Abstract
Study Objectives:
The objective of this study was to determine if positional therapy is a viable treatment alternative for obese children with persistent obstructive sleep apnea (OSA).
Methods:
A retrospective review was performed of children who underwent an adenotonsillectomy for OSA from 2014 to 2017. Children were included if they had a body mass index ≥ 95th percentile and underwent a postoperative polysomnogram. Subjects fell into one of three categories: mixed sleep (the presence of ≥ 30 minutes of both nonsupine and supine sleep), nonsupine sleep, and supine sleep. Cure was defined as an OSA/apnea-hypopnea index of < 1 events/h. Paired t tests were used to assess the differences, and a linear model adjusting for obesity class, age at procedure, and sex was performed to assess the differences between nonsupine and supine sleep.
Results:
There were 154 children who met the inclusion criteria. Using a paired t test, supine sleep position had a significantly higher average OSA/apnea-hypopnea index (7.9 events) compared with nonsupine (OSA/apnea-hypopnea index of 4.1); P value was < .01 for the 60 children with mixed sleep. Forty-three children had predominantly nonsupine sleep and 33 predominantly supine sleep, and a McNemar’s test comparing these children showed that those sleeping in the nonsupine position were significantly more likely to be cured than those in the supine position (P < .001).
Conclusions:
Sleep physicians and otolaryngologists should be cognizant of positional treatment when consulting with families and note that the postoperative polysomnography may be inaccurate if it does not include supine sleep. Positional therapy as a potential treatment option for obese children with persistent OSA after adenotonsillectomy warrants further investigation.
Citation:
Tholen K, Meier M, Kloor J, Friedman, N. Persistent OSA in obese children: does body position matter? Clin J Sleep Med. 2021;17(2):227–232.
Keywords: obstructive sleep apnea, sleep position, positional obstructive sleep apnea, child, obesity, tonsillectomy, polysomnogram, sleep study
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obese children are more likely to have persistent obstructive sleep apnea after an adenotonsillectomy. The primary treatment option is noninvasive ventilation, but adherence is suboptimal. For adults, positional therapy is an alternative to noninvasive ventilation; however, positional therapy is infrequently recommended in children.
Study Impact: In our investigation, obese children with persistent obstructive sleep apnea after an adenotonsillectomy have more severe obstructive sleep apnea in the supine position, suggesting that positional therapy as a potential treatment option for obese children with persistent obstructive sleep apnea after warrants further investigation.
INTRODUCTION
Obstructive sleep apnea (OSA) affects 1%–3% of children1 and may produce significant cardiovascular and neurobehavioral comorbidities.2,3 The American Academy of Pediatrics clinical guideline on OSA recommends adenotonsillectomy (T&A) as first-line therapy for OSA.4 In the only randomized control trial for T&A, about 80% of children were cured after surgery.5
Besides adenotonsillar hypotrophy, obesity is also a significant risk factor. Obese children are at a higher risk of developing OSA and are less likely to be cured by T&A alone.4,6–9 A 2009 meta-analysis reported that up to 88% of obese children still have persistent OSA after a T&A.10 In the Childhood Adenotonsillectomy Trial, which excluded patients with severe obesity (z score ≥ 3), surgical cure following a T&A for obese children was achieved in only 67%.5 For those who have persistent OSA after T&A, continuous positive airway pressure (CPAP) is a treatment option. Unfortunately, compliance to CPAP is low in the pediatric population.11,12
For some patients with OSA, obstructive respiratory events occur more frequently in the supine position rather than in the nonsupine position. Positional obstructive sleep apnea (POSA) is characterized by an apnea-hypopnea index that is at least twice as high in the supine position compared with the nonsupine position.13 POSA is present in about 53% of adult patients and in about 19% of children; however, the prevalence of POSA in children after a T&A is higher at 32%13–15 Of note, although POSA does not affect sleep architecture, it does have a significant effect on gas exchange and breathing patterns in children.16,17
Because T&A success is variable, positional therapy may be another treatment option for children with persistent OSA.15 Positional therapy aims to keep patients in the nonsupine position to alleviate OSA symptoms that would occur in the supine position.18 In adults, the sleep position trainer is a device that is worn around the chest and gives a gentle vibration when a patient is in the supine position.19 The adult adherence rate for sleep position trainer use is relatively high and is as effective as oral appliance therapy in reducing the apnea-hypopnea index in POSA patients.20–22
For children who struggle with CPAP compliance, positional therapy could be beneficial; however, it is infrequently recommended in children compared with adults.23,24 Recognizing that POSA is common in adults and that positional therapy is a treatment option, the objective of this study was to evaluate the role positional therapy might play for obese children, who are less likely to be cured after a T&A.
