Abstract
Background
No reliable biomarkers exist to guide glucocorticoid (GC) replacement treatment in autoimmune Addison’s disease (AAD), leading to overtreatment with alarming and persistent side effects or undertreatment, which could be fatal.
Objective
To explore changes in gene expression following different GC replacement doses as a means of identifying candidate transcriptional biomarkers to guide GC replacement in AAD.
Methods
Step 1: Global microarray expression analysis on RNA from whole blood before and after intravenous infusion of 100 mg hydrocortisone (HC) in 10 patients with AAD. In 3 of the most highly upregulated genes, we performed real-time PCR (rt-PCR) to compare gene expression levels before and 3, 4, and 6 hours after the HC infusion. Step 2: Rt-PCR to compare expression levels of 93 GC-regulated genes in normal versus very low morning cortisol levels in 27 patients with AAD.
Results
Step 1: Two hours after infusion of 100 mg HC, there was a marked increase in FKBP5, MMP9, and DSIPI expression levels. MMP9 and DSIPI expression levels correlated with serum cortisol. Step 2: Expression levels of CEBPB, DDIT4, FKBP5, DSIPI, and VDR were increased and levels of ADARB1, ARIDB5, and POU2F1 decreased in normal versus very low morning cortisol. Normal serum cortisol levels positively correlated with DSIPI, DDIT4, and FKBP5 expression.
Conclusions
We introduce gene expression as a novel approach to guide GC replacement in AAD. We suggest that gene expression of DSIPI, DDIT4, and FKBP5 are particularly promising candidate biomarkers of GC replacement, followed by MMP9, CEBPB, VDR, ADARB1, ARID5B, and POU2F1.
Keywords: Addison’s disease, primary adrenal insufficiency, biomarkers, gene expression, glucocorticoid
In autoimmune Addison’s disease (AAD), patients suffer from deficiency of glucocorticoids (GCs) and mineralocorticoids due to autoimmune destruction of the adrenal cortex, a fatal condition if left untreated [1]. Current treatment strategies rely on replacement of cortisol (the main GC) and aldosterone (the main mineralocorticoid), usually twice or thrice oral hydrocortisone (HC) or cortisone acetate in combination with once daily oral fludrocortisone and salt. Conventional treatment strategies fail to restore good health in patients with AAD, evident by lower quality of life [2, 3], increased prevalence of cardiovascular disease, metabolic syndrome, and infections as well as overall higher mortality rates [4-7].
In addition to the lethal threat of acute adrenal crisis, current evidence points to 2 main causes of the deleterious health outcomes in AAD. First, none of the currently used treatment modalities perfectly mimic the physiological circadian rhythm of GC production. Second, there is no biomarker available to aid physicians in determining the correct GC replacement dosage for each individual [8, 9]. Instead, GC dosages are adjusted based on the patient’s symptoms, signs, and general well-being. Overtreatment may be especially challenging to identify, as clinical clues of too-high GC replacement are nonspecific and slow to develop, including weight gain, metabolic syndrome, hypertension, and sleep disturbances [10].
Despite numerous attempts, measurement of GCs and adrenocorticotropic hormone (ACTH) in blood, urine, and saliva fall short to guide GC replacement [11]. In the last decade, hair cortisol concentration has emerged as a promising tool for assessing GC exposure over time [9]. On the downside, this approach requires a relatively large hair sample (1 cm thick), and is therefore not suited for frequent assessment, and in bald patients, not at all. Although measurement of GC levels in body fluids or tissue will provide information on the bioavailability of the exogenous GC in AAD, it does not reflect the actual physiological effect [12].
Gene expression is an alternative avenue that allows for evaluation of GC effects at a transcriptional level in the individual patient. Through binding to the GC receptor, GCs regulate the expression of several hundred genes involved in vital physiological processes, including metabolic hemostasis, stress response, and immunity [13]. Previous studies suggest that expression levels of GC-regulated genes align with the reported use and dosage of exogenous GC [14]. Yet, its potential use for guiding GC treatment in AAD remains an unexplored landscape.
We conceived this study in an aspiration to improve health and quality of life in patients with AAD by personalizing GC replacement dosages. In order to foresee positive and adverse effects of different GC dosages, we need access to biomarkers that can act as sensors of GC treatment. Here, we set out to identify candidate GC biomarkers among GC-responsive genes, characterized by change in gene expression levels as a response to different levels of GC exposure.
Patients and Methods
Study Design
In a two-step approach, we investigated the expression of GC-regulated genes in different settings of GC replacement in patients with AAD: first, after a high-dose stress test with intravenous 100 mg HC, and second, at normal morning cortisol compared to very low morning cortisol levels. In the second step, normal morning cortisol was achieved through near-physiological replacement with continuous subcutaneous hydrocortisone infusion (CSHI) and very low morning cortisol levels through an over-night (> 15 hour) fast from conventional oral hydrocortisone replacement treatment (OHC).
In the first step, we wanted to explore the immediate effects of high-dose HC exposure on 3 selected genes. In the second step, we compared expression levels of a selected large range of GC-regulated genes in patients with AAD with normal morning cortisol versus very low morning cortisol levels.
