differ from predicted during long-term pegvisomant therapy. Intrahepatic lipid rose from 1.75% to 3.04 % (P = .04). Although lean tissue mass decreased significantly, skeletal muscle (SM) did not change. IGF-1 levels normalized, and homeostasis model assessment insulin resistance and HbA1C were lowered.
Conclusion
Long-term pegvisomant therapy is accompanied by increases in VAT and SAT mass that do not differ from predicted, stable SM mass and improvements in glucose metabolism. Long-term pegvisomant therapy does not produce a GH deficiency-like pattern of body composition change.
Keywords: acromegaly, body composition, pegvisomant
Growth hormone (GH) and insulin-like growth factor 1 (IGF-1) are important regulators of body composition and metabolism [1, 2], but in acromegaly both are abnormal due to GH and IGF-1 excess. In particular, the lipolytic, anabolic, and sodium and water retaining effects of GH excess underlie key features of the acromegaly phenotype [3, 4]. We and others have shown that fat mass, especially that of visceral adipose tissue (VAT) and intrahepatic lipid (IHL) are reduced in active acromegaly and rise with surgical therapy [5-10]. Interestingly, active acromegaly presents a unique constellation, an acromegaly-specific lipodystrophy, characterized by lower adiposity with insulin resistance (IR) and, after surgical treatment, of increased adiposity yet a lowering of IR.
Pegvisomant, a GH receptor (GHR) antagonist, treats acromegaly by blocking GH action in peripheral tissues and the liver [11, 12], thus lowering IGF-1 levels. Normalization of circulating IGF-1 levels with pegvisomant therapy is associated with improvements in the clinical and metabolic abnormalities of acromegaly [13-15]. However, since pegvisomant is a potent antagonist at all GH receptors, it could be questioned whether, if in sufficient doses, some degree of functional GH deficiency could be manifest in tissues with its long-term use that is not reflected in serum IGF-1 levels. Since GH deficiency is associated with increased central adiposity, IR, and reduced muscle mass [16, 17], an investigation of the long-term effects of pegvisomant on body composition was warranted to determine if this normalizes. Prior small, short-term studies showed a rise in abdominal fat with pegvisomant therapy [18] and a rise in hepatic lipid with this added to a somatostatin analog [19], but the long-term effects of GHR antagonism on adipose tissue (AT) mass, skeletal muscle (SM) mass, or the effects of pegvisomant alone on hepatic or muscle lipid have not been reported. Therefore, we investigated, for the first time, the long-term effects of pegvisomant treatment of acromegaly on AT mass and distribution as assessed by both total body magnetic resonance imaging (MRI) and dual-energy x-ray absorptiometry (DXA), and ectopic lipid deposition in liver and muscle by proton magnetic resonance spectroscopy (1HMRS). We also aimed to determine how changes in metabolic abnormalities and GH and IGF-1 levels relate to body composition changes with long-term pegvisomant therapy.
Materials and Methods
Subjects with Acromegaly
We prospectively studied 21 patients with acromegaly (13 males, 8 females), median age 48 years (range 19-62 years) who were beginning pegvisomant therapy. Acromegaly had been diagnosed biochemically based on elevated IGF-1 levels, nadir GH after oral glucose >1 µg/L, and characteristic clinical features. Maximal tumor diameter at diagnosis was 20 mm (median) (range 8-45 mm); 1 was a microadenoma and 20 were macroadenomas. All patients had prior therapy for acromegaly (Table 1). Twenty had noncurative transsphenoidal surgery from 0.75 to 13 years (median 3.05 years), 8 had radiotherapy from 1.15 years (range 0.4-10.5 years), and 19 had received other medical therapies that did not normalize their IGF-1 prior to starting pegvisomant (Table 1). Somatostatin analogs and dopamine agonists were last taken at least 3 months prior to baseline testing, except in 1 patient who continued cabergoline along with pegvisomant for treatment of hyperprolactinemia. The gonadal function of patients is shown in Table 1. Two patients had hypothyroidism on stable replacement therapy for the duration of the study. Six patients had type 2 diabetes mellitus, 4 were treated with oral hypoglycemic agents, and 2 were treated with insulin. All patients had active acromegaly as defined by a high serum IGF-1 level at the start of pegvisomant therapy (Table 2). None received medical therapy for acromegaly, radiotherapy, or additional surgery during the study period. All were ambulatory outpatients with normal renal function and no liver disease. The study was approved by the Institutional Review Board of Columbia University Medical Center and all subjects gave written informed consent before participation.
Table 1.
Characteristics of the 21 acromegaly patients at the time of baseline prepegvisomant testing
| Male/Female | 13/8 |
| Age years median (range) | 48 (19-62) |
| Hypopituitarism | |
| Hypogonadism | |
| Males | |
| Testosterone treateda | 7 (33) |
| Untreated | 1 (5) |
| Females | |
| Postmenopauseb | 3 (14) |
| Secondary amenorrheab | 3 (14) |
| Hypothyroidism | 2 (9.5) |
| Comorbidities | |
| Hypertension | 12 (57) |
| Diabetes mellitus | 6 (29)d |
| Osteoarthritis | 6 (29) |
| Hyperlipidemia | 5 (23) |
| Sleep apnea | 5 (23) |
| Carpal tunnel syndrome | 2 (9.5) |
| Prior therapy | |
| Transsphenoidal surgery | 20 (95) |
| Radiotherapy | 8 (38) |
| Somatostatin analog | 18 (86) |
| Cabergoline | 13 (62) |
| Bromocriptine | 5 (24) |
| Pergolide | 1 (5) |
| Years from surgeryc | 3.05 (0.75-13) |
| Years from radiotherapyc | 1.15 (0.4-10.5) |
Data are presented as n (%) unless stated otherwise.
a Testosterone level within normal range on testosterone therapy for duration of study.
b Not on hormone replacement therapy for duration of study.
c Median and range of years from surgery or radiotherapy to the baseline testing in this study.
d Four treated with medical therapy, 2 with insulin.
Table 2.
Endocrine data and anthropometric measures at baseline testing (prepegvisomant) and at the last study visit and testing while on pegvisomant therapy
| Baseline | On pegvisomanta | P value | |
|---|---|---|---|
| IGF-1 percent ULN | 201.6 (132-337) | 84 (44-119) | <.001 |
| GH (µg/L) | 4.125 (1.2-73) | 14.85 (3.8-125) | .03 |
| Leptin (ng/mL) | 8.5 (1.2-24.7) | 17.5 (2-53) | <.001 |
| HOMA IRb | 2.17 (0.73-8.17) | 1.48 (0.37-6.53) | .001 |
| QUICKIb | 0.53 (0.33-1.22) | 0.63 (0.36-1.77) | .08 |
| HbA1C (%) | 5.85 (4.7-7.6) | 5.4 (4.69-8.1) | .04 |
| Weight (kg) | 94.5 (59.2-137.7) | 96.8 (63.7-139) | .61 |
| BMI (kg/m2) | 29.9 (23.2-40.8) | 30.75 (23.8-40.3) | .69 |
| Waist circumference (cm) | 103.7 (73.7-125.3) | 107.5 (78-139) | .004 |
| Waist/Hip ratio | 0.931 (0.812-1.053) | 0.949 (0.788-1.058) | .51 |
| Duration of IGF-1 elevation prior to pegvisomant therapy (yr)c | 3 (1.5-10) | ||
| Pegvisomant daily dose (mg)d | 20 (5-40) | ||
| Duration of pegvisomant therapy and follow-up in study (yr) | 5.73 (1-13.4) |
Data are given as median (range).
