Abstract
Objective
To investigate the role of low-frequency repetitive transcranial magnetic stimulation (rTMS) along with conventional physiotherapy in the functional recovery of patients with subacute ischemic stroke.
Design
Double-blind, parallel group, randomized controlled trial.
Setting
The outpatient department of a tertiary hospital participants: first ever ischemic stroke patients (N=96) in the previous 15 days were recruited and were randomized after a run-in period of 75±7 days into real rTMS (n=47) and sham rTMS (n=49) groups.
Intervention
Conventional physical therapy was given to both the groups for 90±7 days postrecruitment. Total 10 sessions of low-frequency rTMS on contralesional premotor cortex was administered to real rTMS group (n=47) over a period of 2 weeks followed by physiotherapy regime for 45-50 minutes.
Main Outcome Measures
The primary efficacy outcomes were change in modified Barthel Index (mBI) score (pre- to postscore) and proportion of participants with mBI score more than 90, measured at 90±7 days postrecruitment. The secondary outcomes were change in Fugl-Meyer Assessment–upper extremity, Fugl-Meyer Assessment–lower extremity, Hamilton Depression Scale, modified Rankin Scale, and National Institute of Health and Stroke Scale (pre- to post-rTMS) scores at 90±7 days post recruitment.
Results
Modified intention to treat analysis showed a significant increase in the mBI score from pre- to post-rTMS in real rTMS group (4.96±4.06) versus sham rTMS group (2.65±3.25). There was no significant difference in proportion of patients with mBI>90 (55% vs 59%; P=.86) at 3 months between the groups.
Conclusion
In patients with subacute ischemic stroke, 1-Hz low-frequency rTMS on contralesional premotor cortex along with conventional physical therapy resulted in significant change in mBI score.
Keywords: Rehabilitation, Stroke, Transcranial magnetic stimulation
List of abbreviations: BI, Barthel Index; EEG, electroencephalogram; HAMD, Hamilton Depression Scale; mBI, modified Barthel Index; MCID, minimal clinically important difference; MEP, motor evoked potential; mRS, modified Rankin Scale; NIHSS, National Institutes of Health and Stroke Scale; RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation; TMS, transcranial magnetic stimulation
Stroke is the leading etiology for severe adult disability in the world.1 The stroke recovery may not be always complete. Though the acute stroke interventions have improved dramatically in last few decades with mechanical thrombectomy and thrombolytic therapy, very few studies are conducted in newer treatment modalities for stroke recovery. Repetitive transcranial magnetic stimulation (rTMS), a noninvasive approach, enhances cortical excitability which helps in the motor and functional outcomes of stroke. In stroke patients, the inhibitory activity from the unaffected hemisphere also contributes to the disruption in the affected hemisphere. Hence, 2 potential targets for transcranial magnetic stimulation (TMS) are either by suppressing the inhibitory activity of unaffected (contralesional) motor cortex via low-frequency rTMS (≤1 Hz) or by enhancing the excitability of affected cortex (ipsilesional) by high-frequency rTMS (≥1 Hz). But the therapeutic potential of rTMS is nonconclusive. A Cochrane systematic review and meta-analysis concluded that further evidence is required regarding efficacy of rTMS in stroke.2 Another meta-analysis claimed that rTMS had a positive effect on stroke motor recovery, and low-frequency rTMS may be better than high-frequency rTMS.3 There were severe methodological limitations in included studies especially not studying a clinically meaningful primary outcome such as activity of daily living score. Since there was a clinical equipoise, we conducted a randomized double-blind sham-controlled trial to test the hypothesis whether in patients with subacute ischemic stroke (<3 mo), the use of low-frequency rTMS along with standard physiotherapy will lead to better functional outcome compared to those receiving standard physiotherapy alone.