METHODS
The Colorado Multiple Institutional Review Board reviewed and approved this research study (COMIRB no. 19-2031). Our primary objective was to analyze patients with persistent OSA to determine whether body position plays a significant role.
Study participants
Obese children who underwent T&A from 2014 to 2017 for OSA at Children’s Hospital of Colorado were identified retrospectively through an electronic medical record search. Subjects were included if they were obese with a body mass index (BMI) percentile ≥ the 95th percentile. Our primary analysis included children who had at least 30 minutes of sleep in the both the nonsupine and supine position, that is, the mixed sleep position. The secondary analysis included children who had predominantly nonsupine sleep (ie, < 30 minutes of supine sleep) and predominantly supine sleep (ie, < 30 minutes of nonsupine sleep). Cure was defined as an obstructive apnea-hypopnea index (OAHI) < 1 event/h. Individual charts were reviewed to confirm inclusion criteria. Children were excluded if they were not obese, had Down syndrome, or had neuromuscular conditions.
Measures
For each patient, the following demographic information was recorded: age, sex, race, ethnicity, date of surgery, indication for surgery, height, weight, and obesity severity. Obesity was divided into classes, with the BMI expressed as a percentage of the 95th percentile by sex and age: class 1, BMI 100%–120% of the 95th percentile; class 2, BMI ≥ 120% to < 140% of the 95th percentile; and class 3, BMI ≥ 140% of the 95th percentile.25
Polysomnogram (PSG) metrics collected include total sleep time, time in stage R, mean asleep oxygen saturation, saturation nadir, OAHI, and rapid eye movement (REM) OAHI. All metrics were reported overall and for those with mixed sleep, predominantly nonsupine (prone and lateral combined) sleep, and predominantly supine sleep. PSGs were scored using the American Academy of Sleep Medicine guidelines.26,27 At our institution, each study is performed with all-night video, and the sleep technologists continuously monitor and mark body position changes on the PSG rather than relying on a position sensor. After the acquisition, a scoring technologist scores and reviews the study data, including the body position before the PSG is interpreted.
All data were stored in a secure REDCap (Research Electronic Data Capture) database hosted at the University of Colorado Anschutz Medical Campus.28 REDCap is a secure web-based application designed to support data capture for research studies, providing the following: (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.
Data analysis
Demographic data and sleep characteristics were reported using means with standard deviations for continuous variables and frequencies and percentages for categorical variables. Paired t-tests or McNemar’s tests were used to assess the differences between supine and nonsupine sleep, depending on the distribution of the variables. Change in OAHI from supine to nonsupine position was used as the outcome for a linear regression model, adjusting for obesity status, age at procedure, and sex. A P value of .05 was used for as a cutoff for significance. All summaries and analyses were done using R (R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.r-project.org/foundation).29
RESULTS
A total of 154 children were identified who had a postoperative PSG after T&A and a BMI ≥ 95th percentile. Of those, 78 children had “mixed sleep” with at least 30 minutes each in the supine and nonsupine sleep positions, 43 children had predominantly nonsupine sleep, and 33 had predominantly supine sleep. Table 1 includes their demographic information. The mean age was 9.4 years (SD ± 3.9), 60% (n = 92) were male, and 40% (n = 62) were female. Forty-four percent (n = 67) of patients were considered to have class 1 obesity, 30% (n = 46), class 2 obesity, and 26% (n = 41) class 3 obesity. Table 2 includes patients’ PSG findings: total sleep time, asleep oxygenation, REM OAHI, and overall OAHI stratified by each sleep position.
Table 1.