Step 1: Gene Expression in Response to 100 mg Hydrocortisone
Patients
Ten patients (50% females) with verified AAD were recruited from the National Registry for Addison’s disease (ROAS). Their median daily cortisone acetate dose was 37.5 mg/day (12.5-50 mg/day), equivalent to 30 mg/day HC (10-40 mg/day). Blood samples were collected at 8 to 9 am (time point 0), after having abstained from cortisone acetate treatment for 18 hours, and repeated at 2 (2h), 4 (4h), and 6 (6h) hours after intravenous infusion of 100 mg HC. Samples were anonymized. All subjects provided written informed consent and ethical permission was granted prior to study start (Norway REK Vest no. 014.03).
RNA purification from blood
Whole blood was sampled into Tempus Blood RNA tubes at time points 0, 2h, 4h, and 6h, and RNA extraction from leukocytes performed on the 6100 Nucleic Acid PrepStation (Applied Biosystems, USA). Amount and quality of the extracted RNA was verified by the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA) and the Agilent 2100 Bioanalyzer (Agilent Technologies, USA). All RNA samples were qualitatively adequate with RNA integrity numbers between 6.9 and 9.7.
Global transcriptional analysis in blood using RNA expression microarray
Initially, RNA samples from time points 0 and 2h from all 10 patients were included. Microarray experiments were performed using the Applied Biosystems 1700 Expression Array system. An amount of 800 ng of total RNA from each sample was reverse transcribed, amplified, and DIG-labeled (DIG-dUTP; Roche, Germany), using the Applied Biosystems NanoAmp RT-IVT Labeling Kit. The amount (82-147 μg) and the quality of the DIG-labeled cRNA was controlled by both NanoDrop spectrophotometer and Agilent 2100 Bioanalyzer. All samples except one passed the qualitative analysis, and both samples from this individual were removed. Hence, we proceeded with 18 samples. Then, 20 μg of DIG-labeled cRNA was hybridized to the Applied Biosystems Human Genome Survey Microarray v.1.0. The chemiluminescent signal detection, image acquisition, and image analysis of the microarrays were performed on the Applied Biosystems 1700 Chemiluminescent Microarray Analyzer. In addition to the QC report generated by the AB1700 scanner software, the integrated R software was used to control data integrity graphical outputs. Data was loaded onto BioArray Software Enviroment (BASE), and PANTHER was used to annotate the probe IDs.
Among the 150 most upregulated genes in the global transcription analysis, we selected 3 for further investigation (Supplementary appendix 1 [15]): FK506 binding protein 51 (FKBP5), matrix metallopeptidase 9 (MMP9), and delta sleep inducing peptide immunoreactor (DSIPI; also known as TSC22D3 and GILZ). FKBP5 was selected as it is an important short-loop feedback inhibitor of GC action [16]; MMP9 because mounting evidence points at its potential as a biomarker in multiple conditions, including cardiovascular [17, 18] and inflammatory diseases [19, 20]; and DSIPI for being a multi-tissue biomarker of GC action [21].
Real-time PCR verification of differentially expressed genes following 100 mg HC intravenously
Real-time PCR (rt-PCR) verification of the microarray-data at time points 0, 2h, 4h, and 6h was done for FKBP5, MMP9, and DSIPI using the High-Capacity RNA-to-cDNA Kit and commercially available Taqman-probes. Glyceraldehyde 3-phosphate dehydrogenase (GADPH) was applied as housekeeping gene. The PCR amplification protocol was denaturation at 95 °C for 10 minutes, followed by 40 amplification cycles each at 95 °C in 15 seconds and 60 °C for 1 minute. All samples were run in triplicates on the 7900HT PCR system. The ΔΔCt method was applied to calculate differences between the time points [22].
Hormone analysis
Venous blood was collected at time points 0 and 2h for analysis of serum cortisol and plasma ACTH. The hormone analyses were done using immunoassay kits from Diagnostic Products Corp (Los Angeles, CA, USA; Siemens, Cat# L5KCO2, RRID:AB_2877715, and Siemens, Cat# L5KAC2, RRID:AB_2877714) at Haukeland University Hospital, Bergen, Norway, both assays with a coefficient of variation (CV%) <10%.
Step 2: Gene Expression in Normal Versus Very Low Morning Cortisol Levels
Patients and treatment modalities
Patient selection and study design has been described in detail elsewhere [23]. In short, a prospective, randomized, cross-over study was conducted, comparing 12 weeks treatment with OHC versus CSHI. The 2 treatment periods were separated by a minimum 8 weeks washout, in which the patient followed his or her usual GC replacement. At the end of each of the 12-week treatment period, the patients returned to the hospital for blood sampling at 8 am.
For the present study, we used the 2 treatment modalities as a means of controlling morning GC exposure. When treated with OHC, patients were asked to take their final HC dose before 5 pm the day before testing and were practically cortisol depleted upon testing. When treated with CSHI, however, patients continued to receive HC infusions, meaning the blood samples were collected shortly after the simulated morning cortisol peak. For the rest of this paper, we therefore refer to the 2 different GC replacement situations as very low cortisol (>15 hour fasting from OHC) and normal cortisol (CSHI).
Our study cohort consisted of 27 patients with AAD, among whom 18 patients were from Norway and 9 from Sweden (Table 1). The original study cohort included 5 additional patients who were not included here due to lack of RNA samples. All participants provided written informed consent, and ethical approval was granted in both countries before study start (EudraCT #2009-010917-61; NCT 01063569).
Table 1.
