Abstract
Introduction:
The purpose of this systematic review is to provide supporting evidence for a clinical practice guideline on the use of behavioral and psychological treatments for chronic insomnia disorder in adult populations.
Methods:
The American Academy of Sleep Medicine commissioned a task force of 9 experts in sleep medicine and sleep psychology. A systematic review was conducted to identify randomized controlled trials that addressed behavioral and psychological interventions for the treatment of chronic insomnia disorder in adults. Statistical analyses were performed to determine if the treatments produced clinically significant improvements in a range of critical and important outcomes. Finally, the Grading of Recommendations Assessment, Development, and Evaluation process was used to evaluate the evidence for making specific treatment recommendations.
Results:
The literature search identified 1,244 studies; 124 studies met the inclusion criteria, and 89 studies provided data suitable for statistical analyses. Evidence for the following interventions is presented in this review: cognitive-behavioral therapy for insomnia, brief therapies for insomnia, stimulus control, sleep restriction therapy, relaxation training, sleep hygiene, biofeedback, paradoxical intention, intensive sleep retraining, and mindfulness. This review provides a detailed summary of the evidence along with the quality of evidence, the balance of benefits vs harms, patient values and preferences, and resource use considerations.
Citation:
Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17(2):263–298.
Keywords: chronic insomnia disorder, behavioral treatments, psychological treatments, clinical practice guideline
INTRODUCTION
This systematic review is intended to provide supporting evidence for a clinical practice guideline1 on the behavioral and psychological treatments of chronic insomnia disorder in adults. This systematic review is an update of the evidence review conducted for the previously published American Academy of Sleep Medicine (AASM) guideline.2 The AASM published a separate clinical practice guideline on the pharmacological treatment of chronic insomnia in 2017.3
BACKGROUND
Diagnosis, prevalence, course, and etiology
The International Classification of Sleep Disorders, third edition (ICSD-3)4 diagnostic manual describes chronic insomnia disorder as a report of difficulty initiating or maintaining sleep or waking up too early with associated daytime consequences, occurring despite adequate opportunity and circumstances for sleep. The sleep difficulties must occur at least 3 times per week for at least 3 months. Historically, insomnia disorders have been divided into primary and secondary (comorbid) subtypes, based on the clinician’s diagnostic assessment of the role of medical and/or psychiatric comorbidities in the genesis and maintenance of the insomnia disorder. However, with the publication of ICSD-3 and the Diagnostic and Statistical Manual of Mental Disorders, fifth edition,5 this nosological dichotomy is no longer utilized. The decision to eliminate this distinction was based on the observation that it is often difficult to discern cause-effect relationships between insomnia and co-occurring disorders and that insomnia often becomes an independent disorder even if it is initially caused by another medical or psychiatric condition. Notably, many studies included in this systematic review employed the historical distinction between primary and secondary (comorbid) subtypes in identifying patients for inclusion in treatment trials.
Insomnia symptoms occur in a high percentage of the adult population, with estimates ranging from 35%–50%.6–8 Chronic insomnia disorder, defined by specific diagnostic criteria, has an estimated prevalence of 5%–15%.4,5,8 Chronic insomnia disorder is more common among women, those with lower socioeconomic status, and those with medical or psychiatric illness.6–8 The course of chronic insomnia disorder is typically measured in years or even decades, with spontaneous remission rates generally less than 50%.9 Isolated sleep-onset difficulties are less common than sleep maintenance difficulties, although a substantial proportion of people with insomnia report difficulties with both sleep onset and sleep maintenance.10
Chronic insomnia disorder is associated with daytime fatigue, depressed mood, increased incidence of nonremitting depression with increased suicide risk, impairment in social/vocational functioning, and reduced quality of life.11–14 Studies have shown that insomnia contributes to increased health care costs and utilization15–17 and to lower worker productivity.18 In fact, more than 90% of insomnia-related costs are attributable to work absences and reduced productivity.19
The etiology of chronic insomnia disorder is multifactorial. Emerging research indicates that some individuals may be genetically predisposed to insomnia as a result of clock gene polymorphisms or other genetic factors.20 As noted in the above paragraph, numerous medical and psychiatric disorders are associated with a high risk for insomnia. Some disorders, such as major depressive disorders, show rates of concurrent insomnia as high as 80%–90%.21 A variety of maladaptive cognitions and behaviors play a critical role in the development and maintenance of chronic insomnia.22 These include performance anxiety and negative expectations regarding sleep, with associated worry about potential consequences of not sleeping and unhelpful beliefs and attitudes around sleep. In addition, unhelpful behaviors can have a direct impact on the physiological systems controlling sleep. For example, variability in the timing of sleep-wake behaviors can create circadian dysregulation, and excessive time in bed can diffuse the homeostatic drive for deep sleep and can also lead to conditioned arousal. Finally, psychophysiological studies indicate an increased 24-hour metabolic rate, elevated cortisol levels particularly in the presleep and early sleep period, elevated fast (waking) electroencephalogram activity, and heightened regional brain activity during sleep among individuals with insomnia.6,23,24 These findings collectively support the theory that physiological hyperarousal is an additional significant factor for many patients in the etiology of this sleep disorder.
Definition of behavioral and psychological treatments
Several options are available for treating insomnia, including a range of pharmacotherapies and nonpharmacological approaches. Various psychological and behavioral therapies have been specifically developed for insomnia treatment, and a number of complementary and alternative strategies (eg, dietary supplements, acupuncture) have also been used. This review focuses on psychological and behavioral therapies for insomnia. The nature and focus of these treatments vary considerably, but they are all designed to reduce or eliminate 1 or more of the putative factors that perpetuate insomnia, including sleep-disruptive arousal and/or habits and conditioning factors that sustain insomnia over time. Among these therapies are a range of single-component therapies, each of which targets a specific subset of insomnia-perpetuating factors. Second-generation therapies that evolved from the various single-component therapies combine several such treatments to constitute a more comprehensive, multicomponent intervention approach. Table 1 provides a brief description of each of the therapies considered in this review.
Table 1.
Summary of interventions.
| Intervention | Treatment Typea | Description |
|---|---|---|
| CBT-I | Multicomponent | CBT-I combines 1 or more of the cognitive therapy strategies with education about sleep regulation plus stimulus control instructions and sleep restriction therapy. CBT-I also often includes sleep hygiene education, relaxation training, and other counterarousal methods. Treatment progresses using information typically gathered with sleep diaries completed by the patient throughout the course of treatment (typically 4–8 sessions). |
| BTIs | Multicomponent | BTIs include abbreviated versions of CBT-I (typically 1–4 sessions) emphasizing the behavioral components. BTIs typically consist of education about sleep regulation, factors that influence sleep, and behaviors that promote or interfere with sleep, along with a tailored behavioral prescription based on stimulus control and sleep restriction therapy and on information typically derived from a pretreatment sleep diary. Some therapies include brief relaxation or cognitive therapy elements. |
| Stimulus control | Single-component | A set of instructions designed to (1) extinguish the association between the bed/bedroom and wakefulness to restore the association of bed/bedroom with sleep, and (2) establish a consistent wake-time. Stimulus control instructions: (1) go to bed only when sleepy, (2) get out of bed when unable to sleep, (3) use the bed/bedroom for sleep and sex only (eg, no reading or watching television in bed), (4) wake up at the same time every morning, and (5) refrain from daytime napping. |
| Sleep restriction therapy | Single-component | A method designed to enhance sleep drive and consolidate sleep by limiting time in bed equal to the patient’s sleep duration, typically estimated from daily diaries. Time in bed is initially limited to the average sleep duration and is subsequently increased or decreased based on sleep efficiency thresholds until sufficient sleep duration and overall sleep satisfaction are achieved. |
| Relaxation therapy | Single-component | Structured exercises designed to reduce somatic tension (eg, abdominal breathing, progressive muscle relaxation, autogenic training) and cognitive arousal (eg, guided imagery training, meditation) that may perpetuate sleep problems. |
| Cognitive therapy | Single-component | A set of strategies including structured psychoeducation, Socratic questioning, use of thought records, and behavioral experiments designed to identify and modify unhelpful beliefs about sleep that may support sleep-disruptive habits and/or raise performance anxiety about sleeping. |
| Sleep hygiene | Single-component | A set of general recommendations about lifestyle (eg, diet, exercise, substance use) and environmental factors (eg, light, noise, temperature) that may promote or interfere with sleep. Sleep hygiene may include some education about what constitutes “normal” sleep and changes in sleep patterns with aging. |
| Biofeedback | Single-component | A variant of relaxation training that employs a device capable of monitoring and providing ongoing feedback on some aspect of the patient’s physiology. This technique has most commonly employed continuous monitoring of frontalis electromyography activity to assess the overall level of muscle tension. Typically, the biofeedback device produces an ongoing auditory tone to train the patient to relax by learning how to alter the auditory feedback tone in the desired direction (eg, reduced muscle tension). |
| Paradoxical intention | Single-component | The patient is instructed to remain awake as long as possible after getting into bed. The patient is instructed to purposefully engage in the feared activity (staying awake) to reduce performance anxiety and conscious intent to sleep that confound associated goal-directed behavior (falling asleep). This method alleviates both the patient’s excessive focus on sleep and anxiety over not sleeping; as a result, sleep becomes less difficult to initiate. |
| Intensive sleep retraining | Single-component | This newly described treatment is designed to markedly enhance homeostatic sleep drive to reduce both sleep onset difficulties and sleep misperception. After a night wherein the patient limits time in bed to no more than 5 hours, the treatment includes a 24-hour laboratory protocol in which the patient is given an opportunity to fall asleep every 30 minutes in sleep-conducive conditions. If sleep occurs, then the patient is awakened after 3 minutes and remains awake until the subsequent 30-minute trial. For each sleep opportunity, the patient is given feedback as to whether or not sleep occurred. |
| Mindfulness | Multicomponent or single-component | Mindfulness approaches are used as a form of meditation emphasizing a nonjudgmental state of heightened or complete awareness of one’s thoughts, emotions, or experiences on a moment-to-moment basis. Mindfulness therapies are typically administered in a group format. Structured exercises teach momentary awareness, self-acceptance, and muted reactivity. Home practice of mindfulness exercises is required. When applied to people with insomnia, standard mindfulness is often combined with other insomnia therapies such as stimulus control, sleep restriction therapy, and sleep hygiene (described above). |
Multicomponent treatment is a combination of approaches, and single-component treatment is delivered in isolation. BTIs = brief therapies for insomnia, CBT-I = cognitive-behavioral therapy for insomnia.
Many of the interventions described herein can be delivered using a variety of methods. In describing delivery methods, we use the term “in-person, one-on-one,” which involves a therapist providing a patient with treatment in individual, one-on-one therapy visits. However, the in-person group format has also been used, in which such treatment is provided by a therapist to a group of patients. The self-help format can include self-help books or other written materials that provide treatment instruction, audio recordings, or prerecorded video treatment sessions. Internet-based delivery has also been used for one-on-one or group delivery and for self-help interventions. Telephone and telehealth delivery have also been used in the delivery of insomnia treatments, either with the patient traveling to a clinic with telehealth services (with the provider in a different location) or with the patient at home engaging with the provider using a telephone or online service for real-time interactions. This review includes all delivery modalities for each intervention.
Measurement of insomnia treatment outcomes
A variety of approaches can be taken to measure the effects of behavioral and psychological treatments for insomnia, including questionnaires, daily sleep diaries, polysomnography (PSG), and wrist actigraphy. To address this variability in measurement approaches, standardized assessment instruments have been proposed for insomnia research.25 Current definitions of insomnia disorder include reports of both nighttime symptoms (difficulties with sleep initiation, maintenance, and/or duration) and daytime consequences attributed to insomnia (eg, fatigue, mood disturbance, memory impairment). Thus, treatment measures assessing the impact of behavioral and psychological treatments on insomnia should capture both domains.2 Global measures of sleep disturbances provide an index of the nature and severity of insomnia and can be administered longitudinally to measure treatment response. The Pittsburgh Sleep Quality Index (PSQI)26 and the Insomnia Severity Index (ISI)27 are the two most widely used tools to assess patient-reported sleep disturbances. The PSQI is a measure of global sleep quality, and the ISI more specifically measures self-reported insomnia symptom severity, but both are categorical scales and provide total scores that can be evaluated across treatment and accepted scale-specific criteria for defining treatment response and remission.26–28 These categorical measures, especially insomnia remission, are increasingly recognized as being of primary importance for evaluating the benefits of treatment.
In the study of insomnia treatments, nighttime sleep and insomnia symptoms are most commonly measured with daily sleep diaries,29 which capture information about the timing of sleep (bedtime, rise time) in addition to individual sleep parameters, such as sleep latency (time to fall asleep initially), wake after sleep onset (WASO; duration of nighttime wakefulness), and early morning awakenings (waking in advance of the desired rise time) that are commonly the primary symptoms targeted in insomnia treatments. Additional summary metrics commonly derived from daily sleep diaries include total sleep time and sleep efficiency (total sleep time/time in bed*100%). Daytime napping/sleeping behaviors are also commonly tracked in daily diaries when delivering treatment. The primary advantage of sleep diaries is that they allow for the daily collection of information on nighttime symptoms, making them less subject to recall bias than questionnaires. Treatment effects are most commonly assessed with aggregated mean-level changes in individual sleep diary parameters across time, generally every 1 or 2 weeks, but increasingly, the variability of these parameters across days is also being viewed as clinically important.
The objective evaluation of nighttime sleep and insomnia parameters with PSG and/or actigraphy provides complementary information to sleep diaries and allows for a multimethod comprehensive assessment. However, objective evidence of sleep disturbance is not required to establish a diagnosis of insomnia disorder. When objective information is deemed necessary, wrist actigraphy is a suggested option for clinicians to consider.30
Daytime impairments associated with insomnia commonly include fatigue and/or sleepiness, mood disturbances, impaired cognitive abilities, and overall reduced quality of life. Although discrepancies may exist between the magnitude of self-reported and objectively measured daytime impairments,31 daytime impairment from insomnia is what often leads patients to seek treatment. Thus, perceptions about daytime functioning are important to target with behavioral and psychological treatments. These daytime correlates of insomnia can be measured by a variety of methods, but a limited number of valid and reliable self-report instruments have been recommended for insomnia research.25 Because daytime fatigue is among the most common daytime symptoms of insomnia, various self-report questionnaires designed to assess daytime fatigue have been included in the studies included in this systematic review. Finally, the Dysfunctional Beliefs and Attitudes About Sleep (DBAS) scale32 is a sleep-specific scale that is often included in clinical insomnia trials to determine changes in unhelpful sleep-related beliefs that can serve to perpetuate insomnia.
Statements and recommendations regarding treatment of insomnia disorder
Assessment and treatment of chronic insomnia in adults has been addressed in numerous recent practice guidelines and clinical recommendation statements.3,30,33–36 Recent guidelines and statements that address comprehensive treatment for chronic insomnia uniformly support the use of cognitive-behavioral therapies (CBTs) as first-line treatment for the disorder.34,35,37,38 A report from the American College of Physicians34 recommended that all adult patients receive CBT for insomnia (CBT-I) as the first-line treatment method for chronic insomnia disorder. Likewise, in 2017 the Australian Sleep Association developed recommendations for a limited set of psychological and behavioral treatments for insomnia disorder, noting that CBT-I should be considered first-line treatment.39 The Australian Sleep Association also noted emerging evidence for mindfulness-based treatments for insomnia. The British Association for Psychopharmacology’s recent consensus statement also notes that CBT-I should be considered a first-line approach.38
The current AASM guideline differs from previous guidelines in 2 significant ways.1 First, it is a comprehensive review of both single-component and multicomponent psychological and behavioral interventions. Second, it is designed to complement the existing AASM guidelines specifically related to pharmacological treatments for insomnia disorder, which were published in 2017.3
Meta-analytic reviews
Individual, group, Internet-based, and self-help CBT-I methods have also been the subject of numerous meta-analyses.40–52 A recent comprehensive meta-analysis of individual, group, and self-help cognitive and behavioral therapies49 showed robust clinical improvements in numerous sleep-related outcomes including questionnaires (ISI and PSQI) and sleep diary metrics (eg, sleep efficiency, WASO, and sleep onset latency). Comparable improvements were also shown in a meta-analysis of insomnia comorbid with medical or psychiatric conditions,50 although the magnitude of improvement was greater among patients with psychiatric vs medical comorbidities. Several meta-analyses have also found clinically significant improvements with internet-based CBT-I,40,48,51,52 suggesting that multiple delivery modalities can be used to provide treatment to patients with insomnia disorder.
Previous AASM practice guidelines for and behavioral and psychological treatments of insomnia
The initial (1999) practice parameters53 found the strongest evidence for stimulus control therapy (identified as a “treatment standard”); somewhat weaker evidence in support of relaxation therapy, paradoxical intention, and biofeedback (identified as a “guideline”); and the weakest evidence for multicomponent CBT and sleep restriction therapy (identified as an “option”), reflecting treatment trends and the existing literature of that time period. An update of those parameters was published in 2006.54 Based on review of the evidence since the previous publication, stimulus control, relaxation training, and CBT (with or without relaxation) were recommended in the update as showing the strongest evidence for efficacy (defined as a “standard”). Sleep restriction therapy, multicomponent behavioral therapy (without cognitive therapy), biofeedback and paradoxical intention were also found to be “individually effective therapies in the treatment of chronic insomnia (Guideline).” Sleep hygiene alone was not identified as an effective single-component therapy in any of these reports. To date, there have been no specific guidelines that address the superiority of one behavioral or psychological treatment over another based on direct comparisons, and this remains a limitation of the current guidelines.
The present guidelines represent a further advancement in the establishment of clinical practice guidelines in that the specific recommendations offered herein are based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process (see “Grade Assessment for Developing Recommendations” below), which uses efficacy data and an assessment of the quality of the evidence, patient values and preferences, benefits vs harms, and resource utilization to inform the final recommendation statements, with a goal of improving patient-centered care. We believe that these guidelines are based on the most comprehensive review of available evidence and analysis to date. All single-component therapies (eg, stimulus control alone) and multicomponent therapies (ie, CBT-I and BTI) for which evidence was available were examined in our review. In addition to an analysis of efficacy data for pooled patient populations, we attempted to determine the efficacy of treatment for subgroups of patients such as those with and without identified comorbidities that might affect sleep (ie, medical or psychiatric conditions). We also attempted to examine whether outcomes varied across delivery methods and which delivery method seemed most efficacious. Limitations in the available evidence, including heterogeneity across study samples and methods, did not allow for comparative meta-analysis or specific recommendations; however, delivery methods were still considered in formulating the recommendations and are discussed in detail.
METHODS
Expert task force
The AASM commissioned a task force (TF) of sleep medicine clinicians and researchers with expertise in behavioral and psychological treatments of chronic insomnia disorder. The TF professionals were required to disclose all potential conflicts of interest, per the AASM’s conflicts of interest policy, before being appointed to the TF and throughout the research and writing of these documents. In accordance with the AASM’s conflicts of interest policy, individuals were not allowed to be appointed to the TF if they reported a professional or financial conflict that might diminish the integrity, credibility, or ethical standards of the guideline. Individuals reporting professional or financial conflicts that represented potential bias but did not prohibit participation in the development of the guideline agreed to recuse themselves from discussion or writing responsibilities related to the conflicts. All relevant conflicts of interest are listed in the Disclosures section.
Patient, Intervention, Comparison, and Outcomes questions and clinical significance thresholds
Patient, Intervention, Comparison, and Outcomes (PICO) questions were developed by the TF to assess (1) the efficacy of interventions and (2) the efficacy of different delivery methods (Table 2). The AASM board of directors approved the final list of questions before the literature searches.
Table 2.
PICO questions.
| 1. In adults with chronic insomnia disorder,a which behavioral and psychological treatments,b compared with the control condition,c lead to clinically significant improvements in sleep quality, sleep latency, wake after sleep onset, remission rates, and responder rates? |
| 2. In adults with chronic insomnia disorder,a how do different delivery methodsd for behavioral and psychological insomnia treatmentsc compare for improving the above outcomes? |
When data were available, treatment efficacy was examined in the following subgroups: (1) insomnia without comorbidities, (2) insomnia with medical comorbidities, and (3) insomnia with psychiatric comorbidities. bThe efficacy of the following behavioral and psychological treatments was evaluated: biofeedback, BTIs, CBT-I, cognitive therapy, intensive sleep retraining, mindfulness, relaxation therapy, paradoxical intention, sleep hygiene, sleep restriction therapy, and stimulus control. cControl conditions examined: (1) sleep hygiene or sleep education, (2) pharmacologic—placebo drug, (3) quasi-desensitization, (4) usual care, and (5) wait list. dDelivery methods for behavioral and psychological treatments: in-person one-on-one visit with a trained CBT-I specialist, group behavioral and psychological treatment, telephone delivery, self-help book, and Internet delivery. BTIs = brief therapies for insomnia, CBT-I = cognitive-behavioral therapy for insomnia, PICO = Patient, Intervention, Comparison, and Outcomes.
Through consensus, the TF then developed a list of patient-oriented, clinically relevant outcomes to determine the efficacy of the interventions and delivery methods. The outcomes and/or measurement tools that were employed in the research literature were rated by relative importance for clinical decision-making; outcomes deemed most important for decision-making were considered “critical,” and the remaining outcomes were considered “important.”
The TF set a threshold for each outcome/measurement tool to determine whether the mean treatment vs control differences in the outcomes assessed at posttreatment were clinically significant. The clinical significance threshold was defined as the minimum level of improvement in the outcome of interest that would be considered clinically important to clinicians and patients. For the PICO question on the efficacy of interventions, thresholds based on the mean difference between treatment and control at posttreatment were used for most outcomes; however, standardized mean differences were used when the TF concluded that the interpretation of effect sizes would be more meaningful (Table 3). For the PICO question on the efficacy of different delivery methods, thresholds were set for these methods. Clinical significance thresholds were determined based on a TF literature review of commonly used thresholds including past AASM guidelines.3 Where no clearly established threshold values could be determined, the TF used the literature review, clinical judgment, and experience to establish a clinical significance threshold based on consensus. A summary of the clinical significance thresholds for the outcome measures is presented in Table 3.
Table 3.
