Abstract
Study Objectives:
Person-centered obstructive sleep apnea (OSA) care is a collaborative approach that is respectful of an individual’s health priorities. Informed decision-making is essential to person-centered care, especially as patients age. In a feasibility study, we evaluated the effects of a new decision aid (Decide2Rest) on OSA treatment decision-making in older adults.
Methods:
Patients (aged ≥ 60 years) with newly diagnosed OSA were recruited from two health care systems and randomized either to Decide2Rest or to a control program. Postintervention outcomes included 1) Decisional Conflict Scale (0–100, where 0 = low and 100 = high conflict), which measures perceptions of uncertainty, whether decisions reflect what matters most to patients, and whether patients feel supported in decision-making; 2) Preparation for Decision-Making scale (0–100, where 0 = least and 100 most prepared); and 3) OSA knowledge (0–100, where 0 = poor and 100 = outstanding). Multivariable linear regression models examined relationships between Decide2Rest and outcomes (Decisional Conflict Scale, Preparation for Decision-Making, OSA knowledge).
Results:
Seventy-three patients were randomized to Decide2Rest (n = 36; mean age, 69 years; 72% male) vs control (n = 37; mean age, 69 years; 70% male). Results from the regressions, controlling for study site, indicate that the Decide2Rest program resulted in less decisional conflict (20.5 vs 32.7 on the Decisional Conflict Scale; P = .014), more preparedness for decision-making (87.8 vs 66.2 on the Preparation for Decision-Making scale; P < .001), and greater OSA knowledge (75.1 vs 65.3 OSA knowledge score; P = .04) scores than in the control group.
Conclusions:
The Decide2Rest program promotes person-centered OSA decision-making for older patients with newly diagnosed OSA. Future studies are needed to optimize implementation of the program.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, Name: Improving Older Adults’ Decision-Making for OSAT (eDecide2Rest); URL: https://clinicaltrials.gov/ct2/show/NCT03138993; Identifier: NCT03138993.
Citation:
Fung CH, Martin JL, Liang LJ, et al. Efficacy of a patient decision aid for improving person-centered decision-making by older adults with obstructive sleep apnea. J Clin Sleep Med. 2021;17(2):121–128.
Keywords: obstructive sleep apnea, aging, decision-making, patient autonomy
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is a growing interest in tools to promote person-centered care for older adults, who often have multiple chronic conditions requiring polypharmacy and numerous treatments. This decision-aid program was designed to be respectful and responsive to the treatment preferences of older adults and promote their management of obstructive sleep apnea.
Study Impact: A patient decision-aid program, entitled Decide2Rest, improves decision-making for older adults with newly diagnosed OSA. It is a promising approach to improving decision- making for older adults with obstructive sleep apnea and promoting person-centered care.
INTRODUCTION
A person-centered approach to health care includes informing patients of their treatment options, providing information on the ways different treatments (or the choice for no treatment) may or may not meet their preferences and needs and giving patients the opportunity to voice their treatment preferences so that ultimately the selected strategy optimizes benefits, minimizes harm, aligns with their overarching health priorities, and enhances quality of life.1 This approach to care is associated with more self-management and patient satisfaction.2 Individuals with obstructive sleep apnea (OSA) may not consistently receive person-centered care,3 and only about a third of older adults have specific conversations with their health care provider about their priorities that guide their decision-making.4
Patient decision aids are one strategy for promoting person-centered care and aligning individual priorities with health care decisions.5 Decision aids provide information on health care options and help patients clarify and communicate the value they associate with different option features to facilitate shared decision-making and patient participation in health care decision-making.6 A wide variety of patient decision aids have been developed and tested. Studies demonstrate that decision aids improve patients’ knowledge about therapies and encourage them to actively weigh the risks and benefits of each therapy, preparing patients to make decisions and reducing decisional conflict (ie, personal uncertainty about which course of action to take when choice among the options involves risk, regret, or challenge to personal life values7).8 A recent study found that low decisional conflict and more shared decision-making are associated with higher levels of adherence to OSA treatment.9
Research on decision aids for adults with OSA is limited,10,11 and a patient decision aid for OSA management designed for older adults (for whom person-centered health care is considered a key goal12 owing to the high prevalence of comorbidity) is needed. In the context of a feasibility study (results previously reported),13 we assessed the effects of a decision aid on decisional outcomes related to OSA treatment among older adults with newly diagnosed OSA and explored the effects of providing support for the decision aid in-person compared with the telephone (remote).