Step 2: Baseline Patient Characteristics
| Female, n (%) | 20 (74%) |
|---|---|
| Age, years (range) | 46 (20-66) |
| HC-eqv dosage, mg/ day (range) | 25 (15-66) |
| BMI, kg/m 2 (range) | 24.8 (18.3-37.4) |
Abbreviations: BMI, body mass index; HC-eqv dosage; hydrocortisone equivalent dosage.
Transcriptional and hormonal analysis in blood
All patients provided whole blood, serum, and plasma samples before and after 12 weeks in each treatment arm. Serum cortisol was analyzed by liquid chromatography mass spectrometry [24] and plasma ACTH by chemiluminescent immunometric assay (Immulite 2000; Siemens AG, Munich, Germany; Siemens Cat# L2KAC2, RRID:AB_2783635) with a CV% of ≤10.1% and ≤8%, respectively.
RNA extraction from leukocytes was done using the Tempus Spin RNA Isolation Kit (Applied Biosystems). The cDNA was made using the RT2 First Strand Kit (Qiagen). A customized version of the RT2 Profiler PCR Array Human Glucocorticoid Signaling kit (Qiagen) was then used in order to profile expression of genes of relevance to GC activity. Two of the controls in the panel occurred in triplets, and we replaced the 4 spare copies with SPP1, ARNTL, ARNTL2, and MMP9 (Version 1). In a pilot analysis of version 1, we found 6 genes with gene expression levels below the threshold limit; these were replaced by genes previously shown to be significantly altered in patients with adrenal insufficiency (Version 2; ADM, MMP12, CASP8, EN-RAGE, RETN, CXCL1) [5]. The complete overview of the 93 genes included in Version 2 is listed in the Supplementary Appendix (S2) [15].
We analyzed the gene expression levels in 2 samples from each of the 27 patients, collected at the end of each 12-week treatment period. The assays were run according to the protocol of the manufacturer on the 7900HT PCR system (Applied Biosystems). Data was analyzed with the ΔΔCt method using the mean of 4 housekeeping genes as reference (GADPH, HPRT1, RPLP0, and B2M) [22].
Statistics
Descriptive data are presented as median with range. In step 1, normalized signal levels of each probe from the global transcription analysis were log2 transformed and then quartile normalized (Limma package, R software). To account for any negative intensity values produced by the normalization steps, all values had the lowest negative value added to make all intensity values zero or greater, which would then allow for fold change calculations to be carried out. A positive Log2 fold change value represents an increase in fold change where a value of 1 is equal to a doubling of the original value. Next, we performed t tests analysis along with a false discovery rate (FDR) adjustment. The 150 most upregulated genes were ranked according to log2 fold change after excluding genes with FDR above 0.01. Fold change for rt-PCR results was calculated as the ratio between the final over the initial