Summary of outcomes and clinical significance thresholds.
| Outcome and Tool | Critical Outcome | Clinical Significance Thresholds | Desired Direction Posttreatment Difference | |
|---|---|---|---|---|
| Intervention vs Control (Differences) | Delivery Method vs Delivery Method (Differences) | |||
| Sleep qualitya | ||||
| Diary | Yes | 0.5 SMD | 0.5 SMD* | Higher |
| PSQI | 0.5 SMD | 0.5 SMD* | Lower | |
| Sleep latency | ||||
| Diary | Yes | 20 min | 10 min | Lower |
| PSG | 20 min | 10 min | Lower | |
| WASO | ||||
| Diary | Yes | 20 min | 15 min | Lower |
| Actigraphy | 20 min | 15 min | Lower | |
| PSG | 20 min | 15 min | Lower | |
| Remission rateb | ||||
| ISI | Yes | ≥ 10% patients with < 8 points | ≥ 10% patients with < 8 points | Higher |
| Diary | Yes | ≥ 10% patients with < 31 min sleep latency and/or WASO | 10% patients with < 31 min sleep latency and/or WASO | Higher |
| PSQI | Yes | ≥ 10% patients with ≥ 5 points | ≥ 10% patients with ≥ 5 points | Higher |
| Responder rateb | ||||
| ISI | Yes | ≥ 10% patients with ≥ 8-point drop | ≥ 10% patients with ≥ 8-point drop | Higher |
| Diary | Yes | ≥ 10% patients with ≥ 0.5 SD improvement over baseline sleep latency and/or WASO | ≥ 10% patients with ≥ 0.5 SD improvement over baseline sleep latency and/or WASO | Higher |
| Total wake time | ||||
| Diary | 30 min | 20 min | Lower | |
| Actigraphy | 30 min | 20 min | Lower | |
| PSG | 30 min | 20 min | Lower | |
| Nights with hypnotic use | ||||
| Diary | 2 nights/wk | 2 nights/wk | Lower | |
| Total sleep time | ||||
| Diary | 15 min | 15 min | Higher | |
| Actigraphy | 15 min | 15 min | Higher | |
| PSG | 15 min | 15 min | Higher | |
| Number of nighttime awakenings | ||||
| Diary | 0.5 awakenings/night | 0.5 awakenings/night | Lower | |
| Sleep efficiency (%) | ||||
| Diary | 10 | 5 | Higher | |
| Actigraphy | 10 | 5 | Higher | |
| PSG | 10 | 5 | Higher | |
| Beliefs and attitudes about sleep | ||||
| DBASc | 0.5 SMD | 0.5 SMD | Lower | |
| Daytime fatigue domain | ||||
| All fatigue-specific toolsd | 0.5 SMD | 0.5 SMD | Lower | |
| Insomnia severity | ||||
| ISI | 0.5 SMD | 0.5 SMD | Lower | |
| ISQ | 0.5 SMD | 0.5 SMD | Lower | |
The order of outcomes in the table does not reflect relative weight or importance assigned to the outcomes. aTools to assess sleep quality: daily sleep diary (higher scores indicate higher sleep quality) and PSQI (higher scores indicate worse sleep quality). bClinical cutoff of ≤ 8 indicating no insomnia, PSQI with a clinical cutoff of ≤ 5 indicating normative sleep quality. The task force considered remission and responder rates as the most influential critical outcomes. cHigher scores reflect greater dysfunctional beliefs about sleep. dDaytime fatigue tools (all grouped together): Fatigue Severity Scale, Multidimensional Fatigue Inventory, Profile of Mood States Fatigue subscale, Fatigue Symptom Index, and Flinders Fatigue Scale. For all scales, higher scores indicate greater fatigue. *For SMD, an effect size of 0.5 was considered clinically significant (based on Hedge’s G). DBAS = Dysfunctional Beliefs and Attitudes About Sleep, ISI = Insomnia Severity Index, ISQ = Insomnia Severity Questionnaire, PSG = polysomnography, PSQI = Pittsburgh Sleep Quality Index, SD = standard deviation, SMD = standardized mean difference, WASO = wake after sleep onset.
Literature searches, evidence review, and data extraction
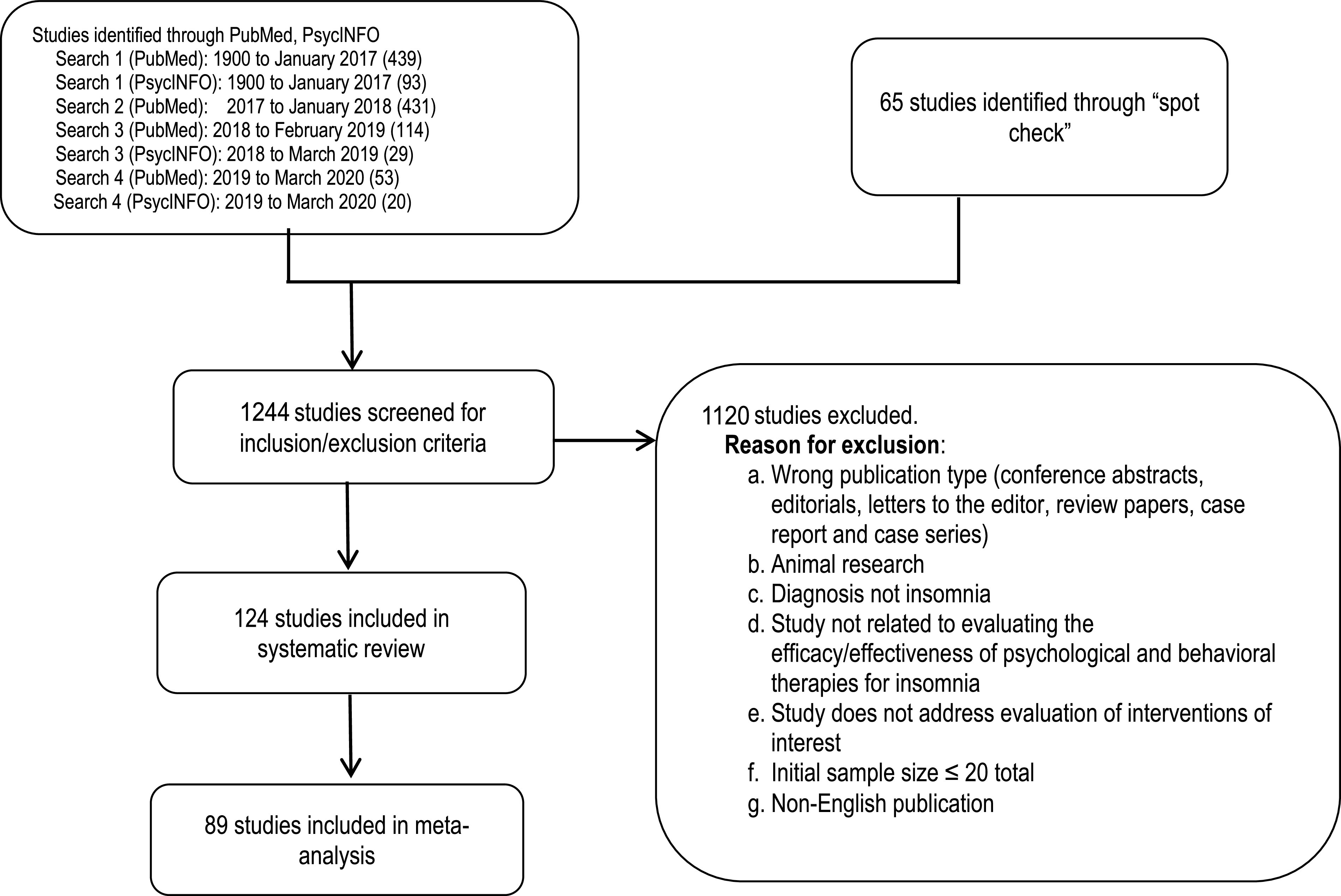
Literature searches were performed using the PubMed and PsycINFO databases for each PICO question (see supplemental material for search strings). The initial search was performed in January 2017 with no date limits, resulting in 532 unique hits. The publications cited in the 2006 review54 were also included if they met the inclusion criteria for this systematic review. An updated literature search was performed in January 2018, resulting in 431 additional unique hits. In February 2019, a subsequent literature search was conducted to identify recently published literature, dating from December 2016 to January 2019, resulting in 114 unique hits. The PsycINFO search between January 2018 and March 2019 identified an additional 29 publications. A final literature search was performed in February 2020, using both the PubMed and PsycINFO databases, and resulted in 73 unique hits. Finally, the TF reviewed previously published guidelines, systematic reviews, and meta-analyses to identify references that may have been missed during the prior searches. The TF identified 65 additional articles through this spot-check process for a total of 1,274 articles that were screened for inclusion/exclusion in the systematic review.
Initial screening by title and abstract was performed by pairs of TF members. Discrepancies were resolved by a third reviewer. Articles were included for further review if they focused on the efficacy of behavioral or psychological treatments for chronic insomnia in adults, addressed at least 1 of the PICO questions, and included at least 1 of the outcomes of interest. Full publications were reviewed by pairs of TF members and were excluded if they did not provide evidence for any PICO questions. The full inclusion/exclusion criteria are listed in the supplemental materials.
A total of 124 articles from the literature searches were accepted and considered for meta-analysis and evidence grading. Specific data elements of all accepted studies were extracted into evidence tables (not published) to address each PICO question. Upon review of these articles, 89 studies were determined to be suitable for meta-analysis and/or the GRADE process. An evidence base flow diagram is presented in Figure 1.
Figure 1. Evidence base flow diagram.
Statistical methods, meta-analysis, and interpretation of clinical significance
Meta-analyses were performed on outcomes of interest for each PICO question (Table 2), using Review Manager 5.3 software (The Cochrane Collaboration, London, United Kingdom) when at least 3 studies with the relevant outcome of interest were available for data pooling. Depending on the number of studies, data analysis proceeded in 1 of 2 ways:
If 3 or more studies were available, a meta-analysis was conducted. The meta-analysis results were then subgrouped by delivery methods (Table 4), and pooled results are reported for each outcome in the Results section.
When fewer than 3 studies were available, studies are described individually and were not subjected to meta-analysis.
Results were also subgrouped by patient populations, which included patients with insomnia with and without comorbidities (Table 5). Data were excluded from the subgroup analyses if studies included a mixed population where the data could not be categorized in any 1 of the subgroups. For delivery method comparisons, the TF chose to compare all alternative delivery methods to in-person, one-on-one delivery, which historically has been the most common and standard delivery method employed. As we have noted, when fewer than 3 studies were available for any delivery method, studies are described individually and were not subjected to meta-analysis.
Table 4.
Description of delivery methods considered by the task force.
| Delivery Method | Description of Method in Included Studies |
|---|---|
| In-person one-on-one delivery | Treatment is provided individually to the patient in a clinical setting by a trained health care provider. |
| In-person group delivery | Treatment is provided to a group of participants in the clinical setting by a trained health care provider. |
| Internet delivery | Treatment is provided via the Internet using email interaction, audio-video recordings, and/or visual graphics and animations used by patients in their homes. Clinical support or health care provider interaction may or may not be included. |
| Self-help delivery | Treatment is provided by reading materials and/or audio recordings used by patients in their homes. No clinical support or health care provider interaction is necessary. |
| Telephone delivery | Treatment is provided via live telephone interaction with a trained health care provider. |
| Video delivery | Treatment is delivered via a recorded video, often including self-help booklets as part of the treatment package used by patients in their homes. No clinical support or health care provider interaction is necessary. |
| Telehealth delivery | Treatment is provided in real time by a trained health care provider using an interactive audio-video telecommunications system. |
Table 5.
Descriptions of patient populations for subgroup analyses.
| Patient Populationa | Description |
|---|---|
| Patients with insomnia and no comorbidities | Patients diagnosed with chronic insomnia disorder (1) in the absence of identified sleep-disruptive comorbidities, or (2) who met the criteria for “primary insomnia” based on earlier diagnostic systems (eg, DSM-IIIR, DSM-IV, and DSM-IV-TR) |
| Patients with insomnia and psychiatric comorbidities | Patients diagnosed with both chronic insomnia disorder and concurrent psychiatric comorbidities (eg, depression, posttraumatic stress disorder, anxiety, or alcohol and substance use) |
| Patients with insomnia and medical comorbidities | Patients diagnosed with chronic insomnia disorder and have concurrent medical comorbidities (eg, cancer, fibromyalgia, osteoarthritis) |
Study populations that do not meet a above descriptions, or were a combination of the patient populations were not included in the subgroup analyses.
For each outcome, unadjusted posttreatment data were used for all statistical analyses. Mean differences were calculated for all outcomes with the exception of sleep quality, ISI, daytime fatigue, and the DBAS scale, for which standardized mean differences were calculated. Some studies had data presented as standard errors, and in these cases, the data were converted into standard deviations so that the study could be included. There were also some studies that reported data in the form of median and interquartile range. These, too, were converted into data expressed as means and standard deviations.55,56 The pooled results for each continuous outcome measure are expressed herein as the mean difference or standardized mean differences between the intervention and comparator groups. The pooled results for the dichotomous outcome measures are expressed as the risk difference between the intervention and comparator. All analyses were performed using a random-effects model with the results displayed as a forest plot. If outcome data were not presented in the format necessary for statistical analysis (ie, mean, standard deviation, and sample size), or data were presented only in graphical formats, then the authors were contacted to obtain the necessary data.
Interpretation of clinical significance for the outcomes of interest was conducted by comparing the mean difference in effect size, or the risk difference for dichotomous outcomes, of each treatment approach to the clinical significance threshold.
GRADE assessment for developing recommendations
The assessment of evidence quality was performed according to the GRADE process for the purposes of making clinical practice recommendations.57,58 GRADE assessment was only performed for the first PICO question on the efficacy of interventions; the TF determined that there was insufficient evidence for delivery method comparisons (the second PICO question) to warrant recommendations. Quality of evidence was assessed only for the studies reporting data that could be included in the meta-analysis. The TF assessed the following 4 components to determine the direction and strength of a recommendation:
Quality of evidence: Based on an assessment of the overall risk of bias (blinding, allocation concealment, selective reporting), imprecision (95% confidence interval [CI] relative to the clinical significance threshold, total sample size < 200), inconsistency (I2 cutoff of 50%), indirectness (study population), and publication bias (funding sources), the members of the TF determined that their overall confidence that the estimated effect found in the body of evidence was representative of the true treatment effect that typical adult patients with insomnia would experience. The overall quality of the evidence was based on outcomes that the TF deemed critical for decision-making. The TF considered remission and responder rates as the most influential critical outcomes in determining the quality of evidence.
Benefits vs harms: Based on any harms/adverse effects reported within the accepted literature and on the clinical experience and expertise of the TF, the TF determined whether the beneficial outcomes of using each intervention outweighed any harms.
Patient values and preferences: Based on the clinical experience and expertise of the TF members and any data published on the topic relevant to patient preferences for behavioral and psychological interventions for insomnia, the TF determined whether patient values and preferences would be consistent across the majority of patients and whether patients would use the interventions based on the body of evidence.
Resource use: Based on the clinical experience and expertise of the TF members, the TF determined whether the accessibility and costs associated with each treatment approach compared favorably to those associated with alternative treatments. Information on costs to both patients and the health care system were considered.
A summary of each GRADE domain is provided herein after the detailed evidence review for each intervention.
Public comment and final approval
Drafts of the systematic review with supplemental materials and accompanying clinical practice guideline1 were made available for public comment for a 2-week period on the AASM website. AASM members, the general public, and other relevant stakeholders were invited to provide feedback on the drafts. The TF considered all the comments received and made decisions about whether to revise the draft based on the scope and feasibility of the comments. The public comments and revised documents were submitted to the AASM board of directors, which subsequently approved the final documents for publication.
The AASM expects this systematic review to have an impact on professional behavior, patient outcomes, and possibly health care costs. This review reflects the state of knowledge at the time of publication and will be reviewed and updated as new information becomes available.
RESULTS
The aims of the current systematic review and data analyses were to inform PICO questions assessing the efficacy of behavioral and psychological treatments for chronic insomnia and treatment efficacy across delivery methods. We found evidence for the following interventions: CBT-I, BTIs, stimulus control, sleep restriction therapy, relaxation training, sleep hygiene, biofeedback, paradoxical intention, intensive sleep retraining (ISR), and mindfulness. No studies meeting our inclusion criteria were found for cognitive therapy as a single-component treatment.
Below are detailed summaries of the evidence identified in the literature searches and the statistical analyses performed by the TF to inform recommendations within the clinical practice guideline.1 All figures and a summary of the study characteristics can be found in the supplemental materials. All values of the critical outcomes results are reported in the following text. For important outcomes results, values are only reported if the results met the clinical significance threshold. Each evidence summary is accompanied by a discussion of the quality of evidence, balance of benefits and harms, patient values and preferences, and resource use considerations that contributed to the development of the clinical practice recommendations, which are provided in the accompanying clinical practice guideline.
Cognitive Behavioral Therapy for Insomnia (CBT-I)
Our review of the literature identified 66 randomized controlled trials (RCTs)59–122 which could be included in the meta-analyses examining the effect of CBT-I vs control in adult patients with chronic insomnia. Forty-nine studies59–61,63–75,77–107,118,119 reported at least 1 of the critical outcomes (Table 3). In addition, 17 RCTs123–139 provided data not suitable for meta-analyses but were included as supporting evidence. The delivery formats of CBT-I in these studies included in-person one-on-one, in-person group, Internet-based delivery, self-help, and video delivery. The control groups received treatment as usual, wait list control, minimal intervention (eg, sleep hygiene education), placebo behavioral treatment (eg, quasi-desensitization), or placebo drug.
The related figures and tables are Figures S1–S76 (2.7MB, pdf) and Tables S1–S28 (2.7MB, pdf) in the supplemental material. Summaries of the meta-analyses conducted are provided in Tables S29–S32 (2.7MB, pdf) . A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section for sleep quality, sleep latency, and WASO.
Sleep quality:
Meta-analysis of 19 studies60,61,63,69,71,74,75,79,80,82,84,86,94,98,100,101,103,105,140 reporting posttreatment comparisons of diary-determined sleep quality between CBT-I and control showed an effect size of 0.44 (95% CI, 0.28—0.61 higher) favoring CBT-I compared with control; these results did not reach the threshold for clinical significance established by the TF (Figure S1 (2.7MB, pdf) ).
In subgroup analyses of patient populations, diary-determined sleep quality was reported in 2 studies of patients with insomnia in the absence of comorbidities.98,101 One study101 showed a clinically significant posttreatment difference, with an effect size of 1.48 (95% CI, 0.64–2.32 higher) favoring CBT-I compared with control (Table S1 (2.7MB, pdf) ). The other study98 did not show a clinically significant posttreatment difference between CBT-I and control, with an effect size of 0.16 (95% CI, 0.29 lower–0.61 higher) (Table S1 (2.7MB, pdf) ). One study in patients with insomnia and comorbid psychiatric conditions61 showed a clinically significant posttreatment difference with an effect size of 0.85 (95% CI, 0.23–1.46 higher) favoring CBT-I over control (Table S2 (2.7MB, pdf) ). Meta-analysis of 3 studies in patients with insomnia and comorbid medical conditions69,79,140 reported an effect size of 0.14 (95% CI, 0.60 lower–0.88 higher) favoring CBT-I over control; these results did not reach the threshold for clinical significance established by the TF69 (Figure S2 (2.7MB, pdf) ).
Meta-analysis of 21 studies59–61,64,72–75,86,91–93,101,104,106,109,112–115,119 reporting PSQI-determined sleep quality (ie, PSQI total score) showed an effect size of 0.66 (95% CI, 0.54–0.78 lower) favoring CBT-I over control (Figure S3 (2.7MB, pdf) ); this effect was above the clinical significance threshold established by the TF.
Two studies64,101 reporting PSQI-determined sleep quality for patients with insomnia and no comorbidities showed clinically significant posttreatment differences, with effect sizes of 0.55 (95% CI, 1.24 lower–0.14 higher) and 1.67 (95% CI, 0.80–2.53 lower), respectively, favoring CBT-I over control (Table S3 (2.7MB, pdf) ). Similarly, a meta-analysis of 4 studies61,73,93,106 in patients with insomnia and comorbid psychiatric conditions showed a clinically significant posttreatment difference with an effect size of 0.80 (95% CI, 0.53–1.06 lower) favoring CBT-I over control (Figure S4 (2.7MB, pdf) ). Meta-analysis of 5 studies,76,91,114,115,119 including patients with insomnia and comorbid medical conditions, showed an effect size of 0.86 (95% CI, 0.60–1.13 lower), which met the clinical significance threshold favoring CBT-I over control (Figure S5 (2.7MB, pdf) ). The quality of evidence for sleep quality ranged from low to moderate because of imprecision and a risk of bias.
Seven studies67,89,104,126,131,133,137 reporting diary-determined sleep quality and 5 studies92,99,125,128,136 reporting PSQI-determined sleep quality compared with control were not included in the current meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
For direct comparisons of delivery methods, 5 studies61,84,100,141,142 reporting diary-determined sleep quality were included in the meta-analysis of in-person one-on-one delivery compared with another delivery modality (Figure S6 (2.7MB, pdf) ). One study141 comparing in-person one-on-one delivery with group delivery showed an effect size of 0.46 favoring in-person one-on-one delivery over group delivery (95% CI, 0.25 lower–1.18 higher). Three studies84,100,142 comparing in-person delivery with Internet delivery reported an effect size of 0.06 favoring in-person one-on-one delivery over Internet delivery (95% CI, 0.99 lower–1.12 higher) (Figure S6 (2.7MB, pdf) ). Out of 2 studies141,142 comparing in-person with telephone delivery, 1 study141 reported an effect size of 0.57 favoring in-person one-on-one delivery over telephone delivery (95% CI, 0.18 lower–1.31 higher), which met the clinical significance threshold (Figure S6 (2.7MB, pdf) ). The other study142 had an effect size of 0.27 (95% CI, 0.75 lower–0.21 higher) favoring telephone delivery and did not meet the clinical significance threshold when compared with in-person one-on-one delivery. One study61 comparing in-person one-on-one with self-help delivery met the clinical significance threshold, with an effect size of 0.65 (95% CI, 0.33 lower–1.64 higher) favoring in-person one-on-one delivery of CBT-I over self-help (Figure S6 (2.7MB, pdf) ).
Two studies59,143 comparing in-person one-on-one delivery with in-person group delivery reported clinically significant mean effect sizes of 0.66 (95% CI, 0.27–1.05 lower) and 1.79 (95% CI, 1.09–2.50 lower), respectively, for PSQI-determined sleep quality favoring in-person one-on-one delivery over group delivery (Figure S7 (2.7MB, pdf) ). One study61 comparing in-person one-on-one delivery with self-help delivery also met the clinical significance threshold with an effect size of 0.61 (95% CI, 1.34 lower–0.11 higher), favoring in-person one-on-one delivery over self-help delivery (Figure S7 (2.7MB, pdf) ).
Sleep latency:
Meta-analysis of 4759–61,63–66,68–75,77,78,80–86,88–105,107,118,119,140,144 studies reporting diary-determined sleep latency showed a mean difference of 12.68 minutes lower (95% CI, 10.48–14.88 minutes lower) for CBT-I compared with control, which did not meet the clinical significance threshold (Figure S8 (2.7MB, pdf) ).
