Abstract
Glycosylation of IgG Fc domains is a central mechanism in the diversification of antibody function. Modifications to the core Fc glycan impact antibody function by shifting the balance of Type I and Type II Fc gamma receptors (FcγR) that will be engaged by immune complexes. This, in turn, modulates the effector cells and functions that can be recruited during immune activation. Critically, humans have evolved to regulate Fc glycan modifications for immune homeostasis. Dysregulation in Fc glycan modifications can lead to loss of immune tolerance, symptomatic autoimmunity, and susceptibility to infectious diseases. Here, we discuss IgG Fc glycosylation and its role in human health and disease.
1. Introduction
Recent studies have defined the considerable heterogeneity that exists among people in specific IgG Fc glycan modifications that impact antibody activity in vivo (Selman et al. 2012a, b; Wang et al. 2015, 2017; Mahan et al. 2016; Mahan et al. 2015). Intriguingly, individuals produce distinct basal repertoires of Fc glycoforms which are quite stable over periods of weeks to months (Wang et al. 2015). Because Fc glycan modifications directly affect the ability of IgGs to recruit various effector cell populations, this heterogeneity is likely a significant driver of immune diversity across the population.
The ability of IgG antibodies to mediate effector functions arises from their capacity to bridge antigen binding through the Fab domain with the recruitment of effector cells through interactions between the Fc domain and FcγRs. Because the majority of FcγRs have low affinity for monomeric IgGs, Fc-FcγR interactions occur when multivalent IgG-antigen immune complexes are formed, thus enabling avidity-based interactions and conferring specificity to the effector cell response.
The structure of the Fc domains contained in a given immune complex determines which effector cells and FcγRs can be engaged by the complex. Fc structure, in turn, is determined by two variables: the IgG subclass and the composition of a complex biantennary glycan that is present on all IgG heavy chains within the CH2 domain. Four IgG subclasses are found in humans (IgG1–4), with IgG1 and IgG3 having highest affinity for activating Type I FcγRs (FcγRI, FcγRIIa, FcγRIIIa). In contrast, IgG2 has highest affinity of all subclasses for the inhibitory FcγR, FcγRIIb (Fig. 1).
Fig. 1. Heterogeneity in the human IgG Fc domain repertoire.
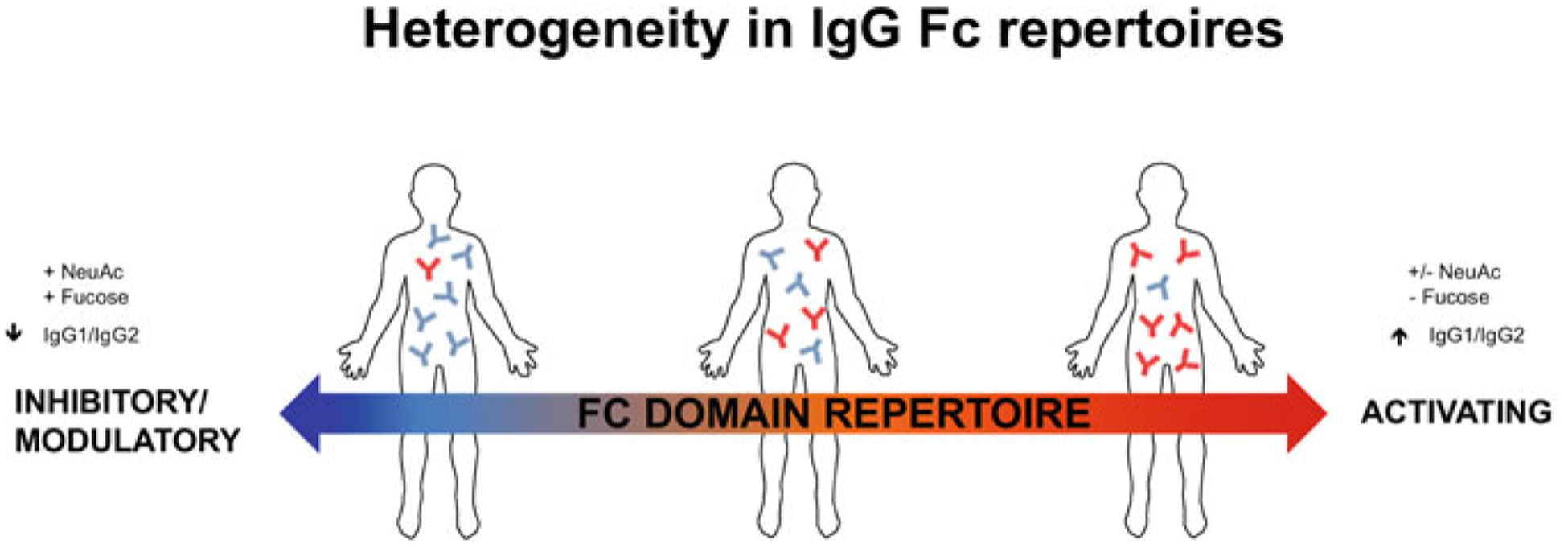
IgG repertoires vary across the population by ratios of activating to inhibitory IgG subclasses ((IgG1+IgG3)/IgG2) and in the abundance of Fc glycoforms that impact Fc domain structure and antibody function. Fucosylated, sialylated Fc glycoforms impart reduced Type I FcγR binding activity and enable binding to the Type II FcγRs. Afucosylated, sialylated or asialylated Fc glycans mediate pro-inflammatory effector functions by virtue of increased affinity for the activating Type I FcγR, FcγRIIIa
The activity of different IgG subclasses is further tuned by modifications to the Fc glycan which can impart diverse and potent effector functions to IgG1s and likely to the other subclasses, though how Fc glycosylation impacts the activity of IgG2, IgG3 and IgG4 has yet to be described. Overall, the composition of IgG subclasses and Fc glycans within immune complexes determines whether they will trigger pro- or anti-inflammatory effector cell activity and regulates the quality of the adaptive immune response against the antigen(s) in complex (Wang et al. 2015; Regnault et al. 1999; Getahun et al. 2004; de Jong et al. 2006; Ding et al. 2016; Hjelm et al. 2008).
