Abstract
Study Objectives:
It is well known that a family history of diabetes (FHD) is a definitive risk factor for type 2 diabetes. It has not been known whether sleep-disordered breathing (SDB) increases the prevalence of diabetes in those with an FHD.
Methods:
We assessed SDB severity in 7,477 study participants by oximetry corrected by objective sleep duration determined by wrist actigraphy. Glycated hemoglobin ≥6.5% and/or current medication for diabetes indicated the presence of diabetes. In addition to the overall prevalence, the prevalence of recent-onset diabetes during the nearly 5 years before the SDB measurements were made was investigated.
Results:
Of the 7,477 participants (mean age: 57.9; range: 34.2–80.7; SD: 12.1 years; 67.7% females), 1,569 had an FHD. The prevalence of diabetes in FHD participants with moderate-to-severe SDB (MS-SDB) was higher than in those without SDB (MS-SDB vs without SDB: all, 29.3% vs 3.3% [P < .001]; females, 32.6% vs 1.9% [P < .001]; males, 26.2% vs 11.7% [P = .037]). However, multivariate analysis showed that MS-SDB was significantly associated with a higher prevalence of diabetes only in FHD-positive females (odds ratio [95% confidence interval]: females, 7.43 [3.16–17.45]; males, 0.92 [0.37–2.31]). Among the FHD-positive participants, the prevalence of recent-onset diabetes was higher in those with MS-SDB than those without SDB, but only in females (MS-SDB vs without SDB: 21.4% vs 1.1%; P < 0.001).
Conclusions:
MS-SDB was associated with diabetes risk in females with an FHD, and future studies are needed on whether treatment of SDB in females with an FHD would prevent the onset of diabetes.
Citation:
Minami T, Matsumoto T, Tabara Y, et al. Impact of sleep-disordered breathing on glucose metabolism among individuals with a family history of diabetes: the Nagahama study. J Clin Sleep Med. 2021;17(2):129–140.
Keywords: heritability, gene, environment, obstructive sleep apnea, effect modification
BRIEF SUMMARY
Current Knowledge/Study Rationale: A family history of diabetes is a strong risk factor for type 2 diabetes. Although it is said that sleep-disordered breathing is also a risk factor for diabetes, whether there is an additional increase in the prevalence of diabetes in individuals with a family history of diabetes is not known.
Study Impact: In our large community-based study, moderate-to-severe sleep-disordered breathing was significantly associated with an increased prevalence of type 2 diabetes and of recent-onset type 2 diabetes during a period of nearly 5 years, especially in females with a family history of diabetes. Thus, moderate-to-severe sleep-disordered breathing might be associated with an increased prevalence of type 2 diabetes in females with a family history of diabetes.
INTRODUCTION
Sleep-disordered breathing (SDB), especially obstructive sleep apnea (OSA), is a common condition characterized by recurrent intermittent hypoxia during sleep and sleep fragmentation. Individuals with SDB have a high prevalence of comorbidities such as cardiometabolic disorders, particularly with concurrent obesity.1,2 Of the comorbidities associated with SDB, abnormal glucose metabolism has been identified in both public health and clinical settings. Indeed, several studies have shown that the prevalence of moderate-to-severe OSA exceeds 30% in patients with diabetes worldwide.3–5 Furthermore, longitudinal observational studies and meta-analyses showed that OSA was an independent risk factor for incident diabetes,6–13 and increased insulin resistance.14–16
Previously, we showed that the impact of SDB on the prevalence of diabetes was modified by sex and menopausal status.17 These findings implied that the effect of SDB might vary according to an individual’s genetic characteristics. Of the various factors related to glucose metabolism, a positive family history of diabetes (FHD) is a well-established strong risk factor for development of diabetes,18 which also exhibits synergistic interactions in the presence of concurrent obesity.19,20 Nevertheless, whether SDB modifies the impact of an FHD on glucose metabolism has not been addressed. Recently, much attention has been paid to the role of hypoxia-mediated epigenetic regulation in several diseases.21 Therefore, intermittent hypoxia induced by OSA might contribute to the development of diabetes in individuals with an FHD.
In the present study, we hypothesized that SDB would augment the risk for abnormal glucose metabolism in those with an FHD. To test this hypothesis we investigated the associations between SDB and the prevalence of diabetes, the prevalence of recent-onset diabetes determined in the study’s most recent 5 years, and insulin resistance among study participants with or without an FHD in the Nagahama cohort.17 Preliminary findings have been previously reported in abstract form.22
METHODS
Participants
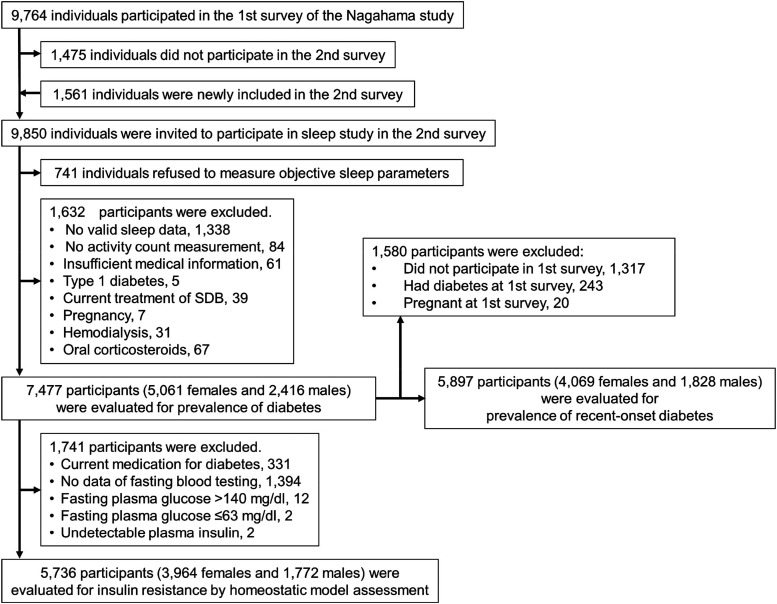
The Nagahama study is an ongoing prospective cohort study in the general population (Figure 1).17 During this cohort study, the first survey was conducted from 2008 to 2010 and the second survey was conducted from 2013 to 2016. We evaluated objective sleep parameters among the participants at the second survey of the Nagahama study (see Detailed Methods S1 (293.1KB, pdf) in the supplemental material). Figure 1 is a flow chart of the present analyses. We investigated the associations among SDB, FHD, and prevalence of type 2 diabetes and homeostatic model assessment–insulin resistance (HOMA-IR) in participants who had fasting blood data and who were not being treated for diabetes. In addition, we investigated the prevalence of recent-onset type 2 diabetes during 5 years prior to SDB measurements in the second survey. The ethics committee of Kyoto University and Nagahama City approved the study, and all participants signed a consent form.
Figure 1. Flowchart of enrollment of participants in the present study.
SDB = sleep-disordered breathing.