Abbreviations: BMI, body mass index; HOMA IR, homeostasis model assessment insulin resistance; IGF, insulin-like growth factor; QUICKI, quantitative insulin sensitivity check index; ULN, upper limit of normal.
a On pegvisomant values were those at the last testing time point in the study.
b HOMA IR = (fasting serum insulin [µU/mL] × fasting plasma glucose [mmol/L]/22.5); QUICKI = [1/[(log(I0) + log(G0)], where I0 is the fasting plasma insulin (µU/mL), and G0 is the fasting blood glucose (mg/dL)] [14].
c Duration of documented IGF-1 level elevation just prior to the start of pegvisomant therapy in this study. Does not include an estimated duration from prior to diagnosis even if continuous from then.
d Dose of pegvisomant at maintenance.
Nonacromegaly Comparison Groups
A group of 185 females and 130 males, aged 18-84 years, of different ethnicities were studied by total body MRI to develop models of predicted body composition as previously described [8, 9, 20]. A second group of 8 healthy subjects (7 males, 1 female) were studied by 1HMRS of the liver and matched to the 8 subjects with acromegaly who underwent MRS of liver, for sex, age ± 5 years, and body mass index (BMI) ± 3 kg/m2. All were healthy without chronic medical problems or medications, nonsmoking, weight stable, and not heavy exercisers.
Study Design
Patients were studied before and up to 4 additional times at 1 to 2, 3 to 4, 5 to 6, and 8 or more years after starting pegvisomant. At each testing session, they had blood sampling, anthropometric measurements, and body composition testing by whole body MRI and/or DXA: MRI (n = 16), DXA (n = 19), and MRI and DXA (n = 14). Patients began pegvisomant therapy per standard clinical practice guidelines with a loading dose of 40 mg followed by 10 mg daily and the dose was escalated monthly in 5-mg increments until the treatment goal of IGF-1 level normalization was reached. Liver function tests were monitored per clinical guidelines and MRI of the pituitary was monitored yearly. Healthy subjects were studied once by whole body MRI body composition testing.
Study Procedures
Laboratory testing
Blood sampling was done after an overnight fast for IGF-1, GH, insulin, glucose, leptin, and HbA1C. Serum and plasma were frozen at –80°C in multiple aliquots. IR was estimated by homeostasis model assessment (HOMA) scores [21] and by the quantitative insulin sensitivity check index (QUICKI) [22] (Table 2).
Anthropometrics
Body weight by a digital scale to the nearest 0.01 kg, height with a stadiometer to the nearest 0.5 cm and waist circumference to the nearest 0.1 cm were measured.
Total body magnetic resonance imaging and 1HMRS
Total and regional body adipose tissue volumes were measured by whole body multislice MRI on a 1.5 T MR scanner (Achieva, Philips Healthcare) as previously described [8, 20]. The intermuscular adipose tissue compartment (IMAT), defined as the AT located between muscle groups and beneath the muscle fascia [23], is distinct from and does not include intramyocellular lipid (IMCL), namely the lipid within myocytes. Images were analyzed with SliceOmatic image analysis software (TomoVision, Inc., Montreal, Canada) in the Image Analysis Core Lab of the New York Obesity Nutrition Research Center. MRI volume estimates were converted to mass using the assumed density of 0.92 kg/L for AT and 1.04 kg/L for SM. The coefficient of variation (CV) for repeated measurements of the same scan by the same observer of MRI-derived AT volumes is 1.7% for subcutaneous adipose tissue (SAT), 2.3% for VAT, and 5.9% for IMAT [23, 24].
Water suppressed and nonwater suppressed single voxel 1HMRS spectra were acquired by applying point resolved spectroscopy technique to measure intrahepatic lipid (IHL) (n = 8; 7 M, 1 F) and tibialis anterior muscle for measurement of IMCL (n = 7; 6 M, 1 F) as previously described [9].
Dual-energy x-ray absorptiometry
Whole-body and regional lean tissue mass and fat mass were estimated in 19 patients (10 M, 8 F) by DXA (software version 11.4; GE Lunar Prodigy Advance, Madison WI) as previously described [10]. We calculated DXA-estimated IMAT-free SM mass (SMDXA) based on a prediction equation previously validated and found it to agree highly with total body SM mass by MRI in acromegaly as previously described [20]. Non-SM lean tissue (total lean tissue – SMDXA) was also calculated. The CV of repeated daily measurements was 1.7% for leg lean tissue, 2.0% for arm lean tissue, and 2.6% for appendicular lean tissue.
Hormone Assays
Growth hormone
was measured by a chemiluminescent immunometric assay from IMMULITE® (Siemens) that is calibrated to World Health Organization (WHO) International Reference Standard 98/574 and had an intra-assay CV of 3.1% and interassay CV of 6% [25, 26]. Functional sensitivity for GH was 0.05 µg/L.
IGF-1 was measured from 2002 to 2005 using the polyclonal radioimmunoassay (RIA) from the Nichols Institute (San Juan Capistrano, CA) calibrated to WHO 1st International Reference Reagent 1988 (IGF-1 87/518) that had an intra-assay CV of 4% and interassay CV of 11% [27], from 2005 to 2016 by chemiluminescent immunoassay IMMULITE (Siemens) calibrated to WHO IRR NIBSC code 87/518 that has an intra-assay CV of 4% and interassay CV of 5.9% [28], and from 2016 to 2020 by chemiluminescent immunoassay (IDS-iSYS) that is calibrated to the new recombinant standard 02/254 [29, 30] and has an intra-assay CV of 1.3% to 3.7% and interassay CV of 3.4% to 8.7%. IGF-1 levels were compared with the manufacturers’ age- and gender-specific (for IDS-iSYS) normative ranges for each assay. IGF-1 levels were measured at both baseline and follow-up using the Nichols RIA in 5 patients, Immulite in 5, and IDS-iSYS in 1 patient, using the Nichols RIA then Immulite in 4, using the Nichols RIA then Immulite then IDS-iSYS in 2, and using the Immulite then IDS-iSYS in 4 patients.
Insulin was measured by IMMULITE (Siemens), glucose by the hexokinase method, and leptin using a sensitive sandwich enzyme-linked immunosorbet assay (R&D Systems), which has a sensitivity is 7 pg/mL.