2. Structure and Assembly of Fc Glycans
Mature Fc glycoforms are N-linked, complex biantennary structures, present at asparagine 297 of all heavy chains (Kao et al. 2015). The core Fc glycan is composed of seven saccharides and is required for maximal binding to FcγRs: 4 N-acetylglucosamine (GlcNAc) and 3 mannose (Man) residues (Lux et al. 2013). This core glycan can be modified by additional sugars, including a core fucose (Fuc), bisecting GlcNAc, galactose (Gal) at one or both arms and, in the presence of galactose, N-acetylneuraminic acid (NeuAc) or sialic acid (Fig. 2). Two modifications to the IgG1 Fc, fucosylation and sialylation, have well defined functions in vivo and will be discussed in more detail below. How bisecting GlcNAc impacts IgG function is not yet well understood. Some studies indicate a role for bisection in modulation of FcγRIIIa-mediated activities, however data on this are not consistent and any phenotype related to FcgRIIIa binding is clearly less pronounced than what can be achieved through afucosylation of Fc glycans (Hodoniczky et al. 2005; Shinkawa et al. 2003). Galactosylation of the Fc is significant as a precursor to sialylation but does not significantly limit the abundance of Fc sialylation as galactosylation occurs with several fold greater frequency than sialylation (Wang et al. 2015; Wuhrer et al. 2015). A direct role for galactosylated Fc glycans in modulation of immune function has not been defined. Overall, 11 distinct complex biantennary Fc glycoforms comprise ~90% of the human IgG1 repertoire (Table 1). In addition to complex biantennary glycans, up to ~2% of Fc glycans on serum IgG1 may be a combination of hybrid and high mannose forms (Flynn et al. 2010 and Wang, unpublished observation).
Fig. 2. Structure of IgG Fc glycans.
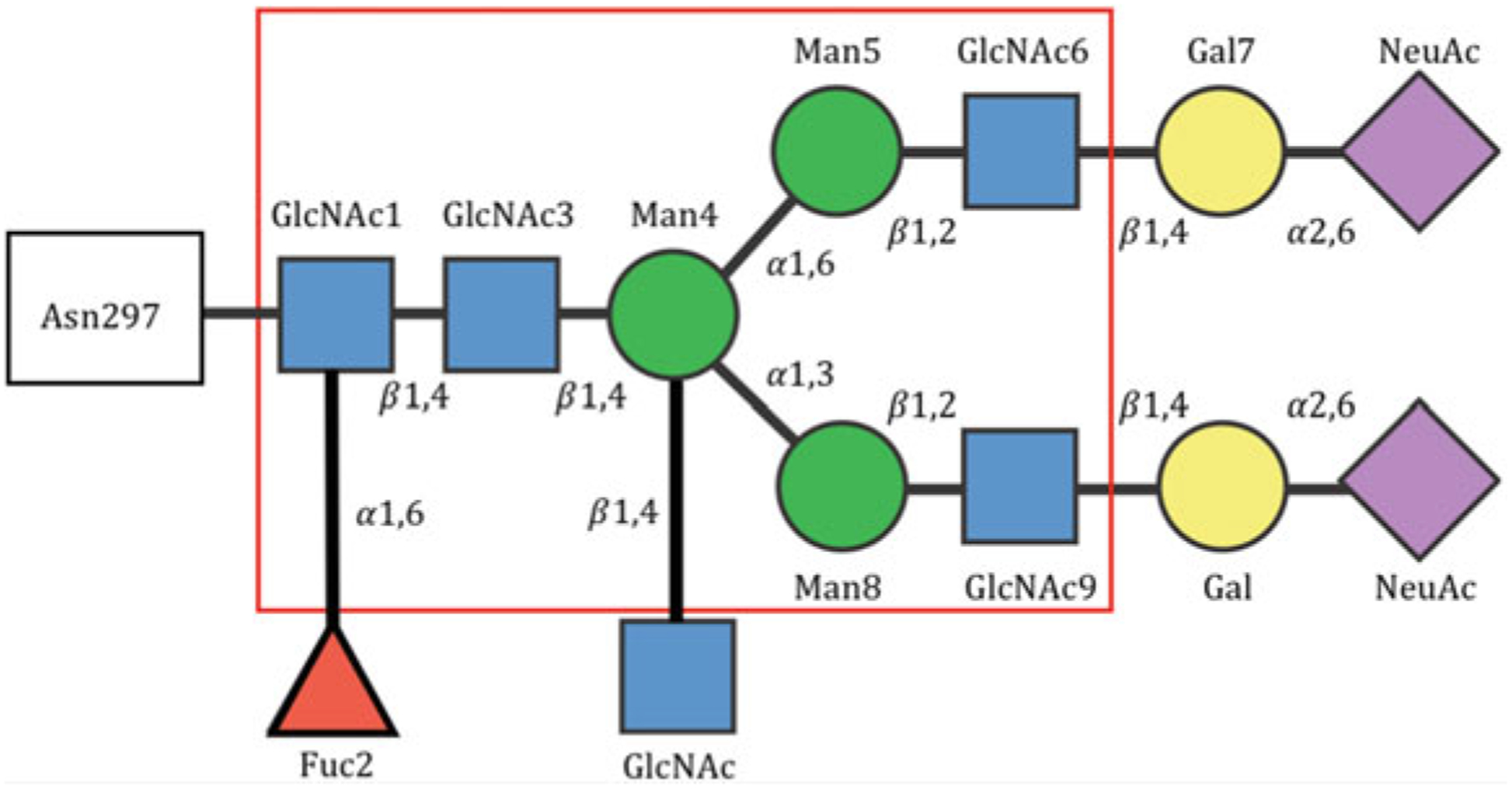
Fc glycoforms are present at Asn 297 within CH2 domains and are complex biantennary glycans. The core Fc glycan (boxed in red) is composed of seven saccharides: 4 N-acetylglucosamine (GlcNAc) and 3 mannose (Man) residues. This core glycan can be modified by a core fucose (Fuc), bisecting GlcNAc, galactose (Gal) at one or both arms and, in the presence of galactose, N-acetyl-neuraminic acid or sialic acid (NeuAc)