METHODS
Framework
This work was designed and performed based on National Institutes of Health Stage Model stage II research activities, which involve experimental testing of promising behavioral interventions in research settings with research-based providers. Stage II activities (intervention generation, refinement, modification, and adaptation) have previously been described.3,13,14
Participants
Participants were recruited from two sites (one Department of Veterans Affairs [VA] site and one university site). Patients scheduled for a sleep study were mailed a letter about the study. Patients who did not opt out were contacted by telephone by our research team. Patients who met the initial inclusion criteria were invited for an in-person consent appointment and baseline assessment. The study flow is summarized in Figure 1.
Figure 1. Study flow diagram.
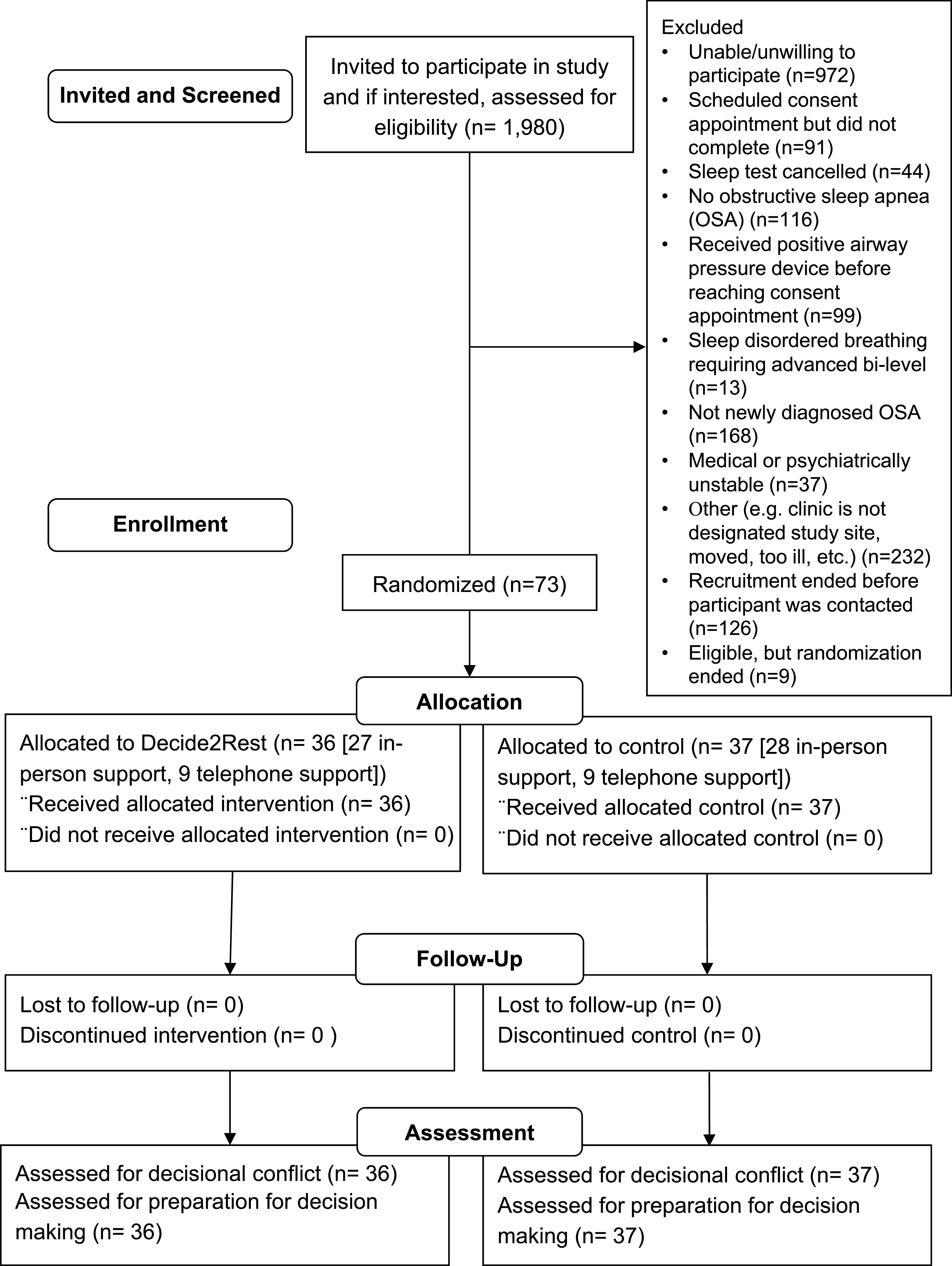
Inclusion criteria for the current study were (1) age ≥ 60 years, (2) apnea-hypopnea index ≥ 5 and newly diagnosed with OSA by a sleep physician based on an overnight sleep study (home-based or in-laboratory), and (3) patient agreed to be scheduled for a sleep clinic appointment.