Methods
Participants, setting, and study design
Ours is as a single center, randomized, parallel group, double-blind, sham-controlled trial. We included patients aged 18-75 years, with first ever acute ischemic stroke, within last 15 days documented by computed tomography or magnetic resonance imaging scan of the head and National Institutes of Health Stroke Scale (NIHSS) of 4-20. We excluded participants who were medically unstable, pregnant, had coexistent brain lesions (tumor, infection), comatose, mechanical ventilation, history of epilepsy, or having any surgical implant or pacemaker. The study was registered under clinical trials with CTRI number CTRI/2016/02/006620 at www.clinicaltrials.gov.
Procedure
Prerandomization run-in period
The baseline clinical assessment of the recruited participants was done by first author who is certified in NIHSS. Baseline data including demographics, risk factor profile, biochemical parameters, imaging parameters, stroke subtype, NIHSS, modified Rankin Scale (mRS), modified Barthel Index (mBI), Hamilton Depression Scale (HAMD), and Fugl-Meyer Assessment of upper and lower extremity were carried out at the time of recruitment. A trained licensed physiotherapist gave standard physical therapy to all the recruited participants till study completion (90±7d).
All participants received standard of care that includes passive, active, and active assisted exercises. Participants were encouraged to continue standard therapy at home after discharge, and the total number of hours of an individual exercise was not monitored.
Randomization
Participants were randomly assigned in a 1:1 ratio to receive either real rTMS or sham rTMS. Randomization was done using a computer-generated randomization sequence. Allocations were concealed using sealed, opaque, numbered envelops and were opened only during the time of randomization phase. The investigator, physiotherapist, and participants were masked to the treatment allocation. Laboratory technician who was not part of the study was responsible for the random allocation of participants to sham or real rTMS group. The outcome assessor was also blinded to allocation.
Interventions
Low frequency rTMS was performed using Magstim Rapid2 stimulatora equipped with air cooled figure of 8 coil (70 mm), ie, biphasic pulse was used for rTMS. The resting motor evoked potential (MEP) was ascertained using an electromyogram, recording from the abductor pollicis brevis in keeping with the International Federation of Clinical Neurophysiology recommendations.4 The coil was placed tangentially to the scalp over the hand area of the primary motor cortex to calculate hot spot. Hot spot was defined as the location on the scalp where stimulation of a slightly supra threshold intensity elicited the largest MEP in the abductor pollicis brevis muscle. After the hot spot was identified, resting motor threshold was determined using the lowest stimulus intensity to produce MEP of >50μV peak to peak amplitude in 5 of 10 subsequent trails. If no MEP was obtained at the time of hot spot calculation in the affected ipsilesion M1, then the hotspot was defined as the symmetric location to the contralesion M1. The stimulation parameters were chosen in accordance with the safety guidelines for rTMS.4 Total 750 pulses, 75 trains using low frequency (1 Hz) with inter train interval of 45 seconds at calculated intensity of 110% resting motor threshold (Fc3/Fc4), was administered to the randomized patient by a qualified technician in the Institute TMS Laboratory. Localization was done using 10-20 electroencephalogram (EEG) method. Sham rTMS pulses were administered using the same stimulation parameters.
Over the contralesion premotor cortex area with the figure of 8 coil angled at 90 degrees from the scalp. Patient was awake throughout the rTMS administration. The rTMS sessions on each day was followed by 45-minute conventional physical therapy regime given by a trained physiotherapist.
Monitoring for complications
All the participants were monitored for any adverse events. A checklist of previously reported side effects was used to report any possible adverse events.
Outcomes
The coprimary efficacy outcomes were changes in mBI score (pre- to post-rTMS) and functional independence score (mBI) >90, measured at 3 months of stroke onset. The secondary efficacy outcomes were changes in Fugl-Meyer Assessment of upper extremity, Fugl-Meyer Assessment of lower extremity, mRS, and NIHSS scores (pre- to post-rTMS) at 3 months.
Sample size
Sample size was calculated for primary clinical efficacy outcome, mBI with a superiority hypothesis assumption. Calculation was based on the study done by Khedr et al,5 who reported 34.6% and 7.7% of good or excellent outcome under rTMS and sham rTMS, respectively, which yielded a sample size of 45 in each arm (90% power and a 2-sided α level of 5%). Adjusting for 10% attrition we estimated a sample size of 100 (50 in each arm).