Demographics stratified by sleep position.
| Demographic Characteristic | Overall (n = 154) | Mixed (n = 78) | Predominantly Nonsupine (n = 43) | Predominantly Supine (n = 33) |
|---|---|---|---|---|
| Mean age at T&A (y) | 9.4 (3.9) | 9.6 (4.0) | 9.2 (3.8) | 9.1 (3.8) |
| Child’s sex (female/male) | 62 (40%)/92 (60%) | 30 (38%)/48 (62%) | 19 (44%)/24 (55%) | 13 (30%)/20 (60%) |
| Obesity class | ||||
| Class 1 | 67 (44%) | 35 (45%) | 21 (49%) | 11 (33%) |
| Class 2 | 46 (30%) | 24 (31%) | 14 (33%) | 8 (24%) |
| Class 3 | 41 (26%) | 19 (24%) | 8 (18%) | 14 (43%) |
| Race | ||||
| White | 37 (24%) | 17 (22%) | 13 (30%) | 7 (21%) |
| Hispanic | 95 (62%) | 47 (60%) | 23 (54%) | 25 (76%) |
| Black/African American | 13 (8%) | 6 (8%) | 6 (14%) | 1 (3%) |
| Asian | 2 (1%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Other | 7 (5%) | 6 (8%) | 1 (2%) | 0 (0%) |
Age, sex, and race/ethnicity of our cohort stratified by mixed sleep, predominantly nonsupine sleep, and predominantly supine sleep. For obesity class, class 1 = body mass index (BMI) 100–120% of the 95th percentile, class 2 = BMI > 120% to < 140% of the 95th percentile, and class 3 = >140% of the 95th percentile.
Table 2.
Sleep characteristics.
| Sleep Characteristic | Overall (n = 154) | Mixed Sleep (n = 78) | Predominantly Nonsupine (n = 43) | Predominantly Supine (n = 33) | |
|---|---|---|---|---|---|
| Sleep time < 90% SpO2 (%) | 1.2 (4.3) | 1.4 (4.8) | 0.55 (2.4) | 1.7 (4.9) | |
| SpO2 nadir of TST (%) | 84.6 (4.9) | 83.7 (5.3) | 85.3 (4.4) | 85.9 (4.4) | |
| Mean SpO2 of TST (%) | 95.4 (1.3) | 95.5 (1.4) | 95.5 (1.2) | 95.3 (1.4) | |
| Nonsupine | Supine | ||||
| TST (min) | 421.7 (79.2) | 189.1 (99.1) | 220.5 (103.6) | 433.3 (67.8) | 427.9 (76.3) |
| Stage R sleep (%) | 17.6 (5.5) | 16.3 (6.1) | 18.3 (5.7) | 16.7 (5.2) | 18.9 (5.9) |
| OAHI (events/h) | 3.3 (4.9) | 4.1 (5.5) | 7.9 (12.1) | 1.4 (2.3) | 2.8 (3.1) |
| REM OAHI (events/h) | 8.7 (12.5) | 14.3 (15.0) | 17.1 (17.0) | 2.7 (3.2) | 7.8 (9.7) |
REM = rapid eye movement. Total sleep time (TST), oxygen saturations (SpO2), and obstructive apnea-hypopnea index (OAHI) stratified by mixed sleep, predominantly nonsupine sleep, and predominantly supine sleep.