Assessment of objective sleep duration and activity count
Participants were requested to wear a pulse oximeter (PULSOX-Me300; Konica Minolta, Inc, Tokyo, Japan) for 4 consecutive nights and an actigraph (Actiwatch 2 or the Actiwatch Spectrum Plus; Philips Respironics, Murrysville, PA) for 1 week on the nondominant wrist concurrently to assess SDB and objective sleep duration. In our other study, as we measured the mean sleep duration for an entire week, participants were requested to wear the actigraph for 1 week.17 Bed-in time and bed-out time were set by well-trained investigators and manually confirmed based on sleep diaries and the light sensor in the actigraph. Total sleep duration (from sleep-onset time to wake-up time) and actual sleep duration (sleep duration after exclusion of wake time after sleep onset from total sleep duration) were determined using the standard factory-default algorithm.17 Objective sleep duration was categorized into <5 hours, 5–7 hours, and ≥7 hours per night averaged over a period of 1 week. To assess objective daily physical activity, we used the activity count per minute while awake as recorded by an actigraph.17
Assessment of SDB
Each oximetry tracing was reviewed by trained staff members. Start and end times of sleep were set according to actigraphy, and actual sleep duration determined from assessments of objective sleep duration was used to calculate the oxygen desaturation index (3% oxygen desaturation index modified by sleep duration derived from actigraphy [Acti-ODI3%]). A minimum of the average of 2 nights of oximetry data was required for analysis. SDB severity was defined by Acti-ODI3% indices as follows: normal, <5 per hour; mild, 5 to <15 events/h; moderate, 15 to <30 events/h; and severe, ≥30 events/h. As shown in the previous study,23 Acti-ODI3% was more comparable to the apnea-hypopnea index (AHI) derived from attended polysomnography in 32 patients (r = .99, P < .001; AHI = Acti-ODI3% × 1.04 + 1.45) than simply measured ODI3% without actigraphy modification (r = .92, P < .001; AHI = usual ODI3% × 1.27 + 2.06).
FHD, diabetes, HOMA-IR, and recent-onset diabetes
FHD was defined as having 1 or more first-degree relatives with diabetes among biological parents, siblings, and children. Because not all participants had fasting blood test results, we used glycated hemoglobin (%) to classify glucose metabolism status as follows: normal, <6.5; diabetes, ≥6.5 and/or current medication for diabetes.24 The HOMA-IR was estimated from fasting insulin and glucose levels (Detailed Methods S1 (293.1KB, pdf) in the supplemental material).
Recent-onset diabetes was defined as diabetes that developed de novo during the interval between the first and second surveys prior to SDB measurements.
Other variables
Body mass index (BMI) was calculated as body weight (kg) divided by height (m) squared. Waist circumference was measured on the umbilicus level while standing. These variables were measured at the study site with the participant in a fasting state. Smoking habits, alcohol consumption, menopausal status in females, hypertension, and results of the Japanese version of the Epworth Sleepiness Scale25 were obtained from questionnaires. From responses in the questionnaires about luxury grocery items, we obtained weekly consumption of alcohol according to units of Japanese sake. One unit of Japanese sake contains about 22 g ethanol. We calculated daily consumption of ethanol (g/d) as (22 × weekly consumption of alcohol by units of Japanese sake)/7. Moderate alcohol intake may have a preventive effect on the development of diabetes.26 We classified consumption of ethanol = 0 g/d, 0 g/d < ethanol ≤ 25 g/d, and ethanol >25 g/d as a categorized variable in addition to ethanol (g/d) as a continuous variable. We defined hypertension as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg and/or current use of antihypertensive agents.
Data analysis
Values are expressed as means (SDs), medians [interquartile range], or percentages. We used the Student’s t test, analysis of variance, Mann-Whitney test, or the Kruskal-Wallis test to compare continuous values as appropriate and the chi-square test to compare categorical values. Tukey-Kramer and Dunn-Bonferroni tests were used as appropriate to perform post hoc pairwise comparisons among groups.
First, we comprehensively investigated the associations between FHD and the prevalence of diabetes in all participants. Next, we evaluated the impact of SDB on the prevalence of diabetes, HOMA-IR, and the prevalence of recent-onset diabetes among participants with or without an FHD. Our analyses also included the total participant population and females and males separately.17 In the analyses of the prevalence of diabetes and HOMA-IR, we showed these values according to SDB severity and BMI categories among those with an FHD (FHD+) or without FHD (FHD−). BMI was categorized from 17.5 to 27.5 kg/m2 in 2.5-kg/m2 increments. The upper category of BMI was ≥27.5 kg/m2 because of the low number of participants with BMI ≥ 30.0 in the present analysis.
In the analysis of the prevalence of diabetes, we conducted multivariate logistic analyses after adjusting for possible confounders such as age and obesity-related parameters. In the analysis of HOMA-IR, we used natural log-transformed values to approximate Acti-ODI3% and HOMA-IR to a normal distribution to finally perform multivariate regression analysis. We added 1 to “HOMA-IR” and “Acti-ODI3%,” respectively, before log transformation to a positive value. In the multivariate regression analysis for log-(HOMA-IR), log-(Acti-ODI3%) was included as a continuous variable in those with or without an FHD.
For each analysis, we evaluated the joint contributions of SDB and FHD by using interaction terms as follows: FHD × SDB severity and FHD × log-(Acti-ODI3%) (Detailed Methods S1 (293.1KB, pdf) in the supplemental material).
P values <.05 were considered significant in all analyses. Analyses were conducted by using SPSS software, version 25 (SPSS, Inc, Chicago, IL).
RESULTS
Number of participants in each analysis
Figure 1 shows the number of participants for each analysis. The second survey of the Nagahama study had 9,850 participants. After excluding 741 individuals who refused a sleep study and 1,632 participants without sufficient objective sleep data, activity count, or medical information and with comorbidities, we evaluated 7,477 participants in the analysis. For the analysis of insulin resistance, we evaluated 5,736 participants after excluding 1,741 participants undergoing diabetic treatment, without fasting blood data, and/or with prominently high or low plasma glucose or insulin values from the above 7,477 participants. Of the 7,477 participants described above, 6,160 participated in the first survey. After excluding 243 participants with diabetes and 20 pregnant female participants at the first survey, we investigated the prevalence of recent-onset diabetes in 5,897 participants.
Associations between FHD and the prevalence of diabetes
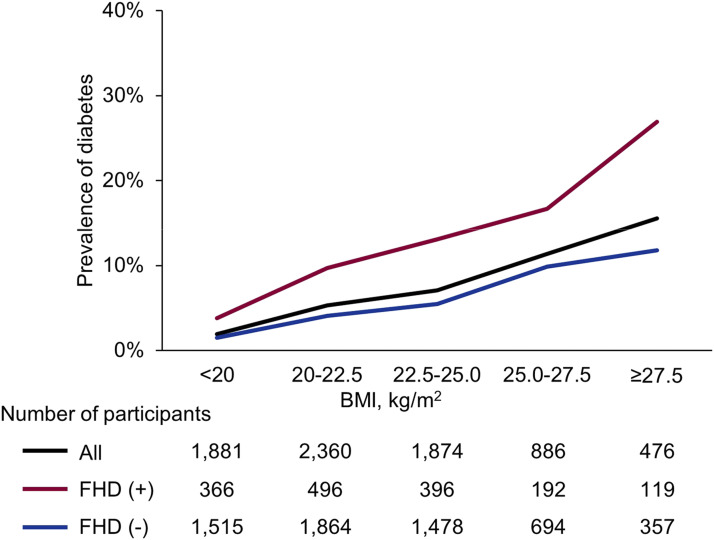
Of the 7,477 eligible participants (5,061 females and 2,416 males) (Figure 1 and Table 1), 1,569 (21.0%) had an FHD. FHD+ participants were younger, had a higher BMI, greater waist circumference, had a larger proportion of menopausal females, higher random plasma glucose and/or glycated hemoglobin, and a greater prevalence of diabetes than FHD− participants. There was no difference in the proportions of degrees of SDB severity between FHD+ and FHD− participants (Table 1). Figure 2 shows the prevalence of diabetes by FHD status and BMI categories. A positive FHD led to a higher prevalence of diabetes than a negative FHD in each BMI category, and the prevalence of diabetes steeply increased in FHD+ participants according to BMI increases.