Table 1. Set of 11 modifications to the core Fc glycan which, together, comprise ~90% of human IgG1 Fc glycoforms. Relative quantification of the 11 modifications is shown.
G: Galactose; 0,1,2 indicate the number of Gal residues; F: Fucose; N: bisecting GlcNAc; S: sialic acid. Data are from a commercial IVIg preparation
| Core glycan modification | IgG1 Fc (% total) |
|---|---|
| G0 | 0.18 |
| G1 | 2.04 |
| G2 | 3.16 |
| G0F | 1.72 |
| G1F | 27.11 |
| G2F | 38.28 |
| G0FN | 0.72 |
| G1FN | 7.00 |
| G2FN | 0.81 |
| G1FS | 1.03 |
| G2FS | 17.96 |
| 100.00 |
Assembly of Fc glycans begins with oligomannose forms that are pre-assembled on a lipid, polyprenol dolichol pyrophosphate. The precursor Fc glycan is assembled first by transfer of sugar residues on the cytoplasmic face of the endoplasmic reticulum (ER), followed by transport of the partial precursor across the ER membrane to the luminal side where the full oligomannose N-glycan precursor is assembled. The triantennary precursor is comprised of two GlcNAc, nine Man and three glucose (Glc) residues. Within the ER, this Glc3Man9GlcNac2 oligosaccharide is transferred to Asn 297 of the IgG heavy chain. The Fc glycan then undergoes remodeling by membrane-anchored glycosidases and glycosyltransferases within the ER and the golgi apparatus, ultimately forming the mature, complex, biantennary glycan structure (Fig. 2). Addition of bisecting GlcNAc is accomplished by the activity of β-1,4-N-Acetylglucosaminyltransferase III (GNT-III) while addition of a core fucose is performed by α-1,6-fucosyltransferase (FUT8). It has been observed that FUT8 activity is inhibited by the presence of bisecting GlcNAc. Thus, fucosylation is likely the initial modification in glycans that are both fucosylated and bisected. Increasing the abundance of afucosylated Fc glycoforms in in vitro expression systems has been achieved by increasing expression of GNT-III and/or by engineering localization of GNT-III to the upper golgi where it can act prior to FUT8 (Ferrara et al. 2006). Galactosylation of one or both arms of the bisected Fc glycan is performed by β-1,4-galactosyltransferase 1 (B4GALT1) and, in the presence of galactosylation, terminal sialylation is catalyzed by α-2,6-sialyltransferase 1 (ST6GAL1) which preferentially adds sialic acid to the α-1,3 arm of the biantennary glycan (Barb et al. 2009).