In subgroup analyses of patient populations, diary-determined sleep latency was reported in 10 studies64,66,78,85,88,90,98,101,107,144 of patients with insomnia and no comorbidities with a mean difference of 12.82 minutes lower (95% CI, 7.56–18.09 minutes lower) for CBT-I compared with control (Figure S9 (2.7MB, pdf) ); this difference did not meet the clinical significance threshold. Five studies61,73,81,99,102 of patients with insomnia and comorbid psychiatric conditions showed a mean difference of 30.60 minutes lower (95% CI, 20.37–40.83 minutes lower) for CBT-I compared with control (Figure S10 (2.7MB, pdf) ). Eleven studies65,69,89,91–93,95–97,119,140 in patients with insomnia and comorbid medical conditions reported a mean difference of 10.63 minutes lower (95% CI, 5.83–15.44 minutes lower) for CBT-I compared with control (Figure S11 (2.7MB, pdf) ). Only the studies1 reporting insomnia in individuals with comorbid psychiatric conditions met the clinical significance threshold.
Meta-analysis of 6 studies77,88,96,97,107,140 reporting PSG-determined sleep latency also did not meet the clinical significance threshold (Figure S12 (2.7MB, pdf) ). One107 out of 2,107 studies reporting PSG-determined sleep latency in patients with insomnia and no comorbidities showed a clinically significant posttreatment difference of 31.50 minutes lower (95% CI, 15.52–47.48 minutes lower) for CBT-I compared with control (Table S4 (2.7MB, pdf) ). The other study88 did not show a clinically significant posttreatment difference favoring CBT-I. Meta-analysis of 3 studies96,97,140 reporting PSG-determined sleep latency in patients with insomnia and comorbid medical conditions did not meet the clinical significance threshold (Figure S13 (2.7MB, pdf) ). The quality of evidence for sleep latency ranged from low to moderate because of imprecision and a risk of bias.
Eight studies92,124,126,127,131,133,134,137 reporting diary-determined sleep latency and 1 study132 reporting PSG-determined sleep latency were not included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
For direct comparisons of delivery methods, 4 studies59,141,143,145 reporting on diary-determined sleep latency were included in a meta-analysis comparing in-person one-on-one delivery with in-person group delivery showed a mean posttreatment difference of 5.94 minutes lower (95% CI, 7.28 minutes lower–22.26 minutes higher) for the one-on-one delivery method (Figure S14 (2.7MB, pdf) ). Three studies84,100,142 comparing in-person one-on-one delivery with Internet delivery reported a mean difference of 3.79 minutes lower (95% CI, 14.31 minutes lower–6.72 minutes higher; Figure S14 (2.7MB, pdf) ) for the in-person one-on-one delivery method. Two studies141,142 comparing in-person one-on-one delivery with telephone delivery reported a mean difference of 11.68 minutes lower (95% CI, 0.28–23.08 minutes lower) and 7.49 minutes higher (95% CI, 7.28 minutes lower–22.26 minutes higher) for the one-on-one delivery method (Figure S14 (2.7MB, pdf) ). One study61 comparing in-person one-on-one delivery with self-help delivery and another study95 comparing in-person one-on-one delivery with video delivery reported mean differences of 2.70 minutes lower (95% CI, 13.25 minutes lower–7.85 minutes higher) and 4.61 minutes lower (95% CI, 0.21–9.01 minutes lower) in the in-person one-on-one delivery arm, respectively (Figure S14 (2.7MB, pdf) ). None of the comparisons reported results that met the clinical significance threshold for in-person delivery compared with the other delivery methods.
WASO:
Meta-analysis of 44 studies59–61,63–66,68–75,77,80–102,105,118,119,140,144 reporting diary-determined WASO showed a posttreatment mean difference of 18.96 minutes lower (95% CI, 15.46–22.46 minutes lower) favoring CBT-I over control, a result that did not meet the clinical significance threshold (Figure S15 (2.7MB, pdf) ).
In subgroup analyses of patient populations, 9 studies64,66,85,87,88,90,98,101,144 reporting diary-determined WASO found a clinically significant posttreatment difference of 22.83 minutes (95% CI, 11.04 minutes–34.63 minutes lower) favoring CBT-I over control in patients with insomnia and no comorbidities (Figure S16 (2.7MB, pdf) ). In contrast, 5 studies61,73,81,99,102 of patients with insomnia and comorbid psychiatric conditions reported a mean posttreatment nonclinically significant difference of 14.55 minutes lower (95% CI, 2.05–26.84 minutes lower; Figure S17 (2.7MB, pdf) ) for CBT-I vs control. Eleven65,69,89,91–93,95–97,119,140 studies of patients with insomnia and comorbid medical conditions showed a mean difference of 19.36 minutes lower (95% CI, 11.90–27.31 minutes lower) for CBT-I compared with control (Figure S18 (2.7MB, pdf) ), which did not meet the clinical significance threshold.
Eleven studies64,65,69,70,72,85,97,99–101,140 reporting actigraphy-estimated WASO did not meet the clinical significance threshold for favoring CBT-I over control (Figure S19 (2.7MB, pdf) ). Three studies64,85,101 in patients with insomnia and no comorbidities, 1 study99 in patients with insomnia and comorbid psychiatric conditions, and 4 studies65,69,97,140 in patients with insomnia and comorbid medical conditions assessed WASO by actigraphy; none of these comparisons met the clinical significance threshold that would favor CBT-I over control (Figure S20 (2.7MB, pdf) and Figure S21, Table S5 (2.7MB, pdf) ).
Meta-analysis of 7 studies77,87,88,96,97,99,140 reporting PSG-determined WASO did not meet the clinical significance threshold for favoring CBT-I over control (Figure S22 (2.7MB, pdf) ). Two studies87,88 assessed WASO by PSG in patients with insomnia and no comorbidities; only 1 reported a clinically significant posttreatment difference of 27.94 minutes lower (95% CI, 6.63–49.25 minutes lower) favoring CBT-I over control (Table S6 (2.7MB, pdf) ). One study99did not report a clinically significant posttreatment difference in PSG WASO between CBT-I and control among patients with insomnia and comorbid psychiatric conditions (Table S7 (2.7MB, pdf) ). Meta-analysis of 3 studies96,97,140 reporting WASO measured by PSG in patients with insomnia and comorbid medical conditions97 did not meet the clinical significance threshold (Figure S23 (2.7MB, pdf) ). The quality of evidence for WASO ranged from low to moderate because of imprecision and a risk of bias.
Eleven studies67,92,124,126,127,129,131,133,134,137,138 were not included in the meta-analyses because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings. These studies showed posttreatment intervention improvements in WASO.
For direct comparisons of delivery methods, 3 studies59,141,145 reporting diary-determined WASO were included in a meta-analysis comparing in-person one-on-one delivery with in-person group delivery (Figure S24 (2.7MB, pdf) ). A posttreatment WASO difference of 0.34 minutes higher (95% CI, 9.75 minutes lower–26.99 minutes higher) was found favoring the group delivery method, a difference that did not meet the clinical significance threshold. Meta-analysis of 3 studies84,100,142 comparing in-person one-on-one delivery with Internet delivery showed a posttreatment difference in WASO of 10.11 minutes lower (95% CI, 2.00–18.23 minutes lower) favoring the in-person one-on-one method; these results also did not meet the clinical significance threshold (Figure S24 (2.7MB, pdf) ). Two studies141,142 comparing in-person one-on-one delivery with telephone delivery reported a mean difference in WASO of 19.23 minutes higher (95% CI, 4.82 minutes lower–43.28 minutes higher) and 8.01 minutes higher (95% CI, 10.18 minutes lower–26.20 minutes higher), respectively, for the in-person one-on-one method at posttreatment (Figure S24 (2.7MB, pdf) ). These results did not meet the clinical significance threshold. One study61 comparing in-person one-on-one delivery with self-help delivery reported a mean difference of 4.00 minutes lower (95% CI, 26.54 minutes lower–18.54 minutes higher) for the in-person one-on-one method (Figure S24 (2.7MB, pdf) ). One study95 comparing in-person one-on-one delivery with video delivery showed a mean difference of 3.16 minutes lower (95% CI, 8.37 minutes lower–2.05 minutes higher) for the in-person one-on-one method (Figure S24 (2.7MB, pdf) ). Neither of the results met the clinical significance thresholds.
For comparisons of delivery methods, 2 studies100,143 reported actigraphy-assessed WASO. One study143 comparing in-person one-on-one delivery with group delivery showed a mean difference of 3.30 minutes lower for group delivery (95% CI, 3.10 minutes lower–9.70 minutes higher) (Table S8 (2.7MB, pdf) ). Another study100 comparing in-person one-on-one delivery with Internet delivery reported a mean posttreatment difference of 4.80 minutes lower (95% CI, 16.27 minutes lower–6.67 minutes higher) for in-person one-on-one delivery (Table S9 (2.7MB, pdf) ). Results did not show clinically significance differences between delivery methods.
Remission rate:
Meta-analysis of 25 studies60,61,63,64,72,73,75,77–80,85,87,89,90,94–97,99–101,106,107,119 reported a clinically significant 33% higher (95% CI, 28–39% higher) remission rate for CBT-I compared with control (Figure S25 (2.7MB, pdf) ).
In subgroup analyses of patient populations, meta-analysis of 7 studies64,78,85,87,90,99,107 consisting of patients with insomnia and no comorbidities showed a clinically significant 46% higher (95% CI, 33–58% higher) remission rate in the CBT-I group than in the control group (Figure S26 (2.7MB, pdf) ). Similarly, clinically significant remission rate differences were noted in the CBT-I group in 4 studies61,73,99,106 that included patients with insomnia and comorbid psychiatric conditions and 8 studies79,89,92,93,95–97,119 that included patients with insomnia and comorbid medical conditions; remission rate differences of 31% higher (95% CI, 13–48% higher) and 35% higher (95% CI, 27–42% higher) favoring CBT-I were found in these comparisons, respectively (Figure S27 (2.7MB, pdf) and Figure S28 (2.7MB, pdf) ). The quality of evidence for remission rate ranged from low to moderate because of imprecision and a risk of bias.
Fourteen studies68,81–84,88,102,104,105,116,118,126,129,131 comparing CBT-I with control could not be included in the meta-analysis because the definition of remission rate used in the studies was not consistent with the TF definition of remission rate or because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Direct comparisons of delivery methods included 3 studies61,95,145 that met the TF’s definition of remission rate (Table 3). One study145 compared in-person one-on-one delivery with in-person group delivery and reported a clinically significant 18% higher remission rate (95% CI, 6% lower–43% higher) for the group method (Figure S29 (2.7MB, pdf) ). Similarly, 1 study61 compared in-person one-on-one delivery with self-help delivery and 1 study95 compared in-person one-on-one delivery with video delivery. The results of both studies met the clinical significance threshold, with the in-person one-on-one method showing 17% higher (95% CI, 14% lower–49% higher) and 10% higher (95% CI, 5% lower–26% higher) remission rates than did the self-help and video delivery methods, respectively (Figure S29 (2.7MB, pdf) ).
Responder rate:
Meta-analysis of 16 studies60,65,66,71,73,75,79,80,82,84,91–94,118,144 showed a clinically significant 45% greater responder rate (95% CI, 39–50% higher) for CBT-I vs control (Figure S30 (2.7MB, pdf) ). In subgroup analyses of patient populations, all subgroups met the clinical significance thresholds favoring the CBT-I group over control. Two studies66,144 including patients with insomnia and no comorbidities reported a clinically significant 26% higher (95% CI, 2% lower to 55% higher) and 44% higher (95% CI, 31–57% higher) responder rate for CBT-I (Table S10 (2.7MB, pdf) ). Three studies73,92,93 included patients with insomnia and comorbid psychiatric conditions, with a clinically significant result of a 49% higher responder rate in the CBT-I group (95% CI, 36–63% higher) (Figure S31 (2.7MB, pdf) ). Similarly, clinically significant differences were noted from a meta-analysis of 3 studies65,79,91 in patients with insomnia and comorbid medical conditions, with a 58% higher (95% CI, 42–73% higher) responder rate for CBT-I group (Figure S32 (2.7MB, pdf) ). The quality of evidence for responder rate ranged from low to moderate because of imprecision and a risk of bias.
Fourteen studies64,67,81,83,85,89,92,96,104,105,118,126,131,133 comparing CBT-I with control could not be included in the meta-analysis because the responder rate definitions in the studies were not consistent with the TF’s definition of responder rate or because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
For the direct comparison of delivery methods, only 1 study84 reported a responder rate that met the definition set by the TF. The study84 showed a 33% higher (95% CI, 8% lower–57% higher) responder rate favoring in-person one-on-one delivery over Internet delivery (Table S11 (2.7MB, pdf) ).
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time.
Beliefs and attitudes about sleep:
Sixteen studies60,75,80,91–93,95,98,100,101,103,105,109,113,140,144 reported data acquired from the DBAS scale for CBT-I vs control. Studies used different versions of the DBAS, including the 30-, 28-, 20-, 16-, and 10-item versions. Results met the clinical significance threshold with an effect size of 0.81 (95% CI, 0.38–1.24 lower), favoring CBT-I compared with control (Figure S33 (2.7MB, pdf) ).
In subgroup analyses of patient populations, DBAS scale results were reported in 3 studies98,101,144 in patients with insomnia and no comorbidities. A meta-analysis showed clinically significant improvements in the treatment group, with effect sizes of 1.21 lower (95% CI, 0.65–1.76 lower) favoring CBT-I over control (Figure S34 (2.7MB, pdf) ). Meta-analysis of 5 studies91–93,95,140 including patients with insomnia and comorbid medical conditions showed an effect size of 1.20 favoring the CBT-I group (95% CI, 0.74–1.67 lower) over control for lowering DBAS scores (Figure S35 (2.7MB, pdf) ). These results met the clinical significance threshold favoring CBT-I when compared with control. The quality of evidence for beliefs and attitudes about sleep ranged from very low to low because of imprecision, inconsistency, and a risk of bias.
Four studies92,107,133,134 were not included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
For the direct comparison of delivery methods, of the 3 studies95,100,145 comparing delivery methods that reported posttreatment DBAS comparisons, only 195 met the clinical significance threshold favoring in-person one-on-one delivery over video delivery, with an effect size of 0.85 (95% CI, 0.53–1.17 lower; Figure S36 (2.7MB, pdf) ). The other 2 studies,100,145 which compared in-person one-on-one delivery with group and Internet delivery, did not meet the clinical significance threshold (Figure S36 (2.7MB, pdf) ).
Daytime fatigue:
Ten studies60,62,85,90,101,105,111,114,120,122 reported data on daytime fatigue. Results of various tools such as the Fatigue Severity Scale, the Multidimensional Fatigue Inventory, the Profile of Mood States Fatigue subscale, the Fatigue Symptom Index, and the Flinders Fatigue Scale were pooled. A meta-analysis of 10 studies60,62,85,90,101,105,111,114,120,122 showed an effect size of 0.56 (95% CI, 0.25–0.87 lower), favoring CBT-I over control and meeting the clinical significance threshold (Figure S37 (2.7MB, pdf) ).
In subgroup analyses of patient populations, findings for all 3 patient subgroups met the clinical significance threshold for daytime fatigue improvements favoring the CBT-I treatment. Two studies85,101 that included patients with insomnia and no comorbidities showed an effect size of 0.96 (95% CI, 0.18–1.74 lower) and 0.62 (95% CI, 0.19–1.06 lower), respectively, favoring CBT-I over control (Table S12 (2.7MB, pdf) ). Only 1 study122 reported on patients with insomnia and comorbid psychiatric conditions, with an effect size of 0.81 (95% CI, 0.19–1.42 lower), favoring CBT-I over control (Table S13 (2.7MB, pdf) ). Meta-analysis of 4 studies62,89,114,120 that included patients with insomnia and comorbid medical conditions showed a mean effect size of 0.53 (95% CI, 0.22–0.84 lower), favoring CBT-I over control (Figure S38 (2.7MB, pdf) ). The quality of evidence for daytime fatigue ranged from moderate to low because of imprecision and a risk of bias.
Nine studies77,80,95,118,125,129,134,136,138 could not be included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Only 1 study142 directly compared in-person one-on-one delivery of CBT-I with Internet and telehealth methods, with no clinically significant differences observed among the delivery methods for reducing daytime fatigue (Table S14 (2.7MB, pdf) ).
Insomnia severity:
Meta-analysis of 30 studies59,60,62,63,73–75,79–81,84–86,90,94–97,99–102,105,108,110,117,118,120,121,144 reporting insomnia severity measured by the ISI showed a clinically significant result with an effect size of 0.95 favoring the CBT-I intervention (95% CI, 0.78–1.13 lower) over control (Figure S39 (2.7MB, pdf) ).
In subgroup analyses of patient populations of ISI-determined insomnia severity, 6 studies85,90,101,110,117,144 reporting on patients with insomnia and no comorbidities, 4 studies73,81,99,102 reporting on patients with insomnia and comorbid psychiatric conditions, and 6 studies62,79,95,97,108,120 reporting on patients with insomnia and comorbid medical conditions resulted in ISI score differences that met the clinical significance threshold favoring CBT-I over control in all 3 groups, with effect sizes of 1.25 (95% CI, 0.95–1.55 lower), 1.61 (95% CI, 1.16–2.05 lower), and 0.67 (95% CI, 0.30–1.04 lower), respectively (Figures S40–S42 (2.7MB, pdf) ).
Three studies64–66 reported insomnia severity measured by the Insomnia Symptom Questionnaire. No clinically significant differences between CBT-I and control were noted using this questionnaire (Figure S43 (2.7MB, pdf) ).
In subgroup analyses of patient populations for the Insomnia Symptom Questionnaire that determined insomnia severity, there were 264,66 studies that included patients with insomnia and no comorbidities. Of those, 1 study66 met the clinical significance threshold, with an effect size of 0.86 (95% CI, 0.15–1.57 lower; Table S15 (2.7MB, pdf) ) favoring CBT-I over control. One study65 reported on patients with insomnia and comorbid medical conditions and did not show clinically significant differences between CBT-I and control at posttreatment (Table S16 (2.7MB, pdf) ). The quality of evidence for insomnia severity ranged from low to moderate because of imprecision, inconsistency, and a risk of bias.
Eight studies68,93,123,127,130,131,135,139 reporting insomnia severity measured by the ISI and 1 study67 measured by the Insomnia Symptom Questionnaire could not be included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
In studies including direct comparisons of delivery methods using the ISI to measure insomnia severity, a meta-analysis of 3 studies84,100,142 comparing in-person one-on-one delivery with Internet delivery showed a clinically significant effect size of 0.61 (95% CI, 0.10–1.11 lower) favoring the in-person one-on-one delivery method over the Internet method (Figure S44 (2.7MB, pdf) ). One study141 comparing in-person one-on-one delivery with telephone delivery and another95 comparing in-person one-on-one delivery with video-delivered CBT-I both met the clinical significance threshold, with effect sizes of 0.67 (95% CI, 1.42 lower–0.09 higher) and 0.57 (95% CI, 0.21–0.93 lower), respectively, favoring the in-person one-on-one delivery method (Figure S44 (2.7MB, pdf) ). Clinical significance thresholds were not met in 2 studies59,141 that compared in-person one-on-one delivery with group delivery and in 1 study142 comparing in-person one-on-one delivery with telehealth delivery (Figure S44 (2.7MB, pdf) ).
Nights with hypnotic use:
Our literature search identified 5 studies83,91,92,103,109 reporting diary-determined nights per week of hypnotic use; results of the meta-analysis did not meet the clinical significance thresholds for CBT-I vs control comparisons (Figure S45 (2.7MB, pdf) ).
In subgroup analyses of patient populations, 1 study88 that included patients with insomnia and no comorbidities and a second92 that included patients with insomnia and comorbid psychiatric conditions did not meet the clinical significance threshold that would favor CBT-I over control for reducing hypnotic use (Table S17 (2.7MB, pdf) and Table S18 (2.7MB, pdf) ). The quality of evidence for nights with hypnotic use ranged from low to moderate because of imprecision, inconsistency, and a risk of bias.
Data from 6 studies72,84,92,98,114,136 reporting hypnotic use could not be included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings. No studies were found for direct comparisons of in-person one-on-one delivery with the other delivery methods.
Number of nighttime awakenings:
A total of 19 studies60,61,63,68,75,81–85,89,90,94,98,100,101,104,105,119 that reported diary-determined data for the number of nighttime awakenings were included in a meta-analysis. Results did not meet the clinical significance threshold (Figure S46 (2.7MB, pdf) ) for comparisons of CBT-I and control.
In subgroup analyses of patient populations, 4 studies85,90,98,101 that included patients with insomnia and no comorbidities reported no clinically significant differences in the treatment group when compared with control (Figure S47 (2.7MB, pdf) ). Two studies61,81 included patients with insomnia and comorbid psychiatric conditions; 161 was clinically significant with 0.86 fewer awakenings favoring CBT-I (95% CI, 1.73–0.01 higher) when compared with control (Table S19 (2.7MB, pdf) ). Two studies89,119 reported patients with insomnia and comorbid medical conditions; 1119 study met the clinical significance threshold of 0.70 fewer awakenings favoring CBT-I (95% CI, 1.86 lower–0.46 higher) when compared with control (Table S20 (2.7MB, pdf) ). The quality of evidence for nighttime awakenings ranged from low to moderate because of imprecision and a risk of bias.
Data from 4 studies118,134,137,138 reporting the diary-determined number of nighttime awakenings could not be included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
In direct comparisons of delivery methods using diary-determined data for the number of nighttime awakenings, a meta-analysis of 3 studies84,100,142 comparing in-person one-on-one delivery with Internet delivery, 1 study145 comparing in-person one-on-one delivery with in-person group delivery and 1 study142 comparing in-person one-on-one delivery with telehealth delivery did not meet the clinical significance threshold for treatment group differences (Figure S48 (2.7MB, pdf) ). One study61 comparing in-person one-on-one delivery with self-help delivery showed a clinically significant result of 0.70 fewer awakenings (95% CI, 0.06–1.34 lower), favoring the in-person one-on-one delivery method (Figure S48 (2.7MB, pdf) ).
Sleep efficiency:
Our literature search identified 50 studies59–61,63–66,68–75,77,78,80–105,107,116,119–121,140,144 reporting diary-determined sleep efficiency that were included in the meta-analyses. Results did not meet the clinical significance threshold when comparing CBT-I with control (Figure S49 (2.7MB, pdf) ).
Similarly, in subgroup analyses of patient populations, no clinically significant CBT-I vs control differences were seen in a meta-analysis of 11 studies64,66,78,85,87,88,90,98,101,107,144 reporting diary-determined efficiency in patients with insomnia and no comorbidities (Figure S50 (2.7MB, pdf) ). No clinically significant differences between CBT-I and control were observed in meta-analysis of 5 studies61,73,81,99,102 reporting diary-determined efficiency in patients with insomnia and comorbid psychiatric conditions (Figure S51 (2.7MB, pdf) ). In addition, meta-analysis of 12 studies65,69,89,91–93,95–97,119,120,140 reporting diary-determined sleep efficiency in patients with insomnia and comorbid medical conditions did not meet the clinical significance threshold when comparing CBT-I with control (Figure S52 (2.7MB, pdf) ).
Eleven studies59,64–66,69,70,72,91,97,100,140 reporting sleep efficiency measured by actigraphy did not meet the clinical significance threshold (Figure S53 (2.7MB, pdf) ) in the CBT-I vs control comparisons.