Statistical analysis
Statistical analysis was performed using Stata version 14.1b and was based on modified intention to treat principle. Normal distribution was tested using Kolmogorov-Smirnov statistic. Study data were not after normal distribution; hence, nonparametric tests were applied. For categorical data, 2 × 2 table was generated. Chi-square test or Fisher exact test was applied to compare the properties in the 2 groups. Between-group comparisons were carried out using Wilcoxon rank-sum test. Wilcoxon signed-rank test was used to assess changes in the scores within the same group. McNemar test was applied for the categorical variable.
Results
Patient characteristics
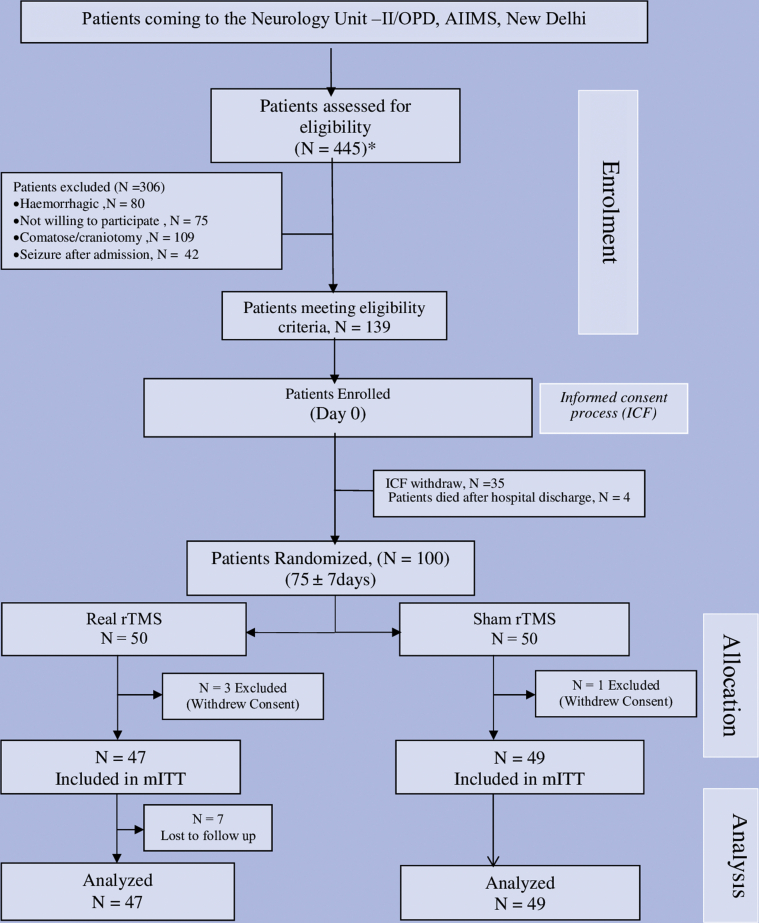
Between August 2012 and February 2016, 445 participants with acute ischemic stroke were screened and 139 participants fulfilling the eligibility criteria were recruited into the pre-rTMS run-in phase. During the run-in period, 35 participants withdrew consent and 4 died after discharge from hospital. Randomization was done in 100 participants (fig 1). Four participants were excluded after randomization (consent withdrawn after randomization). The baseline characteristics were comparable between real rTMS (n=47) and sham rTMS (n=49) (table 1). All study participants received standard of care and 10 sessions (5d in a week) of real or sham rTMS for consecutively 2 weeks. Total 750 pulses with intertrain interval of 45 seconds with calculated intensity of 110% resting motor threshold was administered to real rTMS group. Seven participants in real rTMS were lost to follow-up. One participant had seizure who was managed symptomatically. The institute ethics committee was informed and unblinding was done in that participant. Modified intention to treat analysis was done for 47 participants in real and 49 participants in sham arm.
Fig 1.