Exclusion criteria were (1) OSA treatment for more than 30 days before participation; (2) comorbid sleep-related breathing disorder requiring treatment with an advanced bilevel device; (3) severe, unstable medical/psychiatric illness; (4) cognitive impairment (eg, diagnosis of dementia, abnormal Clock Draw Test15 or Mini-Mental State Examination16 total score < 24); (5) unable to use English-language decision aid (eg, unable to read English language); or (6) a history of failing to come to scheduled appointments (based on research and medical record documentation).
All procedures were approved by the VA Greater Los Angeles Healthcare System Institutional Review Board (PCC no. 2013-081086) and University of California, Los Angeles Institutional Review Board (16-001482). Informed consent was obtained from all participants. Clinical trial registration was completed for the feasibility study.
Intervention
Decide2Rest patient decision aid program
We developed Decide2Rest13 as a patient decision-aid program designed for older adults with newly diagnosed OSA. It was delivered in a voice-narrated (ie, “virtual coach”) web-based format with an accompanying patient workbook. Components of the Decide2Rest program are listed in Table 1. Written narratives described the experiences of hypothetical individuals who had chosen a treatment option, including the strategies used to adhere to the selected treatment. Using the patient workbook, participants explored their treatment options and identified the aspects of treatment that mattered most to them. In the workbook, they also identified their health priorities, reflected on their level of OSA knowledge and support for making decisions, and considered whether others in their lives are involved in making OSA decisions. The Decide2Rest program concludes with a list of resources and instructions to follow up with the sleep provider and a recommendation to bring the workbook with them to the visit so that they can discuss treatment options with their provider. Participants recorded responses to the questions posed on the web pages in a paper workbook that was provided to them at the beginning of the session.
Table 1.
Decide2Rest decision aid components.
| Decision Aid Topic | Description |
|---|---|
| Cover and frontmatter | Provides goals of the decision aid and links to funders/authors of the decision aid |
| Learn about OSA | Provides information about OSA and its effects on health |
| My sleep test results | Form with space to write down OSA severity and oxygen nadir (this information was populated by research staff at the VA site). |
| Types of treatment-related decisions | (1) Whether or not to treatment OSA, (2) which treatment to choose, 3) whether or not to use treatment nightly |
| Learn about treatment options | Provides overview of common OSA treatments |
| Compare treatment benefits | Provides comparison of treatment benefits across treatments |
| Compare treatment risks | Includes risks of common treatment options. Informs patients that insurance coverage and out-of-pocket costs vary for OSA treatments |
| Identify my long-term health goals | Exercise for patient to identify long-term health goals |
| Explore my decisions | Exercise for patient to identify risks and benefits of OSA treatments and what matters most to him or her |
| Resources | Provides other resources for learning about OSA |
| My next steps | Prompts patient to discuss treatment options and preferences with sleep doctor and to ready to receive more information from doctor |
OSA = obstructive sleep apnea; VA = Department of Veterans Affairs.
Control program (general sleep education program)
The control condition was similarly presented in a web-based format plus paper workbook. The control condition involved general information about sleep and resources for learning about sleep. The paper workbook included questions about sleep schedule, sleep quality, and sleep history.