3. IgG Fc Glycans in Inflammation
Antibody-mediated inflammatory responses are central in protection against infectious diseases and malignancies. While inflammatory effector functions can protect against disease, dysregulation of the response can result in autoimmunity or other pathologic inflammation. Balanced signaling through Type I and Type II FcγRs is required for moderation of inflammatory responses. This balance is achieved, in part, through regulated sialylation of Fc glycans, which is the determinant of binding to Type II FcγRs; it is based on the ability to interact with sialylated immune complexes that Type I and Type II FcγRs are distinguished. Fc sialylation has the effect of reducing binding to Type I FcγRs while enabling engagement of Type II FcγRs.
Increased Type II FcγR signaling due to the presence of sialylated Fc glycans can trigger potent anti-inflammatory activity. A classic example of this is observed with administration of high dose intravenous immunoglobulin (IVIg) during acute inflammatory diseases such as immune thrombocytopenia, Kawasaki disease, chronic inflammatory demyelinating polyneuropathy and Guillain-Barre syndrome. IVIg is pooled IgG from thousands of donors and its anti-inflammatory activity is mediated by the minor subset of IgGs within the pool that contain sialylated Fcs (Imbach et al. 1981; Debre et al. 1993; Kaneko et al. 2006). Anti-inflammatory activity can be increased by enhancing the abundance of sialylated Fcs within the IgG pool (Washburn et al. 2015). Sialylated Fcs in IVIg signal through the Type II FcγR DC-SIGN on regulatory macrophages leading to production of interleukin 33 (IL-33). IL-33, in turn, triggers release of IL-4 by basophils, resulting in increased FcγRIIb expression on effector myeloid cells, including monocytes and macrophages at sites of inflammation (Anthony et al. 2011; Schwab et al. 2012; Schwab et al. 2014). A recent study of in vivo sialylation by recombinantly produced glycosyltransferases found that increasing sialylation of endogenous IgG could treat autoimmune sequelae in a similar pathway that required DC-SIGN and FcγRIIb (Pagan et al. 2017). Regulated expression of FcγRIIb constitutes a powerful mechanism for controlling inflammation, with increased FcγRIIb expression elevating the threshold of cellular activation and effectively reducing the inflammatory response by these cells (Santiago-Raber et al. 2009; Dhodapkar et al. 2007; Kaneko et al. 2006; Boruchov et al. 2005; Lehmann et al. 2012). In addition to the DC-SIGN/FcγRIIb pathway, IL-33 release induced by administration of sialylated Fcs can trigger a potent regulatory T cell response that is sufficient to control T cell mediated inflammation in the experimental autoimmune encephalomyelitis model (Fiebiger et al. 2015). Fc glycan sialylation is thus a central regulator of inflammation. Interestingly, differences in baseline Fc sialylation among people can exceed 20% in health, a magnitude well within the range that is associated with changes in Type II FcγR-mediated processes and inflammatory activity (Wang et al. 2015; Pfeifle et al. 2016; Maamary et al. 2017; Fokkink et al. 2014). This implicates Fc sialylation as a driver of diversity in inflammatory thresholds among people.
Another mechanism by which IgG Fc glycoforms act in homeostatic regulation of inflammation involves the Type I FcγR, FcγRIIIa. This activating receptor is expressed on NK cells and subsets of monocytes and macrophages where it mediates activities including cell cytotoxicity, effector cell activation, inflammatory cytokine production and is a minor mediator of phagocytosis (relative to FcγRIIa) (Rafiq et al. 2013; Shalova et al. 2012; Yeap et al. 2016). Dysregulated FcγRIIIa activity is associated with pathologic sequelae such as alloantibody mediated diseases in neonates (Kapur et al. 2014; Sonneveld et al. 2016; Wuhrer et al. 2009) and progression to severe disease and autoimmune pathology during dengue infection. A critical mechanism for controlling signaling through FcγRIIIa in vivo is through regulation of Fc glycan fucosylation. Specifically, IgG1 Fc glycans lacking a core fucose residue (afucosylated Fc glycans) have higher affinity for FcγRIIIa (and FcγRIIIb) due to an unusual, stabilizing sugar-sugar interaction (Ferrara et al. 2011). While afucosylation impacts the affinity of both FcγRIIIa and FcγRIIIb, FcγRIIIb is expressed solely on neutrophils and lacks signaling capacity; thus, the major role of fucosylation on antibody activity is through modulation of FcγRIIIa binding. Afucosylated IgG1 antibodies have approximately 5-fold or 2-fold enhanced affinity for the low (F158) and high (V158) isoforms of FcγRIIIa, respectively.