Statistical Analysis
MRI AT depot and SM values for patients with acromegaly were compared with predicted values using the Wilcoxon signed rank test. Prediction equations were developed for mass of total adipose tissue, VAT, SAT, and SM compartments accounting for gender, age, height, weight, and race using generalized linear models from data in the body composition comparison group as previously described [8, 20], and IMAT as developed by Gallagher et al. [23]. Fisher’s exact test was used to compare the proportion of subjects with values above or below predicted before and after pegvisomant therapy. The Wilcoxon signed rank test was used to compare MRI and DXA values before pegvisomant therapy and Friedman test to compare values at different time points on pegvisomant. IHL in acromegaly was compared with that in controls using the Wilcoxon rank sum test. Spearman’s correlation was used to assess the relationships between absolute and percent change in body composition measurements and those of IGF-1, GH, leptin, HOMA score, and QUICKI. Data are given as median and range unless stated otherwise. P < .05 was considered significant. Data were analyzed by Prism 8 for MAC and SAS 9.4 (SAS Institute, Cary, NC).
Results
Endocrine and Metabolic Markers, Liver Function, and Pituitary Tumor Monitoring
Pre- and postpegvisomant levels of endocrine and metabolic markers are shown in Table 2. IGF-1 levels were <1.2 times the upper limit of normal in all patients after pegvisomant therapy. None had a rise in liver function tests. Tumor size did not change in 19 (6 with no visible tumor, 7 micro- and 6 macroadenomas) and 2 macroadenomas had nonclinically significant increases in tumor size of 2 mm (at 10 years) and 3 mm (at 2 years) of therapy.
Body Composition Measurements
Anthropometric testing
Waist circumference increased, but weight, BMI, and waist/hip ratio did not change with pegvisomant therapy (Table 2).
Total body magnetic resonance imaging
Visceral adipose tissue.
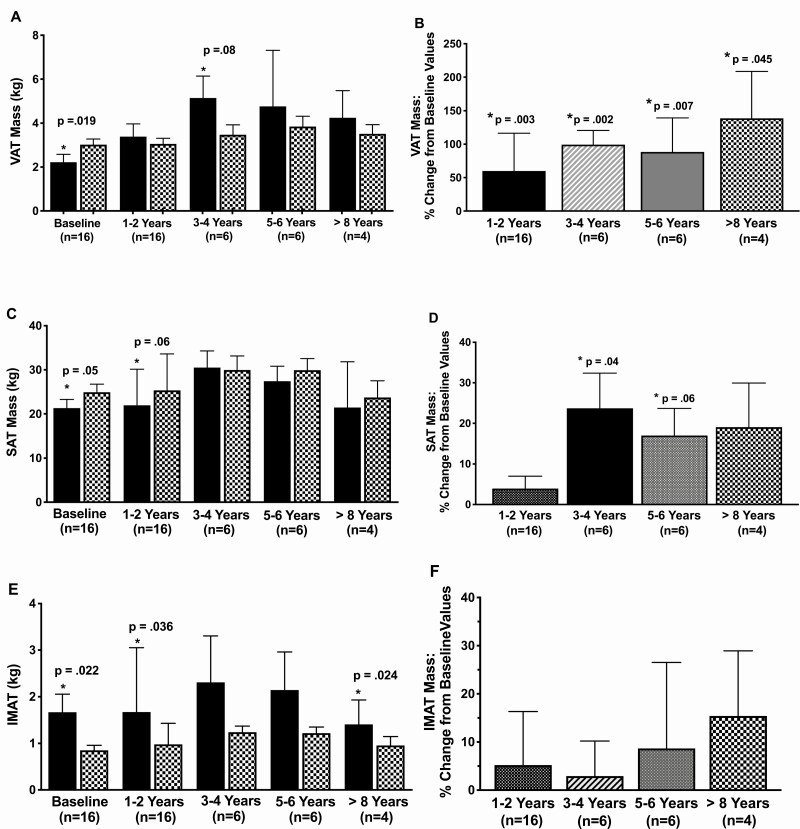
Prepegvisomant, VAT was 66% (median) (range 19-280%) of predicted (Fig. 1A). VAT rose with pegvisomant therapy (Fig. 1B). Prepegvisomant, VAT was below predicted in 14/16 patients, and on pegvisomant it was above predicted in all 16 (P < .0001). VAT increase tended to be larger in males, 110% (1.56-228%), than in females, 27% (19.9-92%) (P = .058). Overall, on pegvisomant, VAT mass did not differ significantly from predicted (Fig. 1A) or between time points of follow-up.
Figure 1.
Total body MRI measured adipose tissue mass before and during pegvisomant therapy. VAT (A), SAT (C) and IMAT (E) mass in acromegaly patients (solid bar) compared to predicted values (patterned bar) at prepegvisomant baseline and at 1 to 2 years, 3 to 4 years, 5 to 6 years, and ≥ 8 years of pegvisomant. P values compare acromegaly to predicted values. Data are mean ± SE. Changes in VAT (B), SAT (D), and IMAT (F) mass from prepregvisomant baseline to VAT mass at 1 to 2 years, 3 to 4 years, 5 to 6 years, and ≥ 8 years of pegvisomant therapy. P-values represent significance of change from prepegvisomant to on pegvisomant time points. AT changes from baseline did not differ between on pegvisomant time points. For example, for VAT mass, for patients followed >8 years mean % change in VAT mass was 128% at 1 to 2 or 3 to 4 years compared with 138% at >8 years The duration of follow-up testing by MRI was 1 to <3 years in 6 patients, 3 to <5 years in 2, 5 to <7 years in 4, and ≥8 to 13.23 years in 4 patients. Data are mean ± SE.
Subcutaneous adipose tissue.
Prepegvisomant SAT was 82.5% (median) (range 52-108%) of predicted (Fig. 1C). SAT rose significantly after 3 to 4 years of pegvisomant therapy (Fig. 1D). The proportion of patients with SAT above predicted did not differ from prepegvisomant, 2/16, to on pegvisomant, 5/16 (all males) (P = .39). Overall, SAT did not differ from predicted or between time points of follow-up on pegvisomant. The percent increase in VAT was greater than that of SAT in all patients combined, VAT 87% (1.56-229%) vs SAT 9% (–17 to 57%) (P < .001), in males VAT 110% (1.56-228)% vs SAT 12%(–17 to 50.3%) (P < .001), but not in females VAT 27% (19.9-92%) vs SAT 5% (–8.9 to 57%) (P = .13).
Intermuscular adipose tissue.
IMAT was above predicted prepegvisomant (P = .02) (Fig. 1E); 198% (median) (range 59-470%) overall, and 200% (59-470%) in males vs 172% (86-227%) in females (P = .44), of predicted. IMAT did not change with pegvisomant therapy (Fig. 1F). The proportion of patients with IMAT that was above predicted did not change from prepegvisomant, 8/11 without and 5/5 with diabetes, to on pegvisomant (P = ns). Four of the 5 patients with DM had a fall in IMAT (range 12-30% decrease) by 1 to 2 years of therapy that was sustained in 2 of them. Overall, IMAT remained significantly above predicted and did not differ between time points of follow-up on pegvisomant.