Table 1.
Characteristics of study participants according to FHD.
| All (n = 7,477) | FHD+(n = 1,569) | FHD−(n = 5,908) | P (FHD+ vs FHD−) | |
|---|---|---|---|---|
| Females, n (%) | 5,061 (67.7) | 1,114 (71.0) | 3,947 (66.8) | .002 |
| Age, years | 57.9 (12.1) | 57.2 (11.7) | 58.1 (12.2) | .007 |
| Body mass index, kg/m2 | 22.3 (3.3) | 22.5 (3.4) | 22.2 (3.3) | .001 |
| Waist circumference, cm | 81.9 (9.4) | 82.5 (9.3) | 81.7 (9.4) | .003 |
| Pack-years of smoking in current/past smoker* | 18.8 [8.0–33.0] | 16.5 [6.0–32.0] | 19.0 [8.0–33.0] | .096 |
| Alcohol consumption | ||||
| Ethanol,* g/d | 0.89 [0.00–12.21] | 0.86 [0.00–10.00] | 0.89 [0.00–12.50] | .056 |
| Ethanol = 0 g/d, n (%) | 3,393 (45.4) | 729 (46.5) | 2,664 (45.1) | .024 |
| 0 g/d< ethanol ≤ 25 g/d, n (%) | 2,917 (39.0) | 630 (40.2) | 2,287 (38.7) | |
| Ethanol > 25 g/d, n (%) | 1,167 (15.6) | 210 (13.4) | 957 (16.2) | |
| Activity count, /minute | 332.5 (99.0) | 334.8 (93.6) | 331.8 (100.4) | .29 |
| Menopausal females, n (%) | 3,324 (65.7) | 698 (62.7) | 2,626 (66.5) | .016 |
| Hypertension, n (%) | 2,849 (38.1) | 600 (38.2) | 2,249 (38.1) | .90 |
| JESS* | 6 [3–8] | 6 [3–8] | 6 [3–8] | .084 |
| Acti-ODI3%,* events/h | 5.91 [3.64–10.09] | 5.86 [3.44–9.93] | 5.94 [3.69–10.11] | .27 |
| SDB severity, n (%) | ||||
| SDB− | 3,074 (41.1) | 662 (42.2) | 2,412 (40.8) | .37 |
| Mild SDB | 3,496 (46.8) | 709 (45.2) | 2,787 (47.2) | |
| MS-SDB | 907 (12.1) | 198 (12.6) | 709 (12.0) | |
| Objective sleep duration | ||||
| <5 hours | 1,048 (14.0) | 214 (13.6) | 834 (14.1) | .82 |
| 5–7 hours | 5,395 (72.2) | 1,142 (72.8) | 4,253 (72.0) | |
| ≥7 hours | 1,034 (13.8) | 213 (13.6) | 821 (13.9) | |
| Current medication for diabetes, n (%) | ||||
| Antihyperglycemic agents | 323 (4.3) | 133 (8.5) | 190 (3.2) | <.001 |
| Insulin use | 23 (0.3) | 14 (0.9) | 9 (0.2) | <.001 |
| Random plasma glucose, mg/dL | 87.6 (13.1) | 89.7 (15.4) | 87.0 (12.3) | <.001 |
| HbA1c, % | 5.56 (0.48) | 5.65 (0.57) | 5.53 (0.45) | <.001 |
| Diabetes, n (%) | ||||
| All | 469 (6.3) | 178 (11.3) | 291 (4.9) | <.001 |
| Females | 211 (4.2) | 88 (7.9) | 123 (3.1) | <.001 |
| Males | 258 (10.7) | 90 (19.8) | 168 (8.6) | <.001 |
Data are expressed as mean (SD), median [interquartile range], or n (%). Diabetes was considered according to HbA1c ≥6.5% and/or current medication for diabetes. FHD+ was defined as having first-degree relative(s) with diabetes. SDB was classified by Acti-ODI3% as follows: no SDB, <5 events/h: mild SDB, 5 to <15 events/h; MS-SDB, ≥15 events/h. Acti-ODI3% = 3% oxygen desaturation index modified by sleep duration derived from actigraphy; FHD = family history of diabetes; HbA1c = glycated hemoglobin; JESS = Japanese version of the Epworth Sleepiness Scale; MS = moderate to severe; SDB = sleep-disordered breathing. *Mann-Whitney test was used to compare medians between groups. Student’s t test was used otherwise.
Figure 2. Prevalence of diabetes according to FHD and categories of BMI.
FHD(+) participants had a higher prevalence of diabetes than FHD(−) participants in each BMI category. Prevalence increased sharply with increases in BMI. Diabetes was considered according to HbA1c ≥6.5% and/or current medication for diabetes. FHD(+) was defined as having a first-degree relative(s) with diabetes. BMI = body mass index; FHD = family history of diabetes; HbA1c = glycated hemoglobin.
Prevalence of diabetes according to FHD status and the severity of SDB
Table 2 shows characteristics of participants according to FHD status and SDB severity. Although the prevalence of diabetes was greater according to the severity of SDB in participants with and without FHD, the degree of the increase was clearly greater in females with FHD (Figure S1 (293.1KB, pdf) in the supplemental material).
Table 2.
Characteristics of participants according to FHD status and SDB severity.