The role of afucosylated Fc glycoforms in modulating inflammatory responses is well demonstrated in dengue infections, where antibodies play a direct role in mediating the severe forms of disease, dengue hemorrhagic fever and dengue shock syndrome. While anti-dengue antibodies can protect, they can also trigger progression to these severe disease states that cause a majority of dengue-associated deaths. Disease progression is largely mediated by virus particles that are bound by IgGs that don’t neutralize the virus. Instead, the virus immune complexes increase virus infection and modulate cytokine production in FcγR-bearing cells (Halstead 2009). Risk for severe dengue is nearly doubled in individuals who produce high levels (≥ 10%) of afucosylated Fc glycans, implicating FcγRIIIa in the pathogenesis of severe dengue (Wang et al. 2017). In healthy adults, afucosylated of IgG1 Fc glycans are nearly always found at levels <10% of all glycoforms but can reach >20% in some individuals. During actue dengue infections, afucosylation of IgG1 is elevated and can approach 30% in some individuals with severe disease (Wang et al. 2017).
One mechanism by which afucosylated IgGs impact dengue disease is through the activity of antibodies that cross-react between the dengue non-structural protein 1 (NS1) and a platelet antigen (Wan et al. 2016). Highly afucosylated anti-NS1 IgGs can mediate platelet loss in vivo, a defining feature of severe dengue disease. Aside from mediating platelet loss, increased afucosylated anti-dengue IgGs during acute infection correlate with increases in hematocrit, the hallmark of plasma leak which defines severe dengue (Wang et al. 2017). This association suggests an additional role for afucosylated Fc glycoforms and FcγRIIIa in severe dengue through an inflammatory cascade that results in vascular permeability. The role of afucosylated IgGs in dengue infection is a clear example of how the basal Fc glycoform repertoire can drive heterogeneity in human disease susceptibilities and outcomes.
4. IgG Fc Glycans and Adaptive Immunity
Immune complex interactions with FcγRs mediate several cellular processes involved in the maturation of high affinity antibody responses. These include: efficient transport of antigen to the germinal center, processing and presentation of antigens to T cells, and selection of B cells based on affinity of the B cell receptor (Wang et al. 2015; Regnault et al. 1999; Getahun et al. 2004; de Jong et al. 2006; Ding et al. 2016; Hjelm et al. 2008). The role of sialylated Fc glycans within immune complexes in B cell selection was recently discovered and involves a mechanism whereby Type I and Type II FcγRs act in cis to promote selection of B cells with high affinity B cell receptors (BCRs) (Wang et al. 2015; Maamary et al. 2017).
The mechanism by which sialylated Fc glycans impact B cell selection relies on the activity of FcγRIIb in regulating thresholds for B cell activation. Expression of activating Type I FcγRs is nearly always coupled to expression of the inhibitory Type I FcγRIIb; this combined expression ensures balanced signaling and is required by most leukocyte types for specificity of cellular maturation and effector activities (Boruchov et al. 2005; Clynes et al. 1999; Dhodapkar et al. 2005; McGaha et al. 2005). B cells represent an important exception to this rule as they express FcγRIIb throughout development, without co-expression of activating FcγRs. FcγRIIb on B cells acts to moderate the cell-activating signals that are triggered by antigen binding to the B cell receptor (BCR). Thus, a key variable in regulation of B cell activation is the expression level of FcγRIIb, which changes with development of B cells, but is also inducible. In the absence of inhibitory FcγRIIb or with low-level expression or signaling, B cells lack appropriate activation thresholds and produce higher titer, low avidity IgGs. The activation thresholds defined through FcγRIIb signaling are in fact required for maintenance of immune tolerance, with autoantibody production observed in FcγRIIb−/− mice and low levels of B cell FcγRIIb found in patients with autoantibody mediated diseases (McGaha et al. 2005; Fukuyama et al. 2005; Kono et al. 2005; Li et al. 2014; Su et al. 2004; Tackenberg et al. 2009; Mackay et al. 2006; Baerenwaldt et al. 2011). FcγRIIb signaling alone on B cells, as can occur in the presence of immune complexes formed from antigens that are not reactive with the BCR, induces pro-apoptotic signals. This pro-apoptotic signaling is attenuated by BCR engagement, ensuring preservation of cells that can respond to relevant antigens (Ono et al. 1997; Pearse et al. 1999).
As FcγRIIb is a critical determinant of B cell selection, regulation of its expression over time is essential. Temporal association of B cell FcγRIIb expression with the presence of antigen is achieved, in part, by regulation of Fc sialyation on antigen-specific IgGs. This was discovered in studies that characterized a shift towards sialylated Fc glycan production on anti-hemagglutinin (HA) IgGs by day 7 post influenza virus vaccination (Selman et al. 2012a; Wang et al. 2015). To determine the role of sialylated immune complexes on maturation of the vaccine response, experiments were done which found that sialylated immune complexes could act through the Type II FcγR, CD23, to induce increased expression of FcγRIIb on B cells. Elevated FcγRIIb, in turn, increases the threshold for activation of B cells that occurs with BCR signaling, resulting in selection of higher affinity, antigen-specific B cells. This mechanism can be recapitulated experimentally by administration of sialylated immune complexes to mice, leading to increased FcγRIIb on germinal center B cells and production of higher affinity antibodies in a CD23-dependent manner (Wang et al. 2015; Maamary et al. 2017). How other modulations in Fc glycoforms that occur following vaccination impact maturation of the vaccine response has yet to be discovered.