gene expression level. We performed Spearman’s correlation (IBM SPSS Statistics) for the relationship between hormone and gene expression levels. Statistical significance was set to 0.05. In step 2, we employed the Wilcoxon signed rank test (IBM SPSS Statistics) to compare normalized gene expression values in normal versus very low morning cortisol, presented as z-score where a score closer to 0 suggests even distribution between the groups. The effect size was calculated by dividing the z-score by the square root of the number of observations (ie, 2 observations for each case, N = 54). To minimize the risk of type 1 error, the significance value was set to P < 0.01. For calculation of fold change, the normalized gene expression value at normal serum cortisol was divided by the very low cortisol for each patient. In both steps, plasma ACTH levels exceeding the upper reference limit (278 pmol/L) were plotted as 278 pmol/L and the lower reference limit (1.1 pmol/L) was plotted as 1.1 pmol/L.
Results
Step 1: Gene Expression in Response to 100 mg Hydrocortisone
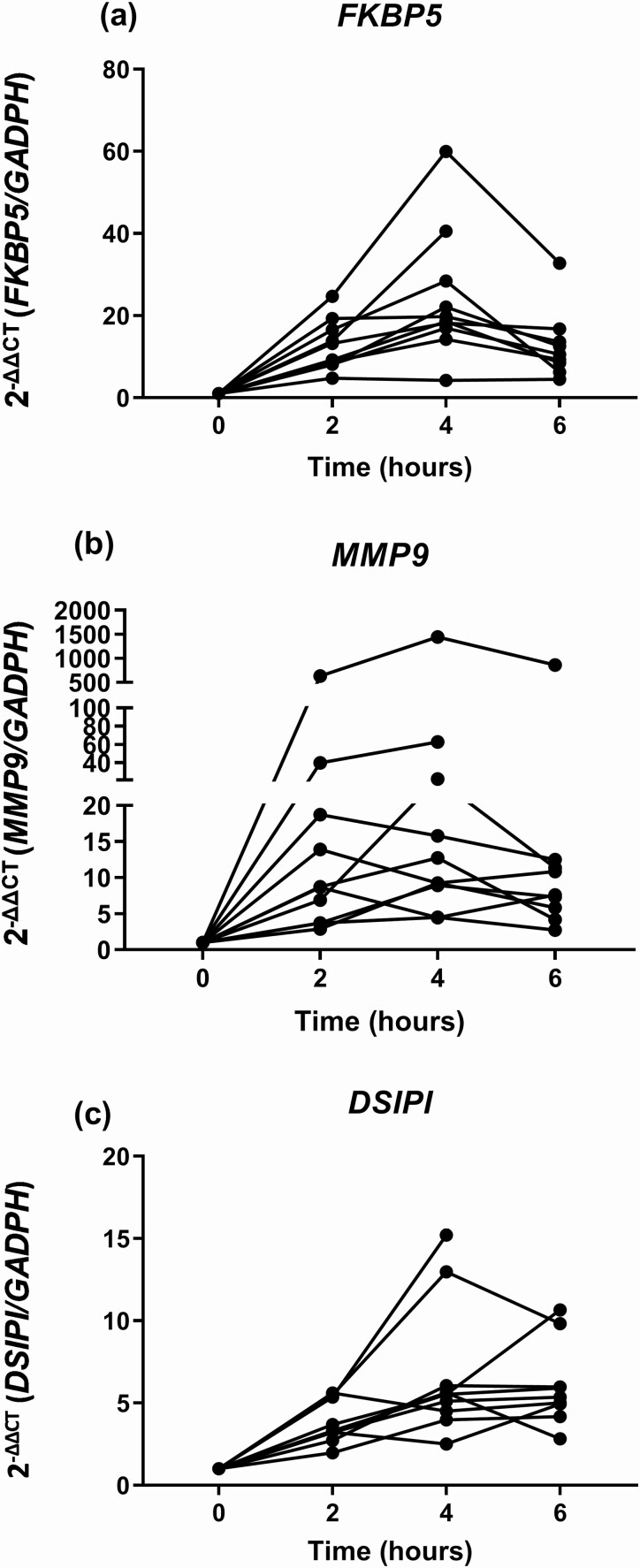
Infusion of 100 mg HC to medication-fasting AAD patients had a profound short-term effect on the expression FKBP5, MMP9, and DSIPI; increased gene expression levels were evident after 2h and had further increased at 4h. At 6h, the expression levels stabilized at an upregulated level compared to before the HC infusion (Fig. 1). Log2 fold change was 0.429 (P < 0.001, FDR < 0.001) for FKBP5, 0.560 (P < 0.001, FDR < 0.002) for MMP9, and 0.274 (P < 0.001, FDR < 0.002) for DSIPI (S1).
Figure 1.
Gene expression of FKBP5 (a), MMP9 (b), and DSIPI (c) before and up to 6 hours after intravenous infusion of 100 mg hydrocortisone.
Individual serum cortisol and plasma ACTH levels are listed in Table 2. At 2h, there was a strong positive correlation between serum cortisol levels and the gene expression levels of DSIPI (r = 0.709, P < 0.022) and MMP9 (r = 0.673, P < 0.033), but not for FKBP5 (r = 0.442, P < 0.200). No significant associations were found between ACTH and gene expression levels (data not shown).
Table 2.
Step 1: Serum Cortisol and Plasma ACTH Before and 2 Hours After Intravenous Infusion of 100 mg Hydrocortisone in Patients With AAD.
| Patient | Before hydrocortisone | 2 hours after hydrocortisone | ||
|---|---|---|---|---|
| Serum cortisol, nmol/L | Plasma ACTH, pmol/L | Serum cortisol, nmol/L | Plasma ACTH, pmol/L | |
| 1 | <28 | >280 | 1879 | >280 |
| 2 | <28 | >280 | 1490 | 104.6 |
| 3 | <28 | >278 | 1862 | 24.2 |
| 4 | <28 | >278 | 1672 | 35.7 |
| 5 | <28 | 39.1 | 2621 | 3.5 |
| 6 | <28 | 15.3 | 2687 | 4.1 |
| 7 | <28 | 178 | 1333 | 11.4 |
| 8 | 109 | 198 | 1708 | 15.1 |
| 9 | <28 | <1.1 | 2759 | 5.5 |
| 10 | <28 | <1.1 | 1873 | <1.1 |
Abbreviations: AAD, autoimmune Addison’s disease; ACTH, adrenocorticotropic hormone.