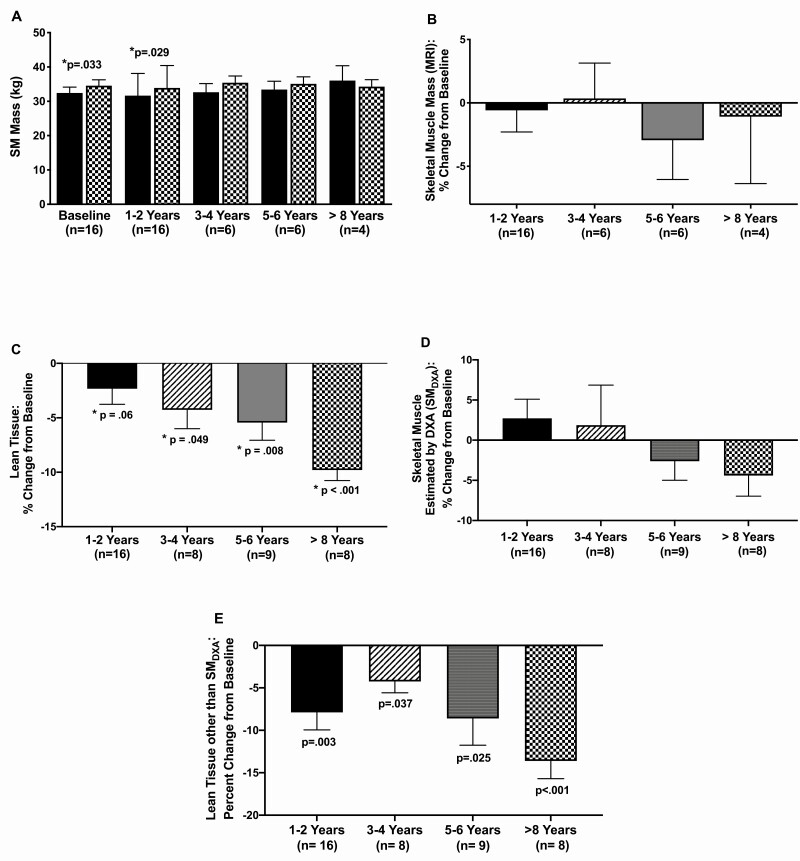
Skeletal muscle.
SM mass was below predicted before (median 95% of predicted) and at 1 to 2 years of pegvisomant therapy (93.7%) (Fig. 2A) and was similarly below predicted in males and females (not shown). Prepegvisomant, SM was below predicted in 12/16 and at or above predicted in 4/16 (range 100-114% predicted) patients. SM mass did not change with pegvisomant therapy (Fig. 2B). At last follow-up, SM change was –1.6% of baseline in males and 0.03% of baseline in females. On pegvisomant, SM was below predicted in 11/16 and at or above predicted in 5/16 (range 100-117%). The proportion of patients above predicted did not change on therapy(P = ns). Overall, SM mass did not differ from predicted or between time points of follow-up on pegvisomant.
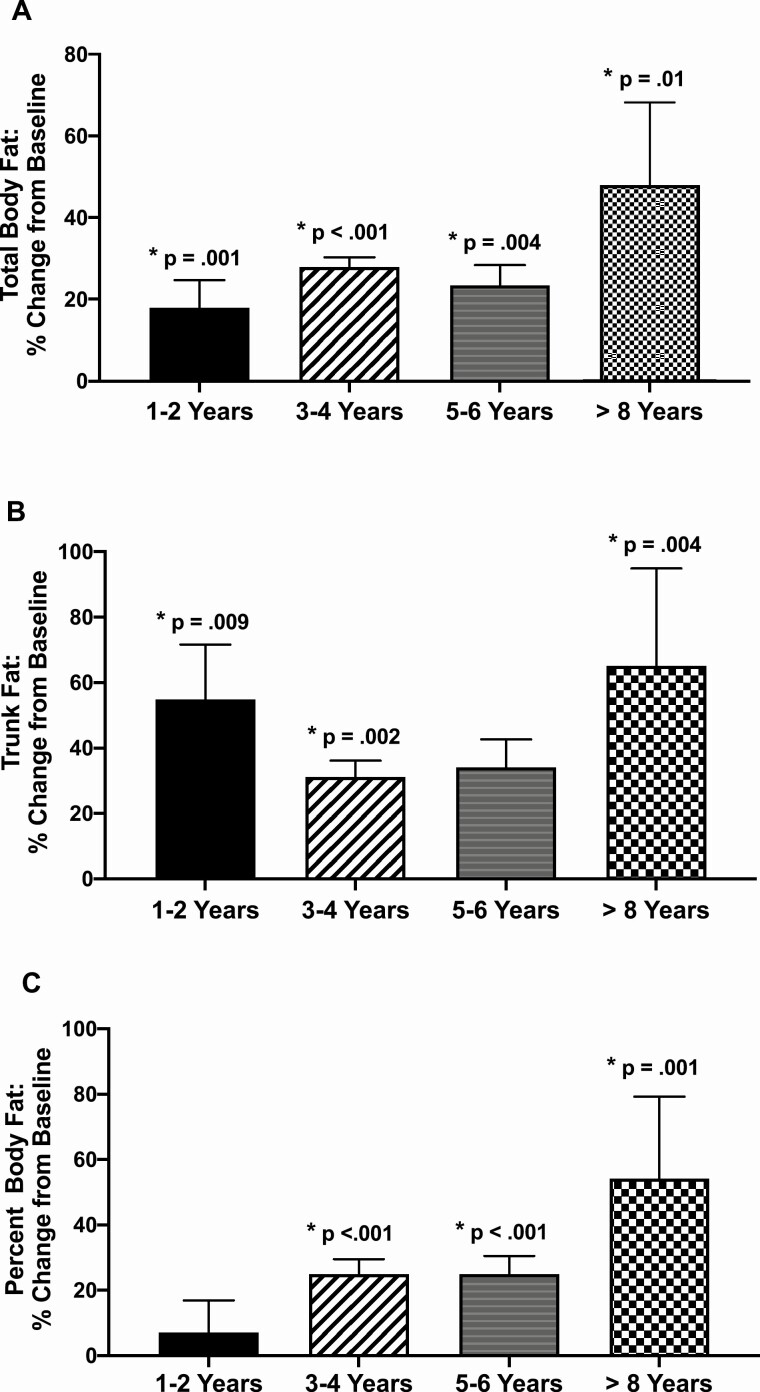
Figure 2.
Dual-energy x-ray absorptiometry (DXA) measured fat before and during pegvisomant therapy. Changes in total body (A) and trunk fat (B) mass and percent body fat (C) from prepegvisomant baseline to 1 to 2 years, 3 to 4 years, 5 to 6 years, and ≥8 years of pegvisomant therapy. P-values represent significance of change from prepegvisomant to on pegvisomant time points. The duration of follow-up by DXA was 1 to <3 years in 4 patients, 2 to <5 years in 4, 5 to <7 years in 3, and ≥8 to 13.44 years in 8 patients. Data are mean ± SE.
Intrahepatic and Intramyocellular lipid.
IHL and IMCL were tested in a subset of 8 patients (1 F, 7 M). IHL rose from 1.75% of water signal (median) (range 0.7-5%) to 3.5% (1.55-10.6%) (P = .04). Compared with matched healthy subjects, IHL showed a trend to be lower in acromegaly patients prepegvisomant, 1.75% (0.7-5%), than in controls, 3.04% (1.57-13.2%) (P = .049), but did not differ in the patients on pegvisomant, 3.5%, from controls, 3.04% (P = .85). There was no change in IMCL/water ratio with pegvisomant therapy (P = .29).