5. Heterogeneity and Regulation of Fc Glycoforms
While the regulators of Fc glycan modifications are not well understood, it is clear that multiple mechanisms impact the Fc glycoform repertoire in humans. One type of regulation is apparent at the level of antigen specificity. An example of this is observed in the antibody response against the influenza HA, in which anti-HA globular head IgGs are significantly more sialylated and fucosylated than stalk-reactive IgGs (Wang et al. 2015). Significant differences in IgG1 Fc sialylation are also observed between IgGs that react with H1 or H3 subtype HA glycoproteins (Wang, unpublished observation). Total IgG Fc glycans (all subclasses) also vary with Fab specificity when comparing IgGs reactive with different HIV proteins in infected individuals (Mahan et al. 2016). Other examples of correlations between Fc glycosylation and Fab specificity have been found in autoimmune diseases such as granulomatosis with polyangiitis (GPA) and rheumatoid arthritis (RA). These diseases are marked by the presence of autoantibodies such as anti— PR3 and anti-ACPA, found in GPA and RA respectively. Anti-PR3 and anti-ACPA can have reduced Fc sialylation over total IgG and may contribute to disease pathogenesis during disease flares through increased Type I FcγR-mediated cellular activation (Wuhrer et al. 2015; Kaneko et al. 2006; Scherer et al. 2010).
Distinctions in Fc glycosylation that are linked to Fab specificity are likely, in part, a consequence of selective glycosylation by different IgG-producing B cell subsets. This is supported by the finding that plasmablasts and memory B cells produce distinct amounts of ST6GAL1 and FUT8, the glycosyltransferases responsible for Fc sialylation and fucosylation, respectively (Wang et al. 2015). Expansion of plasmablasts, which express higher levels of ST6GAL1 and FUT8 in the days following vaccination, is mirrored by production of highly sialylated, fucosylated anti-HA IgGs. In contrast, anti-stalk IgGs, which are likely to be derived from the memory B cell pool, are modified by lower levels of Fc sialylation and fucosylation (Wang et al. 2015).
A second level of Fc glycan regulation occurs with the IgG subclass. Fc glycoforms are distinct depending on the IgG subclass with IgG1 trending toward lower sialylation and fucosylation than other subclasses (Wuhrer et al. 2015; Wang, unpublished observation). The activity of various Fc glycoforms on IgG2, IgG3 and IgG4 subclasses is not yet known however, so how this differential glycosylation contributes to immune homeostasis remains to be discovered.
In addition to differences in Fc glycosylation that occur with Fab specificity or IgG subclass, Fc glycan repertoires vary with the physiologic compartment from which the IgG was isolated. For example, IgGs from the cerebrospinal fluid of patients with multiple sclerosis have reduced sialylation compared with matched donor serum IgGs (Wuhrer et al. 2015). Another study found that Fc galactosylation, the precursor to sialylation, is reduced on synovial fluid IgGs relative to serum IgGs in RA patients (Scherer et al. 2010). The basis of differences in Fc glycosylation associated with physiologic compartments is not known but may involve production by distinct B cell subsets or variation in the availability of saccharide precursors.
While Fc glycan modifications are generally believed to occur within B cells, a topic of some debate is whether any sialylation of Fc glycans occurs extracellularly, in the absence of exogenously administered glycosyltrasferases (Jones et al. 2012, 2016). In favor of sialylation within B cells, human B cell ST6GAL1 and the abundance of sialylation on secreted IgGs can be modulated by cytokines present in cell culture (Selman et al. 2012a; Wang et al. 2011) or increased in vivo by estrogen treatment in postmenopausal patients with rheumatoid arthritis (Engdahl et al. 2018). In addition, human B cell ST6GAL1 expression correlates with Fc sialylation after vaccination (Wang et al. 2015). Another recent study showed that ST6Gal1 is regulated by IL-23 in antibody producing cells (APCs). This regulation was a consequence of IL-23 acting on TH17 cells which suppressed ST6Gal1 expression in an IL-21 and IL-22-dependent pathway (Pfeifle et al. 2016). Overall, data supporting an extracellular mechanism in humans have not yet been described but several factors implicate a dominant B cell intrinsic pathway.
Finally, an important variable among studies of Fc glycosylation in humans relates to the methods used for glycan measurement. In order to interpret data for sound hypothesis generation, the analysis should reflect the Fc domain repertoire that would be present in relevant immune complexes. This requires that the analysis be (1) antigen specific, (2) IgG subclass specific and (3) Fc domain specific. Antigen specific analysis is important because of the segregation of glycans by Fab specificity. Similarly, subclass specific analysis is critical because Fc glycoforms vary by subclass and because the activity of different glycoforms on IgG2, IgG3 and IgG4 antibodies is unknown, as discussed above. Thus, at this time, only data on IgG1 Fc glycosylation is truly interpretable for hypothesis generation. Importantly, bulk IgG glycan analysis cannot be used for hypothesis generation because of the lack of antigen and subclass specificity but also because approximately 20% of IgGs are glycosylated within their Fab domains and those glycoforms are entirely distinct in repertoire from those that modify the Fc (Mimura et al. 2007). Therefore, a detailed study of the methods used for glycan analysis is required of readers who wish to interpret published studies on IgG glycosylation.