Setting, randomization, and delivery (including in-person vs telephone modality)
Patients who met inclusion criteria were randomized within each study site to receive Decide2Rest vs control. At the university site, participants were randomized using simple randomization to one of two groups (decision aid with in-person support; control with in-person support) on the day of the intervention. The Research Electronic Data Capture randomization tool was used to allocate the participants to either the Decide2Rest or control program. At the VA site, participants were randomized to one of four groups (decision aid with in-person support, decision aid with telephone support, control with in-person support, or control with telephone support) on the day of the intervention using a block randomization (block size = 4). The randomization sequence was created using Stata 13.1 (StataCorp LLC, College Station, Texas). A set of opaque, sequentially numbered envelopes was prepared during the setup phase of the study by research team member without direct contact with research participants, and at the time of randomization, the envelopes were opened sequentially by a staff member without contact with the research participants. Each participant completed either Decide2Rest or the control program within 14 days of the scheduled sleep clinic appointment. At the VA site, the program was completed in a research office. At the university site, the program was completed in a private area within the clinic. A research team member (no clinical degree required) was trained in the delivery of the assigned program and followed a standard protocol and a script for introducing the program, the paper booklet, and for concluding the session with the participant. For Decide2Rest, this team member described himself or herself as part of a team that also included the virtual coach narrating the Decide2Rest web-based material. The research team member answered questions about the workbook and assisted participants who needed help with the website.
For the control program, research staff followed a protocol for setting up the control materials but did not provide additional decision support to the participant. At the VA site, the team member remained in the research office with half (n = 18) of the participants and telephoned the participant from another office in the same hallway for the other half (n = 18) of the participants (randomly selected). The purpose of allocating some participants to receive telephone support for their assigned program was to collect additional information about the feasibility of providing remote technological and program support. At the university site, the team member remained in the same room as the participant for the entire program (no telephone support was offered owing to infrastructure limitations at the site).
Measures
Demographic and clinical characteristics
Demographics (age, sex, race, ethnicity, highest level of education, and marital status) were collected at baseline. Baseline apnea-hypopnea index was abstracted from the sleep study report so that statistical analyses could control for the effect of OSA severity (eg, including mild OSA) on outcome measures. The Epworth Sleepiness Scale was administered by research staff at baseline.17
Decisional measures were assessed in all randomized participants postintervention at a separate in-person research visit that occurred after the sleep clinic appointment (typically, the postintervention assessment occurred on the day of the intervention). At the VA site, the assessor was blinded to study arm assignment, whereas at the university site, blinding was not possible because of staffing limitations. The Decisional Conflict Scale (DCS) assesses perceptions of uncertainty, whether decisions reflect what matters most to patients, and whether patients feel supported in decision-making. The DCS has five subscales: Informed, Values Clarity, Support, Uncertainty, and Effective Decision. The primary outcome measure was the Decisional Conflict Scale total score (DCS; 16 items, 5 response options–strongly disagree to strongly agree). Following the user manual instructions, we linearly transformed the average of the 16 items so that the range of the DCS total score was 0 [low conflict]–100 [high conflict]).18 High DCS total scores are associated with decision delay or feeling unsure about moving forward with a decision.18 A secondary outcome measure was the Preparation for Decision-Making Scale (PDM; 10 items, 5 response options: not at all to a great deal; linearly transformed score range: 0 [least prepared]–100 [most prepared]).19 Higher scores indicate feeling more prepared for decision-making. An exploratory outcome was knowledge about OSA, which was assessed using items adapted from a published questionnaire on determinants of positive airway pressure (PAP) adherence; additional items testing knowledge of dental appliances and surgical treatments for OSA were developed and added (23 true/false items; linearly transformed score range 0 [poor]–100 [outstanding]).20
Statistical analyses
All data were analyzed using an intent-to-treat approach. Descriptive statistics and frequencies of demographic and clinical characteristics were summarized by study group. Baseline group comparisons of categorical and continuous characteristics, controlling for study site, were performed using Cochran-Mantel-Haenszel tests and analysis of variance, respectively. A multivariable linear regression model was used to assess the intervention effect between group (Decide2Rest vs control) and site (university vs VA) on the outcome measures (unadjusted model). Study site was included in the regression model as a fixed effect to account for clustering effect. This approach also allowed us to examine whether the intervention effect differed between the two study sites (interaction term). We observed no significant interaction; thus, the final unadjusted model included two factors: group and study site. We estimated mean scores by group from these unadjusted analyses and presented them in side-by-side bar charts. Next, in an adjusted model, the modality of support (in-person vs telephone), preselected demographic characteristics (age, sex, race/ethnicity, education, and marital status), and clinical characteristics (AHI and Epworth Sleepiness Scale total score) were added to the unadjusted model to evaluate whether the intervention effect on each outcome measure (ie, for DCS, PDM, and OSA knowledge) was still significant and to assess whether any of these preselected characteristics (including demographic and clinical) were associated with the decisional and knowledge outcomes. An exploratory analysis using a difference in difference approach (ie, difference in intervention effects between in-person and telephone modes of support) was conducted to evaluate whether the intervention effects on each outcome varied between the two different modalities of support. All statistical analyses were conducted using SAS System for Windows 9.4 (Statistical Analysis Software, Cary, North Carolina), and the graph was generated using the publicly available statistical software R (R Core Team 2020).