Similarly, in subgroup analyses of patient populations, no clinically significant differences were seen in 2 studies64,66 reporting actigraphy-determined sleep efficiency in patients with insomnia and no comorbidities, 1 study91 reporting actigraphy-determined sleep efficiency in patients with insomnia and comorbid psychiatric conditions, and 4 studies65,69,97,140 reporting actigraphy-determined sleep efficiency among patients with insomnia and comorbid medical conditions when comparing CBT-I with control (Table S21 (2.7MB, pdf) and Table S22, Figure S54 (2.7MB, pdf) ).
Eight studies77,87,88,96,97,107,116,140 employing PSG also did not meet the clinical significance threshold (Figure S55 (2.7MB, pdf) ) in the CBT-I vs control comparisons. In subgroup analyses, no clinically significant differences between CBT-I and control were observed in a meta-analysis of 3 studies87,88,107 reporting PSG-determined sleep efficiency in patients with insomnia and no comorbidities (Figure S56 (2.7MB, pdf) ). In addition, meta-analysis of 3 studies96,97,140 measuring sleep efficiency by PSG among patients with insomnia and comorbid medical conditions did not meet the clinical significance threshold when comparing CBT-I with control (Figure S57 (2.7MB, pdf) ). The quality of evidence for sleep efficiency ranged from low to moderate because of imprecision and a risk of bias.
Data from 9 studies67,92,118,124,126,133,134,137,139 measuring sleep efficiency by diary, 2 studies135,139 by actigraphy, and 1 study67 by PSG could not be included in the meta-analysis because the posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Direct comparisons of delivery methods included a meta-analysis of 3 studies59,141,145 reporting diary-determined sleep efficiency that compared in-person one-on-one delivery with group delivery and another 3 studies84,100,142 that compared in-person one-on-one delivery with Internet delivery. None of these studies met the clinical significance threshold for differences among the delivery methods (Figure S58 (2.7MB, pdf) ). Similarly, 1 study61 comparing in-person one-on-one delivery with self-help delivery, another141 comparing in-person one-on-one delivery with telephone delivery, and 1 study95 comparing in-person one-on-one delivery with video delivery did not meet the clinical significance threshold (Figure S58 (2.7MB, pdf) ) for differences among the delivery methods. Two studies59,143 compared in-person one-on-one delivery with group delivery and 1 study100 compared in-person one-on-one delivery with Internet delivery measuring sleep efficiency by actigraphy (Figure S59 (2.7MB, pdf) ). None of these studies met the clinical significance threshold for comparisons among the delivery methods (Figure S59 (2.7MB, pdf) ).
Total wake time:
Meta-analysis of 15 studies59,65,66,71,73,79,84,86,88,95,96,98,101,116,120 reported a clinically significant 39.60 minutes lower (95% CI, 26.07–53.12 minutes lower) posttreatment value of diary-determined total wake time for CBT-I compared with control (Figure S60 (2.7MB, pdf) ).
In subgroup analysis in patients with insomnia and no comorbidities, meta-analysis of 4 studies66,88,98,101 reporting total wake time measured by diary did not meet the clinical significance threshold (Figure S61 (2.7MB, pdf) ) for CBT-I vs control comparisons. Similarly, 1 study73 reporting total wake time measured by diary in patients with insomnia and comorbid psychiatric conditions did not meet the clinical significance threshold for group differences (Table S23 (2.7MB, pdf) ). Meta-analysis of 5 studies65,79,95,96,120 reporting diary-determined total wake time in patients with insomnia and comorbid medical conditions showed a clinically significant 39.51 minutes lower (95% CI, 20.18–58.84 minutes lower) posttreatment total wake time for the CBT-I group (Figure S62 (2.7MB, pdf) ).
Meta-analysis of 3 studies65,66,101 measuring total wake time by actigraphy did not meet the clinical significance threshold for CBT-I vs control comparisons (Figure S63 (2.7MB, pdf) ). In subgroup analysis in patients with insomnia and no comorbidities, 2 studies66,101 measured by actigraphy did not meet the clinical significance threshold for CBT-I vs control comparisons (Table S24 (2.7MB, pdf) ). One study65 reporting total wake time measured by actigraphy in patients with insomnia and comorbid medical conditions also did not meet the clinical significance threshold for group differences (Table S25 (2.7MB, pdf) ).
Three studies88,96,116 reporting total wake time measured by PSG showed a clinically significant 36.98 minutes lower (95% CI, 79.33 lower–5.37 higher) total wake time for CBT-I when compared with control (Figure S64 (2.7MB, pdf) ). In subgroup analysis in patients with insomnia and no comorbidities, 1 study88 measured total wake time by PSG showed a clinically significant 37.92 minutes lower (95% CI, 6.57–69.27 minutes lower) total wake time for CBT-I (95% CI, 6.57–69.27 minutes lower) when compared with control (Table S26 (2.7MB, pdf) ). The quality of evidence for total wake time was low because of imprecision, inconsistency, and risk of bias.
In comparisons of delivery methods, 2 studies59,141 compared diary-determined total wake time of the in-person one-on-one and group delivery methods, 1 study141 compared in-person one-on-one delivery with telephone delivery, and 1 study95 compared in-person one-on-one delivery with video delivery; none of these comparisons met the clinical significance threshold (Figure S65 (2.7MB, pdf) ) for group differences. One study84 comparing in-person one-on-one delivery with Internet delivery showed a clinically significant greater posttreatment difference in total wake time of 29.90 minutes (95% CI, 7.28–52.52 minutes lower), favoring the in-person one-on-one delivery method (Figure S65 (2.7MB, pdf) ).
Total sleep time:
A meta-analysis of 49 studies60,61,63–66,68–75,77–105,107,116,119,120,140,144 comparing CBT-I with control for diary-determined total sleep time did not meet the clinical significance threshold at posttreatment for CBT-I as compared with control (Figure S66 (2.7MB, pdf) ).
In subgroup analyses of patient populations, meta-analysis of 11 studies64,66,78,85,87,88,90,98,101,107,144 that included patients with insomnia and no comorbidities reported total sleep time measured by diary showed results that did not meet the clinical significance threshold for CBT-I vs control (Figure S67 (2.7MB, pdf) ). Meta-analysis of 5 studies61,73,81,99,102 that considered diary-based total sleep time among patients with insomnia and comorbid psychiatric conditions showed a clinically 40.12 minutes higher (95% CI, 19.05–61.19 minutes higher) total sleep time in the CBT-I group (Figure S68 (2.7MB, pdf) ). Meta-analysis of 13 studies65,69,79,89,91–93,95–97,119,120,140 reported diary-determined total sleep time in patients with insomnia and comorbid medical conditions; the results did not meet the clinical significance threshold (Figure S69 (2.7MB, pdf) ).
Meta-analysis of 12 studies64–66,70,72,85,91,97,99–101,140 reporting total sleep time measured by actigraphy reported a clinical significant reduction of total sleep time of 19.15 minutes lower (95% CI: 7.00–31.29 minutes lower) favoring control at the post-treatment time for CBT-I as compared to control (Figure S70 (2.7MB, pdf) ).
In subgroup analyses of patient populations, meta-analysis of 4 studies64,66,85,101 that used actigraphy in patients with insomnia and no comorbidities found a clinically significant 23 minutes lower (95% CI, 51.11 minutes lower–5.11 minutes higher) total sleep time for CBT-I as compared with control (Figure S71 (2.7MB, pdf) ). One study99 estimated total sleep time using actigraphy in patients with insomnia and comorbid psychiatric conditions did not reach the clinical significance threshold for CBT-I at the posttreatment comparison (Table S27 (2.7MB, pdf) ). Meta-analysis of 5 studies65,69,91,97,140 reported actigraphy-determined total sleep time in patients with insomnia and comorbid medical conditions; the results also did not meet the clinical significance threshold (Figure S72 (2.7MB, pdf) ) for group differences.
Nine studies77,87,88,96,97,99,107,116 measured by PSG did not meet the clinical significance threshold at posttreatment for CBT-I as compared with control (Figure S73 (2.7MB, pdf) ). In subgroup analyses of patient populations, meta-analysis of 3 studies87,88,107 reporting total sleep time measured by PSG among patients with insomnia and no comorbidities showed a clinically significant mean difference of 23.38 minutes higher (95% CI, 20.18 minutes lower–66.93 higher) total sleep time, favoring CBT-I over control (Figure S74 (2.7MB, pdf) ). One study99 reporting total sleep time measured by PSG in patients with insomnia and comorbid psychiatric conditions reported a clinically significant 33.60 minutes higher (95% CI, 17.27 minutes lower–84.47 minutes higher) total sleep time for CBT-I at posttreatment compared with control (Table S28 (2.7MB, pdf) ). In patients with insomnia and comorbid medical conditions, meta-analysis of 3 studies96,97,140 reporting PSG-determined total sleep time96,97 did not meet the clinical significance threshold (Figure S75 (2.7MB, pdf) ). The quality of evidence for total sleep time ranged from very low to moderate because of imprecision, inconsistency, and a risk of bias.
Data from 9 studies67,92,118,126,127,133,134,137,138 measuring total sleep time by diary, 1 study126 by actigraphy, and 1 study67 by PSG could not be included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Direct comparisons of in-person one-on-one delivery methods with self-help, group, Internet, video, telephone, and telehealth delivery methods for CBT-I measuring diary-determined total sleep time consisted of 8 studies61,84,95,100,141–143,145 (Figure S76 (2.7MB, pdf) ). Out of these, 1 study141 showed a clinically significant 18.69 minutes higher (95% CI, 22.33 minutes lower–59.71 minutes higher) total sleep time for in-person one-on-one delivery when compared with telephone delivery of CBT-I (Figure S76 (2.7MB, pdf) ). The rest of the results, including 1 study61 comparing in-person one-on-one delivery with self-help delivery, 3 studies141,143,145 comparing in-person one-on-one with group delivery, and 1 study95 comparing in-person one-on-one delivery with video delivery, did not meet the clinical significance threshold for treatment group differences (Figure S76 (2.7MB, pdf) ). Meta-analysis of 3 studies84,100,142 comparing in-person one-on-one delivery with Internet delivery showed clinically greater total sleep time at posttreatment, favoring Internet delivery by 18.28 minutes higher (95% CI, 66.17 minutes lower–29.61 minutes higher) total sleep time (Figure S76 (2.7MB, pdf) ).
Overall quality of evidence
The overall quality of evidence for the use of CBT-I in patients with chronic insomnia was rated as moderate for the critical outcomes because of imprecision and a risk of bias (Table S29 (2.7MB, pdf) ).
Benefits vs harms
The overall benefits of CBT-I for the treatment of insomnia were determined to be moderate based on improvements in WASO, remission rates, and responder rates that met the clinical significance thresholds established by the TF. Improvements in these critical patient outcomes were evident for insomnia patients without comorbidities in addition to patients with comorbid medical and psychiatric disorders, which represent the majority of patients seen for treatment. In addition, substantial evidence exists that treatment gains are durable over the long-term without additional intervention. These benefits, however, need to be considered in the context of potential harms.
Based on available evidence and the TF’s experience, the principal harms associated with CBT-I may include symptoms of daytime fatigue and sleepiness, mood impairment (eg, irritability), and cognitive difficulties (eg, attention problems), primarily restricted to the early stages of treatment when behavioral therapies are introduced. Studies146 have specifically shown that daytime sleepiness is increased, and psychomotor performance is impaired during the initial phase of sleep restriction therapy. At posttreatment, actigraphy TST was shorter in CBT-I vs controls; however, this is sometimes expected as part of sleep restriction therapy, and long-term follow-up data and task force experience suggest that there is a subsequent increase in total sleep time from the end of acute treatment to long term follow up time points. Thus, patients should be routinely warned about the possible dangers associated with daytime sleepiness, such as drowsy driving, when undergoing treatment. There is also the potential risk of nighttime falls when patients are out of bed at night in some patient populations such as older adults, those using sleep medications, or patients with physical disabilities who are following stimulus control instructions. This is likely due to an anticipated reduction in total sleep time in the early weeks of treatment if the patient adheres to the recommendation to reduce time in bed. However, the TF assessed that these harms are generally temporary, resolve as treatment continues, are small in magnitude, and tolerable to most patients. The TF noted that most RCTs of CBT-I did not include assessments of side effects associated with treatment, so adequate data on the direct harms associated with CBT-I are lacking. Based on the available literature and their clinical experience, the TF determined that the overall benefits of CBT-I strongly outweighed the harms for adults with chronic insomnia.
Resource use
Cost effectiveness was considered to favor CBT-I based on ad hoc analysis which suggests significant cost advantage with CBT-I vs estimated costs of untreated chronic insomnia.147 Multiple formats are available for delivering CBT-I, with resource requirements ranging from moderate (eg, in person one-on-one treatment) to minimal (eg, internet-delivered). In-person one-on-one or group CBT-I carries substantial costs, owing to the resources needed to train therapists to deliver the treatment and space required for patients to be seen, but the emergence of other formats for delivering CBT-I, such as via the internet, represents a significant cost savings in terms of resource use. Two cost analysis studies148,149 showed that internet-delivered CBT-I has a high probability of being more cost effective than both treatment as usual and in-person group therapy. Cost-benefit estimates projected a net benefit to the employer of $512 per internet-delivered CBT-I participant and a return on investment of 208%, stemming mostly from the effects on presenteeism.149 Available data are limited by small sample sizes and therefore more systematic work is needed in this area.
Patient values and preferences
Based on their clinical experience, the TF determined that the majority of patients with chronic insomnia would choose CBT-I given its established efficacy and safety. The limited available data indicate that CBT-I is preferred to pharmacological treatment because it is perceived to have better long-term efficacy,150 to benefit daytime symptoms more, and to have fewer side effects.150,151 Furthermore, patients may prefer CBT-I over other single-component therapy options,152 however the relative preference for CBT-I compared to other available treatments may differ by insomnia subgroup.126,153
Brief Therapies for Insomnia (BTIs)
Our review of the literature identified 8 RCTs154–161 that examined the effect of BTIs vs control treatment on adult patients with chronic insomnia that were included in the meta-analyses. In addition, 3 RCTs162–164 were identified that could not be pooled with other studies but were reviewed as supporting evidence. The delivery format of BTIs in the studies included only in-person one-on-one delivery. One study was conducted in the general adult population,161 3 studies154,155,159 were conducted in older adults, 2 studies156,157 were conducted in military veterans, and 1 study156 was conducted in patients with insomnia not related to comorbid conditions. One study155 delivered BTIs over the course of 2 sessions. Four studies154,157,159,161 delivered BTIs over the course of 4 sessions. One study156 also incorporated imagery rehearsal therapy for nightmares into the BTI program, and the combined program was delivered over the course of 8 sessions.
The related figures and tables are Figures S77–S84 (2.7MB, pdf) and Tables S33–S42 (2.7MB, pdf) . A summary of the findings is provided in Table S43. (2.7MB, pdf) A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below. There were insufficient data present for subgroup analyses and delivery method analyses.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (the PSQI, actigraphy, PSG) are also reported in this section.
Sleep quality:
Meta-analysis of 3 studies154,158,161 reporting diary-determined sleep quality showed a clinically significant effect size of 1.73 (95% CI, 0.16 lower–3.62 points higher) favoring BTI when compared with control (Figure S77 (2.7MB, pdf) ). Meta-analysis of 4 studies154–157 reporting PSQI-determined sleep quality also showed a clinically significant treatment group difference with an effect size of 2.10 (95% CI, 4.24 lower–0.04 higher) favoring BTI over control (Figure S78 (2.7MB, pdf) ). The quality of the evidence for sleep quality was low because of imprecision, inconsistency, and a risk of bias.
Data from 2 studies162,164 measuring sleep quality by diary and from 1 study164 measuring sleep quality measured by the PSQI were not included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Sleep latency:
Meta-analysis of 7 studies154–156,158–161 reporting diary-determined sleep latency showed a reduction of 10.54 minutes (95% CI, 9.25–11.83 minutes lower); these results did not meet the clinical significance threshold for the treatment vs control comparisons (Figure S79 (2.7MB, pdf) ). Two studies154,156 reported PSG-determined sleep latency. Neither of them met the clinical significance threshold (Table S33 (2.7MB, pdf) ) for group differences. The quality of the evidence for sleep latency was moderate because of imprecision.
Data from 3 studies162–164 measuring sleep latency by diary and from 2 studies163,164 of sleep latency measured by PSG could not be included because the posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of these findings.
WASO:
Meta-analysis of 7 studies154–156,158–161 reporting diary-determined WASO showed a 16.16-minutes-lower (95% CI, 8.83–23.48 minutes lower) mean value of WASO in the BTI group at posttreatment compared with control; these results did not meet the clinically significant threshold (Figure S80 (2.7MB, pdf) ). Two studies154,158 assessing WASO by actigraphy and 2 studies154,156 employing PSG comparing BTI vs control did not meet the clinical significance threshold for treatment vs control comparisons (Table S34 (2.7MB, pdf) and Table S35 (2.7MB, pdf) ). The quality of the evidence for WASO was low because of imprecision and inconsistency.
Three studies162–164 measuring WASO by diary and 2 studies163,164 measured by actigraphy and PSG were not included in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Remission rate:
Meta-analysis of 5 studies154,155,157–159 reported a clinically significant 34% (95% CI, 22–45% higher) higher remission rate in the BTI group vs the control group (Figure S81 (2.7MB, pdf) ). The quality of the evidence for remission rate was moderate because of imprecision.
Data from 2 studies162,163 measuring remission rate were not included in the analysis because the remission rate definition in the studies did not meet the TF definition of remission rate or because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Responder rate:
Results of 2 studies157,160 reporting responder rates met the clinical significance threshold for responder rate for BTIs compared with control. Results were 26% higher (95% CI, 5% lower–58% higher) and 21% higher (95% CI, 14% lower–56% higher) for BTI, respectively (Table S36 (2.7MB, pdf) ). The quality of the evidence for responder rate was moderate because of imprecision.
Data from 3 studies155,162,163 measuring responder rate were not included in the analysis because the definition in the studies did not meet the TF definition of responder rate or because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for daytime fatigue, nights with hypnotic use, and total wake time.
Beliefs and attitudes about sleep:
Results of 1 study161 reporting beliefs and attitudes about sleep on the DBAS scale did not show any clinically significant BTI vs control differences (Table S37 (2.7MB, pdf) ). The quality of the evidence for beliefs and attitudes about sleep was low because of imprecision and a risk of bias.
Insomnia severity:
Meta-analysis of 4 studies156,157,160,161 reporting insomnia severity on the ISI showed a clinically significant result with an effect size of 0.81 (95% CI, 0.18–1.43 lower) favoring BTI compared with control (Figure S82 (2.7MB, pdf) ). The quality of the evidence for insomnia severity was low because of imprecision and a risk of bias.
Data from 1 study162 were not included in the analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Number of nighttime awakenings:
Two studies159,160 reported the diary-determined number of nighttime awakenings, only 1 of which160 met the clinical significance threshold of 0.50 fewer nighttime awakenings (95% CI, 1.33 lower–0.33 higher) for BTI than for control (Table S38 (2.7MB, pdf) ). The other study159 did not meet the clinical significance threshold. The quality of the evidence for number of nighttime awakenings was moderate because of imprecision.
Sleep efficiency:
Meta-analysis of 7 studies154,155,158–162 reporting diary-determined sleep efficiency showed no clinically significant group differences (Figure S83 (2.7MB, pdf) ). Results of 2 studies154,158 reporting actigraphy-determined sleep efficiency and 2 studies154,156 reporting PSG-determined sleep efficiency also did not meet the clinical significance threshold when BTI was compared with control (Table S39 (2.7MB, pdf) and Table S40 (2.7MB, pdf) ). The quality of the evidence for sleep efficiency was moderate because of imprecision and a risk of bias.
Data from 2 studies162,164 were not included in the analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Total sleep time:
Meta-analysis of 6 studies154,155,158–161reporting diary-determined total sleep time comparing BTI with control reported clinically significant results favoring control over BTI with a 23.89-minutes-lower (95% CI, 9.89–37.88 minutes lower) total sleep time for BTI at posttreatment (Figure S84 (2.7MB, pdf) ). Two studies154,158 reported actigraphy-assessed total sleep time, of which 1 study154 reported a clinically significant 32.28-minutes lower (95% CI, 28.72–35.84 minutes lower) total sleep time at posttreatment for BTI (Table S41 (2.7MB, pdf) ) compared with control. Of the 2 studies154,156 reporting PSG-determined total sleep time, 1 study156 showed a clinically significant difference favoring control over BTI with a 34.10-minutes-lower (95% CI, 77.23 minutes lower–9.03 minutes higher) total sleep time at posttreatment (Table S42 (2.7MB, pdf) ). The quality of the evidence for total sleep time was moderate because of imprecision.
Overall quality of evidence
The overall quality of evidence for the use of BTI in patients with chronic insomnia was moderate for the critical outcomes because of imprecision and a risk of bias (Table S43 (2.7MB, pdf) ).
Benefits vs harms
Based on clinically significant differences in sleep quality and insomnia remission rates compared with control, the TF determined that the efficacy was moderate. In addition, BTI also improved sleep latency and WASO when compared with control, although not at a level that exceeded clinical significance thresholds. At posttreatment, diary-reported total sleep time was shorter for BTI vs control; however, this is sometimes expected as part of sleep restriction therapy, and long-term follow-up data and TF experience suggest that there is a subsequent increase in total sleep time from the end of acute treatment to long-term follow-up timepoints. Notably, our meta-analysis was based only on posttreatment differences. Studies146 have specifically shown that daytime sleepiness is increased and psychomotor performance is impaired during the initial phase of sleep restriction therapy. Thus, patients should be routinely warned about the possible dangers associated with daytime sleepiness, such as drowsy driving, when undergoing treatment. There is also the potential risk of nighttime falls in specific populations such as older adults, those using sleep medications, or patients with disabilities (eg, frail older adults) following stimulus control instructions. Based on their clinical experience, the members of the TF determined that the undesirable effects are minimal and that the balance of benefits vs harms strongly favors the use of BTI.
Resource use
No prior analysis has examined the resource use of BTI. It would stand to reason that BTI requires fewer resources than CBT-I and greater resources than single-component therapies such as sleep restriction therapy or stimulus control.
Patient values and preferences
Based on their clinical experience, the TF determined that most patients would choose BTI treatment given the benefits of treatment and the amount of time that this treatment requires (ie, attending fewer treatment sessions).
Stimulus control
Our review identified 8 RCTs126,165–171 that compared stimulus control to a control condition among adult patients with chronic insomnia. Three studies165,168,169 focused on sleep onset insomnia, and 2 studies enrolled only older adults.126,170 Four studies delivered stimulus control in a group format,126,168–170 and 2 studies165,166 delivered stimulus control in person, using a one-on-one format.