6. Concluding Remarks
IgG Fc glycan modifications are a key regulator of antibody activity in vivo. While Fc sialylation has an active role in anti-inflammatory signaling and in maturation of adaptive immune responses, fucosylation of the Fc is regulated to drive appropriate pro-inflammatory effector cell functions. A critical question for future studies to address is how the human Fc glycoform repertoire is regulated. Understanding this will be necessary to shift endogenous Fc glycosylation for therapeutic benefit, which could potentially be done to treat diseases responsive to IVIg therapy, to enhance the activity of anti-tumor antibodies, to prevent autoantibody-mediated tissue disease, to enhance the quality of antibodies generated during vaccination or to reduce the mortality associated with dengue virus infections. The ability to direct shifts in the Fc domain repertoire, in both IgG subclass distribution and Fc glycosylation, is an exciting prospect for treating and preventing human diseases through tuning of the interaction between antibodies and their receptors.
Acknowledgements
Support was received from Stanford University, the Chan Zuckerberg Biohub and the Searle Scholars Program. Research reported in this publication was supported in part by the Bill & Melinda Gates Foundation (OPP1188461) and the National Institutes of Health (1R01AI139119-01A1, 5K22AI12347802, 5U19AI111825-05, UL1TR001866).
References
- Anthony RM et al. (2011) Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 475(7354):110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerenwaldt A et al. (2011) Fcγ receptor IIB (FcγRIIB) maintains humoral tolerance in the human immune system in vivo. Proc Natl Acad Sci U S A 108(46):18772–18777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW, Brady EK, Prestegard JH (2009) Branch-specific sialylation of IgG-Fc glycans by ST6Gal-I. Biochemistry 48(41):9705–9707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruchov AM et al. (2005) Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest 115(10):2914–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R et al. (1999) Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med 189(1):179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JM et al. (2006) Dendritic cells, but not macrophages or B cells, activate major histocompatibility complex class II-restricted CD4+ T cells upon immune-complex uptake in vivo. Immunology 119(4):499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debre M et al. (1993) Infusion of Fcγ fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet 342(8877):945–949 [DOI] [PubMed] [Google Scholar]
- Dhodapkar KM et al. (2005) Selective blockade of inhibitory Fcγ receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc Natl Acad Sci U S A 102(8):2910–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar KM et al. (2007) Selective blockade of the inhibitory Fcγ receptor (FcγRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med 204(6):1359–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z et al. (2016) IgE-mediated enhancement of CD4(+) T cell responses requires antigen presentation by CD8α(−) conventional dendritic cells. Sci Rep 6:28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endy TP et al. (2004) Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis 189(6):990–1000 [DOI] [PubMed] [Google Scholar]
- Engdahl C et al. (2018) Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res Ther 20(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara C et al. (2006) Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous β1, 4-N-acetylglucosaminyltransferase III and Golgi α-mannosidase II. Biotechnol Bioeng 93(5):851–861 [DOI] [PubMed] [Google Scholar]
- Ferrara C et al. (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 108(31):12669–12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebiger BM et al. (2015) Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs. Proc Natl Acad Sci U S A 112(18):E2385–E2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn GC et al. (2010) Naturally occurring glycan forms of human immunoglobulins G1 and G2. Mol Immunol 47(11–12):2074–2082 [DOI] [PubMed] [Google Scholar]
- Fokkink WJ et al. (2014) IgG Fc N-glycosylation in Guillain-Barre syndrome treated with immunoglobulins. J Proteome Res 13(3):1722–1730 [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Nimmerjahn F, Ravetch JV (2005) The inhibitory Fcγ receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol 6(1):99–106 [DOI] [PubMed] [Google Scholar]
- Getahun A et al. (2004) IgG2a-mediated enhancement of antibody and T cell responses and its relation to inhibitory and activating Fcγ receptors. J Immunol 172(9):5269–5276 [DOI] [PubMed] [Google Scholar]
- Halstead SB (2009) Antibodies determine virulence in dengue. Ann N Y Acad Sci 1171(Suppl 1):E48–E56 [DOI] [PubMed] [Google Scholar]
- Hjelm F, Karlsson MC, Heyman B (2008) A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen. J Immunol 180(10):6604–6610 [DOI] [PubMed] [Google Scholar]
- Hodoniczky J, Zheng YZ, James DC (2005) Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 21(6):1644–1652 [DOI] [PubMed] [Google Scholar]
- Imbach P et al. (1981) High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1(8232):1228–1231 [DOI] [PubMed] [Google Scholar]
- Jones MB et al. (2012) Anti-inflammatory IgG production requires functional P1 promoter in β-galactoside α2,6-sialyltransferase 1 (ST6Gal-1) gene. J Biol Chem 287(19):15365–15370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MB et al. (2016) B-cell-independent sialylation of IgG. Proc Natl Acad Sci U S A 113(26):7207–7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV (2006a) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313(5787):670–673 [DOI] [PubMed] [Google Scholar]