| FHD+ | FHD− | |||||||
|---|---|---|---|---|---|---|---|---|
| No SDB (n = 662) | Mild SDB (n = 709) | MS-SDB (n = 198) | P | No SDB (n = 2,412) | Mild SDB (n = 2,787) | MS-SDB (n = 709) | P | |
| Females, n (%) | 568 (85.8) | 451 (63.6) | 95 (48.0) | <.001 | 2,051 (85.0) | 1,653 (59.3) | 243 (34.3) | <.001 |
| Age, years | 52.1 (10.5) | 59.8 (11.0)† | 64.9 (10.4)†‡ | <.001 | 53.1 (11.3) | 60.8 (11.5)† | 64.8 (10.8) †‡ | <.001 |
| Body mass index, kg/m2 | 20.9 (2.5) | 23.1 (3.2)† | 25.4 (3.7)†‡ | <.001 | 20.9 (2.6) | 22.6 (3.1)† | 24.8 (3.7) †‡ | <.001 |
| Waist circumference, cm | 77.8 (7.5) | 84.6 (8.5)† | 90.5 (8.9)†‡ | <.001 | 77.6 (7.9) | 83.2 (8.8)† | 89.6 (9.3) †‡ | <.001 |
| Pack-years of smoking in current/past smoker* | 12.0 [4.8–24.4] | 19.5 [7.0–34.0]† | 22.5 [12.5–40.0]† | <.001 | 12.0 [5.0–23.0] | 20.0 [10.0–37.0]† | 22.0 [11.3–37.5]† | <.001 |
| Alcohol consumption | ||||||||
| Ethanol,* g/d | 0.71 [0.00–7.50] | 0.89 [0.00–12.21] | 0.89 [0.00–13.50] | .35 | 0.36 [0.00–5.34] | 1.25 [0.00–6.79]† | 5.00 [0.00–33.86]†‡ | <.001 |
| Ethanol = 0 g/d, n (%) | 304 (45.9) | 334 (47.1) | 91 (46.0) | .044 | 1,184 (49.1) | 1,223 (43.9) | 257 (36.2) | <.001 |
| 0 g/d < ethanol ≤ 25 g/d, n (%) | 287 (43.4) | 270 (38.1) | 73 (36.9) | 1,000 (41.5) | 1,048 (37.6) | 239 (33.7) | ||
| Ethanol >25 g/d, n (%) | 71 (10.7) | 105 (14.8) | 34 (17.2) | 228 (9.5) | 516 (18.5) | 213 (30.0) | ||
| Activity count, /minute | 359.0 (95.0) | 323.2 (88.1)† | 295.3 (87.1)†‡ | <.001 | 351.4 (104.9) | 325.7 (95.4) † | 289.4 (87.0)†‡ | <.001 |
| Menopausal females, n (%) | 268 (47.2) | 347 (76.9) | 83 (87.4) | <.001 | 1,063 (51.8) | 1,335 (80.8) | 228 (93.8) | <.001 |
| Hypertension, n (%) | 519 (78.4) | 379 (53.5) | 71 (35.9) | <.001 | 514 (21.3) | 1,236 (44.3) | 499 (70.4) | <.001 |
| JESS* | 6 [3–9] | 6 [3–8] | 6 [3–8] | .16 | 6 [3–9] | 5 [3–8]† | 6 [3–8] | <.001 |
| Acti-ODI3%,* events/h | 3.18 [2.38–3.93] | 8.04 [6.20–10.38]† | 19.95 [17.27–25.56]†‡ | <0.001 | 3.28 [2.45–4.15] | 7.91 [6.27–10.36]† | 20.59 [17.15–26.72]†‡ | <0.001 |
| Objective sleep duration, n (%) | <0.001 | |||||||
| <5 hours | 93 (14.0) | 87 (12.3) | 34 (17.2) | 0.44 | 317 (13.1) | 368 (13.2) | 149 (21.0) | |
| 5–7 hours | 480 (72.5) | 521 (73.5) | 141 (71.2) | 1,753 (72.7) | 2,023 (72.6) | 477 (67.3) | ||
| ≥7 hours | 89 (13.4) | 101 (14.2) | 23 (11.6) | 342 (14.2) | 396 (14.2) | 83 (11.7) | ||
| Current medication for diabetes, n (%) | ||||||||
| Antihyperglycemic agents | 17 (2.4) | 78 (11.0) | 39 (19.7) | <0.001 | 25 (1.0) | 115 (4.1) | 50 (7.1) | <0.001 |
| Insulin use | 0 (0.0) | 5 (0.7) | 9 (4.5) | <0.001 | 1 (0.0) | 8 (0.3) | 0 (0.0) | 0.042 |
| Random plasma glucose, mg/dL | 85.2 (11.4) | 91.7 (16.9)† | 97.9 (16.9)†‡ | <0.001 | 83.7 (8.4) | 88.4 (12.7)† | 93.1 (17.2)†‡ | <0.001 |
| HbA1c, % | 5.46 (0.44) | 5.73 (0.60)† | 5.95 (0.60)†‡ | <0.001 | 5.42 (0.35) | 5.58 (0.47)† | 5.73 (0.58)†‡ | <0.001 |
| Diabetes, n (%) | ||||||||
| All | 22 (3.3) | 98 (13.8) | 58 (29.3) | <0.001 | 40 (1.7) | 168 (6.0) | 83 (11.7) | <0.001 |
| Females | 11 (1.9) | 46 (10.2) | 31 (32.6) | <0.001 | 25 (1.2) | 73 (4.4) | 25 (10.3) | <0.001 |
| Males | 11 (11.7) | 52 (20.2) | 27 (26.2) | 0.037 | 15 (4.2) | 95 (8.4) | 58 (12.4) | <0.001 |
Data are expressed as mean (SD), median [interquartile range], or n (%). Diabetes was considered according to HbA1c ≥6.5% and/or current medication for diabetes. FHD+ was defined as having first-degree relative(s) with diabetes. SDB was classified by Acti-ODI3% as follows: no SDB, <5 events/h; mild SDB, 5 to <15 events/h; MS-SDB, ≥15 events/h. Acti-ODI3% = 3% oxygen desaturation index modified by sleep duration derived from actigraphy; FHD = family history of diabetes; HbA1c, glycated hemoglobin; JESS = Japanese version of the Epworth Sleepiness Scale; MS = moderate to severe; SDB = sleep-disordered breathing. *Kruskal-Wallis test was used to compare differences between groups; analysis of variance was used otherwise. †P < 0.05 vs No SDB, ‡P < 0.05 vs Mild SDB.
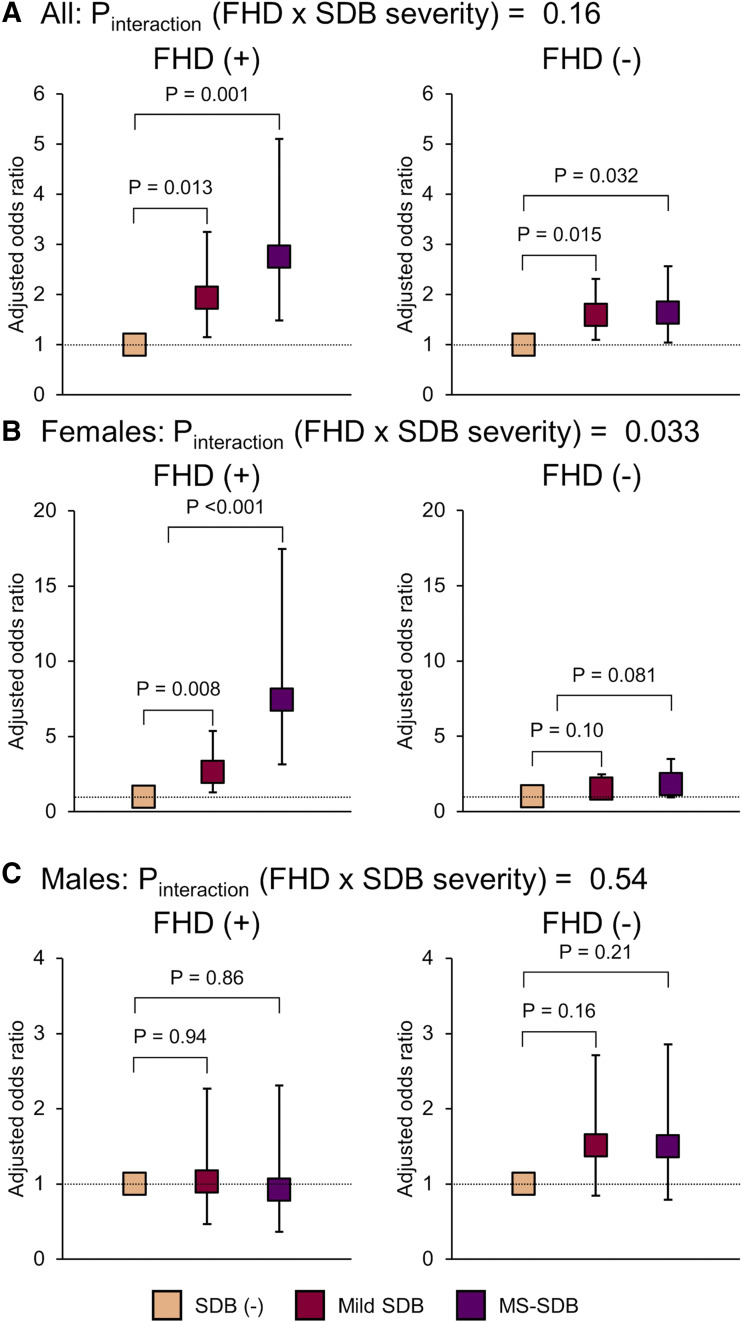
The multivariate logistic regression analyses showed that mild SDB and moderate-to-severe SDB (MS-SDB) were significantly associated with the prevalence of diabetes in all participants and females with FHD but not male participants (odds ratio [95% confidence interval]—overall: mild SDB, 1.93 [1.15–3.25]; MS-SDB, 2.75 [1.48–5.11]; females: mild SDB, 2.63 [1.29–5.37]; MS-SDB, 7.43 [3.16–17.45]; males: mild SDB, 1.03 [0.47–2.27]; MS-SDB, 0.92 [0.37–2.31]) (Figure 3). However, in the analysis of FHD− female and male participants, mild SDB and MS-SDB were not significantly associated with the prevalence of diabetes. There was a significant interaction between FHD and SDB severity only in females (Pinteraction [FHD × SDB severity]: overall, 0.16; females, 0.033, males, 0.54) (Figure 3).