Dual-energy x-ray absorptiometry
Total body, trunk, and percent body fat rose (Fig. 3A-3C) and lean tissue fell with pegvisomant therapy (Fig. 2C). SMDXA did not change (Fig. 3D), but non-SM lean tissue fell with pegvisomant therapy (Fig. 3E). Thus, the reduction in lean tissue with pegvisomant therapy was due to that of non-SM lean tissue, which includes soft tissues, organs and other lean tissues and not to a reduction in SM mass.
Figure 3.
MRI measured skeletal muscle mass and DXA measured lean tissue and estimated skeletal muscle mass. (A) MRI measured SM mass in acromegaly patients (solid bar) compared with predicted values (patterned bar) at prepegvisomant baseline and at 1 to 2 years, 3 to 4 years, 5 to 6 years, and ≥8 years of pegvisomant. P values compare acromegaly with predicted values. (B) Changes in SM mass (MRI) from prepregvisomant baseline to 1 to 2 years, 3 to 4 years, 5 to 6 years, and ≥8 years of pegvisomant therapy. P values represent significance of change from prepegvisomant to on pegvisomant time points. DXA measured changes in lean tissue (C), SMDXA (skeletal muscle estimated by DXA) (D) and lean tissue other than SMDXA (E) from prepregvisomant baseline to 1 to 2 years, 3 to 4 years, 5 to 6 years, and ≥8 years of pegvisomant therapy. P values represent significance of change from prepegvisomant to on pegvisomant time point. Data are mean ± SE.
Relationship of Body Composition to Endocrine Changes
Reduction in IGF-1 level correlated with that of lean tissue (DXA) (r = 0.459, P = .048), weight (r = 0.642, P = .002) and BMI (r = 0.63, P = .003). Percent change in leptin level correlated with that of SAT (r = 0.536, P = .03), IMAT (r = 0.601, P = .014), total body fat (DXA) (r = 0.472, P = .048) and % body fat (DXA) (r = 0.525, P = .025). QUICKI change correlated inversely with change in BMI (r = –0.469, P = .049) and showed a trend to correlate inversely with that of weight (r = –0.422, P = .07).
Discussion
A main finding of this study is that increases in AT mass, especially that of VAT, occur with pegvisomant therapy and are maintained, but do not escalate, during long-term therapy. In accord with this, we found persistent increases in DXA measured total and percent body and trunk fat with pegvisomant treatment. The effects of pegvisomant on body composition have been examined previously in a few small, short-term studies. In one, 7 patients treated with 4 weeks of pegvisomant had a moderate increase in percent body fat assessed by bioimpedance analysis (BIA) [31] and, in another, 5 patients treated for 6 months with pegvisomant, computed tomography–measured intra-abdominal fat mass increased, but SAT did not [18]. Our larger and longer study, by contrast, showed a significant rise in VAT and SAT that persisted over years of pegvisomant therapy. Waist circumference increased, in accord with the increases in central adiposity seen by MRI and DXA. Although our patients’ prior acromegaly therapy might be considered a limitation of our study, they had been unsuccessfully treated for years prior to starting pegvisomant (Table 2) and had biochemical changes with it similar to those that occur with successful surgery [32]. This likely explains why their prepegvisomant body composition pattern was similar to that of newly diagnosed acromegaly [8] and their VAT and SAT changes with pegvisomant were similar or just somewhat less than those observed in a surgical cohort [9]. We also found that VAT and SAT did not differ from predicted with long-term therapy. Use of a predictive model is advantageous to matching acromegaly patients to controls because abnormalities of body composition, including the skeleton, make matching on typical parameters, such as BMI, unreliable [33]. The fact that VAT did not rise above predicted is reassuring that long-term GHR antagonism does not produce a growth hormone deficiency (GHD) like pattern of central adiposity and elevated VAT mass, which might increase cardiovascular risk as in other populations [34, 35]. Long-term outcome studies are needed to investigate this further.
The changes in AT mass we observed with GHR antagonist therapy are consistent with reversal of the mechanisms by which GH reduces AT, in particular VAT, mass [36-41]. The effects of pegvisomant on fat metabolism have been studied in a few prior, short-term studies in acromegaly and healthy subjects and showed no changes [31, 42, 43] or suppression of lipid mobilization and oxidation [44]. Interestingly, cases of localized SAT hypertrophy have been reported with pegvisomant use [45-48]. Biopsied SAT in one such patient showed normal AT [48]. Antagonism of the effects of GH on local adipocyte lipid metabolism and unopposed insulin effects resulting in lipogenesis were suggested to be responsible for this lipohypertrophy [45]. We did not observe localized lipohypertrophy in our patients, but similar generalized effects on AT metabolism could have contributed to produce the increased AT mass we observed.
Our study also importantly shows that pegvisomant monotherapy leads to a rise in IHL. IHL was lower than in controls in active disease and similar to controls on pegvisomant, suggesting that it returns to expected levels on therapy. The magnitude of change of IHL was less than VAT, suggesting a possible predominance of the mechanisms by which GH exerts its lipid reducing effects in VAT compared with liver as has been shown for VAT compared with SAT [38-40], but the small number of subjects studied is a limitation of this analysis. In a 24-week study of patients well controlled on long-acting somatostatin analog therapy, pegvisomant added to this increased IHL in 9 patients compared with that in 9 who continued somatostatin analog alone [19]. We and others previously showed that IHL increases with surgical treatment of acromegaly and is higher than in controls years after surgery [9, 10, 49, 50]. Other data in rodents and patients with GHD support a role for GH in regulating IHL accumulation [51-53]. Taken together, these results suggest that in active acromegaly IHL is low and rises to normal with therapy.
We also found that IR improved with pegvisomant therapy in parallel with the rise in adiposity and IHL. The patterns of greater IR with lower adiposity/IHL in active and less IR yet higher adiposity/IHL in treated acromegaly contrasts with other populations where these correlate positively [54]. Pegvisomant treatment of acromegaly improves hepatic and peripheral insulin sensitivity [42, 55] and glycemic control [13, 55-58]. In our study, HOMA, QUICKI, and HbA1C improved during pegvisomant therapy and no patients had a worsening of these parameters of glucose metabolism despite increases in VAT and IHL. Since liver and muscle IR developed in a model of pegvisomant-induced GHD in healthy adults [59] and pegvisomant-induced GHD may manifest as a combination of increased adiposity and glucose intolerance [18], our data are not consistent with GHD during pegvisomant therapy. We also found that plasma leptin levels rise with pegvisomant therapy, in accord with another prospective study [60]. As expected, since leptin correlates positively with fat mass [61, 62], we found that percent change in leptin correlated with that of SAT and IMAT masses and % body fat by DXA. Interestingly, however, leptin changes did not correlate inversely with measures of IR as in other populations, likely because mechanisms for IR other than fat mass are most important to IR in acromegaly. The small number of subjects with diabetes in our study precluded a separate analysis of them, but they showed trends for all body composition changes in accord with those of the subjects without DM.