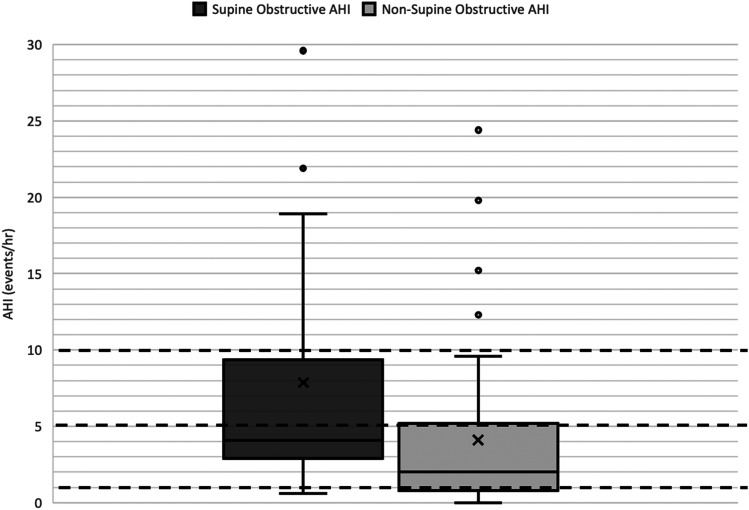
For patients with mixed sleep, the mean age was 9.6 years (SD ± 4.0), and 14 (18%) children were considered “cured” with an overall OAHI < 1. Sixty-four patients (82%) had persistent OSA after T&A. Figure 1 highlights the OAHI by supine and nonsupine sleep. The supine position had a significantly higher mean OAHI at 7.9 events/h as compared with nonsupine sleep, with a mean OAHI of 4.1 (P value = .004). For the mixed group, no significant difference was found in the REM OAHI between supine and nonsupine sleep. Overall, 32 children (50%) had more severe OSA in the supine compared with the nonsupine position. When analyzing supine or nonsupine OAHI rather than overall OAHI, only one patient experienced a “cure” (OAHI < 1) in the supine position compared with 19 patients (30%) in the nonsupine position, which was significant (P value < .0001). Twenty-nine children (48%) met the rigid definition for POSA with an OAHI that was at least twice as high in the supine position compared with the nonsupine position. The overall OAHI for supine and nonsupine sleep was analyzed, and adjusting for obesity level, age, and sex, no significant difference was found in OAHI status when comparing age, sex, and class 1 with higher levels of obesity (Table 3).
Figure 1. Obstructive apnea-hypopnea index (OAHI) category by supine and nonsupine position for the mixed sleep position group.
The dark black line indicates the median, the X indicates the mean, and the circular data points indicate outlier values. Dashed lines have been placed to indicate important clinical OAHI cutoffs (OAHI = 1, 5, 10). The supine position had a significantly higher mean OAHI at 7.9 events/h as compared nonsupine sleep with a mean OAHI of 4.1, P value = .01.
Table 3.
Difference between nonsupine obstructive apnea-hypopnea index (AHI) and supine obstructive AHI (OAHI).
| Predictors | Estimates | 95% Confidence Interval | P value |
|---|---|---|---|
| Obesity class 1 vs 2/3 | −.09 | −2.40–2.22 | .941 |
| Age at procedure | −.22 | −.52–.08 | .160 |
| Male | .41 | −1.95–2.77 | .734 |
Linear regression model for the nonsupine and supine obstructive apnea hypopnea index (OAHI) adjusted for age at procedure, sex, and obesity class. There is no significant difference in OAHI status when comparing age, sex, or class 1 to higher levels of obesity.
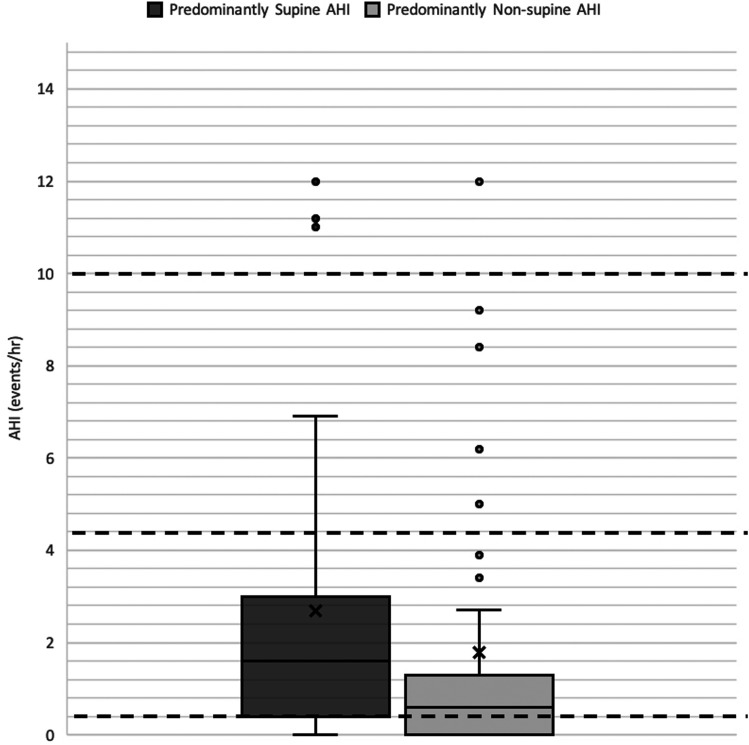
Figure 2 highlights the OAHI outcomes of children who slept predominantly nonsupine compared with those who slept supine. Twenty-seven children (63%) with nonsupine sleep were cured, whereas only 15 children (45%) who had predominantly supine sleep were cured. A McNemar’s test showed that those who sleep in the nonsupine position are significantly more likely to be cured than those who sleep in the supine position (P value < .001). Table 2 shows that our cohort had about the same amount of stage R sleep in both positions, and REM OAHI was significantly higher in the predominantly supine group compared with the predominantly nonsupine group (P value < .01).