CONSORT flow diagram of randomized controlled trial study. mITT, modified intention to treat analysis.
Table 1.
Showing baseline characteristics between real rTMS group, n=47 and sham rTMS group, n=49
| Variable | Sham rTMS n=49 | Real rTMS n=47 | P |
|---|---|---|---|
| Age (y), mean ± SD (95% CI) | 52.89±14.95 (48.60-57.19) | 54.85±13.39 (50.91-58.78) | .50 |
| Sex, n (%) | .93 | ||
| Men | 34 (69) | 33 (77) | |
| Women | 15 (31) | 14 (30) | |
| Hypertension, n (%) | 32 (65) | 35 (74) | .60 |
| Diabetes, n (%) | 13 (26) | 11 (23) | .45 |
| Smoking, n (%) | 16 (33) | 15 (32) | .55 |
| Tobacco chewing, n (%) | 6 (12) | 4 (8) | .39 |
| IV-tPA, n (%) | 6 (12) | 6 (12) | .59 |
| Stroke subtype, n (%) | .98 | ||
| Large artery | 12 (25) | 12 (26) | |
| Lacunar | 6 (12) | 4 (8) | |
| Cardioembolic | 7 (14) | 6 (13) | |
| Others | 24 (49) | 25 (53) | |
| Onset to enrollment, n (%) | .33 | ||
| ≤7 | 41 (84) | 36 (76) | |
| 8-15 | 8 (16) | 11 (24) | |
| Mean ± SD | 4.68±1.26 | 4.96±1.56 | |
| Onset to TMS, n (%) | .91 | ||
| 60-75 | 10 (20) | 16 (34) | |
| 76-83 | 39 (80) | 31 (66) | |
| Mean ± SD | 77.34±10.21 | 77.51±5.38 |
Abbreviation: 95% CI, 95% confidence interval; tPA, tissue plasminogen activator.
Primary outcomes
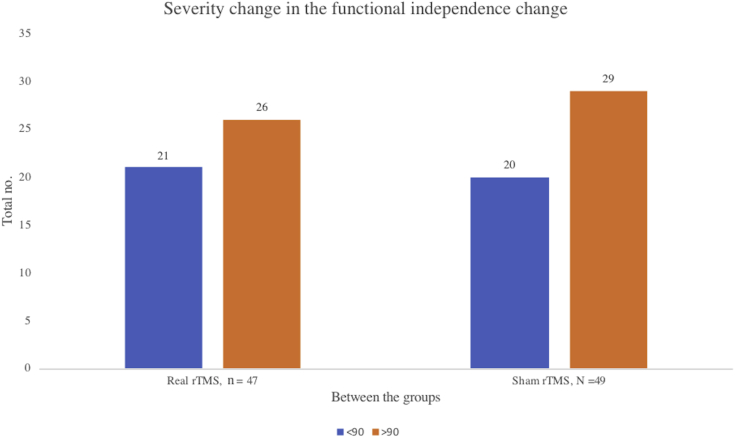
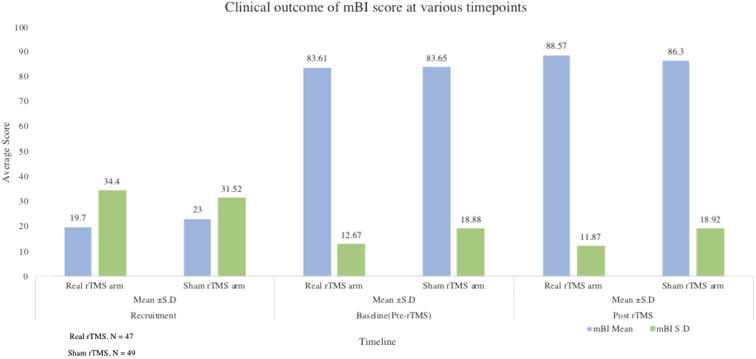
There was a significant change in mBI score from pre- to post-rTMS, showing an increase in the real rTMS (4.96±4.06) compared to sham rTMS (2.65±3.25) group (P<.001) (table 2) (fig 2). The proportion of participants who were functionally independent, defined as a score on mBI>90, did not differ between the groups at 3 months of stroke onset, 55% in real rTMS versus 59% in sham rTMS arm, P=.86 (Table 3, Table 4, Table 5) (fig 3).