RESULTS
Descriptive
The mean age (69 years) was similar in both groups, which were primarily male (70% control, 72% Decide2Rest). There were no significant differences between study groups in sample characteristics (see Table 2).
Table 2.
Participant characteristics and outcome measures.
| Variable | Control (n = 37) | Decide2Rest Decision Aid (n = 36) |
|---|---|---|
| Research site, n (%) | ||
| University | 18 (48.7) | 18 (50.0) |
| Department of Veterans Affairs | 19 (51.3) | 18 (50.0) |
| Age, mean (SD) | 68.8 (7.0) | 69.4 (5.7) |
| 60–65 y, n (%) | 15 (40.5) | 11 (30.6) |
| 66–70 y, n (%) | 9 (24.3) | 9 (25.0) |
| 70+ y, n (%) | 13 (35.1) | 16 (44.4) |
| Male, n (%) | 26 (70.3) | 26 (72.2) |
| Non-Hispanic White, n (%) | 27 (73.0) | 23 (63.9) |
| Education, n (%) | ||
| High school (≤ 12 y) | 5 (13.5) | 4 (11.1) |
| Some college (13–15 y) | 15 (40.5) | 11 (30.6) |
| College or higher (16+ y) | 17 (46.0) | 21 (58.3) |
| Marital status, n (%) | ||
| Married or living as married | 21 (56.8) | 19 (52.8) |
| Single / Never married | 5 (13.5) | 6 (16.7) |
| Other (divorced/separated/widowed) | 11 (29.7) | 11 (30.5) |
| Baseline apnea-hypopnea index, mean (SD) | 18.3 (11.6) | 20.1 (17.1)a |
| Epworth Sleepiness Scale, mean (SD) | 7.51 (4.93) | 8.22 (4.99) |
Missing one value. Demographic and clinical characteristics (controlling for study site) at baseline were comparable between treatment and control groups (P > .05; Cochran-Mantel-Haenszel tests [categorical] and analysis of variance [continuous]). SD = standard deviation.
Main analyses of intervention effect
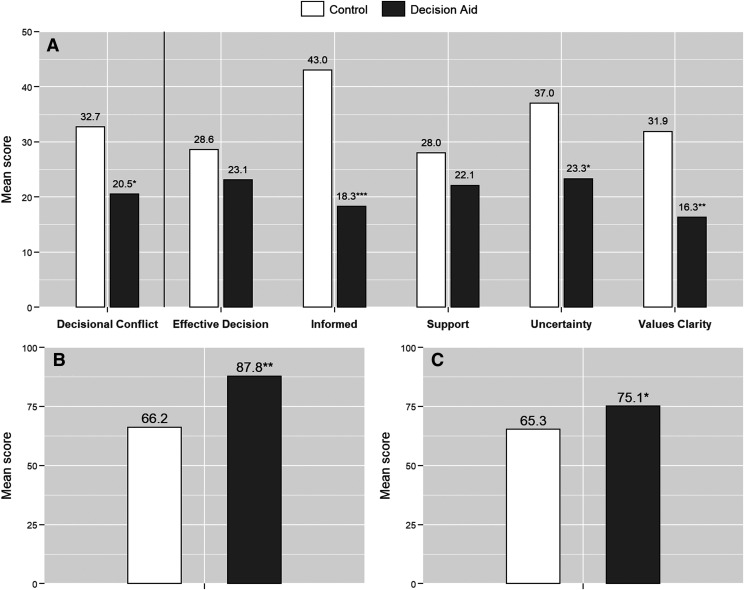
The primary outcome, DCS total score, was more favorable in the Decide2Rest group (estimated mean = 20.5, SE = 3.41) than the control group (estimated mean = 32.7, SE = 3.47; P = .014; see Figure 2). Compared with those in the control group, participants in the Decide2Rest group showed significantly lower (more favorable) mean scores on the DCS subscales: Informed (estimated mean: 18.3 vs 43.0; P < .001), Values Clarity (estimated mean: 16.3 vs 31.9; P = .002), and Uncertainty (estimated mean: 23.3 vs 37.0; P = .015), which is shown in Figure 2A. No significant differences were observed between Decide2Rest and control groups for the DCS-Support and DCS-Effective Decision subscales.