We also studied, for the first time, the effect of pegvisomant on IMAT [8]. We found, unexpectedly, that despite improved insulin sensitivity, IMAT was not lowered and remained above predicted during long-term pegvisomant therapy. Increased IMAT in active acromegaly suggests that GH-induced AT lipolysis leads to lipid movement out of VAT and SAT and into IMAT, thus ectopic lipid deposition in muscle. In our study, IMCL also did not change. In another study, IMCL did not differ in 7 pegvisomant treated acromegaly patients and controls [50], but in another IMCL decreased with the addition of pegvisomant to somatostatin analog therapy [19]. Other data suggest that GH excess produces muscle lipid accumulation. In acromegaly and GH use, free fatty acids (FFA) flux and muscle uptake are increased [63-68] and in supraphysiologic GH increased IMCL on SM biopsy [69]. Ectopic lipid deposition in and around SM may contribute to IR in acromegaly. In other settings, higher IMCL [67] and IMAT mass correlate with IR [70-74] and in a combined acromegaly and control cohort IMCL correlated inversely with insulin sensitivity [50]. We did not directly test IR in SM in our study and the reason why IMCL and IMAT did not change with pegvisomant while IR improved is unknown.
Another important finding in our study is that SM mass did not change with long-term pegvisomant therapy. No prior study has reported on the effects of pegvisomant on SM mass. Stability of SMDXA, which correlates highly with SM measured by MRI in acromegaly [20], further supports our MRI findings. The small number of subjects at some time points and variations in their duration of follow-up and magnitude of body composition changes should be considered in the interpretation of this analysis and others and are limitations of our study. Although lean tissue decreased, this could be accounted for by that of its non-SM component, which includes soft tissues and organs, and not SMDXA. Reports of the effects of acromegaly and its treatment on muscle mass, strength, and performance vary [6, 7, 61, 75-80]. Pegvisomant increased protein oxidation in acromegaly patients [31], but did not change this over 36 hours in healthy subjects [81] and attenuated exercise performance in healthy males [43]. Adult GHD is associated with reduced muscle strength, at least partly due to reduced muscle mass [16, 82, 83]. Since pegvisomant therapy, in sufficient doses, could potentially cause a functional GHD in tissues, and serum IGF-1 level is not a reliable indicator of GHD in adults [84], investigation of potential SM changes was warranted. While muscle mass does not necessarily predict its quality or performance, lack of reduction and stability at predicted levels seem reassuring that GHD in SM does not occur with long-term pegvisomant use. Weight and BMI did not change with pegvisomant therapy, as has been reported by others [15], likely reflecting that gains in AT were matched by losses in lean tissue other than SM, but some component of loss of bone mass is also possible. As after surgery, lean tissue losses likely represent those of extracellular water [4], other soft tissue, or vital organ mass [7]. Further study is needed to discern components of lean tissue change with pegvisomant therapy. Although IGF-1 values were expressed relative to assay-specific normal ranges, our use of 3 different IGF-1 assays is a limitation of our study.
In conclusion, this study provides novel evidence of a sustained increase in adiposity, but no change in SM mass with long-term pegvisomant therapy. The stability of SM mass and the facts that AT mass does not rise above predicted or escalate over time and IR improves, suggest that years of GHR antagonism does not result in a GHD pattern of body composition. Pegvisomant therapy leads to a seemingly less favorable body composition profile and rise in cardiovascular risk markers [85] that parallel normalization of IGF-1 levels and improvement in glucose metabolism. Long-term outcome data are needed to confirm that normalization of IGF-1 level on pegvisomant returns excess mortality to normal despite these other effects.
Acknowledgments
Financial Support: Funded by NIH grants R01 DK 110771 and DK 064720 to P.U.F., UL1 TR000040 from NCATS/NIH to Columbia University and in part by P30-DK-26687 and P01-DK42618 to the New York Obesity Research Center.
Glossary
Abbreviations
- 1HMRS
proton magnetic resonance spectroscopy
- AT
adipose tissue
- BMI
body mass index
- CV
coefficient of variation
- DXA
dual-energy X-ray absorptiometry
- GH
growth hormone
- GHR
growth hormone receptor
- HbA1C
glycated hemoglobin
- HOMA
homeostasis model assessment
- IGF-1
insulin-like growth factor 1
- IHL
intrahepatic lipid
- IMAT
intermuscular adipose tissue compartment
- IMCL
intramyocellular lipid
- IR
insulin resistance
- MRI
magnetic resonance imaging
- RIA
radioimmunoassay
- SAT
subcutaneous adipose tissue
- SM
skeletal muscle
- QUICKI
quantitative insulin sensitivity check index
- VAT
visceral adipose tissue
- WHO
World Health Organization
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Ho KK, O’Sullivan AJ, Hoffman DM. Metabolic actions of growth hormone in man. Endocr J. 1996;43 Supp(l):S57-S63. [DOI] [PubMed] [Google Scholar]
- 2. Russell-Jones DL, Weissberger AJ. The role of growth hormone in the regulation of body composition in the adult. Growth Regul. 1996;6(4):247-252. [PubMed] [Google Scholar]
- 3. Reid TJ, Post KD, Bruce JN, Nabi Kanibir M, Reyes-Vidal CM, Freda PU. Features at diagnosis of 324 patients with acromegaly did not change from 1981 to 2006: acromegaly remains under-recognized and under-diagnosed. Clin Endocrinol (Oxf). 2010;72(2):203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Sullivan AJ, Kelly JJ, Hoffman DM, Freund J, Ho KK. Body composition and energy expenditure in acromegaly. J Clin Endocrinol Metab. 1994;78(2):381-386. [DOI] [PubMed] [Google Scholar]
- 5. Bengtsson BA, Brummer RJ, Edén S, Bosaeus I. Body composition in acromegaly. Clin Endocrinol (Oxf). 1989;30(2):121-130. [DOI] [PubMed] [Google Scholar]
- 6. Bengtsson BA, Brummer RJ, Edén S, Bosaeus I, Lindstedt G. Body composition in acromegaly: the effect of treatment. Clin Endocrinol (Oxf). 1989;31(4):481-490. [DOI] [PubMed] [Google Scholar]
- 7. Brummer RJ, Lönn L, Kvist H, Grangård U, Bengtsson BA, Sjöström L. Adipose tissue and muscle volume determination by computed tomography in acromegaly, before and 1 year after adenomectomy. Eur J Clin Invest. 1993;23(4):199-205. [DOI] [PubMed] [Google Scholar]