The related figures and tables are Figures S85–S87 (2.7MB, pdf) and Tables S44–S52. (2.7MB, pdf) A summary of findings is provided in Table S53 (2.7MB, pdf) . A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below.
There were insufficient data to evaluate the efficacy of this treatment among patients with psychiatric or medical comorbidities or to determine the relative efficacy of different delivery methods for this therapy.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section. None of the studies identified in our literature review reported data for responder rate.
Sleep quality:
One study171 reported sleep quality measured by diary. The results reported an effect size of 0.15 favoring stimulus control treatment compared with control (95% CI, 0.15 lower–0.45 higher; Table S44 (2.7MB, pdf) ). Results did not meet the clinical significance threshold.
One study166 reported sleep quality as measured by the PSQI. The results reported an effect size of 0.86 favoring stimulus control treatment compared with control (95% CI, 0.17–1.54 lower; Table S45 (2.7MB, pdf) ). These results met the clinical significance threshold. The quality of evidence for sleep quality was low because of imprecision and a risk of bias.
Data from 4 studies126,165,167,171 reporting diary-determined sleep quality were not included in the analysis because posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings. The studies reported postintervention differences in sleep quality.
Sleep latency:
Meta-analysis of 4 studies reporting sleep latency measured by diary166,168,170,171 comparing stimulus control treatment with control showed a posttreatment difference of 10.48 minutes lower (95% CI, 24.68 minutes lower–3.72 minutes higher) between treatment conditions. Results did not meet the clinical significance threshold (Figure S85 (2.7MB, pdf) ). The quality of evidence for sleep latency was low because of imprecision and risk of bias.
Data from 3 studies126,165,169 reporting diary-determined sleep latency were not included in the analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings. All 3 studies126,165,169 reported improvements in sleep latency with stimulus control compared with control. One of these studies169 also reported a greater improvement in sleep latency compared with the use of a sleep education comparator. However, another study165 found no difference between stimulus control and an imagery relief placebo for improving sleep latency.
WASO:
Meta-analysis of 3 studies166,170,171 reported that WASO measured by diary comparing stimulus control with control showed a posttreatment difference of 18.98 minutes lower (95% CI, 41.78 minutes lower–3.83 minutes higher) between treatment conditions. Results did not meet the clinical significance threshold (Figure S86 (2.7MB, pdf) ). One study166 also reported WASO measured by actigraphy, and results did not meet the clinical significance threshold for posttreatment comparisons (Table S46 (2.7MB, pdf) ). The quality of evidence for WASO was low because of imprecision and a risk of bias.
Data could not be included for 1 study126 reporting WASO measured by diary and actigraphy because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Remission rate:
One study171 reporting remission rate measured by ISI showed a clinically significant 19% (95% CI, 6–31% higher) higher remission rate for stimulus control compared with control (Table S47 (2.7MB, pdf) ). The quality of evidence for remission rate was moderate because of imprecision.
Two studies126,171 reported data on remission rate favoring stimulus control; however, a posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for beliefs and attitude about sleep, daytime fatigue, nights with hypnotic use, and total wake time.
Insomnia severity:
One study171 reporting insomnia severity on the ISI showed a clinically significant result with an effect size of 0.57 (95% CI, 0.26–0.88 lower) favoring stimulus control compared with control (Table S48 (2.7MB, pdf) ).
Data from 2 studies126,171 reporting insomnia severity measured by the ISI were not included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Number of nighttime awakenings:
Two studies170,171 assessed number of nighttime awakenings and reported a clinically significant difference between treatment and control of 0.69 fewer awakenings (95% CI, 1.72 lower–0.34 higher) and 0.70 fewer awakenings (95% CI, 0.13–1.27 lower), respectively, favoring stimulus control (Table S49 (2.7MB, pdf) ). The quality of evidence for number of nighttime awakenings was low because of imprecision and risk of bias.
Sleep efficiency:
Two studies166,171 measured sleep efficiency using a diary, of which only 1166 showed a clinically significant 13.33% higher (95% CI, 6.08–20.58% higher) sleep efficiency for stimulus control group compared with control. The other study171 did not meet the clinical significance threshold (Table S50 (2.7MB, pdf) ).
One study166 reported sleep efficiency measured by actigraphy, and results did not meet the clinical significance threshold (Table S51 (2.7MB, pdf) ). The quality of evidence for sleep efficiency was low because of imprecision and a risk of bias.
Data from 1 study126 could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Total sleep time:
Meta-analysis of 3 studies166,170,171 examined the efficacy of stimulus control on total sleep time measured by diary, results did not meet the clinical significance threshold (Figure S87 (2.7MB, pdf) ). One study166 also measured total sleep time by actigraphy; results did not meet the clinical significance threshold (Table S52 (2.7MB, pdf) ). The quality of evidence for total sleep time was low because of imprecision and risk of bias.
Data from 2 studies126,165 reporting total sleep time could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Overall quality of evidence
The overall quality of evidence for the use of stimulus control in patients with chronic insomnia was rated as low for the critical outcomes because of imprecision and a risk of bias (Table S53 (2.7MB, pdf) ).
Benefits vs harms
The benefit of stimulus control is that it may produce clinically significant improvements in some of the critical and important sleep outcomes vs control conditions. Although the data were limited by small sample sizes and few studies, at least 1 study126 suggested that stimulus control produced a higher insomnia remission rate than control, although note that only 44 participants were randomized to stimulus control in this study. In regard to harms, studies did not report adverse events. Potential risks of this treatment include nighttime falls in specific populations such as older adults, those using sleep medications, or patients with disabilities. Based on their clinical experience, the members of the TF determined that the undesirable effects are minimal and that the balance of benefits vs harms strongly favors the use of stimulus control.
Resource use
Formal cost-effectiveness studies have not been conducted with stimulus control. However, the literature review and TF expertise suggest that the resource use and costs of stimulus control relative to other behavioral and psychological therapies vary but seem to be in line with other single-component therapies.
Patient values and preferences
Based on their clinical experience, the members of the TF determined that most patients would use stimulus control because of the sleep improvements it produces relative to minimal harms because it requires few resources.
Sleep restriction therapy
Our review of the literature identified 4 RCTs63,171–173 that examined the effect of sleep restriction therapy vs control treatment on adult patients with chronic insomnia. Two additional RCTs126,174 were identified that could not be pooled with other studies but were included as supporting evidence. The control treatments included wait list control and minimal intervention (ie, sleep hygiene). One of these studies174 enrolled a general adult sample of insomnia patients, whereas 3 of these studies126,172,173 included samples of older adults with insomnia. The remaining study63 exclusively enrolled women with menopause-associated insomnia. Four studies126,172–174 included participants with insomnia without comorbid conditions. There were insufficient data to evaluate the efficacy of this treatment among patients with psychiatric or medical comorbidities or to determine the relative efficacy of different delivery methods for this therapy.
The related figures and tables are Figures S88–S92 (2.7MB, pdf) and Tables S54–S63 (2.7MB, pdf) . A summary of findings is provided in Table S64 (2.7MB, pdf) . A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) is provided beginning below.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section. None of the studies identified in our literature review reported data for responder rate.
Sleep quality:
Meta-analysis of 3 studies63,171,173 reporting sleep quality measured by diary did not show clinically significant posttreatment differences, with an effect size of 0.49 (95% CI, 0.07–0.92 higher), favoring sleep restriction therapy over control (Figure S88 (2.7MB, pdf) ). The quality of evidence for sleep quality was low because of imprecision and a risk of bias.
Data from 3 studies126,171,174 measuring sleep quality by diary could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Sleep latency:
Meta-analysis of 4 studies63,171–173 reporting sleep latency measured by diary comparing sleep restriction therapy with control treatment showed a posttreatment difference of 6.42 minutes lower (95% CI, 2.87–9.96 minutes lower). Results did not meet the clinical significance threshold (Figure S89 (2.7MB, pdf) ). Similarly, 1 study172 reported sleep latency measured by PSG. Results did not meet the clinical significance threshold when sleep restriction therapy was compared with control treatment (Table S54 (2.7MB, pdf) ). The quality of evidence for sleep latency was moderate because of a risk of bias.
Data from 1 study126 measuring sleep latency by diary could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
WASO:
Four studies63,171–173 included in a meta-analysis reported that WASO measured by diary comparing sleep restriction therapy with control showed a posttreatment difference of 11.67 minutes lower (95% CI, 6.47–16.86 minutes lower). Results did not meet the clinical significance threshold (Figure S90 (2.7MB, pdf) ). Similarly, 1 study172 reported that WASO measured by both actigraphy and PSG did not meet the clinical significance threshold when sleep restriction therapy was compared with control conditions (Table S55 (2.7MB, pdf) and Table S56 (2.7MB, pdf) ). The quality of evidence for WASO was moderate because of a risk of bias.
Data from 1 study126 measuring WASO by diary and 1 study126 measuring WASO by actigraphy could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Remission rate:
Two studies63,171 reporting remission rate measured by diary showed clinically significant 24% (95% CI, 5–43% higher) and 13% (95% CI, 0–26% higher) remission rates, respectively, for sleep restriction therapy compared with control (Table S57 (2.7MB, pdf) ). The quality of evidence for remission rate was low because of imprecision and a risk of bias.
Data from 2 studies126,171 measuring remission rate were not included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for beliefs and attitude about sleep, daytime fatigue, nights with hypnotic use, and total wake time.
Insomnia severity:
Two studies63,171 reported a clinically significant lower posttreatment difference in insomnia severity measured by the ISI, with an effect size of 1.28 (95% CI, 0.85–1.71 lower) and 0.65 (95% CI, 0.33–0.97 lower), respectively, favoring sleep restriction therapy over control (Table S58 (2.7MB, pdf) ). The quality of evidence for insomnia severity was low because of imprecision and a risk of bias.
Data from 1 study126 reporting insomnia severity measured by the ISI could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Number of nighttime awakenings:
Data from 1 study171 reported a clinically significant reduction of 0.60 fewer awakenings (95% CI, 0.01–1.19 lower), favoring sleep restriction therapy over control (Table S59 (2.7MB, pdf) ). The quality of evidence for number of nighttime awakenings was low because of imprecision and a risk of bias.
Sleep efficiency:
Meta-analysis of 4 studies63,171–173 reporting sleep efficiency measured by diary and 1 study172 reporting sleep efficiency measured by actigraphy and PSG did not meet the clinical significance threshold when sleep restriction therapy was compared with control (Figure S91, Table S60 (2.7MB, pdf) , and Table S61 (2.7MB, pdf) ). The quality of evidence for sleep efficiency was low because of imprecision and a risk of bias.
Data from 1 study126 reporting sleep efficiency measured by diary and actigraphy were not included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Total sleep time:
Meta-analysis of 4 studies63,171–173 comparing diary-determined total sleep time reported a clinically significant posttreatment difference favoring control with a 21.34-minutes-lower (95% CI, 9.56–33.13 minutes lower) total sleep time in the sleep restriction treatment group (Figure S92 (2.7MB, pdf) ). One study172 reporting total sleep time measured by actigraphy and PSG also reported clinically significant group differences favoring control with the sleep restriction group showing 40.26 minutes lower (95% CI, 4.36–76.16 minutes lower) and 43.91 minutes lower (95% CI, 90.52 minutes lower–2.70 minutes higher) total sleep time in these comparisons (Table S62 (2.7MB, pdf) and Table S63 (2.7MB, pdf) ). The quality of evidence for total sleep time was low because of imprecision and a risk of bias.
Data from 1 study126 reporting total sleep time measured by diary and actigraphy could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Overall quality of evidence
The overall quality of evidence for the use of sleep restriction therapy in patients with chronic insomnia was rated as low for the critical outcomes because of imprecision and a risk of bias (Table S64 (2.7MB, pdf) ).
Benefits vs harms
The benefits of sleep restriction therapy are that of producing clinically significant improvements in several of the critical and important sleep outcomes compared with control conditions. Regarding harms, 1 study146 found that sleep restriction therapy produced an increase in daytime sleepiness and cognitive impairment as compared with a control condition. This finding was likely because of the reduction in total sleep time in the early weeks of treatment if a patient adhered to the recommendation to reduce time in bed. Such daytime effects could translate into an increased risk for drowsy driving and impairment at work as a result of this treatment. In the experience of TF members, these effects are usually transient and dissipate as treatment progresses and the time-in-bed restriction is eased as sleep improves. Hence the potential harms may occur in the early phases of treatment but decline as treatment progresses. At posttreatment, actigraphy- and PSG-measured total sleep time were shorter for sleep restriction vs control; however, these results are sometimes expected as part of sleep restriction therapy, and long-term follow-up data and TF experience suggest that there is a subsequent increase in total sleep time from the end of acute treatment to long-term follow-up timepoints. Based on their clinical experience, the members of the TF determined that the undesirable effects are minimal and that the balance of benefits vs harms strongly favors the use of sleep restriction therapy.
Resource use
Formal cost-effectiveness studies have not been conducted with sleep restriction therapy. However, the literature review and TF expertise suggest that the resource use and costs of sleep restriction therapy relative to other psychological and behavioral therapies varies but falls in line with other single-component therapies. The use of sleep restriction therapy may result in moderate cost and resource use savings compared to multicomponent therapies such as CBT-I, but such savings would be negligible compared with those stemming from the use of other single-component therapies.
Patient values and preferences
Based on their clinical experience, the TF determined that most patients would use sleep restriction therapy as a treatment for insomnia. However, because restricting time in bed may lead to an average reduction in total sleep time and an increase in daytime sleepiness and reduction in alertness, many patients find it challenging to adhere to initial sleep restriction therapy schedules and are not inclined to choose this treatment.151 Those who tolerate the initial increase in daytime sleepiness and reduced alertness and who can increase their time in bed and gradually increase their sleep time over the treatment period are likely to find this treatment acceptable and be willing to engage in it.
Relaxation therapy
Our review of the literature identified 12 RCTs67,91,165,167,168,170,175–180 that examined the efficacy of relaxation therapy vs control (wait list, quasi-desensitization, or behavioral placebo) on adult patients with chronic insomnia. Of these studies, 767,165,167,176–179 utilized an in-person one-on-one delivery format of the relaxation therapy, 3168,170,180 utilized group therapy, and 2 utilized91,175 audio delivery.
The related figures and tables are Figures S93–S97 (2.7MB, pdf) and Tables S65–S72. (2.7MB, pdf) A summary of findings is provided in Table S73 (2.7MB, pdf) . A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below. There were insufficient data to evaluate the efficacy of this treatment among prespecified patient subgroups or to determine the relative efficacy of differing delivery methods.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section. None of the studies identified in our literature review reported data for remission rate.
Sleep quality:
Two studies175,177 reported sleep quality measured by diary, of which 1177 showed a clinically significant posttreatment difference, with an effect size of 0.99 (95% CI, 0.43–1.54 higher), favoring relaxation therapy compared with control (Table S65 (2.7MB, pdf) ). One study91 reporting sleep quality measured by the PSQI also reported clinically significant results, with an effect size of 0.96 (95% CI, 0.15–1.76 lower), favoring relaxation therapy compared with control (Table S66 (2.7MB, pdf) ). The quality of evidence for sleep quality ranged from low to very low because of imprecision, inconsistency, and a risk of bias.
Four studies165,167,176,178 reported sleep quality measured by diary that could not be added in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Sleep latency:
Meta-analysis of 6 studies91,168,170,175,177,179 reporting diary-determined sleep latency compared with control showed a posttreatment difference of 7.21 minutes (95% CI, 0.60–13.83 minutes lower). These results did not meet the clinical significance threshold (Figure S93 (2.7MB, pdf) ). The quality of evidence for sleep latency was low because of imprecision and a risk of bias.
Three studies165,178,180 also examined the effects of relaxation therapy compared with control treatment on sleep latency but could not be added in the meta-analysis because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
WASO:
Meta-analysis of 4 studies91,170,175,177 reporting diary-determined WASO showed a posttreatment difference of 15.67 minutes (95% CI, 39.15 minutes lower–7.81 minutes higher) between relaxation therapy and control. These results did not meet the clinical significance threshold for posttreatment comparisons (Figure S94 (2.7MB, pdf) ). One study91 reporting WASO assessed by actigraphy showed a clinically significant posttreatment difference of 25.00 minutes (95% CI, 62.89 minutes lower–12.89 minutes higher) favoring relaxation therapy over control treatment (Table S67 (2.7MB, pdf) ). The quality of evidence for WASO was low because of imprecision and a risk of bias.
One study67 reporting WASO assessed by diary and PSG reported data that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Responder rate:
Meta-analysis of 3 studies67,91,165 reported clinically significant 16% higher responder rates (95% CI, 11% lower–43% higher) favoring relaxation therapy compared with control treatment (Figure S95 (2.7MB, pdf) ). The quality of evidence for responder rate was very low because of imprecision, inconsistency, and a risk of bias.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for daytime fatigue and total wake time.
Beliefs and attitudes about sleep:
Two studies91,177 reported DBAS scale results in older adults and college students, respectively. Of these, only 1 study met the clinical significance threshold in DBAS scores, with an effect size of 1.01 (95% CI, 0.20–1.82 lower) favoring relaxation therapy over control therapy (Table S68 (2.7MB, pdf) ).91 The quality of evidence for beliefs and attitudes about sleep was low because of imprecision and a risk of bias.
Insomnia severity:
One study67 examined insomnia severity measured by the Insomnia Severity Questionnaire in response to relaxation therapy vs control. The data were not included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Nights with hypnotic use:
One study91 examined the effect of relaxation therapy vs control on hypnotic medication use; results did not meet the clinical significance threshold (Table S69 (2.7MB, pdf) ). The quality of evidence for nights with hypnotic use was low because of imprecision and a risk of bias.
Number of nighttime awakenings:
One study170 examined the effect of relaxation therapy vs control on number of nighttime awakenings. Results did not meet the clinical significance threshold (Table S70 (2.7MB, pdf) ). The quality of evidence for number of nighttime awakenings was low because of imprecision and a risk of bias.
Sleep efficiency:
Meta-analysis of 3 studies91,175,177 of diary-determined sleep efficiency (Figure S96 (2.7MB, pdf) ) and 1 study91 reporting actigraphy-determined sleep efficiency did not meet the clinical significance threshold between relaxation therapy and control conditions (Table S71 (2.7MB, pdf) ). The quality of evidence for sleep efficiency was low because of imprecision and a risk of bias.
One study67 reporting sleep efficiency measured by diary reported data that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Total sleep time:
Meta-analysis of 3 studies91,170,175 did not meet the clinical significance threshold for total sleep time at posttreatment for relaxation therapy compared with control (Figure S97 (2.7MB, pdf) ). One study91 reporting total sleep time assessed by actigraphy reported a clinically significant result favoring control, with the relaxation condition showing a 27.50-minutes-lower (95% CI. 95.27 minutes lower–40.27 minutes higher) total sleep time at posttreatment (Table S72 (2.7MB, pdf) ). The quality of evidence for total sleep time was low because of imprecision and a risk of bias.
Two studies67,165 reporting total sleep time measured by diary and one study67 assessing total sleep time measured by PSG reported data that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Overall quality of evidence
The overall quality of evidence for the use of relaxation therapy in patients with chronic insomnia was rated as very low for the critical outcomes because of imprecision and a risk of bias (Table S73 (2.7MB, pdf) ).
Benefits vs harms
The TF concluded that for adult patients with insomnia disorder, the modest benefits of relaxation therapy compared to no therapy likely outweigh the potential minimal harms and burdens. This conclusion was based on expert consensus. There were no data available on harm, but the potential harms were considered minimal. Furthermore, the potential benefits of relaxation therapy that go beyond sleep improvement, such as pain reduction or stress management, were also considered and deemed to add further evidence that the benefits outweigh the potential minimal harms or burden.
Resource use
There were no studies on cost-effectiveness identified. However, relaxation therapy can be delivered at relatively low cost and with few resources, particularly given that many therapists and clinical providers have training in relaxation therapies.
Patient values and preferences
A study of 18 combat veterans with traumatic brain injury and their care providers126 found that relaxation therapy was the most acceptable form of behavioral intervention, perhaps underscoring the need to recognize that treatment preference may vary according to specific patient characteristics. Based on their clinical experience, the members of the TF determined that the undesirable effects are trivial and that the balance of benefits vs harms strongly favors the use of relaxation therapy.
Sleep hygiene
Our review of the literature identified 3 RCTs65,181,182 that examined the effect of sleep hygiene therapy vs control therapy on adult patients with chronic insomnia. Delivery of sleep hygiene varied widely and included general education by a therapist in person supplemented with audiocassette and pamphlet educational materials,65 individual therapist weekly educational sessions,181 and 6 sessions of therapist-provided sleep hygiene advice with or without supportive therapy.182 One study included patients with insomnia and comorbid medical conditions,65 and another included older adults.181
The related tables are Tables S74–S83. (2.7MB, pdf) A summary of findings is provided in Table S84 (2.7MB, pdf) . A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below. There were insufficient data to evaluate the efficacy of this treatment among varying patient types or the relative efficacy of differing delivery methods.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section. None of the studies identified in our literature review reported data for remission rate.
Sleep quality:
One study that compared sleep hygiene with control for diary-determined sleep quality182 reported data that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Sleep latency:
One study65 reported sleep latency measured by diary with a mean difference of 0.80 minutes lower (95% CI, 12.98 minutes lower–11.38 minutes higher) in the sleep hygiene group; these results did not meet the clinical significance threshold (Table S74 (2.7MB, pdf) ). The quality of evidence for sleep latency was low because of imprecision and a risk of bias.
Two studies181,182 reported data on diary-determined sleep latency that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
WASO:
One study65 comparing sleep hygiene with wait list control measuring WASO by diary showed a 15.20-minutes-lower sleep WASO in the sleep hygiene group (95% CI, 39.65 minutes lower–9.25 minutes higher). Results did not meet the clinical significance threshold (Table S75 (2.7MB, pdf) ). The same study measured WASO by actigraphy and also did not meet the clinical significance threshold (Table S76 (2.7MB, pdf) ). The quality of evidence for WASO was low because of imprecision and a risk of bias.
One study182 reported data that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Responder rate:
One study65 comparing sleep hygiene with control reported clinically significant 17% higher responder rates (95% CI, 9% lower to 43% higher) favoring sleep hygiene over control (Table S77 (2.7MB, pdf) ). The quality of evidence for responder rate was low because of imprecision and risk of bias.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for beliefs and attitude about sleep, daytime fatigue, insomnia severity, and nights with hypnotic use.
Number of nighttime awakenings:
Two studies181,182 comparing sleep hygiene with control reported number of nighttime awakenings measured by diary, and 1 study182 reported such comparisons measured by actigraphy. Both studies181,182 provided data that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed for these studies.
Sleep efficiency:
One study65 compared sleep hygiene with control measured by diary and actigraphy, but posttreatment comparisons did not meet the clinical significance threshold (Table S78 (2.7MB, pdf) and Table S79 (2.7MB, pdf) ). The quality of evidence for sleep efficiency was low because of imprecision and a risk of bias.