Step 2: Gene Expression in Normal Versus Very Low Morning Cortisol Levels
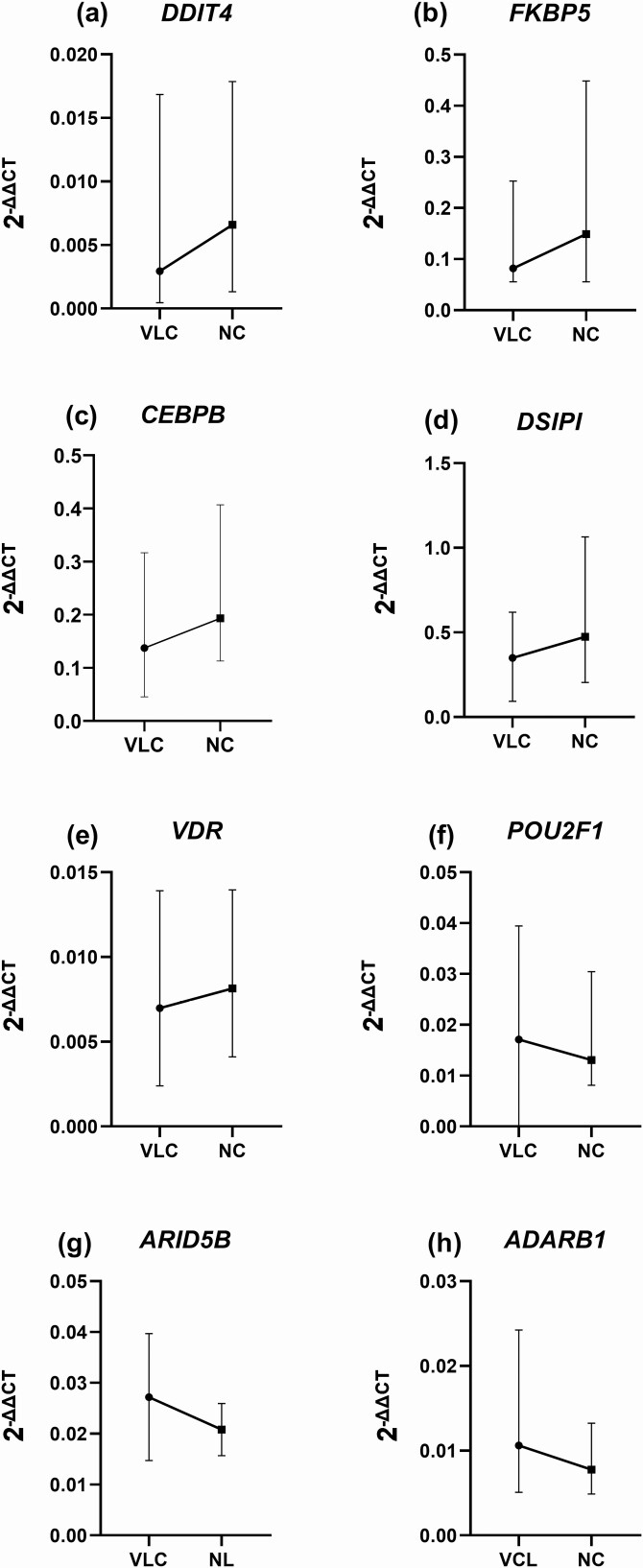
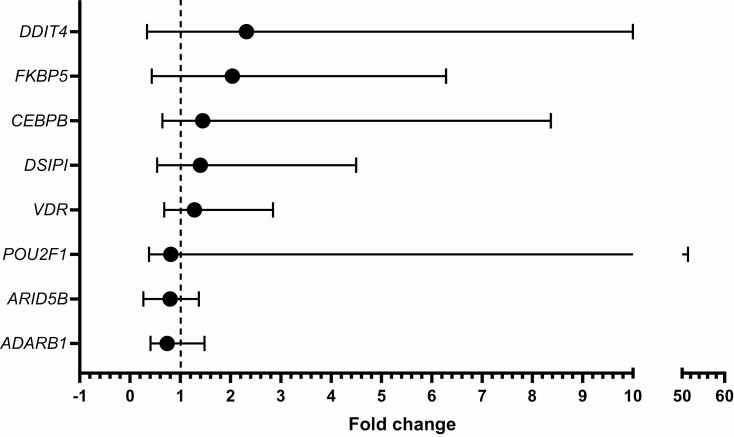
Eight of the 93 investigated genes were significantly different in normal compared to very low cortisol levels (Fig. 2). DDIT4 (z = −3.05, P < 0.002), CEBPB (z = −2.84, P < 0.005), DSIPI (z = −2.82, P < 0.007), FKBP5 (z = −2.79, P < 0.005), and VDR (z = −2.67, P < 0.008) revealed increased expression, while ADARB1 (z = −3.84, P < 0.001), POU2F1 (z = −2.79, P < 0.005), and ARID5B (z = −2.64, P < 0.008) were decreased. The effect size was largest for ADARB1 (r = 0.52), followed by DDIT4 (r = 0.42), CEBPB (r = 0.39), FKBP5 (r = 0.38), POU2F1 (r = 0.38), DSIPI (r = 0.37), VDR (r = 0.36), and ARID5B (r = 0.36). Fold change was greatest for DDIT4 (md = 2.32 [0.34, 10.00]) and FKBP5 (md = 2.03 [0.43, 6.28]) (Fig. 3).
Figure 2.
Genes with significant change in expression levels in very low cortisol (VLC) compared with normal cortisol (NC) levels. The black circles and black squares mark the median values for VLC and NC, respectively, and the whiskers mark the range.
Figure 3.
Gene expression fold change in normal cortisol relative to very low cortisol. The black circles mark the median fold change and the whiskers mark the range.
As expected, median serum cortisol was significantly higher and plasma ACTH significantly lower at normal cortisol exposure compared with very low cortisol (cortisol; 277 nmol/L [7, 467] vs 3 nmol/L [0.2, 32], P < 0.0001, ACTH; 7.3 pmol/L [1.1, 276] vs. 123 pmol/L [1.1, 278], P < 0.0001, respectively). At normal cortisol levels, serum cortisol correlated negatively with plasma ACTH (r = −0.435, P < 0.030) and positively with DDIT4, FKBP5, and DSIPI (r = 0.450, P < 0.021; r = 0.419, P < 0.033; and r = 0.400, P < 0.043, respectively). At very low cortisol levels, no correlations were found for serum cortisol and gene expression levels, but plasma ACTH negatively correlated with gene expression levels of DDIT4 and FKBP5 (r = −0.411, P < 0.033 and r = −0.399, P < 0.039, respectively).