Figure 2. Mean scores.
Mean scores of Decisional Conflict Scale (DCS) with subscales. (A) Higher scores suggest more decisional conflict, Preparation for Decision-Making (PDM) scale, (B) Higher scores are indicative of feeling more prepared for decision-making, and OSA Knowledge score (C) Higher scores are indicative of greater OSA knowledge. The means were estimated from the unadjusted multivariable regression, controlling for study site. * P < .05, ** P < .005, and *** P < .0001.
The Decide2Rest group also had better scores on the PDM (Figure 2B), mean: 87.8 vs 66.2; P < .001).
OSA knowledge score was significantly higher in the Decide2Rest group compared with the control group (Figure 2C), mean: 75.1 vs 65.3; P = .040).
The effects of the Decide2Rest intervention on DCS total score, PDM, and OSA knowledge score persisted after controlling for the intervention mode, the preselected demographic characteristics, OSA severity (apnea-hypopnea index), and Epworth Sleepiness Scale (DCS total score P = .019; PDM P < .001; OSA knowledge score P = .018; Table 3). As shown in Table 3, none of the covariates was significantly associated with the outcomes (all P values ≥ .145).
Table 3.
Multivariable regressionsa for decisional outcome measures.
| Outcome Measure | Primary Outcome | Secondary Outcome | ||
|---|---|---|---|---|
| Decisional Conflict | Preparation for Decision- Making | |||
| Estimate (SE) | P Value | Estimate (SE) | P Value | |
| Intervention effect (DA-CTL) | −12.70 (5.26) | .019 | 22.19 (5.75) | <.001 |
| Covariates | ||||
| Intervention mode (REF = telephone) | ||||
| In-person | −3.45 (7.16) | .632 | −5.43 (7.98) | .499 |
| Age (REF = 70+ y) | ||||
| 60–65 | −4.27 (6.75) | .529 | 4.73 (7.21) | .515 |
| 66–70 | 6.80 (7.12) | .344 | −6.30 (8.02) | .436 |
| Male | −9.15 (7.50) | .228 | 4.62 (8.36) | .583 |
| Non-Hispanic White | 0.16 (5.92) | .978 | -9.53 (6.45) | .145 |
| Education (REF = college or higher) | ||||
| High school (≤ 12 y) | 2.24 (8.36) | .790 | 12.85 (9.37) | .176 |
| Some college (13–15 y) | −3.88 (5.92) | .515 | 7.86 (6.37) | .223 |
| Marital status (REF = others) | ||||
| Married or living as married | 2.58 (6.13) | .675 | −0.15 (6.56) | .982 |
| Single or never married | 0.05 (8.56) | .995 | −3.20 (8.99) | .723 |
| Baseline apnea-hypopnea index | −0.09 (0.19) | .626 | −0.03 (0.20) | .878 |
| Epworth Sleepiness Scale | 0.82 (0.56) | .153 | −0.44 (0.57) | .443 |
Each column represents a multivariable regression, controlling for study site. CTL = control, DA, Decide2Rest decision aid, REF = reference group.
Exploratory analyses of modality (in-person vs telephone support)
Results from the exploratory analyses showed that the intervention effect (ie, the effect of the Decide2Rest program vs control program) on DCS total score for the in-person modality (estimated mean: 20.7 vs 32.6 for DA vs control, respectively) was similar to that of the telephone modality (20.0 vs 33.1). The intervention effect on PDM for the in-person modality (87.4 vs 62.8) appeared to be greater than the telephone modality (89.4 vs 76.9), and on OSA knowledge score, the in-person modality (75.0 vs66.4) was lower than that of the telephone modality (75.4 vs 61.9); however, none of these differences in intervention effects reached statistical significance.