Table 2.
Showing clinical outcome of primary and secondary stroke scales at recruitment, pre, post, and mean absolute change between real rTMS group, n=47 and sham rTMS group, n=49
| mBI Mean ± SD Median (range) |
NIHSS Mean ± SD Median (range) |
mRS Mean ± SD Median (range) |
HAMD Mean ± SD Median (range) |
FMA Upper Mean ± SD Median (range) |
FMA Lower Mean ± SD Median (range) |
|
|---|---|---|---|---|---|---|
| At recruitment, before run-in period (mean ± SD) | ||||||
| Sham | 23.0±31.52 | 11.6±4.83 | 3.8±0.76 | 9.38±4.37 | 15.81±19.02 | 12.14±11.63 |
| n=49 | 0 (0-50) | 11 (8-15) | 4 (3-4) | 10 (6-11) | 4 (0-32) | 12 (0-21) |
| Real | 19.7±34.40 | 12.34±4.70 | 3.7±0.98 | 8.34±4.09 | 13.04±21.66 | 8.51±11.93 |
| n=47 | 0 (0-38) | 12 (9-15) | 4 (3-4) | 8 (6-10) | 1 (0-31) | 1 (0-20) |
| P | .66 | .48 | .61 | .22 | .92 | .30 |
| Baseline, after run-in period (pre-rTMS) | ||||||
| Sham | 83.65±18.88 | 5.57±2.69 | 2.32±0.82 | 5.93±3.32 | 46.79 ±17.06 | 28.53±6.01 |
| n=49 | 90 (80-94) | 5 (4-6) | 2 (2-3) | 6 (3-8) | 52 (35-61) | 31 (27-32) |
| Real | 83.61±12.67 | 5.91±2.32 | 2.38±0.77 | 6.57±3.25 | 43.65±16.04 | 27.61±5.92 |
| n=47 | 85 (79-94) | 6 (4-8) | 3 (2-3) | 7 (4-9) | 50 (28-58) | 29 (25-32) |
| P | .99 | .50 | .73 | .34 | .38 | .35 |
| Post-rTMS score | ||||||
| Sham | 86.30±18.92 | 5.08±2.77 | 2.18±0.88 | 5.34±2.81 | 49.00±16.64 | 29.73±6.10 |
| n=49 | 92 (85-95) | 5 (3-6) | 2 (2-3) | 5 (3-7) | 55 (38-63) | 32 (28-34) |
| Real | 88.57±11.87 | 4.4±2.27 | 1.89±0.84 | 5.25±3.20 | 47.38±15.32 | 30.29±4.73 |
| n=47 | 92 (82-98) | 4 (3-6) | 2 (1-3) | 4 (3-7) | 52 (33-61) | 32 (29-34) |
| P | .75 | .19 | .10 | .88 | .71 | .63 |
| Post-rTMS score improvement (absolute change) | ||||||
| Sham | 2.65±3.25 | 0.49±0.71 | 0.14±0.35 | 0.59±1.38 | 2.20±3.14 | 1.20±1.74 |
| n=49 | 2 (0-3) | 0 (0-1) | 0 (0-0) | 0 (0-1) | 1 (0-3) | 0 (0-2) |
| Real | 4.96±4.06 | 1.51±1.18 | 0.49±0.50 | 1.32±1.30 | 3.72±7.67 | 2.68±2.68 |
| n=47 | 4 (2-6) | 1 (1-2) | 0 (0-1) | 1 (0-2) | 3 (1-6) | 2 (0-4) |
| P | <.001 | <.001 | .001 | <.003 | <.001 | <.001 |
Fig 2.
Change in the primary outcome score mBI>90 between real rTMS, n=47 and sham rTMS, n=49 group.
Table 3.