Expression levels of DSIPI, FKBP5, and DDIT4 strongly correlated with each other at both normal and very low cortisol levels (DSIPI and FKBP5: r = 0.705, P < 0.001 and r = 0.706, P < 0.001; DSIPI and DDIT4: r = 0.695, P < 0.001 and r = 0. 702, P < 0.001; FKBP5 and DDIT4: r = 0.573, P < 0.001 and r = 0.692 P < 0.002, respectively). Other significant correlations were found between CEBPB and DSIPI (r = 0.564, P < 0.001 and r = 0. 584, P < 0.002) and CEBPB and FKBP5 (r = 0.522, P < 0.005 and r = 0.521, P < 0.005) at normal and very low cortisol levels, respectively.
Discussion
Biomarkers reflecting GC replacement in AAD are lacking. In this exploratory study, we mapped the transcriptional landscape of GC exposure and found that DSIPI, DDIT4, and FKBP5—independently and as a triad—are candidate transcriptional biomarkers reflecting levels of GC because (i) all 3 genes were clearly and consistently upregulated after both high-dose HC infusion and in normal cortisol compared to very low cortisol, (ii) gene expression levels significantly correlated with normal cortisol levels, and (iii) they strongly correlated with each other, implying a high degree of co-regulation.
DSIPI, a key mediator of GC anti-inflammatory effects [25], appears as the most precise biomarker since its expression significantly correlated with serum cortisol both after high-dose HC infusion (step 1) and with normal cortisol levels (step 2). DDIT4, an important inhibitor of the mTOR pathway in response to stress [26], and FKBP5, an important short-loop feedback inhibitor of GC action [16], may be more sensitive markers of GC exposure as they revealed the largest fold changes between very low and normal cortisol levels (step 2). Finally, DDIT4 and FKBP5 were the only genes where expression levels significantly correlated with plasma ACTH at very low cortisol.
In addition to DSIPI and FKBP5, we found that that MMP9 had a rapid and sustained increase in gene expression following infusion of 100 mg HC (step 1). This is in contrast to a study by Aljada and coworkers where plasma MMP9 protein levels significantly decreased after 100 mg HC infusion [27], but in line with another study on healthy individuals where MMP9 levels increased in response to 300 mg HC infusion [28]. Obviously, gene expression and plasma protein levels are not directly comparable, but we would expect the direction of the response to HC infusion to correspond. Taken together, this indicates that more research is needed to determine the true effect of high-dose HC exposure on MMP9 gene expression and MMP9 protein levels.
In step 2, 5 of the 93 GC-regulated genes were significantly upregulated (DDIT4, DSIPI, FKBP5, CEBPB, VDR) whereas 3 were downregulated (ADARB1, POU2F1, ARID5B) in normal compared with very low cortisol. Increased CEBPB expression has been suggested as an early marker of efficacious response to GC treatment in inflammatory bowel disease (IBD) and could be used to identify the 20% of IBD patients who are refractory to GC treatment [29]. With this in mind, we suggest that future studies explore whether difference in CEBPB expression could help identify patients with AAD in need of higher GC replacement dosages due to partial GC resistance.
Expression of VDR has previously been shown to increase following GC exposure in a time- and dose-dependent manner, but only in the presence of GC receptor, indicating that GCs directly regulate VDR expression through GC receptor activation [30].
The largest effect size on gene expression change was noted for ADARB1, a gene considered key for circadian rhythmicity [31]. Previous studies disagree on whether GCs increase or decrease ADARB1 expression. In an in vitro study on subcutaneous preadipocytes, expression of ADARB1 was twice as high in cells treated with cortisol compared with untreated cell controls [32]. In contrast, our data adds evidence to the notion that GCs suppress ADARB1 expression, as previously demonstrated in human keratinocytes treated with dexamethasone [33].
For POU2F1, a transcription factor important in stem cell regulation, our findings support that GCs suppress its expression in line with previous studies [34]. The clinical value of POU2F1 has primarily been demonstrated in the setting of cancer, as measurement of POU2F1 protein levels is better than clinical cancer staging in predicting prognosis [34].
Finally, ARID5B has been associated with susceptibility to and treatment outcomes of acute lymphatic leukemia (ALL), and lower ARID5B expression at diagnosis has been linked to increased risk of ALL relapse [35]. To our knowledge, this is the first study to demonstrate the inhibitory effect of GCs on ARID5B expression.
One patient in step 1 was not cortisol deplete before HC infusion. We do not, however, doubt that the diagnosis of AAD was correct as all study patients were recruited from our national quality registry (the National Registry for Addison’s disease [ROAS]) and were carefully characterized both clinically and biochemically. Furthermore, the patient had clearly elevated ACTH (198 pmol/L) before HC infusion that was successfully suppressed after HC infusion (15.1 pmol/L). We propose that this patient had residual GC production, which we recently demonstrated is present in one-third of all patients with AAD [36]. To ensure full privacy protection, all samples were anonymized before analysis, and we were unable to identify the study patient in order to verify residual GC production.
In step 2, the cross-over design allowed us to evaluate changes in gene expression in the same individuals at normal cortisol (the CSHI arm) compared to very low cortisol levels (the OHC arm). We are, however, aware that our results instead could represent changes in gene expression related to the 2 different treatment modalities, CSHI and OHC. This would, however, require that the half-life of gene expression (RNA) levels exceeded 15 hours, corresponding to the minimum length of medication fasting in the OHC arm. Although we do not know the exact half-life of all the included genes, the median half-life of gene expression in general is estimated to be 7.1 hours [37].