- Kaneko Y et al. (2006b) Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med 203(3):789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao D et al. (2015) A monosaccharide residue is sufficient to maintain mouse and human IgG subclass activity and directs IgG effector functions to cellular Fc receptors. Cell Rep 13(11):2376–2385 [DOI] [PubMed] [Google Scholar]
- Kapur R et al. (2014) A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood 123(4):471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H et al. (2005) FcγRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet 14(19):2881–2892 [DOI] [PubMed] [Google Scholar]
- Lehmann B et al. (2012) FcγRIIB: a modulator of cell activation and humoral tolerance. Expert Rev Clin Immunol 8(3):243–254 [DOI] [PubMed] [Google Scholar]
- Li F, Smith P, Ravetch JV (2014) Inhibitory Fcγ receptor is required for the maintenance of tolerance through distinct mechanisms. J Immunol 192(7):3021–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A et al. (2013) Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol 190(8):4315–4323 [DOI] [PubMed] [Google Scholar]
- Maamary J et al. (2017) Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay M et al. (2006) Selective dysregulation of the FcγIIB receptor on memory B cells in SLE.J Exp Med 203(9):2157–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AE et al. (2015) A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. J Immunol Methods 417:34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AE et al. (2016) Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog 12(3):e1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaha TL, Sorrentino B, Ravetch JV (2005) Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science 307(5709):590–593 [DOI] [PubMed] [Google Scholar]
- Mimura Y et al. (2007) Contrasting glycosylation profiles between Fab and Fc of a human IgG protein studied by electrospray ionization mass spectrometry. J Immunol Methods 326(1–2):116–126 [DOI] [PubMed] [Google Scholar]
- Ono M et al. (1997) Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell 90(2):293–301 [DOI] [PubMed] [Google Scholar]
- Pagan JD, Kitaoka M, Anthony RM (2017) Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RN et al. (1999) SHIP recruitment attenuates FcγRIIB-induced B cell apoptosis. Immunity 10(6):753–760 [DOI] [PubMed] [Google Scholar]
- Pfeifle R et al. (2016) Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq S et al. (2013) Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol 190(6):2702–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A et al. (1999) Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med 189(2):371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Raber ML et al. (2009) Fcγ receptor-dependent expansion of a hyperactive monocyte subset in lupus-prone mice. Arthritis Rheum 60(8):2408–2417 [DOI] [PubMed] [Google Scholar]
- Scherer HU et al. (2010) Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum 62(6):1620–1629 [DOI] [PubMed] [Google Scholar]
- Schwab I et al. (2012) IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur J Immunol 42(4):826–830 [DOI] [PubMed] [Google Scholar]
- Schwab I et al. (2014) Broad requirement for terminal sialic acid residues and FcγRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur J Immunol 44(5):1444–1453 [DOI] [PubMed] [Google Scholar]
- Selman MH et al. (2012a) Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics 11(4):M111 014563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman MH et al. (2012b) Fc specific IgG glycosylation profiling by robust nano-reverse phase HPLC-MS using a sheath-flow ESI sprayer interface. J Proteomics 75(4):1318–1329 [DOI] [PubMed] [Google Scholar]
- Shalova IN et al. (2012) CD16 regulates TRIF-dependent TLR4 response in human monocytes and their subsets. J Immunol 188(8):3584–3593 [DOI] [PubMed] [Google Scholar]
- Shinkawa T et al. (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278(5):3466–3473 [DOI] [PubMed] [Google Scholar]
- Sonneveld ME et al. (2016) Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br J Haematol 174(2):310–320 [DOI] [PubMed] [Google Scholar]
- Su K et al. (2004) A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcγRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol 172(11):7186–7191 [DOI] [PubMed] [Google Scholar]
- Tackenberg B et al. (2009) Impaired inhibitory Fcγ receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci U S A 106(12):4788–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan SW et al. (2016) Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages. Thromb Haemost 115(3):646–656 [DOI] [PubMed] [Google Scholar]
- Wang J et al. (2011) Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol Cell Proteomics 10(5):M110 004655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT et al. (2015) Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell 162(1):160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT et al. (2017) IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 355(6323):395–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn N et al. (2015) Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M et al. (2009) Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J Proteome Res 8(2):450–456 [DOI] [PubMed] [Google Scholar]
- Wuhrer M et al. (2015a) Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J Proteome Res 14(4):1657–1665 [DOI] [PubMed] [Google Scholar]
- Wuhrer M et al. (2015b) Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J Neuroinflammation 12:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap WH et al. (2016) CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci Rep 6:34310. [DOI] [PMC free article] [PubMed] [Google Scholar]