DISCUSSION
In the context of a feasibility study, we evaluated a program (Decide2Rest) to promote a person-centered approach to making OSA treatment decisions, which focused on helping patients understand and feel more certain about their OSA treatment choices and eliciting their health priorities. The program reduced decisional conflict, improved preparation for decision-making, and increased participants’ OSA knowledge. These relationships persisted in regression models that adjusted for OSA severity and other participant characteristics. The program has the potential to help older adults make OSA treatment decisions that are better aligned with their health goals and values.
These results suggest that Decide2Rest may be beneficial for older adults with OSA. This is important because some patients either do not receive information about other treatment options or do not recall the treatment options that may have been described during conversations with their provider, which may result in some patients not returning to speak with their provider about treatment alternatives.3 When patients do not follow up with their provider to discuss other treatment options, no treatment becomes the long-term “treatment” plan. Our study shows that patients who used our program, which communicates treatment options in an explicit and structured manner, resulted in patients feeling more informed about OSA treatment options, which could address the issue of no active therapy becoming the default option when patients do not use a prescribed device such as PAP.
The Decide2Rest program can support older patients who prefer to be more involved in the treatment selection process. It provides information about aspects of treatment that are important to patients but that may otherwise be omitted during clinic visit discussions owing to limited time. Incorporating patients’ preferences in OSA treatment decision-making is a departure from a clinical strategy of prescribing PAP therapy first to all patients who are eligible for PAP. Use of the decision aid acknowledges the high rates of PAP nonacceptance and nonadherence as well as the observation that individual response to treatment may vary because of underlying heterogeneity caused by varying levels of device adherence and different physiologic,21 polysomnographic,22,23 and clinical phenotypes.24 This approach strives to match OSA treatment with a patient’s goals and preferences, by providing patients with the knowledge and skills to discuss their treatment options with their providers. The decision aid is a tool to promote discussion (not a substitute for a discussion with a health care provider). As a condition that involves clinical uncertainty, OSA treatment decisions in older adults should be personalized, and patients should be involved and informed when making treatment decisions.
We note several limitations and strengths of the study. First, most of the study participants were men, which may limit generalizability of the findings to women with OSA, and the study was not powered to examine sex differences. Participants who had a history of multiple missed clinical appointments were excluded, which limits generalizability to patients with this pattern of appointment attendance. There were some methodological differences across the two sites resulting from the feasibility goals and logistical issues (ie, the availability of private office space for research staff); however, inclusion of an interaction term between study site and intervention was not statistically significant. The feasibility study’s clinical trial registration sample size reflects only the university site due to changes in registration requirements for feasibility studies during the grant. Of note, although DCS total score was a priori selected as the primary decisional outcome, it was not included in the clinical trial registration, which described feasibility outcomes. Strengths include the randomized, controlled study design and the process used to develop the intervention tested in the study, which include focus groups, interviews, and extensive iterative refinement of the intervention.13
In conclusion, Decide2Rest, which is a patient decision aid for older adults with newly diagnosed OSA, is a potentially promising approach to promoting person-centered sleep care. Although supporting patients’ decision-making processes and ensuring that patients are informed of their treatment options is intrinsically valuable, the program may also prove beneficial in promoting self-management2 and treatment acceptance, which in turn could improve other health outcomes.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was conducted at the Veterans Administration Greater Los Angeles Healthcare System and the UCLA Health System. The authors have no financial or nonfinancial interests that are relevant to the submitted manuscript. This study was funded by the National Institute on Aging of the National Institutes of Health (K23AG045937 to C.H.F., K23AG055668 to Y.S., K23AG049955 to J.D., National Center for Advancing Translational Science, UCLA CTSI Grant UL1TR001881), as well as the American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies (The Beeson Career Development in Aging Research Award Program to C.H.F.). R.D.H. received support from the University of California, Los Angeles Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly under the National Institutes of Health National Institute on Aging Grant P30-AG021684. J.L.M. received support from the National Heart, Lung and Blood Institute at the National Institutes of Health (K24HL143055).
ACKNOWLEDGMENTS
We thank our research staff at the VA Greater Los Angeles and UCLA and the clinical staff at these sites who supported our project.