Figure 3. Multivariate logistic regression analysis of the prevalence of diabetes among participants with or without FHD.
(A) All participants; (B) female participants; (C) male participants. Having mild SDB or MS-SDB was significantly associated with the prevalence of diabetes among FHD(+) participants overall, females, and FHD(−) participants overall; however, Pinteraction (FHD × SDB severity) was significant only in females. Analyses were adjusted for age, sex (only A), body mass index, waist circumference, alcohol consumption (ethanol = 0 g/d, 0 g/d < ethanol ≤ 25 g/d, and ethanol >25 g/d), pack-years of smoking, sleep duration categories, activity count, hypertension, and menopause (only B). Diabetes was considered according to HbA1c ≥6.5% and/or current medication for diabetes. SDB was classified by 3% oxygen desaturation index modified by sleep duration from actigraphy as follows: no SDB [SDB (-)], <5 events/h; mild SDB, 5 to <15 events/h; MS-SDB, ≥15 events/h. FHD = family history of diabetes; HbA1c = glycated hemoglobin; SDB = sleep-disordered breathing; MS = moderate to severe.
HOMA-IR according to the severity of SDB and FHD status
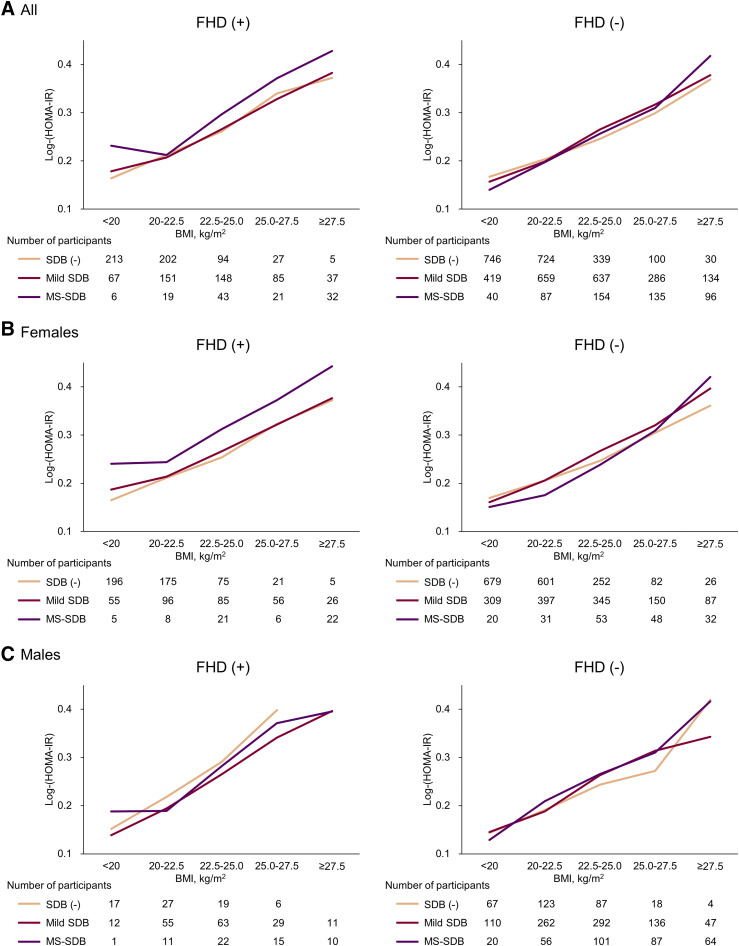
Figure 4 shows log-(HOMA-IR) according to SDB severity and BMI categories among FHD+ and FHD− participants. Only FHD+ females with MS-SDB had consistently higher insulin resistance than those without SDB and mild SDB in each BMI category. Multivariate regression analyses for log-(HOMA-IR) additionally showed that log-(Acti-ODI3%) was positively associated with higher insulin resistance only in FHD+ females (Table 3). Pinteraction (FHD × log-[Acti-ODI3%]) was also significant only in females (overall, 0.051; females, 0.001; males, 0.092).
Figure 4. Insulin resistance by HOMA-IR according to SDB and categories of BMI among participants with or without FHD.
(A) Log-(HOMA-IR) in all participants; (B) log-(HOMA-IR) in female participants; (C) log-(HOMA-IR) in male participants. Among only FHD(+) female participants, those with MS-SDB consistently and clearly showed higher insulin resistance than those without SDB and mild SDB in each BMI category. SDB was classified by the 3% oxygen desaturation index modified by sleep duration from actigraphy as follows: no SDB [SDB (-)], <5 events/h; mild SDB, 5 to <15 events/h; MS-SDB, ≥15 events/h. BMI = body mass index; FHD = family history of diabetes; HOMA-IR = homeostatic model assessment–insulin resistance; MS = moderate to severe; SDB = sleep-disordered breathing.
Table 3.
Multivariate regression analysis for log-(HOMA-IR) according to FHD.
| All | Females | Males | ||||
|---|---|---|---|---|---|---|
| Variables | β | P | β | P | β | P |
| A. Individuals with FHD, n | 1,150 | 852 | 298 | |||
| Age, years | −0.10 | <.001 | −0.10 | .0497 | −0.08 | .14 |
| Body mass index, kg/m2 | 0.39 | <.001 | 0.37 | <.001 | 0.41 | <.001 |
| Waist circumference, cm | 0.20 | <.001 | 0.18 | <.001 | 0.21 | .019 |
| Females | 0.06 | .069 | — | — | — | — |
| Pack-years of smoking | 0.01 | .72 | 0.02 | .56 | 0.002 | .96 |
| Ethanol, g/d | −0.04 | .17 | −0.01 | .71 | −0.04 | .39 |
| Activity count, /minute | −0.09 | <.001 | −0.06 | .032 | −0.13 | .008 |
| Menopause | — | — | −0.01 | .81 | — | — |
| Hypertension | 0.09 | <.001 | 0.09 | .002 | 0.08 | .12 |
| Log-(Acti-ODI3%) | 0.06 | .058 | 0.13 | <.001 | −0.10 | .055 |
| R2 (adjusted R2), % | 36.2 (36.0) | 40.2 (39.6) | 40.9 (39.2) | |||
| B. Individuals without FHD, n | 4,586 | 3,112 | 1,474 | |||
| Age, years | −0.10 | <.001 | −0.11 | <.001 | −0.10 | <.001 |
| Body mass index, kg/m2 | 0.40 | <0.001 | 0.41 | <.001 | 0.34 | <.001 |
| Waist circumference, cm | 0.20 | <0.001 | 0.16 | <.001 | 0.26 | <.001 |
| Females | 0.06 | 0.001 | — | — | — | — |
| Pack-years of smoking | 0.01 | 0.56 | −0.03 | .08 | 0.01 | .60 |
| Ethanol, g/d | −0.07 | <0.001 | −0.03 | .02 | −0.11 | <.001 |
| Activity count, /minute | −0.07 | <0.001 | −0.06 | <.001 | −0.07 | <.001 |
| Menopause | — | — | 0.01 | .59 | — | — |
| Hypertension | 0.09 | <0.001 | 0.10 | <.001 | 0.06 | .011 |
| Log-(Acti-ODI3%) | 0.02 | 0.19 | 0.01 | .68 | 0.02 | .31 |
| R2 (adjusted R2), % | 36.4 (36.3) | 34.7 (34.5) | 39.4 (39.1) | |||
Acti-ODI3% = 3% oxygen desaturation index modified by sleep duration derived from actigraphy; FHD = family history of diabetes; HOMA-IR = homeostatic model assessment–insulin resistance; β = standardized partial regression coefficient.