- 8. Freda PU, Shen W, Heymsfield SB, et al. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J Clin Endocrinol Metab. 2008;93(6):2334-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reyes-Vidal CM, Mojahed H, Shen W, et al. Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J Clin Endocrinol Metab. 2015;100(8):2946-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bredella MA, Schorr M, Dichtel LE, et al. Body composition and ectopic lipid changes with biochemical control of acromegaly. J Clin Endocrinol Metab. 2017;102(11): 4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev. 2002;23(5):623-646. [DOI] [PubMed] [Google Scholar]
- 12. Franck SE, van der Lely AJ, Neggers S. Extra-hepatic acromegaly. Eur Endocrinol. 2013;9(1):66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Lely AJ, Hutson RK, Trainer PJ, et al. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet. 2001;358(9295):1754-1759. [DOI] [PubMed] [Google Scholar]
- 14. Trainer PJ, Drake WM, Katznelson L, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342(16):1171-1177. [DOI] [PubMed] [Google Scholar]
- 15. Kuhn E, Maione L, Bouchachi A, et al. Long-term effects of pegvisomant on comorbidities in patients with acromegaly: a retrospective single-center study. Eur J Endocrinol. 2015;173(5):693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr Rev. 2006;27(3):287-317. [DOI] [PubMed] [Google Scholar]
- 17. Jørgensen JO, Vestergaard E, Gormsen L, et al. Metabolic consequences of GH deficiency. J Endocrinol Invest. 2005;28(5 Suppl):47-51. [PubMed] [Google Scholar]
- 18. Plöckinger U, Reuter T. Pegvisomant increases intra-abdominal fat in patients with acromegaly: a pilot study. Eur J Endocrinol. 2008;158(4):467-471. [DOI] [PubMed] [Google Scholar]
- 19. Madsen M, Krusenstjerna-Hafstrøm T, Møller L, et al. Fat content in liver and skeletal muscle changes in a reciprocal manner in patients with acromegaly during combination therapy with a somatostatin analog and a GH receptor antagonist: a randomized clinical trial. J Clin Endocrinol Metab. 2012;97(4):1227-1235. [DOI] [PubMed] [Google Scholar]
- 20. Freda PU, Shen W, Reyes-Vidal CM, et al. Skeletal muscle mass in acromegaly assessed by magnetic resonance imaging and dual-photon x-ray absorptiometry. J Clin Endocrinol Metab. 2009;94(8):2880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 22. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402-2410. [DOI] [PubMed] [Google Scholar]
- 23. Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim J, Heshka S, Gallagher D, et al. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy x-ray absorptiometry in adults. J Appl Physiol (1985). 2004;97(2):655-660. [DOI] [PubMed] [Google Scholar]
- 25. Freda PU, Post KD, Powell JS, Wardlaw SL. Evaluation of disease status with sensitive measures of growth hormone secretion in 60 postoperative patients with acromegaly. J Clin Endocrinol Metab. 1998;83(11):3808-3816. [DOI] [PubMed] [Google Scholar]
- 26. RRID:AB_2811291. [Google Scholar]
- 27. Freda PU, Nuruzzaman AT, Reyes CM, Sundeen RE, Post KD. Significance of “abnormal” nadir growth hormone levels after oral glucose in postoperative patients with acromegaly in remission with normal insulin-like growth factor-I levels. J Clin Endocrinol Metab. 2004;89(2):495-500. [DOI] [PubMed] [Google Scholar]
- 28. RRID:AB_2756880. [Google Scholar]
- 29. Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin-like growth factor-1 (IGF-I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712-1721. [DOI] [PubMed] [Google Scholar]
- 30. RRID:AB_2861357. [Google Scholar]
- 31. Lindberg-Larsen R, Møller N, Schmitz O, et al. The impact of pegvisomant treatment on substrate metabolism and insulin sensitivity in patients with acromegaly. J Clin Endocrinol Metab. 2007;92(5):1724-1728. [DOI] [PubMed] [Google Scholar]
- 32. Reyes-Vidal C, Fernandez JC, Bruce JN, et al. Prospective study of surgical treatment of acromegaly: effects on ghrelin, weight, adiposity, and markers of CV risk. J Clin Endocrinol Metab. 2014;99(11):4124-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reid TJ, Jin Z, Shen W, et al. IGF-1 levels across the spectrum of normal to elevated in acromegaly: relationship to insulin sensitivity, markers of cardiovascular risk and body composition. Pituitary. 2015;18(6):808-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed). 1984;288(6428):1401-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27(5):996-1003. [DOI] [PubMed] [Google Scholar]
- 36. Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat Rev Endocrinol. 2020;16(3):135-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Møller N, Gjedsted J, Gormsen L, Fuglsang J, Djurhuus C. Effects of growth hormone on lipid metabolism in humans. Growth Horm IGF Res. 2003;13(Suppl (A):S18-S21. [DOI] [PubMed] [Google Scholar]
- 38. Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24(6):276-283. [DOI] [PubMed] [Google Scholar]
- 39. Hoffstedt J, Arner P, Hellers G, Lönnqvist F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J Lipid Res. 1997;38(4):795-804. [PubMed] [Google Scholar]
- 40. Richelsen B, Pedersen SB, Møller-Pedersen T, Bak JF. Regional differences in triglyceride breakdown in human adipose tissue: effects of catecholamines, insulin, and prostaglandin E2. Metabolism. 1991;40(9):990-996. [DOI] [PubMed] [Google Scholar]
- 41. Nam SY, Lobie PE. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev. 2000;1(2):73-86. [DOI] [PubMed] [Google Scholar]
- 42. Higham CE, Rowles S, Russell-Jones D, Umpleby AM, Trainer PJ. Pegvisomant improves insulin sensitivity and reduces overnight free fatty acid concentrations in patients with acromegaly. J Clin Endocrinol Metab. 2009;94(7):2459-2463. [DOI] [PubMed] [Google Scholar]
- 43. Goto K, Doessing S, Nielsen RH, Flyvbjerg A, Kjaer M. Growth hormone receptor antagonist treatment reduces exercise performance in young males. J Clin Endocrinol Metab. 2009;94(9):3265-3272. [DOI] [PubMed] [Google Scholar]
- 44. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177. [DOI] [PubMed] [Google Scholar]
- 45. Bonert VS, Kennedy L, Petersenn S, Barkan A, Carmichael J, Melmed S. Lipodystrophy in patients with acromegaly receiving pegvisomant. J Clin Endocrinol Metab. 2008;93(9):3515-3518. [DOI] [PubMed] [Google Scholar]
- 46. Schreiber I, Buchfelder M, Droste M, et al. ; German Pegvisomant Investigators . Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol. 2007;156(1):75-82. [DOI] [PubMed] [Google Scholar]
- 47. Maffei P, Martini C, Pagano C, Sicolo N, Corbetti F. Lipohypertrophy in acromegaly induced by the new growth hormone receptor antagonist pegvisomant. Ann Intern Med. 2006;145(4):310-312. [DOI] [PubMed] [Google Scholar]
- 48. Marazuela M, Daudén E, Ocón E, Moure D, Nattero L. Pegvisomant-induced lipohypertrophy: report of a case with histopathology. Ann Intern Med. 2007;147(10):741-743. [DOI] [PubMed] [Google Scholar]
- 49. Winhofer Y, Wolf P, Krššák M, et al. No evidence of ectopic lipid accumulation in the pathophysiology of the acromegalic cardiomyopathy. J Clin Endocrinol Metab. 2014;99(11):4299-4306. [DOI] [PubMed] [Google Scholar]