One study182 reported data that could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Total wake time:
One study65 comparing sleep hygiene with control reported total wake time measured by diary and actigraphy and did not show clinically significant results (Table S80 (2.7MB, pdf) and Table S81 (2.7MB, pdf) ). The quality of evidence for total wake time was low because of imprecision and a risk of bias.
Total sleep time:
One study65 comparing sleep hygiene with control reported total sleep time measured by diary and actigraphy and did not show clinically significant differences between treatment conditions (Table S82 (2.7MB, pdf) and Table S83 (2.7MB, pdf) ). The quality of evidence for total sleep time was low because of imprecision and a risk of bias.
Two studies181,182 reported data that could not be included because the posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Overall quality of evidence
The overall quality of evidence for the use of sleep hygiene in patients with chronic insomnia was rated as low for the critical outcomes because of imprecision and a risk of bias (Table S84 (2.7MB, pdf) ).
Benefits vs harms
Based on the meta-analyses and other available evidence, the potential benefits of sleep hygiene were considered by the TF to be minimal compared with control. In the 1 study65 that showed improvement in the sleep hygiene group, patients receiving this treatment made additional behavioral changes such as standardizing their sleep schedules without being told to do so. The same study also showed CBT-I to be superior to sleep hygiene treatment alone. In a second study171 comparing active treatments, sleep hygiene alone was less effective than sleep restriction therapy, stimulus control, or a combined treatment including all 3 of these therapies. The potential harms of utilizing a sleep hygiene intervention as a stand-alone therapy for insomnia disorder may include delayed implementation of effective therapies with continued or worsening insomnia symptoms. Patients with chronic insomnia could potentially elect not to undergo other treatments based on their experience using an ineffective intervention. As such, the TF did not favor the use of sleep hygiene as a stand-alone therapy for chronic insomnia.
Resource use
Cost analyses of sleep hygiene have not been systematically conducted. A systematic review of 6 electronic databases found no data regarding the cost-effectiveness of sleep hygiene interventions.183 Sleep hygiene education is generally considered inexpensive; however, the included studies involved fairly high-intensity sleep hygiene interventions, sometimes delivered by trained clinicians, making the use of resources similar to that of other single-component treatments. The TF judged that any resources utilized for an ineffective intervention may be considered excessive.
Patient values and preferences
Although previous studies have reported that patients prefer sleep hygiene to other elements of CBT-I,151 our analysis shows that it does not produce clinically significant improvements in insomnia symptoms when used as a single-component therapy. Therefore, based on their clinical experience, the members of the TF determined that the majority of informed adults with chronic insomnia would not choose sleep hygiene as stand-alone therapy given its lack of efficacy.
Biofeedback
Our review of the literature identified 4 RCTs reporting on the use of biofeedback as a treatment for chronic insomnia in adults.167,179,184,185 Only 1 of these studies179 provided sufficient data to calculate the mean difference at posttreatment.
The related table is Table S85. (2.7MB, pdf) A summary of findings is provided in Table S86. (2.7MB, pdf) A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below. There were insufficient data to evaluate the efficacy of this treatment among varying patient types or the relative efficacy of differing delivery methods.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section. None of the studies identified in our literature review reported data for sleep quality, responder rate, or remission rate.
Sleep latency:
One study179 reporting diary-determined sleep latency showed a clinically significant posttreatment difference of 52.58 minutes (95% CI, 22.42–82.74 minutes lower), favoring biofeedback over control treatment (Table S85 (2.7MB, pdf) ). The quality of evidence for sleep latency was low because of imprecision and a risk of bias.
Three studies167,184,185 reporting sleep latency measured by diary were not suitable for meta-analysis because of posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
WASO:
One study185 reporting WASO, measured by diary, could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, and total wake time.
Number of nighttime awakenings:
One study184 reporting number of nighttime awakenings measured by diary could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Sleep efficiency:
One study184reporting sleep efficiency measured by diary could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Total sleep time:
Two studies reporting total sleep time184,185 measured by diary could not be included because the posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Overall quality of evidence
The overall quality of evidence for the use of biofeedback in patients with chronic insomnia was rated very low for the critical outcomes because of imprecision and a risk of bias (Table S86 (2.7MB, pdf) ).
Benefits vs harms
There were no harms evaluated or reported in the included studies. Based on their clinical experience, the members of the TF determined that the balance between desirable and undesirable effects likely favors the use of biofeedback.
Resource use
Biofeedback treatment requires expensive psychophysiological monitoring equipment and advanced training, so the costs of prescribing/providing this service would be considered moderate to high and may render this option less appealing or accessible than the other therapies. There were no studies that evaluated the cost-effectiveness of this intervention.
Patient values and preferences
Biofeedback does not require behavior change, which may make it more appealing to some patients. However, the expensive nature of biofeedback and the need to use monitoring equipment may factor negatively in the decision to use it. Because biofeedback is not widely available, and the TF could not identify any published evaluations of patient preferences, the TF determined that patients’ values and preferences for this intervention were uncertain.
Paradoxical intention
Our review of the literature identified 5 RCTs168,169,186–188 that examined the effect of paradoxical intention vs control for adult patients with chronic insomnia. Determination of clinical significance was possible for only 2 of these studies. All the studies were conducted in the adult population. Three studies168,187,188 excluded patients with medical comorbidities, and 1168 also excluded patients with psychiatric comorbidities. One study delivered paradoxical intention over the course of 2 sessions,188 3 studies168,169,186 delivered treatment over 4 sessions, and the final study187 delivered therapy over the course of 8 sessions. Four studies169,186–188conducted their last follow-up at postintervention, and the remaining study168 conducted the last assessment at 12 weeks posttreatment.
The related tables are Table S87 (2.7MB, pdf) and Table S88. (2.7MB, pdf) A summary of findings is provided in Table S89. (2.7MB, pdf) A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below. There were insufficient data to evaluate the efficacy of this treatment among varying patient types or the relative efficacy of differing delivery methods.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section. None of the studies identified in our literature review reported data on WASO, remission rate, or responder rate.
Sleep quality:
Two studies187,188reporting diary-determined sleep quality could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Sleep latency:
Two studies168,186compared paradoxical intention to placebo control using diary-measured sleep latency among adult patients with insomnia disorder. Results of 1 study186 showed a clinically significant 28.25-minutes-lower sleep latency for paradoxical intention (95% CI, 8.37–48.13 minutes) compared with control (Table S87 (2.7MB, pdf) ). The other study168 reported a posttreatment difference of 1.38 minutes lower (95% CI, 19.50 minutes lower–16.74 minutes higher), between paradoxical intention and control that did not meet the clinical significance threshold (Table S87 (2.7MB, pdf) ). The quality of evidence for sleep latency was very low because of imprecision, inconsistency, and risk of bias.
Two studies169,187 reporting diary-determined sleep latency could not be included because the posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention: beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, sleep efficiency, total wake time, and total sleep time.
Number of nighttime awakenings:
One study186 compared paradoxical intention to placebo control for reducing the number of nighttime awakenings reported in sleep diaries. Results of that study showed a clinically significant difference of 0.75 fewer awakenings (95% CI, 0.15–1.35 fewer awakenings) for patients receiving paradoxical intention treatment compared with control treatment (Table S88 (2.7MB, pdf) ). The quality of evidence for number of nighttime awakenings was low because of imprecision and a risk of bias.
Total sleep time:
One study187 reporting diary-determined total sleep time could not be included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Overall quality of evidence
The overall quality of evidence for the use of paradoxical intention in patients with chronic insomnia was rated very low for the critical outcomes because of imprecision, inconsistency, and risk of bias (Table S89 (2.7MB, pdf) ).
Benefits vs harms
The potential benefits of paradoxical intention could include a modest reduction in sleep latency. The results suggest that this treatment did produce a clinically significant reduction in the number of awakenings during the night, an outcome designated as important but not critical by the TF. The harms of this treatment, for the most part, remain unstudied. However, 1 report showed that paradoxical intention combined with feedback about a patient’s accuracy in self-reported sleep latency estimates can lead to an increase rather than a decrease in self-reported sleep latency.168 Given the nature of paradoxical intention, wherein patients are given the instruction to try to stay awake in bed, this intervention could increase anxiety about sleep in some patients.
Resource use
Formal cost-effectiveness studies have not been conducted with paradoxical intention. However, the literature review and TF expertise suggest that there would be negligible costs or savings inherent in this treatment compared with other behavioral and psychological insomnia therapies.
Patient values and preferences
This approach may raise anxiety levels in some patients and may make sleep more difficult. Because our meta-analyses did not show benefits of this treatment vs control treatment for reducing sleep latency, it may not be viewed as a desirable treatment choice for patients with insomnia. Based on their clinical experience, the members of the TF determined that some patients would choose paradoxical intention but that it may be less appealing to patients who already have difficulty falling asleep.
Intensive Sleep Retraining (ISR)
One study166 reported on ISR using an in-person one-on-one delivery method compared with sleep hygiene. Participants consisted of patients with insomnia and no comorbidities.
The related tables are Tables S90–S96. (2.7MB, pdf) A summary of findings is provided in Table S97 (2.7MB, pdf) . A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below. There were insufficient data to evaluate the efficacy of reducing sleep latency using this treatment among varying patient types or the relative efficacy of differing delivery methods.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section. None of the studies identified in our literature review reported data for remission rate or responder rate.
Sleep quality:
One study166 reporting on PSQI-determined sleep quality compared ISR to sleep hygiene control therapy and reported an effect size of 0.76 points (95% CI, 0.07–1.45 points lower), and it thus met the clinical significance threshold favoring ISR (Table S90 (2.7MB, pdf) ). The quality of evidence for sleep quality was low because of imprecision and a risk of bias.
Sleep latency:
The same study166 also reported on diary-determined sleep latency and noted a clinically significant posttreatment difference of 30.24 minutes (95% CI, 11.51–48.97 minutes lower) favoring ISR when compared with control (Table S91 (2.7MB, pdf) ). The quality of evidence for sleep latency was low because of imprecision and a risk of bias.
WASO:
One study166 comparing ISR to control measured WASO by diary; results showed a posttreatment difference of 19.60 minutes (95% CI, 58.35 minutes lower–19.15 minutes higher; Table S92 (2.7MB, pdf) ) favoring ISR, which did not meet the clinical significance threshold. The quality of evidence for WASO was low because of imprecision and risk of bias.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention; beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for beliefs and attitude about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, and total wake time.
Sleep efficiency:
One study166 reported a clinically significant posttreatment difference of 11.61% (95% CI, 3.77%–19.45%) for diary-determined sleep efficiency when compared with control (Table S93 (2.7MB, pdf) ). The same study166 also reported sleep efficiency measured by actigraphy, and the results did not meet the clinical significance threshold (Table S94 (2.7MB, pdf) ). The quality of evidence for sleep efficiency was low because of imprecision and a risk of bias.
Total sleep time:
One study166 reported clinically significant posttreatment differences for total sleep time measured by diary of 52.97 minutes higher (95% CI, 8.32–97.62 minutes higher) and differences measured by actigraphy of 23.78 minutes higher (95% CI, 21.70 minutes lower–69.26 minutes higher), favoring the ISR intervention (Table S95 (2.7MB, pdf) and Table S96 (2.7MB, pdf) ). The quality of evidence for total sleep time was low because of imprecision and a risk of bias.
Overall quality of evidence
The overall quality of evidence for the use of ISR in patients with chronic insomnia was rated low for the critical outcomes because of imprecision and a risk of bias (Table S97 (2.7MB, pdf) ).
Benefits vs harms
The potential benefits of ISR were based on 1 study166 that revealed clinically significant improvement in critical outcomes, including self-reported sleep latency, sleep quality, and response rate. These desirable effects were judged by the TF to be moderate. ISR was delivered in a brief 25-hour sleep deprivation period, so treatment resulted in rapid sleep improvements.166 These potential benefits need to be considered in relation to possible harms, which may include cognitive impairment, fatigue, and increased sleepiness resulting from the procedure. The TF assessed these undesirable effects as minor. Based on their clinical experience, the members of the TF determined that the undesirable effects are minimal and that the balance of benefits vs harms favors the use of ISR.
Resource use
Formal cost-effectiveness studies have not been conducted with ISR.This intervention, as conducted by Harris, Lack, Kemp, and colleagues,166 would require a PSG laboratory with intensive monitoring by a trained technologist throughout the treatment and would be conducted during periods that may not be typically staffed by laboratory personnel. The resource use and costs related to ISR would likely surpass other forms of chronic insomnia treatments. Future research investigating the utilization of a self-administered version of ISR at home could potentially result in substantial resource reductions for ISR treatment.
Patient values and preferences
Based on their clinical experience, the members of the TF determined that some adults with chronic insomnia would choose ISR therapy. The short-term nature of this intervention could be appealing to some adults with chronic insomnia. However, some patients may not want to engage in ISR treatment because of its demanding procedure.
Mindfulness
Our review of the literature identified 3 RCTs189–191 that examined the effect of mindfulness vs control on adult patients with chronic insomnia. All 3 studies utilized group therapy. One study191focused on older adults.
The related tables are Tables S98–S111. (2.7MB, pdf) A summary of findings is provided in Table S112. (2.7MB, pdf) A summary of the evidence, the results of the statistical analysis, and whether the results met the clinical significance thresholds for each outcome (Table 3) are provided beginning below. There were insufficient data to evaluate the efficacy of this treatment among varying patient types or the relative efficacy of differing delivery methods.
Critical outcomes
The following outcomes were determined by the TF to be critical for decision-making when recommending the use of this intervention: sleep quality, sleep latency, WASO, remission rate, and responder rate; of these outcomes, remission rate and responder rate were considered the most important. The TF also determined that only diary-reported outcomes were considered critical, but data reported using other tools (PSQI, actigraphy, PSG) are also reported in this section.
Sleep quality:
Only 1 study191 reported sleep quality measured by the PSQI with a clinically significant effect size of 1.04 (95% CI, 0.50–1.50 points lower) at posttreatment, favoring mindfulness over control (Table S98 (2.7MB, pdf) ). The quality of evidence for sleep quality was low because of a low sample size and a risk of bias.
Sleep latency:
One study190 reporting sleep latency measured by sleep diary showed a posttreatment difference of 3.80 minutes lower (95% CI, 15.52 minutes lower–7.92 minutes higher) between treatment and control, results that were not clinically significant (Table S99 (2.7MB, pdf) ). The quality of evidence for sleep latency was low because of imprecision and a risk of bias.
WASO:
Similarly, 1 study190 reporting WASO measured by sleep diary showed a posttreatment difference of 10.00 minutes lower (95% CI, 26.35 minutes lower–6.35 minutes higher) between treatment and control, which did not meet the clinical significance threshold established by the TF (Table S100 (2.7MB, pdf) ). The quality of evidence for WASO was low because of imprecision and risk of bias.
Remission rate:
One study reported remission rate based on the ISI.189 Remission rate differences were clinically significant, with the mindfulness group having a 36% higher rate (95% CI, 11%–61% higher) than the placebo group (Table S101 (2.7MB, pdf) ). The quality of evidence for remission rate was low because of a small sample size and risk of bias.
Data from 1 study190 reporting remission rate measured by the ISI was not included because the posttreatment mean difference could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings.
Responder rate:
Two studies189,190 reporting responder rate measured by the ISI were not included because the posttreatment mean differences could not be calculated. Therefore, the TF could not evaluate the clinical significance of the findings and the quality of evidence was not assessed.
Important outcomes
The following outcomes were determined by the TF to be important outcomes but not critical for decision-making when recommending the use of this intervention; beliefs and attitudes about sleep, daytime fatigue, insomnia severity, nights with hypnotic use, number of nighttime awakenings, sleep efficiency, total wake time, and total sleep time. None of the studies identified in our literature review reported data for beliefs and attitudes on sleep, daytime fatigue, nights with hypnotic use, and number of nighttime awakenings.
Insomnia severity:
Two studies189,190 reported insomnia severity measured by the ISI. Only 1 study189 met the clinical significance threshold, with an effect size of 1.01 (95% CI, 0.30–1.72 lower), favoring the mindfulness treatment (Table S102 (2.7MB, pdf) ). The quality of evidence for insomnia severity was low because of imprecision and a risk of bias.
Sleep efficiency:
Two studies189,190 reported sleep efficiency as measured by sleep diary. The results did not meet the clinical significance threshold (Table S103 (2.7MB, pdf) ). One study189 also reported PSG and actigraphy data, and with both methods, results did not meet the clinical significance threshold (Table S104 (2.7MB, pdf) and Table S105 (2.7MB, pdf) ). The quality of evidence for sleep efficiency was low because of a low sample size, imprecision, and a risk of bias.
Total wake time:
One study189 reported total wake time reported by sleep diary, actigraphy, and PSG. None of the measures met the clinical significance thresholds established by the TF (Tables S106–S108 (2.7MB, pdf) ). The quality of evidence for total wake time was low because of imprecision and a risk of bias.
Total sleep time:
Two studies reported total sleep time measured by diary and actigraphy.189,190 Neither reported clinically significant results (Table S109 (2.7MB, pdf) and Table S110 (2.7MB, pdf) ). The same study189 also reported PSG data that showed a clinically significant posttreatment difference favoring control, with the mindfulness treatment having 22.82 minutes lower (95% CI, 53.40 minutes lower–7.76 minutes higher) total sleep time at posttreatment (Table S111 (2.7MB, pdf) ). The quality of evidence for total sleep time was very low because of a low sample size, considerable imprecision, and risk of bias.
Overall quality of evidence
The overall quality of evidence for the use of mindfulness in patients with chronic insomnia was rated low for the critical outcomes because of imprecision and risk of bias (Table S112 (2.7MB, pdf) ).
Benefits vs harms
The TF determined that undesirable effects were no different from any that occurred with control treatment. On balance, the benefits of mindfulness likely favor treatment over control.
Resource use
Resource use does not favor mindfulness or control, but consideration should be given to the considerable time investment needed to become an accomplished mindfulness provider, the greater length of treatment sessions and home practice compared with typical CBT-I, and the absence of data indicating that mindfulness is amenable to cost-efficient delivery formats such as online or application-based delivery.
Patient values and preferences
Based on their clinical experience, the members of the TF determined that the majority of patients with chronic insomnia would favor mindfulness given the benefits. However, the time investment required for mindfulness therapy may reduce patient adherence to this treatment. The limited data and the 1 study favoring control suggest that patient’s preferences for this treatment are unclear.189
DISCUSSION AND FUTURE DIRECTIONS
Chronic insomnia disorder is a common sleep disorder among adults. It is known to cause and exacerbate physical and psychological morbidity for patients and is associated with significant financial costs at the societal level. There have been decades of research studying behavioral and psychological treatments for insomnia disorder in adults, and several recent international guidelines have recommended CBT-I as first-line therapy for patients with insomnia.34,36,38 This evaluation of behavioral and psychological treatments for insomnia in adults is a comprehensive summary of the evidence to date and is intended to provide clinicians and researchers with a resource to guide their treatment of insomnia and to guide future research. Note that the conclusions drawn by this review are limited to the published data emerging from research on this subject and are inherently limited by issues with the study design of trials reported within those publications. Limitations observed across the body of available literature are outlined below, noting that individual studies may have had other limitations as well.
Limitations
Several issues were noted that spanned multiple studies reviewed by the TF, across treatment modalities, that limited the TF’s ability to draw definitive conclusions about subgroups of patients, various methods of treatment delivery, or the relative strengths and weaknesses of different behavioral and psychological treatments.
Variability in the control conditions: Numerous different control conditions were used across studies, including wait list or no treatment, minimal interventions, and sham interventions. Sleep hygiene was used as the control condition in a number of trials, especially in studies testing CBT-I; however, there was limited discussion of the actual content of the sleep hygiene condition and how it was delivered to participants, making it difficult to understand the potential potency of the different control conditions used. These circumstances limited the ability of the TF to interpret variability across studies in terms of the benefits of some treatments for some outcomes.
Variability of the intervention content and intervention delivery method across studies: Although studies generally described the components of treatment, there was not a sufficient number of studies to compare outcomes based on variations in content. For example, studies of CBT-I varied; for example, some included relaxation therapy as a component of the treatment package and some did not. In addition, the cognitive therapy strategies used across studies varied. Likewise, multiple biofeedback methods were used across studies, but there was not a sufficient number of studies to evaluate each specific biofeedback method relative to control. The TF therefore could not make specific recommendations about intervention content. Variability in delivery method was considered by the TF, and it generally seemed that behavioral and psychological treatments are effective across delivery methods; however, there was not a sufficient number of comparative effectiveness trials to make statements about the relative benefits across delivery methods. The TF considered conducting a network meta-analysis to compare the various delivery methods, but because of the heterogeneity of samples included in the various studies considered, the TF decided against conducting such an analysis.
Small number of studies evaluating single-component therapies, with key missing data in some published trials: Many of the single-component therapy studies were conducted more than 10 years ago, and there was not always sufficient information about the study methods or outcomes of interest. For example, in the scant literature on paradoxical intention, most studies only reported changes in sleep onset latency; however, the TF was interested in other critical outcomes. In addition, there were few studies of relaxation therapy to conduct meta-analyses for most outcomes, limiting the TF’s ability to evaluate some potential benefits.
Small sample sizes in studies of some single-component therapies (stimulus control, sleep restriction therapy, biofeedback, paradoxical intention, and relaxation therapy): The quality of evidence for a number of studies was downgraded because of small sample size. As noted in number 3 above, this primarily was a concern for studies that were conducted more than 10 years ago.
Dropout rates not considered: The analyses conducted did not consider treatment dropout rates and whether these rates differed between treatment and control conditions.
Lack of data concerning adverse effects of treatments: In general, adverse events/effects from the treatments were not assessed or reported in the majority of studies included in this systematic review. Thus, such effects remain in question.
Some of the limitations of the available literature can be explained by when certain treatment approaches were developed and when the treatment was of interest to researchers. For example, the first ISR trial192 was published in 2007, the first BTI trial was published in 2011,154 and the first mindfulness trial was published in 2014.189 Because of the relatively recent development of these treatments, there are few studies on their efficacy and much of the research has been conducted by a small number of research groups. Thus, even with promising data, more studies conducted by different centers/researchers are needed to ensure replicability and generalizability. In contrast to these emerging treatments, some treatments (eg, biofeedback, relaxation therapy) emerged decades ago and thus reflect clinical conventions of those times, such as a focus on sleep-onset insomnia and conceptualization of most insomnia as a symptom of another disorder; therefore, they do not reflect current diagnostic or assessment standards. Thus, the data informing the efficacy of treatment modalities would benefit from evaluation in the context of current diagnostic criteria and current measurement, reporting, and statistical approaches. To date, there are no formal evaluations of cost-effectiveness to compare different behavioral and psychological treatments for insomnia in adults. Some of the modalities vary greatly in the resources they require.