Prevalence of recent-onset diabetes according to the severity of SDB and FHD status
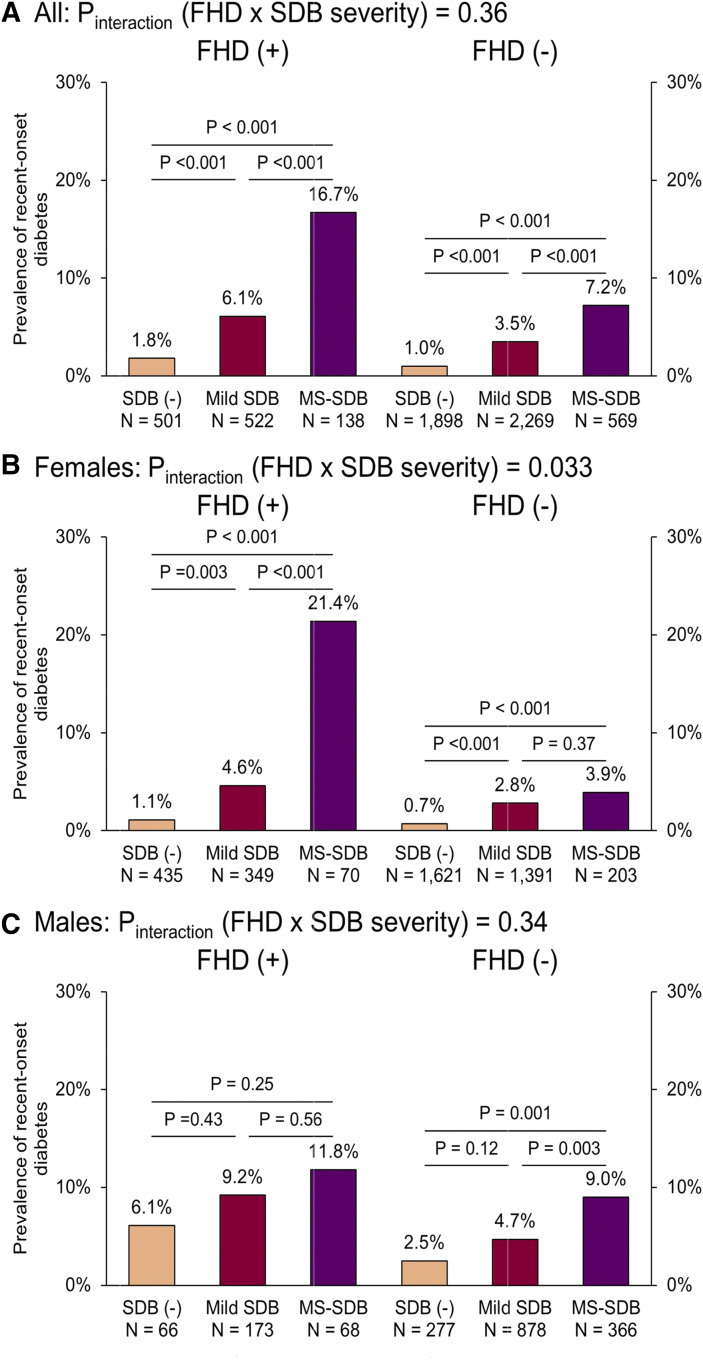
Of the above 5,897 participants, 204 (3.5%) had recent-onset diabetes by the time of the second survey; the mean (SD) interval between the first and second surveys was 4.96 (0.36) years. Figure 5 shows the prevalence of recent-onset diabetes according to SDB severity among FHD+ or FHD− participants. Although the prevalence of recent-onset diabetes increased significantly according to the severity of SDB among the overall population, there were significant differences among the overall FHD+ participants, FHD+ females, and FHD− participants overall regarding the prevalence of recent-onset diabetes. However, a significant Pinteraction (FHD × SDB severity) was seen only in females (P = .033).
Figure 5. Prevalence of recent-onset diabetes according to SDB among participants with or without FHD.
(A) All participants; (B) female participants; (C) male participants. Among FHD(+) participants overall, females, and FHD(−) participants overall, the increase in SDB severity led to a significantly high prevalence of recent-onset diabetes at each degree of SDB severity. However, Pinteraction (FHD × SDB severity) was significant only in females. Recent-onset diabetes was considered according to HbA1c ≥6.5% and/or current use of medication for diabetes at the second survey in participants without diabetes at the first survey. SDB was classified by the 3% oxygen desaturation index modified by sleep duration from actigraphy as follows: no SDB [SDB(−)], <5 events/h; mild SDB, 5 to <15 events/h; MS-SDB, ≥15 events/h. The chi-square test was used to compare the prevalence of recent-onset diabetes between each SDB severity. FHD = family history of diabetes; HbA1c = glycated hemoglobin; MS = moderate to severe; SDB = sleep-disordered breathing.
DISCUSSION
In the present study of a large prospective cohort, we first evaluated associations between the prevalence of diabetes and SDB in the context of an FHD. SDB was associated with the prevalence of type 2 diabetes only in females with FHD. Also, there was a significant interaction between SDB and FHD only in females. Furthermore, in females with an FHD, SDB was related to an increase in insulin resistance. In addition, although the data were not prospective, females with FHD and MS-SDB had the highest prevalence of recent-onset diabetes during the nearly 5 years before SDB measurements. Taken together, MS-SDB might have an association with the increased prevalence of type 2 diabetes in females with FHD.
Clinical and epidemiological studies have consistently reported independent associations between OSA and cardiovascular and metabolic dysfunction as well as the modulation of the adverse impact of obesity in this context.1,2,27–30 FHD is also a well-established risk factor for the development of diabetes.18 However, the potential contributions of SDB on the impact of FHD on diabetes risk have not been explored. Here, the presence of such a contribution was detected among the whole cohort, with clear differences between sexes, whereby SDB did not appear to influence the effect of an FHD in men but displayed a robust effect among women. We considered 2 possible explanations for this sex difference. The first is the effect of menopause. The mean age of the participants was almost 58 years (Table 1); thus, many peri- to postmenopausal females were in the study cohort. Menopausal females generally have multiple risk factors for developing diabetes (eg, central adiposity, androgenicity, and mood disorder), making older females vulnerable to diabetes.31 Second, sex hormone (progesterone and estrogen) levels decrease dramatically following menopause. Female hormones strongly affect hypoxic sensitivity, which is significantly associated with diabetes.32 These 2 factors might account for the sex difference in this study.