Figure 2. Patients in predominantly supine sleep and predominantly nonsupine sleep.
The dark black line indicates the median, the X indicates the mean, and the circular data points indicate outlier values. Dashed lines have been placed to indicate important clinical OAHI cutoffs (OAHI = 1, 5, 10). Those who sleep predominantly nonsupine are more likely to be cured than those who sleep predominantly supine (P < .001). OAHI = obstructive apnea hypopnea index.
DISCUSSION
Obese children are more likely to have persistent OSA.5,9,10,30,31 The primary treatment for these children is noninvasive ventilation; however, the compliance rate for children to these therapies is low.11,12,32–34 In addition, there is a risk of craniofacial changes caused by the mask interface, depending on the child’s age.34 Besides weight reduction programs and noninvasive ventilation, other management options for persistent OSA include oral appliances, medications, drug-induced sleep endoscopy, and revision surgery.24 Despite the comprehensive review of therapies from Bluher et al., positional therapy is not listed as an option.
The incidence of persistent OSA after T&A is variable and has been reported to be as high as 88%.10 The Childhood Adenotonsillectomy Trial study’s rate of persistent OSA for obese children was lower, but children with severe obesity (BMI z score 3) were excluded.4 In our analysis, 82% of obese children experienced persistent OSA; however, the cohort may exhibit a selection bias and is most likely an overestimate. Being a retrospective study, it is unknown how many obese children underwent T&A during the study period and did not have a postoperative PSG, so there is no proper denominator. Furthermore, the PSG practice patterns of providers are unknown. Potentially, providers routinely request a postoperative PSG only for severe OSA or persistent symptoms.
Recognizing that POSA is common in adults and that positional therapy is a treatment option, we wondered what role positional therapy might play for obese children, who are less likely to be cured after a T&A. The original criteria for POSA was an apnea-hypopnea index that is at least twice as high in the supine position as in the nonsupine position.13 More recently, some studies include a minimum duration of time spent in supine sleep before being diagnosed with POSA.15,21 In our investigation, 48% of the children with persistent OSA were diagnosed with POSA under Cartwright’s definition of an OAHI at least twice as high in the supine position compared with the nonsupine position. Verhelst et al. published a prevalence of only 19% also using Cartwright’s definition of POSA. In their investigation, they included children with the following conditions: nonobese, obese, Down syndrome, surgically naïve (no T&A), and those who had a previous T&A. POSA was present in 32% (7/22) of the T&A cohort, of which only 13 children were obese. The inclusion criteria required a child to have moderate or severe OSA (OAHI > 5 events/h), in contrast to our investigation, which included mild OSA (OAHI ≥ 1 events/h). Inclusion of children with mild OSA enables clinicians to be aware of how positional status matters for any child with OSA. Overall, the supine OAHI was significantly higher, with a mean value of 7.9 compared with a nonsupine mean OAHI of 4.1 events/h.
Because about 50% of our cohort did not have sufficient sleep in both supine and nonsupine positions to be included in the primary analysis, where each child served as his or her own control, a secondary analysis comparing children with predominantly nonsupine to predominantly supine sleep was performed. Of note, children who slept predominantly nonsupine were more likely to be cured (63% vs 45%). In addition, patients were more likely to have a lower REM OAHI in predominantly nonsupine sleep. These findings suggest that body position does matter after a T&A in obese children, and if a child’s postoperative PSG has minimal supine sleep, one may falsely assume that the T&A was curative.