- 50. Szendroedi J, Zwettler E, Schmid AI, et al. Reduced basal ATP synthetic flux of skeletal muscle in patients with previous acromegaly. PLoS One. 2008;3(12):e3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937-19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takahashi Y, Iida K, Takahashi K, et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology. 2007;132(3):938-943. [DOI] [PubMed] [Google Scholar]
- 53. Takano S, Kanzaki S, Sato M, Kubo T, Seino Y. Effect of growth hormone on fatty liver in panhypopituitarism. Arch Dis Child. 1997;76(6):537-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(23):2237-2238. [DOI] [PubMed] [Google Scholar]
- 55. Drake WM, Rowles SV, Roberts ME, et al. Insulin sensitivity and glucose tolerance improve in patients with acromegaly converted from depot octreotide to pegvisomant. Eur J Endocrinol. 2003;149(6):521-527. [DOI] [PubMed] [Google Scholar]
- 56. Jørgensen JO, Feldt-Rasmussen U, Frystyk J, et al. Cotreatment of acromegaly with a somatostatin analog and a growth hormone receptor antagonist. J Clin Endocrinol Metab. 2005;90(10):5627-5631. [DOI] [PubMed] [Google Scholar]
- 57. Feola T, Cozzolino A, Simonelli I, et al. Pegvisomant improves glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab. 2019;104(7):2892-2902. [DOI] [PubMed] [Google Scholar]
- 58. Rose DR, Clemmons DR. Growth hormone receptor antagonist improves insulin resistance in acromegaly. Growth Horm IGF Res. 2002;12(6):418-424. [DOI] [PubMed] [Google Scholar]
- 59. Muller AF, Janssen JA, Hofland LJ, et al. Blockade of the growth hormone (GH) receptor unmasks rapid GH-releasing peptide-6-mediated tissue-specific insulin resistance. J Clin Endocrinol Metab. 2001;86(2):590-593. [DOI] [PubMed] [Google Scholar]
- 60. Parkinson C, Whatmore AJ, Yates AP, et al. The effect of pegvisomant-induced serum IGF-I normalization on serum leptin levels in patients with acromegaly. Clin Endocrinol (Oxf). 2003;59(2):168-174. [DOI] [PubMed] [Google Scholar]
- 61. Homem TS, Guimarães FS, Soares MS, Kasuki L, Gadelha MR, Lopes AJ. Balance control and peripheral muscle function in aging: a comparison between individuals with acromegaly and healthy subjects. J Aging Phys Act. 2017;25(2): 218-227. [DOI] [PubMed] [Google Scholar]
- 62. Bolanowski M, Milewicz A, Bidzińska B, Jedrzejuk D, Daroszewski J, Mikulski E. Serum leptin levels in acromegaly – a significant role for adipose tissue and fasting insulin/glucose ratio. Med Sci Monit. 2002;8(10):CR685-CR689. [PubMed] [Google Scholar]
- 63. Rabinowitz D, Klassen GA, Zierler KL. Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest. 1965;44:51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nielsen S, Møller N, Christiansen JS, Jørgensen JO. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes. 2001;50(10):2301-2308. [DOI] [PubMed] [Google Scholar]
- 65. Møller N, Jørgensen JO, Alberti KG, Flyvbjerg A, Schmitz O. Short-term effects of growth hormone on fuel oxidation and regional substrate metabolism in normal man. J Clin Endocrinol Metab. 1990;70(4):1179-1186. [DOI] [PubMed] [Google Scholar]
- 66. Møller N, Butler PC, Antsiferov MA, Alberti KG. Effects of growth hormone on insulin sensitivity and forearm metabolism in normal man. Diabetologia. 1989;32(2):105-110. [DOI] [PubMed] [Google Scholar]
- 67. Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113-116. [DOI] [PubMed] [Google Scholar]
- 68. Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612-1617. [DOI] [PubMed] [Google Scholar]
- 69. Krag MB, Gormsen LC, Guo Z, et al. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab. 2007;292(3):E920-E927. [DOI] [PubMed] [Google Scholar]
- 70. Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372-379. [DOI] [PubMed] [Google Scholar]
- 71. Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82(6):1210-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Albu JB, Kenya S, He Q, et al. Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am J Clin Nutr. 2007;86(1):100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Joy T, Grinspoon SK. Adipose compartmentalization and insulin resistance among obese HIV-infected women: the role of intermuscular adipose tissue. Am J Clin Nutr. 2007;86(1):5-6. [DOI] [PubMed] [Google Scholar]
- 74. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885-892. [DOI] [PubMed] [Google Scholar]
- 75. Mastaglia FL, Barwich DD, Hall R. Myopathy in acromegaly. Lancet. 1970;2(7679):907-909. [DOI] [PubMed] [Google Scholar]
- 76. Lopes AJ, Ferreira AS, Walchan EM, Soares MS, Bunn PS, Guimarães FS. Explanatory models of muscle performance in acromegaly patients evaluated by knee isokinetic dynamometry: Implications for rehabilitation. Hum Mov Sci. 2016;49:160-169. [DOI] [PubMed] [Google Scholar]
- 77. Füchtbauer L, Olsson DS, Bengtsson BÅ, Norrman LL, Sunnerhagen KS, Johannsson G. Muscle strength in patients with acromegaly at diagnosis and during long-term follow-up. Eur J Endocrinol. 2017;177(2):217-226. [DOI] [PubMed] [Google Scholar]
- 78. Tominaga A, Arita K, Kurisu K, et al. Effects of successful adenomectomy on body composition in acromegaly. Endocr J. 1998;45(3):335-342. [DOI] [PubMed] [Google Scholar]
- 79. Nagulesparen M, Trickey R, Davies MJ, Jenkins JS. Muscle changes in acromegaly. Br Med J. 1976;2(6041):914-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khaleeli AA, Levy RD, Edwards RH, et al. The neuromuscular features of acromegaly: a clinical and pathological study. J Neurol Neurosurg Psychiatry. 1984;47(9):1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moller L, Norrelund H, Jessen N, et al. Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. J Clin Endocrinol Metab. 2009;94(11):4524-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Janssen YJ, Doornbos J, Roelfsema F. Changes in muscle volume, strength, and bioenergetics during recombinant human growth hormone (GH) therapy in adults with GH deficiency. J Clin Endocrinol Metab. 1999;84(1):279-284. [DOI] [PubMed] [Google Scholar]
- 83. Johannsson G, Grimby G, Sunnerhagen KS, Bengtsson BA. Two years of growth hormone (GH) treatment increase isometric and isokinetic muscle strength in GH-deficient adults. J Clin Endocrinol Metab. 1997;82(9):2877-2884. [DOI] [PubMed] [Google Scholar]
- 84. Paisley AN, Trainer PJ. The challenges of reliance on insulin-like growth factor I in monitoring disease activity in patients with acromegaly. Horm Res. 2004;62 Suppl (1):83-88. [DOI] [PubMed] [Google Scholar]
- 85. Sesmilo G, Fairfield WP, Katznelson L, et al. Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist pegvisomant. J Clin Endocrinol Metab. 2002;87(4):1692-1699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.