In addition, current findings suggest that the impact of the genetic and environmental components of FHD on impaired glucose regulation (probably due to increased insulin resistance) was augmented by SDB. Since diabetes is a polygenic disease, many single nucleotide polymorphisms have been identified as being related to the pathogenesis of the disease. Nevertheless, single nucleotide polymorphisms could not fully explain the heritability of FHD in isolation,33,34 and gene–environment interactions are considered as critical contributors to diabetes risk along with epigenetic modifications. Indeed, some environmental factors provide not only an additive but also a synergistic effect on specific genetic variants through an epigenetic modification such as DNA methylation.35 There is growing evidence that physiological features of SDB have the potential to evoke such epigenetic modifications. For example, exposures of pregnant rodents to intermittent hypoxia and sleep fragmentation induce epigenetic changes to affect metabolic dysfunction in their offspring that only become manifest in late adulthood.36,37 These epigenetic factors are also affected by sex hormones.38 Future research efforts appear warranted to unravel possible mechanistic pathways involving SDB, genetic variants (particularly those associated with insulin resistance), and epigenetic modifications linked to abnormal glucose metabolism.
Clinically, a recent epidemiological study in a similar Japanese cohort showed that SDB was associated with the prevalence of diabetes only in females.17 Indeed, although several longitudinal studies showed that OSA was associated with incident diabetes,7,9,11 treatment of OSA did not necessarily improve glycemic control. Our current findings suggesting that the impact of SDB on glucose metabolism is stronger in females than in males raise the possibility that sex differences might have influenced the outcomes of such trials that included a vast majority of male participants.27,28,39
The combined impact of FHD and SDB on worsening of glucose metabolism could be of clinical importance. Indeed, while FHD includes both genetic and environmental risk factors inherited and shared among family members, it is estimated that genetic components predominantly account for an FHD.33,40 FHD is readily accessible clinical information; however, the genetic aspects of an FHD are unmodifiable. In contrast, SDB is a treatable disease. In clinical practice, physicians should pay close attention to the potential incidence or the high prevalence of abnormal glucose tolerance status in females with SDB and an FHD and recognize that such populations may benefit through glycemic control by SDB treatment, a proposition that will require corroboration by appropriate clinical trials.
We acknowledge several limitations in this study. First, analyses were cross-sectional, and the findings cannot be considered as providing causal inferences. Although data were not prospective, the higher prevalence of recent-onset diabetes in female participants with both an FHD and MS-SDB than with either one of those factors would support the hypothesis that MS-SDB might induce diabetes in female participants with an FHD. Second, we did not assess objective sleep parameters by polysomnography and could not distinguish the SDB type. However, although the participants with SDB in our cohort could include not only those with OSA but also those with other types of SDB, the prevalence of other types of SDB (eg, central sleep apnea) is exceedingly less common than OSA in the general population.41 Third, recall bias of FHD may exist. Participants without diabetes may not have remembered their FHD precisely. Fourth, we analyzed a homogeneous ethnic group (only Japanese). As shown in a prior study, racial differences may have existed with regard to the impact of SDB on glucose metabolism.42 Frequencies of genetic variants associated with diabetes and lifestyle vary across ethnicities, and therefore expansion of studies like the present one to other racial backgrounds is of importance.
In summary, SDB was significantly associated with a high prevalence of diabetes and a recent incidence of diabetes in females with an FHD in this cohort. Therefore, if women with OSA and an FHD are identified in clinical settings, careful evaluation of their glucose metabolism would be recommended. Future studies on genetic factors that influence the clinical phenotype and the beneficial effects of SDB treatment on glycemic control in the presence or absence of underlying FHD appear warranted.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Kyoto University and Nagahama Municipal Office. This study was funded by a University Grant; a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (26293198, 17H04182, 20K17860); the Center of Innovation Program; and the Global University Project from Japan Science and Technology Agency, Japan Agency for Medical Research and Development (AMED) under grant numbers dk0207006, ek0109070, ek0109283, ek0109196, ek0109348, kk0205008, ek0210066, and ek0210096, ek0210116; grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology; the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group from the Ministry of Health, Labor, and Welfare of Japan; the Research Foundation for Healthy Aging, and the Ministry of Health, Labour, and Welfare Sciences Research grants; Research on Region Medical (H30-31-iryo-ippan-009). D.G. is supported by National Institutes of Health grants HL130984 and HL140548. The Department of Respiratory Care and Sleep Control Medicine is funded by endowments from Philips-Respironics, ResMed, Fukuda Denshi, and Fukuda Lifetec Keiji to Kyoto University. Takuma Minami reports personal fees from Teijin Zaitakuiryou, outside the submitted work. Yasuharu Tabara reports grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan and grants from Japan AMED, during the conduct of the study. Kimihiko Murase, and Hirofumi Takeyama report grants from Philips-Respironics, grants from ResMed, grants from Fukuda Denshi, grants from Fukuda Lifetec Keiji, and grants from Teijin Pharma, outside the submitted work. Naomi Takahashi and Yoshinari Nakatsuka report grants from Philips-Respironics, grants from ResMed, grants from Fukuda Denshi, and grants from Fukuda Lifetec Keiji, outside the submitted work. Toru Oga reports grants from Philips-Respironics, ResMed, Fukuda Denshi, and Fukuda Lifetec Keiji, during the conduct of the study (he previously belonged to the Department of Respiratory Care and Sleep Control Medicine, Kyoto University, which has been funded by endowments from these companies). Fumihiko Matsuda reports grants from Kyoto University; grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan; grants from Japan AMED; and grants from the Takeda Medical Research Foundation, during the conduct of the study. Kazuo Chin reports grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; grants from the Center of Innovation Program and the Global University Project from Japan Science and Technology Agency; the Japan AMED, during the conduct of the study; grants and personal fees from Philips-Respironics; grants and personal fees from ResMed; grants and personal fees from Fukuda Denshi; grants and personal fees from Fukuda Lifetec Keiji; grants and personal fees from Teijin Pharma; grants from Kyorin Pharmaceutical Co, Ltd; grants from Nippon Boehringer Ingelheim Co, Ltd; grants and personal fees from GlaxoSmithKline; personal fees from MSD; personal fees from Astellas Pharma; personal fees from Eisai Co, Ltd; grants and personal fees from Resmed Japan; personal fees from Mylan EPO; Research Grants on Region Medical (H30-31-iryo-ippan-009) from the Ministry of Health, Labor and Welfare in Japan; and grants from the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group from the Ministry of Health, Labor and Welfare of Japan, outside the submitted work. The other authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank Nagahama City Office and the Zeroji Club, a nonprofit organization, for their assistance in conducting the Nagahama study. The authors thank Yoshiro Toyama, Masanori Azuma, Ryo Tachikawa, and Morito Inouchi (Department of Respiratory Medicine, Graduate School of Medicine, Kyoto University) for their assistance in collecting data. We also thank Chiaki Tojo and Makoto Yamashita (Nursing Science, Human Health Sciences, Graduate School of Medicine, Kyoto University) for their assistance in analyzing the data of actigraphy.