The current study has a few limitations. The study was retrospective, the cohort was relatively small, and a disproportionate number of the children were Hispanic. In addition, body position documentation relies on the acquiring sleep technologist. Although this documentation is reviewed by both the scoring technologist and the interpreting physician, some of the documentation is potentially inaccurate; however, the technologist-to-patient ratio is low (1.2:1), so the technologist is not overextended. The strengths of the study are that by requiring an inclusion criterion for mixed sleep of at least 30 minutes of sleep in both nonsupine and supine sleep, the risk of a sampling error in the OAHI is reduced and would occur only in the event of a few events occurring during a short sleep period in a specific position. Also, analyzing the data by obesity severity allowed us to better stratify the potential benefits of positional therapy.
Although persistent OSA symptoms after T&A are often treated with CPAP, compliance is suboptimal in the pediatric population.11,12 Because most of our cohort had more severe OSA in the supine position after a T&A, positional therapy may be a viable treatment option. Positional therapy is infrequently recommended in children compared with adults, but our results demonstrate that further investigation of positional therapy as a management option for persistent OSA is warranted.
CONCLUSIONS
Sleep physicians and otolaryngologists should be cognizant of positional treatment when consulting with families and note that the postoperative PSG may be inaccurate if it does not include supine sleep. Positional therapy as a potential treatment option for obese children with persistent OSA after T&A warrants further investigation.
DISCLOSURE STATEMENT
This work was performed at Children’s Hospital of Colorado and University of Colorado Anschutz Medical Campus. Funding was provided by the Children’s Hospital Center for Research in Outcomes in Children's Surgery. Norman Friedman is a member of the American Board of Internal Medicine (ABIM) Board of Directors and the ABIM Internal Medicine Exam Committee. To protect the integrity of Board Certification, ABIM strictly enforces the confidentiality and its ownership of ABIM exam content, and Dr. Friedman has agreed to keep ABIM exam content confidential. No ABIM exam content is shared or otherwise disclosed in this article. All authors have seen and approved the manuscript. The authors have no financial relationships or support relevant to this manuscript. The authors have no conflicts of interest to report.
ABBREVIATIONS
- BMI
body mass index
- CPAP
continuous positive airway pressure
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PSG
polysomnogram
- POSA
positional obstructive sleep apnea
- REM
rapid eye movement
- T&A
adenotonsillectomy
REFERENCES
- 1.Gozal D, Kheirandish-Gozal L. Childhood obesity and sleep: relatives, partners, or both?--a critical perspective on the evidence. Ann N Y Acad Sci. 2012;1264(1):135–141. 10.1111/j.1749-6632.2012.06723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 3.Weinstock TG, Rosen CL, Marcus CL, et al. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep. 2014;37(2):261–269. 10.5665/sleep.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. 10.1542/peds.2012-1671 [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Moore RH, Rosen CL, et al. Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly A, Marcus CL. Childhood obesity, inflammation, and apnea: what is the future for our children? Am J Respir Crit Care Med. 2005;171(3):202–203. 10.1164/rccm.2412001 [DOI] [PubMed] [Google Scholar]
- 7.Xu Z, Jiaqing A, Yuchuan L, Shen K. A case-control study of obstructive sleep apnea-hypopnea syndrome in obese and nonobese Chinese children. Chest. 2008;133(3):684–689. 10.1378/chest.07-1611 [DOI] [PubMed] [Google Scholar]
- 8.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149(6):803–808. 10.1016/j.jpeds.2006.08.067 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg. 2007;137(1):43–48. 10.1016/j.otohns.2007.03.028 [DOI] [PubMed] [Google Scholar]
- 10.Costa DJ, Mitchell R. Adenotonsillectomy for obstructive sleep apnea in obese children: a meta-analysis. Otolaryngol Head Neck Surg. 2009;140(4):455–460. 10.1016/j.otohns.2008.12.038 [DOI] [PubMed] [Google Scholar]