Generalizability of findings
Across the treatment modalities, study samples tended to be relatively homogeneous demographically, and the delivery of most interventions was conducted in-person, with 1 trained sleep interventionist treating 1 participant at a time. We know less about the efficacy of the behavioral and psychological treatments for insomnia among key patient subgroups, such as ethnic/racial minorities, those living in rural areas, and older adults. Because of the variation across studies, such as different comparator arms and different patient populations, we were not able to compare effect sizes across delivery modalities (eg, in-person vs use of technology/Internet). We also did not have sufficient data to compare outcomes across various settings (eg, in-clinic vs the community) or across different types of interventionists (eg, CBT-I specialists vs nonsleep specialists). Recently, there has been a focus on insomnia “phenotypes,” such as insomnia with and without short objective sleep duration. These different types of insomnia have not been systematically evaluated in intervention trials.
Future research directions
Additional research would inform the field of behavioral and psychological treatments for insomnia in adults in several key areas:
Across behavioral and psychological treatments, there is a need for noninferiority and other comparative effectiveness studies evaluating patient outcomes. Implementation studies that examine different delivery methods and settings and different types of clinical providers with a range of backgrounds/professional experiences are needed. More studies that evaluate the utility of objective sleep monitoring, including PSG, actigraphy, and consumer sleep technologies, are needed. Although objective monitoring is not required for the diagnosis of insomnia disorder, technological advances and the increasing number of consumer-facing devices create a need for systematic research in this area to identify novel phenotypes.
Future trials of CBT-I should more consistently incorporate assessment of daytime symptoms and daytime functional impairments associated with insomnia along with quality of life and other important sleep-related outcomes (eg, hypnotic use).
Studies to better understand the risks of behavioral and psychological interventions, including daytime sleepiness and other potential adverse effects (eg, cognitive effects and gait/balance issues) typically associated with pharmacotherapy for insomnia, which has been reported in observation studies, are needed. Methods to mitigate the potential risks associated with treatment also need to be systematically evaluated, such as using alternatives to sleep restriction therapy or using other methods to attenuate sleepiness.
Studies of the relative efficacy of treatments in patient subgroups, including those with early morning awakenings, different insomnia phenotypes, racial/ethnic minority groups, patients with low health literacy or cognitive impairment, patients who require assistance with activities of daily living, and patients living in institutional settings (eg, nursing homes), along with the impact among different cultural groups, are needed.
Studies are also needed to improve our understanding of the moderators and mediators of treatment response and methods to target CBT-I components based on patient presentation and insomnia characteristics.
BTIs represent a potential method to increase access to care, and studies that directly compare BTIs to CBT-I, particularly among patients with complex comorbid conditions, are needed.
Although there is evidence of the long-term sustained benefits of CBT-I, similar data are not widely available for single-component treatments. There is limited research evaluating the long-term benefits of single-component treatments. Further, there is limited research examining any follow-up treatments after the delivery of a single-component therapy. Sleep hygiene is one of the oldest treatment approaches for insomnia in adults; however, recent evidence shows that it is no longer supported as a single-component therapy. Given that sleep hygiene is commonly delivered as single-component therapy in current practice, often without systematic follow-up, studies to develop and evaluate dissemination strategies for educating patients and providers about more effective approaches are needed.
ISR may represent an alternative to longer-term treatments and could be appealing to some patients (eg, those who require quick treatment and/or cannot tolerate the temporary increase in sleepiness that can occur during CBT-I). More research is needed to determine optimal patient selection for ISR compared with other insomnia therapies (eg, CBT-I) and to balance cost/resource utilization for this approach. Future studies should also test whether alternative forms of ISR implementation (eg, utilizing self-monitoring devices at home) or variations of the therapy are efficacious.
Mindfulness-based approaches represent a recent addition to the insomnia treatment literature. Future studies should incorporate standard measures used to evaluate insomnia treatments, such as sleep diaries, actigraphy, and/or PSG. In addition, studies should explore whether briefer mindfulness-based approaches preserve therapeutic benefits and whether mindfulness-based concepts can be incorporated with other approaches (eg, sleep restriction therapy and stimulus control) to enhance treatment benefits.
Cognitive therapy approaches (without behavioral treatment) could not be evaluated because of insufficient evidence; however, studies of the potential benefits of cognitive therapy alone may be informative. Understanding which patient groups are most likely to benefit from cognitive approaches is also worthy of future consideration.
Incorporating patient-centered approaches and engaging key stakeholders in the design of intervention trials to determine patient uptake and preferences for available treatments would also be useful.
To date, no specific guidelines have addressed the superiority of 1 psychological or behavioral treatment over another based on direct comparisons, and this remains a limitation of the current guidelines as well because there are few comparative effectiveness studies upon which to base such recommendations.
The TF noted that most randomized clinical trials do not include assessments of adverse effects associated with these psychological/behavioral therapies, so adequate data on the direct harms associated with them are lacking.
Other considerations
When considering the various behavioral and psychological treatments evaluated herein, it is important to consider a number of factors that may represent barriers or facilitators to their ongoing use in clinical venues. One of those is patient acceptance of these therapies. The limited available evidence does suggest that patients’ acceptance of behavioral and psychological therapies is greater than their acceptance of pharmacological therapies150,193,194; however, not all patients will be interested in these approaches. Among the available psychological treatments themselves, it seems that patients may initially believe sleep restriction therapy to be undesirable; however, those who improve with this treatment rate it positively.151,195 There remains a problem with the accessibility/scalability of these treatments. In fact, data would suggest that patients may have limited access to CBT-I, which has a strong evidence base of support. This limited access may result from patients’ lack of knowledge of this treatment, providers’ perceptions that such treatment is not acceptable or accessible for their patients, and issues related to the stigma of using mental health treatments overall.196,197 Because the behavioral and psychological treatments for insomnia have proven to be cost-effective relative to care as usual (ie, treatment in primary care mainly with medications) in terms of improving quality of life and presenteeism at worksites,198 improving communication between patients and providers about CBT-I and other behavioral and psychological treatments is an important priority. Although effective Internet-based interventions designed to disseminate CBT-I more broadly to patients who may not have access to a trained provider are available, it does not seem that these interventions have achieved their broadest use at this juncture, and it is not yet clear which patients can benefit from these self-directed approaches and which patients require the support of a skilled provider.
When considering the findings of this systematic review, a number of limitations should be noted. The TF accepted a larger number of studies in the systematic review than were eventually included in our meta-analyses, primarily because of how data were reported (ie, investigators not reporting means and standard deviations, or reporting “adjusted” means and standard deviations or standard errors at posttreatment time points). It also should be noted that the GRADE process we used to evaluate available evidence required the TF to establish a threshold for “clinical significance,” representing a meaningful difference between active treatment and control conditions for the critical and important outcomes. These thresholds were established by consensus of the TF based on their expertise and experience because there are no commonly accepted or empirically based thresholds of this nature in the literature. In consensus, the TF defined thresholds that were considered reasonable, given what is known about insomnia treatment at this time and their clinical expertise. We recognize that these thresholds may evolve with information from future research on patient-centered outcomes of insomnia treatment.
Summary
In summary, there is a large evidence base to support the use of behavioral and psychological treatments, particularly CBT-I, for patients with insomnia disorder. Although there are challenges to the delivery of these interventions and a need for additional research to understand the optimal delivery modalities and benefits achieved by patient subgroups, clinicians should provide a recommended treatment to patients with chronic insomnia disorder, and programs to train providers in the delivery of these approaches should be continued and expanded.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The development of this article was funded by the American Academy of Sleep Medicine. The following include all the conflicts managed throughout guideline development. Dr. Edinger received funding from Merck for an insomnia research study, and his research program was loaned portable polysomnography recorders from Philips/Respironics to support a National Institute of Mental Health–funded research grant. Dr. Arnedt was loaned U.S. Food & Drug Administration–cleared device treatment for insomnia by Ebb (2018–-present), served as a consultant to Magna Seating Systems Engineering until 2016 to develop a report on drowsy driving, served on a contract project with the National Highway Safety Transportation Board on drowsy driving (2018–present), and was a coinvestigator for a study funded by the National Basketball Association and contracted with the University of Michigan to evaluate the validity of commercially available devices to measure sleep (ended 2019). Dr. Bertisch was a coinvestigator on an insomnia research project funded by Merck (ended 2017), serves as an unpaid member of the board of directors of the Alliance of Sleep Apnea Partners, a non -profit patient centered group. Dr. Lichstein is a member of the insomnia advisory board for Merck, the topic of which is unrelated to the behavioral treatments of insomnia. Dr. Sateia served as a consultant for Physicians Seal for melatonin in 2018. Dr. Troxel was a coinvestigator on a study about the prevalence and risk factors for nocturia using data from the British Healthy Workers (ended March 2020), served as a consultant for Guidepoint as a subject matter expert on sleep to industry clients (January 2018–present), has received research funding from the National Institutes of Health, was principal investigator on a grant funded by Feelmore Labs to review a clinical trial design and protocol for an investigational neurostimulation device (ended June 2020), and serves on the scientific advisory board for Feelmore Labs. Mr. Heald was employed by the American Academy of Sleep Medicine during his work on this article, but his employment by the American Academy of Sleep Medicine ended in July 2020. Dr. Martin serves on the American Academy of Sleep Medicine board of directors. Ms. Kazmi is employed by the American Academy of Sleep Medicine. The remaining authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The TF thanks and acknowledges the early contributions of Charles M. Morin, PhD, and Vanessa Gonzalez, MPH. Dr. Morin served as a member of the TF during the initial stages of the systematic review, which served as the basis for this clinical practice guideline, and Ms. Gonzalez was the staff for the initial phase of the project. The TF also thanks Daniel J. Buysse, MD; Rachel Manber, PhD; and Colin E. Espie, PhD, DSc, FRSM, FBPsS for serving as external reviewers of the document and providing valuable feedback.
ABBREVIATIONS
- AASM =
American Academy of Sleep Medicine
- BTI =
brief therapy for insomnia
- CBT =
cognitive-behavioral therapy
- CBT-I =
cognitive-behavioral therapy for insomnia
- CI =
confidence interval
- DBAS =
Dysfunctional Beliefs and Attitudes About Sleep
- GRADE =
Grading of Recommendations Assessment, Development, and Evaluation
- ISI =
Insomnia Severity Index
- ISR =
intensive sleep retraining
- PICO =
Patient, Intervention, Comparison, and Outcomes
- PSG =
polysomnography
- PSQI =
Pittsburgh Sleep Quality Index
- RCT =
randomized clinical trial
- TF =
task force
- WASO =
wake after sleep onset
REFERENCES
- 1.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255–262. 10.5664/jcsm.8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. 10.5664/jcsm.27286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 6.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. 10.1093/sleep/31.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. 10.1016/j.sleep.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 9.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–453. 10.1001/archinternmed.2008.610 [DOI] [PubMed] [Google Scholar]
- 10.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34(8):997–1011. 10.5665/SLEEP.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deguchi Y, Iwasaki S, Ishimoto H, et al. Relationships between temperaments, occupational stress, and insomnia among Japanese workers. PLoS One. 2017;12(4):e0175346. 10.1371/journal.pone.0175346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16(1):375. 10.1186/s12888-016-1075-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olfson M, Wall M, Liu SM, Morin CM, Blanco C. Insomnia and impaired quality of life in the United States. J Clin Psychiatry. 2018;79(5):17m12020. 10.4088/JCP.17m12020 [DOI] [PubMed] [Google Scholar]
- 14.Vargas I, Perlis ML, Grandner M, et al. Insomnia symptoms and suicide-related ideation in U.S. Army service members. Behav Sleep Med. 2020;18(6):820–836. [DOI] [PubMed] [Google Scholar]
- 15.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273. 10.1093/sleep/30.3.263 [DOI] [PubMed] [Google Scholar]
- 16.Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(Suppl 2):S386–S393. [PubMed] [Google Scholar]
- 17.Wickwire EM, Tom SE, Scharf SM, Vadlamani A, Bulatao IG, Albrecht JS. Untreated insomnia increases all-cause health care utilization and costs among Medicare beneficiaries. Sleep. 2019;42(4):zsz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34(9):1161–1171. 10.5665/SLEEP.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- 20.Lane JM, Jones SE, Dashti HS, et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51(3):387–393. 10.1038/s41588-019-0361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soehner AM, Kaplan KA, Harvey AG. Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. J Affect Disord. 2014;167:93–97. 10.1016/j.jad.2014.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis J, Hampson SE, Cropley M. The role of dysfunctional beliefs and attitudes in late-life insomnia. J Psychosom Res. 2007;62(1):81–84. 10.1016/j.jpsychores.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 23.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. 10.1176/appi.ajp.161.11.2126 [DOI] [PubMed] [Google Scholar]
- 24.Levenson JC, Kay DB, Buysse DJ. The pathophysiology of insomnia. Chest. 2015;147(4):1179–1192. 10.1378/chest.14-1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. 10.1093/sleep/29.9.1155 [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 27.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 28.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14(7):1209–1230. 10.5664/jcsm.7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedel BW, Lichstein KL. Insomnia and daytime functioning. Sleep Med Rev. 2000;4(3):277–298. 10.1053/smrv.1999.0074 [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16). Sleep. 2007;30(11):1547–1554. 10.1093/sleep/30.11.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2018;14(7):1231–1237. 10.5664/jcsm.7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 35.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- 36.Mansfield D, Grima NA, Bei B. Insomnia management. Aust J Gen Pract. 2019;48(4):198–202. [PubMed] [Google Scholar]
- 37.National Institutes of Health. NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1–30. [PubMed] [Google Scholar]
- 38.Wilson S, Anderson K, Baldwin D, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J Psychopharmacol. 2019;33(8):923–947. 10.1177/0269881119855343 [DOI] [PubMed] [Google Scholar]
- 39.Ree M, Junge M, Cunnington D. Australasian Sleep Association position statement regarding the use of psychological/behavioral treatments in the management of insomnia in adults. Sleep Med. 2017;36(Suppl 1):S43–S47. 10.1016/j.sleep.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 40.Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom. 2012;81(4):206–216. 10.1159/000335379 [DOI] [PubMed] [Google Scholar]
- 41.Cheong MJ, Lee GE, Kang HW, et al. Clinical effects of mindfulness meditation and cognitive behavioral therapy standardized for insomnia: a protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(51):e13499. 10.1097/MD.0000000000013499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrand P, Woodford J. Impact of support on the effectiveness of written cognitive behavioural self-help: a systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2013;33(1):182–195. 10.1016/j.cpr.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 43.Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54–67. 10.1016/j.smrv.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 44.Ho FY, Chung KF, Yeung WF, et al. Self-help cognitive-behavioral therapy for insomnia: a meta-analysis of randomized controlled trials. Sleep Med Rev. 2015;19:17–28. 10.1016/j.smrv.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 45.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. 10.1016/j.smrv.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 46.Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6–16. 10.1016/j.smrv.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–1180. 10.1176/ajp.151.8.1172 [DOI] [PubMed] [Google Scholar]
- 48.Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. 10.1371/journal.pone.0149139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3–16. 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 50.Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. 10.1001/jamainternmed.2015.3006 [DOI] [PubMed] [Google Scholar]
- 51.Ye YY, Chen NK, Chen J, et al. Internet-based cognitive-behavioural therapy for insomnia (ICBT-i): a meta-analysis of randomised controlled trials. BMJ Open. 2016;6(11):e010707. 10.1136/bmjopen-2015-010707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. 10.1016/j.smrv.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 53.Chesson AL Jr, Anderson WM, Littner M, et al. Practice parameters for the nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 1999;22(8):1128–1133. 10.1093/sleep/22.8.1128 [DOI] [PubMed] [Google Scholar]
- 54.Morgenthaler T, Kramer M, Alessi C, et al.American Academy of Sleep Medicine . Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415–1419. 10.1093/sleep/29.11.1415 [DOI] [PubMed] [Google Scholar]
- 55.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 56.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 58.Morgenthaler TI, Deriy L, Heald JL, Thomas SM. The evolution of the AASM clinical practice guidelines: another step forward. J Clin Sleep Med. 2016;12(1):129–135. 10.5664/jcsm.5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alessi C, Martin JL, Fiorentino L, et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc. 2016;64(9):1830–1838. 10.1111/jgs.14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnedt JT, Cuddihy L, Swanson LM, Pickett S, Aikens J, Chervin RD. Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia. Sleep. 2013;36(3):353–362. 10.5665/sleep.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99(9):1121–1132. 10.1111/j.1360-0443.2004.00835.x [DOI] [PubMed] [Google Scholar]
- 62.Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61(6):664–675. 10.1111/j.1365-2648.2007.04560.x [DOI] [PubMed] [Google Scholar]
- 63.Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32(4):499–510. 10.1093/sleep/32.4.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med. 2005;165(21):2527–2535. 10.1001/archinte.165.21.2527 [DOI] [PubMed] [Google Scholar]
- 66.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep. 2007;30(2):203–212. 10.1093/sleep/30.2.203 [DOI] [PubMed] [Google Scholar]
- 67.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–1864. 10.1001/jama.285.14.1856 [DOI] [PubMed] [Google Scholar]
- 68.Ellis JG, Cushing T, Germain A. Treating acute insomnia: a randomized controlled trial of a “single-shot” of cognitive behavioral therapy for insomnia. Sleep. 2015;38(6):971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum. 2007;34(5):E51–E59. 10.1188/07.ONF.E51-E59 [DOI] [PubMed] [Google Scholar]
- 70.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. 10.1200/JCO.2007.13.9006 [DOI] [PubMed] [Google Scholar]
- 71.Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769–781. 10.5665/sleep.1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30(5):574–584. 10.1093/sleep/30.5.574 [DOI] [PubMed] [Google Scholar]
- 73.Harvey AG, Soehner AM, Kaplan KA, et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: a pilot randomized controlled trial. J Consult Clin Psychol. 2015;83(3):564–577. 10.1037/a0038655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho FY, Chung KF, Yeung WF, Ng TH, Cheng SK. Weekly brief phone support in self-help cognitive behavioral therapy for insomnia disorder: relevance to adherence and efficacy. Behav Res Ther. 2014;63:147–156. 10.1016/j.brat.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 75.Horsch CH, Lancee J, Griffioen-Both F, et al. Mobile phone-delivered cognitive behavioral therapy for insomnia: a Randomized waitlist controlled trial. J Med Internet Res. 2017;19(4):e70. 10.2196/jmir.6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou Y, Hu P, Liang Y, Mo Z. Effects of cognitive behavioral therapy on insomnia of maintenance hemodialysis patients. Cell Biochem Biophys. 2014;69(3):531–537. 10.1007/s12013-014-9828-4 [DOI] [PubMed] [Google Scholar]
- 77.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37(9):1543–1552. 10.5665/sleep.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164(17):1888–1896. 10.1001/archinte.164.17.1888 [DOI] [PubMed] [Google Scholar]
- 79.Jansson-Fröjmark M, Linton SJ, Flink IK, Granberg S, Danermark B, Norell-Clarke A. Cognitive-behavioral therapy for insomnia co-morbid with hearing impairment: a randomized controlled trial. J Clin Psychol Med Settings. 2012;19(2):224–234. 10.1007/s10880-011-9275-y [DOI] [PubMed] [Google Scholar]
- 80.Jernelöv S, Lekander M, Blom K, et al. Efficacy of a behavioral self-help treatment with or without therapist guidance for co-morbid and primary insomnia—a randomized controlled trial. BMC Psychiatry. 2012;12(1):5. 10.1186/1471-244X-12-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jungquist CR, O’Brien C, Matteson-Rusby S, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010;11(3):302–309. 10.1016/j.sleep.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lancee J, Eisma MC, van Straten A, Kamphuis JH. Sleep-related safety behaviors and dysfunctional beliefs mediate the efficacy of online CBT for insomnia: a randomized controlled trial. Cogn Behav Ther. 2015;44(5):406–422. 10.1080/16506073.2015.1026386 [DOI] [PubMed] [Google Scholar]