ABBREVIATIONS
- Acti-ODI3%
3% oxygen desaturation index modified by sleep duration derived from actigraphy
- BMI
body mass index
- FHD
family history of diabetes
- HOMA-IR
homeostatic model assessment–insulin resistance
- OSA
obstructive sleep apnea
- MS-SDB
moderate-to-severe sleep-disordered breathing
- SDB
sleep-disordered breathing
REFERENCES
- 1.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. 10.1016/j.jacc.2013.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C-J, Kao T-W, Chang Y-W, Chen W-L. Examining the association between obstructive sleep apnea and cardiometabolic risk factors in the elderly. Sleep Biol Rhythms. 2018;16(2):231–237. 10.1007/s41105-018-0145-y [DOI] [Google Scholar]
- 3.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. 10.1164/rccm.200909-1423OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186(5):434–441. 10.1164/rccm.201112-2135OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada Y, Oga T, Chin K, et al. Differences in relationships among sleep apnoea, glucose level, sleep duration and sleepiness between persons with and without type 2 diabetes. J Sleep Res. 2012;21(4):410–418. 10.1111/j.1365-2869.2012.00997.x [DOI] [PubMed] [Google Scholar]
- 6.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. 10.1164/rccm.200504-637OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122(12):1122–1127. 10.1016/j.amjmed.2009.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall NS, Wong KKH, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5(1):15–20. 10.5664/jcsm.27387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celen YT, Hedner J, Carlson J, Peker Y. Impact of gender on incident diabetes mellitus in obstructive sleep apnea: a 16-year follow-up. J Clin Sleep Med. 2010;6(3):244–250. 10.5664/jcsm.27821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindberg E, Theorell-Haglöw J, Svensson M, Gislason T, Berne C, Janson C. Sleep apnea and glucose metabolism: a long-term follow-up in a community-based sample. Chest. 2012;142(4):935–942. 10.1378/chest.11-1844 [DOI] [PubMed] [Google Scholar]
- 11.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes: a historical cohort study. Am J Respir Crit Care Med. 2014;190(2):218–225. 10.1164/rccm.201312-2209OC [DOI] [PubMed] [Google Scholar]
- 12.Huang T, Lin BM, Stampfer MJ, Tworoger SS, Hu FB, Redline S. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. cohorts. Diabetes Care. 2018;41(10):2111–2119. 10.2337/dc18-0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Bi Y, Zhang Q, Pan F. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology. 2013;18(1):140–146. 10.1111/j.1440-1843.2012.02267.x [DOI] [PubMed] [Google Scholar]
- 14.Ip MSM, Lam B, Ng MMT, Lam WK, Tsang KWT, Lam KSL. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. 10.1164/ajrccm.165.5.2103001 [DOI] [PubMed] [Google Scholar]
- 15.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators . Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. 10.1093/aje/kwh261 [DOI] [PubMed] [Google Scholar]
- 16.Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11(4):475–485. 10.5664/jcsm.4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto T, Murase K, Tabara Y, et al. Impact of sleep characteristics and obesity on diabetes and hypertension across genders and menopausal status: the Nagahama study. Sleep. 2018;41(7):1–10. [DOI] [PubMed] [Google Scholar]
- 18.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. 10.1016/j.smrv.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 19.van Dam RM, Boer JM, Feskens EJ, Seidell JC. Parental history of diabetes modifies the association between abdominal adiposity and hyperglycemia. Diabetes Care. 2001;24(8):1454–1459. 10.2337/diacare.24.8.1454 [DOI] [PubMed] [Google Scholar]
- 20.Sargeant LA, Wareham NJ, Khaw KT. Family history of diabetes identifies a group at increased risk for the metabolic consequences of obesity and physical inactivity in EPIC-Norfolk: a population-based study. Int J Obes Relat Metab Disord. 2000;24(10):1333–1339. 10.1038/sj.ijo.0801383 [DOI] [PubMed] [Google Scholar]
- 21.Chen Y-C, Hsu P-Y, Hsiao C-C, Lin M-C. Epigenetics: a potential mechanism involved in the pathogenesis of various adverse consequences of obstructive sleep apnea. Int J Mol Sci. 2019;20(12):2937. 10.3390/ijms20122937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minami T, Matsumoto T, Tanizawa K, et al. Impact of sleep disordered breathing on diabetes based on presence or absence of family history of diabetes: the Nagahama study. Abstract. Am J Respir Crit Care Med. 2018;197:A6220. [Google Scholar]
- 23.Matsumoto T, Murase K, Tabara Y, et al. Sleep disordered breathing and metabolic comorbidities across sex and menopausal status in East Asians: the Nagahama study. Eur Respir J. 2020;56(2):1902251. 10.1183/13993003.02251-2019 [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes–2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- 25.Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009;10(5):556–565. 10.1016/j.sleep.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 26.Waki K, Noda M, Sasaki S, et al. ; JPHC Study Group . Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet Med. 2005;22(3):323–331. 10.1111/j.1464-5491.2004.01403.x [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Cerón E, Barquiel B, Bezos A-M, et al. Effect of CPAP on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. a randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–485. 10.1164/rccm.201510-1942OC [DOI] [PubMed] [Google Scholar]
- 28.Shaw JE, Punjabi NM, Naughton MT, et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med. 2016;194(4):486–492. 10.1164/rccm.201511-2260OC [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Liu Z, Yang H, Luo Q. Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2013;17(1):33–38. 10.1007/s11325-012-0680-8 [DOI] [PubMed] [Google Scholar]
- 30.Toyama Y, Murase K, Azuma M, et al. Impact of long-term continuous positive airway pressure on liver fat in male obstructive sleep apnea patients with fatty liver. Sleep Biol Rhythms. 2018;16(1):117–124. 10.1007/s41105-017-0133-7 [DOI] [Google Scholar]
- 31.Kim C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Wom Health Lond. 2012;8(2):155–167. 10.2217/WHE.11.95 [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15(1):21–32. 10.1038/s41574-018-0096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornelis MC, Zaitlen N, Hu FB, Kraft P, Price AL. Genetic and environmental components of family history in type 2 diabetes. Hum Genet. 2015;134(2):259–267. 10.1007/s00439-014-1519-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott RA, Langenberg C, Sharp SJ, et al. ; InterAct Consortium . The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: the EPIC-InterAct study. Diabetologia. 2013;56(1):60–69. 10.1007/s00125-012-2715-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davegårdh C, García-Calzón S, Bacos K, Ling C. DNA methylation in the pathogenesis of type 2 diabetes in humans. Mol Metab. 2018;14:12–25. 10.1016/j.molmet.2018.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalyfa A, Cortese R, Qiao Z, et al. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol Lond. 2017;595(8):2551–2568. 10.1113/JP273570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortese R, Khalyfa A, Bao R, Andrade J, Gozal D. Epigenomic profiling in visceral white adipose tissue of offspring of mice exposed to late gestational sleep fragmentation. Int J Obes Lond. 2015;39(7):1135–1142. 10.1038/ijo.2015.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62(11):969–974. 10.1136/thx.2006.074351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemminki K, Li X, Sundquist K, Sundquist J. Familial risks for type 2 diabetes in Sweden. Diabetes Care. 2010;33(2):293–297. 10.2337/dc09-0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353–1359. 10.5665/sleep.5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakker JP, Weng J, Wang R, Redline S, Punjabi NM, Patel SR. Associations between obstructive sleep apnea, sleep duration, and abnormal fasting glucose the multi-ethnic study of atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2015;192(6):745–753. 10.1164/rccm.201502-0366OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.