- 11.Beebe DW, Byars KC. Adolescents with obstructive sleep apnea adhere poorly to positive airway pressure (PAP), but PAP users show improved attention and school performance. PLoS One. 2011;6(3):e16924. 10.1371/journal.pone.0016924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkins SMM, Jensen EL, Simon SL, Friedman NR. Correlates of pediatric CPAP adherence. J Clin Sleep Med. 2016;12(6):879–884. 10.5664/jcsm.5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright RD, Diaz F, Lloyd S. The effects of sleep posture and sleep stage on apnea frequency. Sleep. 1991;14(4):351–353. 10.1093/sleep/14.4.351 [DOI] [PubMed] [Google Scholar]
- 14.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhelst E, Clinck I, Deboutte I, Vanderveken O, Verhulst S, Boudewyns A. Positional obstructive sleep apnea in children: prevalence and risk factors. Sleep Breath. 2019;23(4):1323–1330. 10.1007/s11325-019-01853-z [DOI] [PubMed] [Google Scholar]
- 16.Zhang XW, Li Y, Zhou F, Guo CK, Huang ZT. Association of body position with sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Acta Otolaryngol. 2007;127(12):1321–1326. 10.1080/00016480701242451 [DOI] [PubMed] [Google Scholar]
- 17.Walter LM, Dassanayake DUN, Weichard AJ, Davey MJ, Nixon GM, Horne RSC. Back to sleep or not: the effect of the supine position on pediatric OSA: sleeping position in children with OSA. Sleep Med. 2017;37:151–159. 10.1016/j.sleep.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 18.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. 10.5664/jcsm.27497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 2013;17(2):771–779. 10.1007/s11325-012-0764-5 [DOI] [PubMed] [Google Scholar]
- 20.Beyers J, Dieltjens M, Kastoer C, et al. Evaluation of a trial period with a sleep position trainer in patients with positional sleep apnea. J Clin Sleep Med. 2018;14(4):575–583. 10.5664/jcsm.7048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benoist L, de Ruiter M, de Lange J, de Vries N. A randomized, controlled trial of positional therapy versus oral appliance therapy for position-dependent sleep apnea. Sleep Med. 2017;34:109–117. 10.1016/j.sleep.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 22.de Ruiter MHT, Benoist LBL, de Vries N, de Lange J. Durability of treatment effects of the sleep position trainer versus oral appliance therapy in positional OSA: 12-month follow-up of a randomized controlled trial. Sleep Breath. 2018;22(2):441–450. 10.1007/s11325-017-1568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baugh RF, Archer SM, Mitchell RB, et al. American Academy of Otolaryngology-Head and Neck Surgery Foundation . Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1)(Suppl:S1–S30. 10.1177/0194599810389949 [DOI] [PubMed] [Google Scholar]
- 24.Bluher AE, Ishman SL, Baldassari CM. Managing the child with persistent sleep apnea. Otolaryngol Clin North Am. 2019;52(5):891–901. 10.1016/j.otc.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 25.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr. 2014;168(6):561–566. 10.1001/jamapediatrics.2014.21 [DOI] [PubMed] [Google Scholar]
- 26.Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 27.Berry RB, Brooks R, Gamaldo CE, et al. ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Version 2.2. Darien, IL: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. R: A language and enviroment for statistical computing. Vienna, Austira: R Foundation for Statistical Computing R Core Team; 2014. Available from: https://www.r-project.org/foundation. Accessed July 1, 2020. [Google Scholar]
- 30.Keefe KR, Patel PN, Levi JR. The shifting relationship between weight and pediatric obstructive sleep apnea: A historical review. Laryngoscope. 2019;129(10):2414–2419. 10.1002/lary.27606 [DOI] [PubMed] [Google Scholar]
- 31.O’Brien LM, Sitha S, Baur LA, Waters KA. Obesity increases the risk for persisting obstructive sleep apnea after treatment in children. Int J Pediatr Otorhinolaryngol. 2006;70(9):1555–1560. 10.1016/j.ijporl.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 32.Uong EC, Epperson M, Bathon SA, Jeffe DB. Adherence to nasal positive airway pressure therapy among school-aged children and adolescents with obstructive sleep apnea syndrome. Pediatrics. 2007;120(5):e1203–e1211. 10.1542/peds.2006-2731 [DOI] [PubMed] [Google Scholar]
- 33.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117(3):e442–e451. 10.1542/peds.2005-1634 [DOI] [PubMed] [Google Scholar]
- 34.Roberts SD, Kapadia H, Greenlee G, Chen ML. Midfacial and dental changes associated with nasal positive airway pressure in children with obstructive sleep apnea and craniofacial conditions. J Clin Sleep Med. 2016;12(4):469–475. 10.5664/jcsm.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]