- 83.Lancee J, van den Bout J, van Straten A, Spoormaker VI. Internet-delivered or mailed self-help treatment for insomnia? A randomized waiting-list controlled trial. Behav Res Ther. 2012;50(1):22–29. 10.1016/j.brat.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 84.Lancee J, van Straten A, Morina N, Kaldo V, Kamphuis JH. Guided online or face-to-face cognitive behavioral treatment for insomnia: a randomized wait-list controlled trial. Sleep. 2016;39(1):183–191. 10.5665/sleep.5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lovato N, Lack L, Wright H, Kennaway DJ. Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. Sleep. 2014;37(1):117–126. 10.5665/sleep.3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morin CM, Beaulieu-Bonneau S, LeBlanc M, Savard J. Self-help treatment for insomnia: a randomized controlled trial. Sleep. 2005;28(10):1319–1327. 10.1093/sleep/28.10.1319 [DOI] [PubMed] [Google Scholar]
- 87.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–999. 10.1001/jama.281.11.991 [DOI] [PubMed] [Google Scholar]
- 88.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61(1):137–146. 10.1037/0022-006X.61.1.137 [DOI] [PubMed] [Google Scholar]
- 89.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21(7):695–705. 10.1002/pon.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an Internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. 2009;66(7):692–698. 10.1001/archgenpsychiatry.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol Aging. 2002;17(2):288–298. 10.1037/0882-7974.17.2.288 [DOI] [PubMed] [Google Scholar]
- 92.Rybarczyk B, Lopez M, Schelble K, Stepanski E. Home-based video CBT for comorbid geriatric insomnia: a pilot study using secondary data analyses. Behav Sleep Med. 2005;3(3):158–175. 10.1207/s15402010bsm0303_4 [DOI] [PubMed] [Google Scholar]
- 93.Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73(6):1164–1174. 10.1037/0022-006X.73.6.1164 [DOI] [PubMed] [Google Scholar]
- 94.Sandlund C, Hetta J, Nilsson GH, Ekstedt M, Westman J. Improving insomnia in primary care patients: a randomized controlled trial of nurse-led group treatment. Int J Nurs Stud. 2017;72:30–41. 10.1016/j.ijnurstu.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 95.Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37(8):1305–1314. 10.5665/sleep.3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–6096. 10.1200/JCO.2005.09.548 [DOI] [PubMed] [Google Scholar]
- 97.Smith MT, Finan PH, Buenaver LF, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. 2015;67(5):1221–1233. 10.1002/art.39048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ström L, Pettersson R, Andersson G. Internet-based treatment for insomnia: a controlled evaluation. J Consult Clin Psychol. 2004;72(1):113–120. 10.1037/0022-006X.72.1.113 [DOI] [PubMed] [Google Scholar]
- 99.Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. 10.5665/sleep.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taylor DJ, Peterson AL, Pruiksma KE, Young-McCaughan S, Nicholson K, Mintz J; STRONG STAR Consortium . Internet and in-person cognitive behavioral therapy for insomnia in military personnel: a randomized clinical trial. Sleep. 2017;40(6):1–12. [DOI] [PubMed] [Google Scholar]
- 101.Taylor DJ, Zimmerman MR, Gardner CE, et al. A pilot randomized controlled trial of the effects of cognitive-behavioral therapy for insomnia on sleep and daytime functioning in college students. Behav Ther. 2014;45(3):376–389. 10.1016/j.beth.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 102.Taylor HL, Rybarczyk BD, Nay W, Leszczyszyn D. Effectiveness of a CBT intervention for persistent insomnia and hypnotic dependency in an outpatient psychiatry clinic. J Clin Psychol. 2015;71(7):666–683. 10.1002/jclp.22186 [DOI] [PubMed] [Google Scholar]
- 103.van Straten A, Cuijpers P, Smit F, Spermon M, Verbeek I. Self-help treatment for insomnia through television and book: a randomized trial. Patient Educ Couns. 2009;74(1):29–34. 10.1016/j.pec.2008.07.050 [DOI] [PubMed] [Google Scholar]
- 104.van Straten A, Emmelkamp J, de Wit J, et al. Guided Internet-delivered cognitive behavioural treatment for insomnia: a randomized trial. Psychol Med. 2014;44(7):1521–1532. 10.1017/S0033291713002249 [DOI] [PubMed] [Google Scholar]
- 105.Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32(6):807–815. 10.1093/sleep/32.6.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagley JN, Rybarczyk B, Nay WT, Danish S, Lund HG. Effectiveness of abbreviated CBT for insomnia in psychiatric outpatients: sleep and depression outcomes. J Clin Psychol. 2013;69(10):1043–1055. 10.1002/jclp.21927 [DOI] [PubMed] [Google Scholar]
- 107.Wu R, Bao J, Zhang C, Deng J, Long C. Comparison of sleep condition and sleep-related psychological activity after cognitive-behavior and pharmacological therapy for chronic insomnia. Psychother Psychosom. 2006;75(4):220–228. 10.1159/000092892 [DOI] [PubMed] [Google Scholar]
- 108.Bjorvatn B, Berge T, Lehmann S, Pallesen S, Saxvig IW. No effect of a self-help book for insomnia in patients with obstructive sleep apnea and comorbid chronic insomnia—a randomized controlled trial. Front Psychol. 2018;9:2413. 10.3389/fpsyg.2018.02413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bjorvatn B, Fiske E, Pallesen S. A self-help book is better than sleep hygiene advice for insomnia: a randomized controlled comparative study. Scand J Psychol. 2011;52(6):580–585. 10.1111/j.1467-9450.2011.00902.x [DOI] [PubMed] [Google Scholar]
- 110.Blom K, Jernelöv S, Rück C, Lindefors N, Kaldo V. Three-year follow-up of insomnia and hypnotics after controlled Internet treatment for insomnia. Sleep. 2016;39(6):1267–1274. 10.5665/sleep.5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Espie CA, Emsley R, Kyle SD, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry. 2019;76(1):21–30. 10.1001/jamapsychiatry.2018.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaku A, Nishinoue N, Takano T, et al. Randomized controlled trial on the effects of a combined sleep hygiene education and behavioral approach program on sleep quality in workers with insomnia. Ind Health. 2012;50(1):52–59. 10.2486/indhealth.MS1318 [DOI] [PubMed] [Google Scholar]
- 113.Mao H, Wu J, Xu Y, Liu Y, Tang X. Effectiveness of sleep self-management group intervention in Chinese patients with insomnia disorder. Perspect Psychiatr Care. 2018;54(2):156–161. 10.1111/ppc.12215 [DOI] [PubMed] [Google Scholar]
- 114.Martínez MP, Miró E, Sánchez AI, et al. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. 2014;37(4):683–697. 10.1007/s10865-013-9520-y [DOI] [PubMed] [Google Scholar]
- 115.Miró E, Lupiáñez J, Martínez MP, et al. Cognitive-behavioral therapy for insomnia improves attentional function in fibromyalgia syndrome: a pilot, randomized controlled trial. J Health Psychol. 2011;16(5):770–782. 10.1177/1359105310390544 [DOI] [PubMed] [Google Scholar]
- 116.Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851–2858. 10.1001/jama.295.24.2851 [DOI] [PubMed] [Google Scholar]
- 117.Bernstein AM, Allexandre D, Bena J, et al. “Go! to Sleep”: A web-based therapy for insomnia. Telemed J E Health. 2017;23(7):590–599. 10.1089/tmj.2016.0208 [DOI] [PubMed] [Google Scholar]
- 118.Bothelius K, Kyhle K, Espie CA, Broman JE. Manual-guided cognitive-behavioural therapy for insomnia delivered by ordinary primary care personnel in general medical practice: a randomized controlled effectiveness trial. J Sleep Res. 2013;22(6):688–696. 10.1111/jsr.12067 [DOI] [PubMed] [Google Scholar]
- 119.Currie SR, Wilson KG, Pontefract AJ, deLaplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol. 2000;68(3):407–416. 10.1037/0022-006X.68.3.407 [DOI] [PubMed] [Google Scholar]
- 120.Pigeon WR, Moynihan J, Matteson-Rusby S, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: a pilot study. Behav Res Ther. 2012;50(11):685–689. 10.1016/j.brat.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thiart H, Lehr D, Ebert DD, Berking M, Riper H. Log in and breathe out: internet-based recovery training for sleepless employees with work-related strain— results of a randomized controlled trial. Scand J Work Environ Health. 2015;41(2):164–174. 10.5271/sjweh.3478 [DOI] [PubMed] [Google Scholar]
- 122.Thorndike FP, Ritterband LM, Gonder-Frederick LA, Lord HR, Ingersoll KS, Morin CM. A randomized controlled trial of an internet intervention for adults with insomnia: effects on comorbid psychological and fatigue symptoms. J Clin Psychol. 2013;69(10):1078–1093. 10.1002/jclp.22032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Batterham PJ, Christensen H, Mackinnon AJ, et al. Trajectories of change and long-term outcomes in a randomised controlled trial of internet-based insomnia treatment to prevent depression. BJPsych Open. 2018;3(5):228–235. 10.1192/bjpo.bp.117.005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cape J, Leibowitz J, Whittington C, Espie CA, Pilling S. Group cognitive behavioural treatment for insomnia in primary care: a randomized controlled trial. Psychol Med. 2016;46(5):1015–1025. 10.1017/S0033291715002561 [DOI] [PubMed] [Google Scholar]
- 125.Chen HY, Chiang CK, Wang HH, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2008;52(2):314–323. 10.1053/j.ajkd.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 126.Epstein DR, Sidani S, Bootzin RR, Belyea MJ. Dismantling multicomponent behavioral treatment for insomnia in older adults: a randomized controlled trial. Sleep. 2012;35(6):797–805. 10.5665/sleep.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39(1):45–60. 10.1016/S0005-7967(99)00157-6 [DOI] [PubMed] [Google Scholar]
- 128.Feuerstein S, Hodges SE, Keenaghan B, Bessette A, Forselius E, Morgan PT. Computerized cognitive behavioral therapy for insomnia in a community health setting. J Clin Sleep Med. 2017;13(2):267–274. 10.5664/jcsm.6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fleming L, Randell K, Harvey CJ, Espie CA. Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psychooncology. 2014;23(6):679–684. 10.1002/pon.3468 [DOI] [PubMed] [Google Scholar]
- 130.Freeman D, Sheaves B, Goodwin GM, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. 2017;4(10):749–758. 10.1016/S2215-0366(17)30328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Freeman D, Waite F, Startup H, et al. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): a prospective, assessor-blind, randomised controlled pilot trial. Lancet Psychiatry. 2015;2(11):975–983. 10.1016/S2215-0366(15)00314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guilleminault C, Palombini L, Poyares D, Chowdhuri S. Chronic insomnia, premenopausal women and sleep disordered breathing: part 2. Comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of chronic insomnia. J Psychosom Res. 2002;53(1):617–623. 10.1016/S0022-3999(02)00463-4 [DOI] [PubMed] [Google Scholar]
- 133.Jansson M, Linton SJ. Cognitive-behavioral group therapy as an early intervention for insomnia: a randomized controlled trial. J Occup Rehabil. 2005;15(2):177–190. 10.1007/s10926-005-1217-9 [DOI] [PubMed] [Google Scholar]
- 134.Matthews EE, Berger AM, Schmiege SJ, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncol Nurs Forum. 2014;41(3):241–253. 10.1188/14.ONF.41-03AP [DOI] [PubMed] [Google Scholar]
- 135.McCurry SM, Shortreed SM, Von Korff M, et al. Who benefits from CBT for insomnia in primary care? Important patient selection and trial design lessons from longitudinal results of the Lifestyles trial. Sleep. 2014;37(2):299–308. 10.5665/sleep.3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morgan K, Gregory P, Tomeny M, David BM, Gascoigne C. Self-help treatment for insomnia symptoms associated with chronic conditions in older adults: a randomized controlled trial. J Am Geriatr Soc. 2012;60(10):1803–1810. 10.1111/j.1532-5415.2012.04175.x [DOI] [PubMed] [Google Scholar]
- 137.Soeffing JP, Lichstein KL, Nau SD, et al. Psychological treatment of insomnia in hypnotic-dependent older adults. Sleep Med. 2008;9(2):165–171. 10.1016/j.sleep.2007.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vincent N, Walsh K, Lewycky S. Determinants of success for computerized cognitive behavior therapy: examination of an insomnia program. Behav Sleep Med. 2013;11(5):328–342. 10.1080/15402002.2012.700662 [DOI] [PubMed] [Google Scholar]
- 139.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947–956. 10.1111/jgs.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCrae CS, Williams J, Roditi D, et al. Cognitive behavioral treatments for insomnia and pain in adults with comorbid chronic insomnia and fibromyalgia: clinical outcomes from the SPIN randomized controlled trial. Sleep. 2019;42(3):zsy234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bastien CH, Morin CM, Ouellet MC, Blais FC, Bouchard S. Cognitive-behavioral therapy for insomnia: comparison of individual therapy, group therapy, and telephone consultations. J Consult Clin Psychol. 2004;72(4):653–659. 10.1037/0022-006X.72.4.653 [DOI] [PubMed] [Google Scholar]
- 142.Holmqvist M, Vincent N, Walsh K. Web- vs. telehealth-based delivery of cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep Med. 2014;15(2):187–195. 10.1016/j.sleep.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 143.Yamadera W, Sato M, Harada D, et al. Comparisons of short-term efficacy between individual and group cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2013;11(3):176–184. 10.1111/sbr.12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hagatun S, Vedaa Ø, Nordgreen T, et al. The short-term efficacy of an unguided Internet-based cognitive-behavioral therapy for insomnia: a randomized controlled trial with a six-month nonrandomized follow-up. Behav Sleep Med. 2019;17(2):137–155. 10.1080/15402002.2017.1301941 [DOI] [PubMed] [Google Scholar]
- 145.Verbeek IH, Konings GM, Aldenkamp AP, Declerck AC, Klip EC. Cognitive behavioral treatment in clinically referred chronic insomniacs: group versus individual treatment. Behav Sleep Med. 2006;4(3):135–151. 10.1207/s15402010bsm0403_1 [DOI] [PubMed] [Google Scholar]
- 146.Kyle SD, Miller CB, Rogers Z, Siriwardena AN, Macmahon KM, Espie CA. Sleep restriction therapy for insomnia is associated with reduced objective total sleep time, increased daytime somnolence, and objectively impaired vigilance: implications for the clinical management of insomnia disorder. Sleep. 2014;37(2):229–237. 10.5665/sleep.3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wickwire EM, Shaya FT, Scharf SM. Health economics of insomnia treatments: the return on investment for a good night’s sleep. Sleep Med Rev. 2016;30:72–82. 10.1016/j.smrv.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 148.De Bruin EJ, van Steensel FJ, Meijer AM. Cost-effectiveness of group and Internet cognitive behavioral therapy for insomnia in adolescents: results from a randomized controlled trial. Sleep. 2016;39(8):1571–1581. 10.5665/sleep.6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thiart H, Ebert DD, Lehr D, et al. Internet-based cognitive behavioral therapy for insomnia: a health economic evaluation. Sleep. 2016;39(10):1769–1778. 10.5665/sleep.6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morin CM, Gaulier B, Barry T, Kowatch RA. Patients’ acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15(4):302–305. 10.1093/sleep/15.4.302 [DOI] [PubMed] [Google Scholar]
- 151.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24(4):411–417. 10.1093/sleep/24.4.411 [DOI] [PubMed] [Google Scholar]
- 152.Sidani S, Miranda J, Epstein DR, Bootzin RR, Cousins J, Moritz P. Relationships between personal beliefs and treatment acceptability, and preferences for behavioral treatments. Behav Res Ther. 2009;47(10):823–829. 10.1016/j.brat.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sedov ID, Goodman SH, Tomfohr-Madsen LM. Insomnia treatment preferences during pregnancy. J Obstet Gynecol Neonatal Nurs. 2017;46(3):e95–e104. 10.1016/j.jogn.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 154.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. 10.1001/archinternmed.2010.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. 2006;2(4):403–406. 10.5664/jcsm.26654 [DOI] [PubMed] [Google Scholar]
- 156.Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89–96. 10.1016/j.jpsychores.2011.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Germain A, Richardson R, Stocker R, et al. Treatment for insomnia in combat-exposed OEF/OIF/OND military veterans: preliminary randomized controlled trial. Behav Res Ther. 2014;61:78–88. 10.1016/j.brat.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McCrae CS, Curtis AF, Williams JM, et al. Efficacy of brief behavioral treatment for insomnia in older adults: examination of sleep, mood, and cognitive outcomes. Sleep Med. 2018;51:153–166. 10.1016/j.sleep.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCrae CS, McGovern R, Lukefahr R, Stripling AM. Research evaluating brief behavioral sleep treatments for rural elderly (RESTORE): a preliminary examination of effectiveness. Am J Geriatr Psychiatry. 2007;15(11):979–982. 10.1097/JGP.0b013e31813547e6 [DOI] [PubMed] [Google Scholar]
- 160.Pigeon WR, Funderburk J, Bishop TM, Crean HF. Brief cognitive behavioral therapy for insomnia delivered to depressed veterans receiving primary care services: a pilot study. J Affect Disord. 2017;217:105–111. 10.1016/j.jad.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 161.Wang J, Wei Q, Wu X, Zhong Z, Li G. Brief behavioral treatment for patients with treatment-resistant insomnia. Neuropsychiatr Dis Treat. 2016;12:1967–1975. 10.2147/NDT.S110571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–182. 10.1093/sleep/26.2.177 [DOI] [PubMed] [Google Scholar]
- 163.Troxel WM, Conrad TS, Germain A, Buysse DJ. Predictors of treatment response to brief behavioral treatment of insomnia (BBTI) in older adults. J Clin Sleep Med. 2013;9(12):1281–1289. 10.5664/jcsm.3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tyagi S, Resnick NM, Perera S, Monk TH, Hall MH, Buysse DJ. Behavioral treatment of chronic insomnia in older adults: does nocturia matter? Sleep. 2014;37(4):681–687. 10.5665/sleep.3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Espie CA, Lindsay WR, Brooks DN, Hood EM, Turvey T. A controlled comparative investigation of psychological treatments for chronic sleep-onset insomnia. Behav Res Ther. 1989;27(1):79–88. 10.1016/0005-7967(89)90123-X [DOI] [PubMed] [Google Scholar]
- 166.Harris J, Lack L, Kemp K, Wright H, Bootzin R. A randomized controlled trial of intensive sleep retraining (ISR): a brief conditioning treatment for chronic insomnia. Sleep. 2012;35(1):49–60. 10.5665/sleep.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hughes R, Hughes H. Insomnia: effects of EMG biofeedback, relaxation training, and stimulus control. Behav Eng. 1978;5:67–72. https://psycnet.apa.org/record/1980-21649-001 [Google Scholar]
- 168.Lacks P, Bertelson AD, Gans L, Kunkel J. The effectiveness of three behavioral treatments for different degrees of sleep onset insomnia. Behav Ther. 1983;14(5):593–605. 10.1016/S0005-7894(83)80052-5 [DOI] [Google Scholar]
- 169.Ladouceur R, Gros-Louis Y. Paradoxical intention vs stimulus control in the treatment of severe insomnia. J Behav Ther Exp Psychiatry. 1986;17(4):267–269. 10.1016/0005-7916(86)90062-5 [DOI] [PubMed] [Google Scholar]
- 170.Morin CM, Azrin NH. Behavioral and cognitive treatments of geriatric insomnia. J Consult Clin Psychol. 1988;56(5):748–753. 10.1037/0022-006X.56.5.748 [DOI] [PubMed] [Google Scholar]
- 171.Sidani S, Epstein DR, Fox M, Collins L. Comparing the effects of single- and multiple-component therapies for insomnia on sleep outcomes. Worldviews Evid Based Nurs. 2019;16(3):195–203. 10.1111/wvn.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13(1):17–27. 10.1177/089198870001300103 [DOI] [PubMed] [Google Scholar]
- 173.Riedel BW, Lichstein KL, Dwyer WO. Sleep compression and sleep education for older insomniacs: self-help versus therapist guidance. Psychol Aging. 1995;10(1):54–63. 10.1037/0882-7974.10.1.54 [DOI] [PubMed] [Google Scholar]
- 174.Fernando A 3rd, Arroll B, Falloon K. A double-blind randomised controlled study of a brief intervention of bedtime restriction for adult patients with primary insomnia. J Prim Health Care. 2013;5(1):5–10. 10.1071/HC13005 [DOI] [PubMed] [Google Scholar]
- 175.Creti L, Libman E, Bailes S, Fichten CS. Effectiveness of cognitive-behavioral insomnia treatment in a community sample of older individuals: more questions than conclusions. J Clin Psychol Med Settings. 2005;12(2):153–164. 10.1007/s10880-005-3275-8 [DOI] [Google Scholar]
- 176.Greeff AP, Conradie WS. Use of progressive relaxation training for chronic alcoholics with insomnia. Psychol Rep. 1998;82(2):407–412. 10.2466/pr0.1998.82.2.407 [DOI] [PubMed] [Google Scholar]
- 177.Means MK, Lichstein KL, Epperson MT, Johnson CT. Relaxation therapy for insomnia: nighttime and day time effects. Behav Res Ther. 2000;38(7):665–678. 10.1016/S0005-7967(99)00091-1 [DOI] [PubMed] [Google Scholar]
- 178.Nicassio P, Bootzin R. A comparison of progressive relaxation and autogenic training as treatments for insomnia. J Abnorm Psychol. 1974;83(3):253–260. 10.1037/h0036729 [DOI] [PubMed] [Google Scholar]
- 179.Nicassio PM, Boylan MB, McCabe TG. Progressive relaxation, EMG biofeedback and biofeedback placebo in the treatment of sleep-onset insomnia. Br J Med Psychol. 1982;55(Pt 2):159–166. 10.1111/j.2044-8341.1982.tb01494.x [DOI] [PubMed] [Google Scholar]
- 180.Woolfolk RL, Carr-Kaffashan L, McNulty TF, Lehrer PM. Meditation training as a treatment for insomnia. Behav Ther. 1976;7(3):359–365. 10.1016/S0005-7894(76)80064-0 [DOI] [Google Scholar]
- 181.Engle-Friedman M, Bootzin RR, Hazlewood L, Tsao C. An evaluation of behavioral treatments for insomnia in the older adult. J Clin Psychol. 1992;48(1):77–90. [DOI] [PubMed] [Google Scholar]
- 182.Hauri PJ. Can we mix behavioral therapy with hypnotics when treating insomniacs? Sleep. 1997;20(12):1111–1118. 10.1093/sleep/20.12.1111 [DOI] [PubMed] [Google Scholar]
- 183.Chung KF, Lee CT, Yeung WF, Chan MS, Chung EW, Lin WL. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 2018;35(4):365–375. 10.1093/fampra/cmx122 [DOI] [PubMed] [Google Scholar]
- 184.Hauri P. Treating psychophysiologic insomnia with biofeedback. Arch Gen Psychiatry. 1981;38(7):752–758. 10.1001/archpsyc.1981.01780320032002 [DOI] [PubMed] [Google Scholar]
- 185.Sanavio E, Vidotto G, Bettinardi O, Rolletto T, Zorzi M. Behaviour therapy for DIMS: comparison of three treatment procedures with follow-up. Behav Psychother. 1990;18(3):151–167. 10.1017/S0141347300009654 [DOI] [Google Scholar]
- 186.Ascher LM, Turner R. Paradoxical intention and insomnia: an experimental investigation. Behav Res Ther. 1979;17(4):408–411. 10.1016/0005-7967(79)90015-9 [DOI] [PubMed] [Google Scholar]
- 187.Espie CA, Brooks DN, Lindsay WR. An evaluation of tailored psychological treatment of insomnia. J Behav Ther Exp Psychiatry. 1989;20(2):143–153. 10.1016/0005-7916(89)90047-5 [DOI] [PubMed] [Google Scholar]
- 188.Ott BD, Levine BA, Ascher LM. Manipulating the explicit demand of paradoxical intention instructions. Behav Psychother. 1983;11(1):25–35. 10.1017/S014134730000879X [DOI] [Google Scholar]
- 189.Ong JC, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt JK. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–1563. 10.5665/sleep.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wong SY, Zhang DX, Li CC, et al. Comparing the effects of mindfulness-based cognitive therapy and sleep psycho-education with exercise on chronic insomnia: a randomised controlled trial. Psychother Psychosom. 2017;86(4):241–253. 10.1159/000470847 [DOI] [PubMed] [Google Scholar]
- 191.Zhang JX, Liu XH, Xie XH, et al. Mindfulness-based stress reduction for chronic insomnia in adults older than 75 years: a randomized, controlled, single-blind clinical trial. Explore (NY). 2015;11(3):180–185. 10.1016/j.explore.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 192.Harris J, Lack L, Wright H, Gradisar M, Brooks A. Intensive sleep retraining treatment for chronic primary insomnia: a preliminary investigation. J Sleep Res. 2007;16(3):276–284. 10.1111/j.1365-2869.2007.00595.x [DOI] [PubMed] [Google Scholar]
- 193.Cheung JMY, Bartlett DJ, Armour CL, Saini B, Laba TL. Patient preferences for managing insomnia: a discrete choice experiment. Patient. 2018;11(5):503–514. 10.1007/s40271-018-0303-y [DOI] [PubMed] [Google Scholar]
- 194.Culver NC, Song Y, Kate McGowan S, et al. Acceptability of medication and nonmedication treatment for insomnia among female veterans: effects of age, insomnia severity, and psychiatric symptoms. Clin Ther. 2016;38(11):2373–2385. 10.1016/j.clinthera.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ibrahim S, Sidani S. Preferences for behavioral therapies for chronic insomnia. Health. 2013;5:1784–1790. 10.4236/health.2013.511240 [DOI] [Google Scholar]
- 196.Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med. 2018;33(6):955–962. 10.1007/s11606-018-4390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ulmer CS, Bosworth HB, Beckham JC, et al. Veterans affairs primary care provider perceptions of insomnia treatment. J Clin Sleep Med. 2017;13(8):991–999. 10.5664/jcsm.6702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M. Psychological treatment for insomnia in the regulation of long-term hypnotic drug use. Health Technol Assess. 2004;8(8):iii–iv, 1–68. 10.33310/hta8080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