ABBREVIATIONS
- DCS
Decisional Conflict Scale
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PDM
Preparation for Decision-Making scale
- VA
Department of Veterans Affairs
REFERENCES
- 1.Boyd C, Smith CD, Masoudi FA, et al. Decision making for older adults with multiple chronic conditions: executive summary for the American Geriatrics Society guiding principles on the care of older adults with multimorbidity. J Am Geriatr Soc. 2019;67(4):665–673. 10.1111/jgs.15809 [DOI] [PubMed] [Google Scholar]
- 2.Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70(4):351–379. 10.1177/1077558712465774 [DOI] [PubMed] [Google Scholar]
- 3.Fung CH, Alessi C, Truong C, et al. Patient-provider communication with older adults about sleep apnea diagnosis and treatment. Behav Sleep Med. 2017;15:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Case SM, O’Leary J, Kim N, Tinetti ME, Fried TR. Older adults’ recognition of trade-offs in healthcare decision-making. J Am Geriatr Soc. 2015;63(8):1658–1662. 10.1111/jgs.13534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaboration International Patient Decision Aids Standards (IPDAS) [updated June 20, 2012]. What are patient decision aids? https://ipdas.ohri.ca/what.html; 2012; Accessed on September 3, 2015.
- 6.Elwyn G, O’Connor A, Stacey D, et al. International Patient Decision Aids Standards (IPDAS) Collaboration . Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. 10.1136/bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBlanc A, Kenny DA, O’Connor AM, Légaré F. Decisional conflict in patients and their physicians: a dyadic approach to shared decision making. Med Decis Making. 2009;29(1):61–68. 10.1177/0272989X08327067 [DOI] [PubMed] [Google Scholar]
- 8.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broström A, Pakpour AH, Nilsen P, Hedberg B, Ulander M. Validation of CollaboRATE and SURE - two short questionnaires to measure shared decision making during CPAP initiation. J Sleep Res. 2019;28(5):e12808. 10.1111/jsr.12808 [DOI] [PubMed] [Google Scholar]
- 10.Trenaman L, Munro S, Almeida F, Ayas N, Hicklin J, Bansback N. Development of a patient decision aid prototype for adults with obstructive sleep apnea. Sleep Breath. 2016;20(2):653–661. 10.1007/s11325-015-1269-9 [DOI] [PubMed] [Google Scholar]
- 11.Pelletier-Fleury N, Gafni A, Krucien N, Fleury B. The development and testing of a new communication tool to help clinicians inform patients with obstructive sleep apnoea syndrome about treatment options. J Sleep Res. 2012;21(5):577–583. 10.1111/j.1365-2869.2012.01015.x [DOI] [PubMed] [Google Scholar]
- 12.Besdine R, Boult C, Brangman S, et al.; American Geriatrics Society Task Force on the Future of Geriatric Medicine . Caring for older Americans: the future of geriatric medicine. J Am Geriatr Soc. 2005;53(6) (Suppl):S245–S256. [DOI] [PubMed] [Google Scholar]
- 13.Fung CH, Martin JL, Hays RD, et al. Development of a program promoting person-centered care of older adults with sleep apnea. J Am Geriatr Soc. 2019;67(10):2204–2207. 10.1111/jgs.16084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning clinical science: unifying the discipline to improve the public health. Clin Psychol Sci. 2014;2(1):22–34. 10.1177/2167702613497932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman KIG, DP, Cohen CA, Zucchero CA. Clock-drawing and dementia in the community: a longitudinal study. Int J Geriatr Psychiatry. 1993;8(6):487–496. 10.1002/gps.930080606 [DOI] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 18.O’Connor AM. Validation of a decisional conflict scale. Med Decis. Making. 1995;15(1):25–30. 10.1177/0272989X9501500105 [DOI] [PubMed] [Google Scholar]
- 19.Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O’Connor AM. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;78(1):130–133. 10.1016/j.pec.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 20.Stepnowsky CJ, Jr., Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med 2002;3(3):239–247. 10.1016/S1389-9457(01)00162-9 [DOI] [PubMed] [Google Scholar]
- 21.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17(1):99–107. 10.1111/j.1440-1843.2011.02037.x [DOI] [PubMed] [Google Scholar]
- 23.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014;10(2):215–227. 10.5664/jcsm.3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilbert J, Yaggi HK. Patient-centered care in obstructive sleep apnea: a vision for the future. Sleep Med Rev. 2018;37:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]