Finally, since this was an exploratory study, we acknowledge that interpretation of our data must be done with caution. In particular, without a control group of healthy individuals, we cannot know whether the observed differences in gene expression are changes toward a more normal physiological state or quite the reverse. In a future clinical study, we therefore advocate the need for a control group of healthy individuals to establish reference ranges for gene expression levels. Nevertheless, by presenting the data as-is, we provide a basis for future studies to explore the true potential and validity of gene expression as biomarkers for GC replacement in patients with AAD. Specifically, we suggest a repeated-measures design where gene expression levels are measured in the same individuals with AAD on repeated occasions following different GC administration types and doses, compared with healthy controls.
In conclusion, we nominate the gene expression of DSIPI, DDIT4, and FKBP5 as candidate transcriptional biomarkers of GC replacement, followed by CEBPB, VDR, POU2F1, ARID5B, ADARB1, and MMP9.
Acknowledgments
This project received bioinformatics support from ELIXIR Norway, supported by the Research Council of Norway’s grant 270068 and 288022, the University of Bergen, the University of Oslo, the Arctic University of Norway in Tromsø, the Norwegian University of Science and Technology and the Norwegian University of Life Sciences: NMBU. Finally, the authors wish to thank the Norwegian and Swedish patients with autoimmune Addison’s disease who participated in the study.
Financial support: This work was supported by the European Union Seventh Framework Programme Grant 201167, Euradrenal, the KG Jebsen center for autoimmune disorders and Western Norway health authorities. Financial support for the Swedish contribution was also provided through the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet, The Swedish Society for Medical Research, the Swedish Society of Medicine, the NovoNordisk Foundation, the Tore Nilsons Foundation for Medical Research, the Karolinska Institutet, the Åke Wiberg Foundation, and the Fredrik and Ingrid Thuring Foundation.
Glossary
Abbreviations
- AAD
autoimmune Addison’s disease
- ACTH
adrenocorticotropic hormone
- CA
cortisone acetate
- CSHI
continuous subcutaneous hydrocortisone infusion
- CV
coefficient of variation
- FDR
false discovery rate
- GC
glucocorticoid
- HC
hydrocortisone
- OHC
oral hydrocortisone replacement
- rt-PCR
real-time polymerase chain reaction
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Supplementary results are available in the data repositories listed in References.
References
- 1. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ho W, Druce M. Quality of life in patients with adrenal disease: a systematic review. Clin Endocrinol (Oxf). 2018;89(2):119-128. [DOI] [PubMed] [Google Scholar]
- 3. Oksnes M, Bensing S, Hulting AL, et al. Quality of life in European patients with Addison’s disease: validity of the disease-specific questionnaire AddiQoL. J Clin Endocrinol Metab. 2012;97(2):568-576. [DOI] [PubMed] [Google Scholar]
- 4. Skov J, Sundström A, Ludvigsson JF, Kämpe O, Bensing S. Sex-specific risk of cardiovascular disease in autoimmune addison disease-a population-based cohort study. J Clin Endocrinol Metab. 2019;104(6):2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergthorsdottir R, Ragnarsson O, Skrtic S, et al. Visceral fat and novel biomarkers of cardiovascular disease in patients with addison’s disease: a case-control study. J Clin Endocrinol Metab. 2017;102(11):4264-4272. [DOI] [PubMed] [Google Scholar]
- 6. Tresoldi AS, Sumilo D, Perrins M, et al. Increased Infection Risk in Addison’s Disease and Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab. 2020;105(2):418-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bensing S, Brandt L, Tabaroj F, et al. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin Endocrinol (Oxf). 2008;69(5):697-704. [DOI] [PubMed] [Google Scholar]
- 8. Ueland GA, Husebye ES. Metabolic Complications in Adrenal Insufficiency. Front Horm Res. 2018;49:104-113. [DOI] [PubMed] [Google Scholar]
- 9. Staufenbiel SM, Andela CD, Manenschijn L, Pereira AM, van Rossum EF, Biermasz NR. Increased hair cortisol concentrations and BMI in patients with pituitary-adrenal disease on hydrocortisone replacement. J Clin Endocrinol Metab. 2015;100(6):2456-2462. [DOI] [PubMed] [Google Scholar]
- 10. Noppe G, van Rossum EF, Vliegenthart J, Koper JW, van den Akker EL. Elevated hair cortisol concentrations in children with adrenal insufficiency on hydrocortisone replacement therapy. Clin Endocrinol (Oxf). 2014;81(6):820-825. [DOI] [PubMed] [Google Scholar]
- 11. Ross IL, Lacerda M, Pillay TS, et al. Salivary cortisol and cortisone do not appear to be useful biomarkers for monitoring hydrocortisone replacement in addison’s disease. Horm Metab Res. 2016;48(12):814-821. [DOI] [PubMed] [Google Scholar]
- 12. Bhake RC, Kluckner V, Stassen H, et al. Continuous free cortisol profiles-circadian rhythms in healthy men. J Clin Endocrinol Metab. 2019;104(12):5935-5947. [DOI] [PubMed] [Google Scholar]
- 13. Biddie SC, Hager GL. Glucocorticoid receptor dynamics and gene regulation. Stress. 2009;12(3):193-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu Y, Carman JA, Holloway D, et al. Development of a molecular signature to monitor pharmacodynamic responses mediated by in vivo administration of glucocorticoids. Arthritis Rheumatol. 2018;70(8):1331-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sævik ÅB. Replication data for: Potential Transcriptional Biomarkers to Guide Glucocorticoid Replacement in Autoimmune Addison’s Disease. DataverseNO; UiT The Arctic University of Norway. Deposited October 21, 2020. 10.18710/Q3FZAN [DOI] [Google Scholar]
- 16. Winkler BK, Lehnert H, Oster H, Kirchner H, Harbeck B. FKBP5 methylation as a possible marker for cortisol state and transient cortisol exposure in healthy human subjects. Epigenomics. 2017;9(10):1279-1286. [DOI] [PubMed] [Google Scholar]
- 17. Medeiros NI, Gomes JAS, Fiuza JA, et al. MMP-2 and MMP-9 plasma levels are potential biomarkers for indeterminate and cardiac clinical forms progression in chronic Chagas disease. Sci Rep. 2019;9(1):14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther. 2013;139(1):32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gruber BL, Sorbi D, French DL, et al. Markedly elevated serum MMP-9 (gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker. Clin Immunol Immunopathol. 1996;78(2):161-171. [DOI] [PubMed] [Google Scholar]
- 20. Yablecovitch D, Kopylov U, Lahat A, et al. Serum MMP-9: a novel biomarker for prediction of clinical relapse in patients with quiescent Crohn’s disease, a post hoc analysis. Therap Adv Gastroenterol. 2019;12:1756284819881590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayyar VS, DuBois DC, Almon RR, Jusko WJ. Modeling corticosteroid pharmacokinetics and pharmacodynamics, Part III: estrous cycle and estrogen receptor-dependent antagonism of glucocorticoid-induced leucine zipper (GILZ) enhancement by corticosteroids. J Pharmacol Exp Ther. 2019;370(2):337-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 23. Oksnes M, Björnsdottir S, Isaksson M, et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of addison’s disease: a randomized clinical trial. J Clin Endocrinol Metab. 2014;99(5):1665-1674. [DOI] [PubMed] [Google Scholar]
- 24. Methlie P, Hustad SS, Kellmann R, et al. Multisteroid LC-MS/MS assay for glucocorticoids and androgens, and its application in Addison’s disease. Endocr Connect. 2013;2(3):125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee MJ, Yang RZ, Karastergiou K, et al. Low expression of the GILZ may contribute to adipose inflammation and altered adipokine production in human obesity. J Lipid Res. 2016;57(7):1256-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolff NC, McKay RM, Brugarolas J. REDD1/DDIT4-independent mTORC1 inhibition and apoptosis by glucocorticoids in thymocytes. Mol Cancer Res. 2014;12(6):867-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aljada A, Ghanim H, Mohanty P, Hofmeyer D, Tripathy D, Dandona P. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86(12):5988-5991. [DOI] [PubMed] [Google Scholar]
- 28. Dandona P, Ghanim H, Sia CL, et al. A mixed anti-inflammatory and pro-inflammatory response associated with a high dose of corticosteroids. Curr Mol Med. 2014;14(6):793-801. [DOI] [PubMed] [Google Scholar]
- 29. Assas BM, Levison S, Pennock JL. Early induction of C/EBPβ expression as a potential marker of steroid responsive colitis. Sci Rep. 2019;9(1):13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hidalgo AA, Deeb KK, Pike JW, Johnson CS, Trump DL. Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription. J Biol Chem. 2011;286(42):36228-36237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Terajima H, Yoshitane H, Ozaki H, et al. ADARB1 catalyzes circadian A-to-I editing and regulates RNA rhythm. Nat Genet. 2017;49(1):146-151. [DOI] [PubMed] [Google Scholar]
- 32. Bujalska IJ, Quinkler M, Tomlinson JW, Montague CT, Smith DM, Stewart PM. Expression profiling of 11beta-hydroxysteroid dehydrogenase type-1 and glucocorticoid-target genes in subcutaneous and omental human preadipocytes. J Mol Endocrinol. 2006;37(2):327-340. [DOI] [PubMed] [Google Scholar]
- 33. Stojadinovic O, Lee B, Vouthounis C, et al. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. J Biol Chem. 2007;282(6):4021-4034. [DOI] [PubMed] [Google Scholar]
- 34. Portseva TN, Pankratova EV, Stepchenko AG, Georgieva SG. Increased level of Oct-1 protein in tumor cells modulates cellular response to anticancer drugs. Dokl Biochem Biophys. 2016;469(1):269-272. [DOI] [PubMed] [Google Scholar]
- 35. Xu H, Zhao X, Bhojwani D, et al. ARID5B influences antimetabolite drug sensitivity and prognosis of acute lymphoblastic leukemia. Clin Cancer Res. 2020;26(1):256-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sævik ÅB, Åkerman AK, Methlie P, et al. Residual corticosteroid production in autoimmune Addison disease. J Clin Endocrinol Metab. 2020;105(7);105(7):2430-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharova LV, Sharov AA, Nedorezov T, Piao Y, Shaik N, Ko MS. Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res. 2009;16(1):45-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Supplementary results are available in the data repositories listed in References.