Showing severity change in the functional independence score between real rTMS group, n=47 and sham rTMS group, n=49
| Severity Range mBI | Real rTMS n=47 |
Sham rTMS n=49 |
P | ||
|---|---|---|---|---|---|
| % | % | ||||
| <60 | 2 | 4% | 4 | 8% | .71 |
| Moderate dependency 61-90 | 19 | 40% | 16 | 33% | .56 |
| Slight dependency 91-99 | 20 | 43% | 25 | 51% | .53 |
| Independence -100 | 6 | 13% | 4 | 8% | .68 |
| >90 | 26 | 55% | 29 | 59% | .86 |
Table 4.
Showing severity change in the functional independence change between real rTMS group, n=47 and sham rTMS group, n=49
|
Severity range mBI |
Real rTMS n=47 |
Sham rTMS n=49 |
P | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| <90 Severe to moderate dependency | 21 | 45% | 20 | 41% | |
| >90 Slight to independent dependency | 26 | 55% | 29 | 59% | .86 |
Table 5.
Final checklist of study participant particulars
| Final checklist | ||
|---|---|---|
| Were the following participant factors | Reported? | Controlled? |
| Age of individuals | √ | |
| Sex of individuals | √ | |
| Handedness of individuals | √ | |
| Individuals prescribed medication | √ | |
| Use of CNS active drugs (eg, anticonvulsants) | N/A | |
| Presence of neurologic/psychiatric disorders when studying healthy individuals | N/A | |
| Any medical conditions | √ | |
| History of specific repetitive motor activity | N/A | |
| Were the following methodological factors | ||
| Position and contact of electromyogram electrodes | √ | |
| Amount of relaxation or contraction of target muscles | √ | |
| Prior motor activity of the muscle to be tested | √ | |
| Level of relaxation of muscles other than those being tested | N/A | |
| Coil type (size and geometry) | √ | |
| Coil orientation | √ | |
| Direction of induced current in the brain | √ | |
| Coil location and stability (with or without a neuronavigation system) | √ | |
| Type of stimulator used (eg, brand) | √ | |
| Stimulation intensity | √ | |
| Pulse shape (monophasic or biphasic) | √ | |
| Determination of optimal hotspot | √ | |
| The time between MEP trials | √ | |
| Time between days of testing | √ | |
| Subject attention (level of arousal) during testing | √ | |
| Method for determining threshold (active or resting) | √ | |
| Number of MEP measures made | √ | |
| Paired pulse only: intensity of test pulse | N/A | |
| Paired pulse only: intensity of conditioning pulse | N/A | |
| Paired pulse only: interstimulus interval | N/A | |
| Were the following analytical factors | ||
| Method for determining MEP size during analysis | ||
| Size of unconditioned MEP | N/A |
Abbreviations: CNS, central nervous system; N/A, not applicable.
Fig 3.
Change in the modified intention to treat analysis (mITT) primary outcome mBI score from pre-rTMS to post-rTMS between the real rTMS, n=47 and sham rTMS group, n=49.
Secondary outcomes
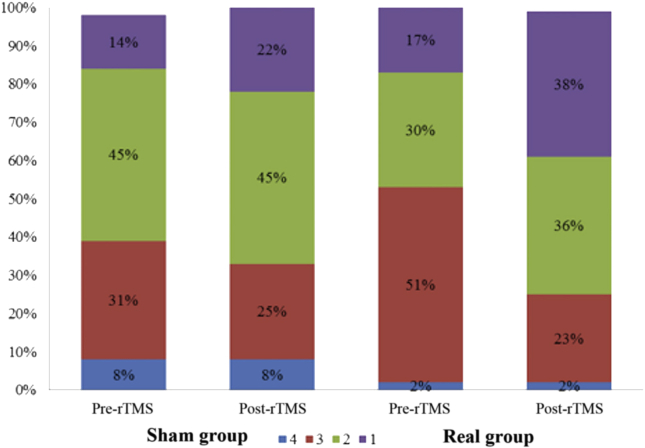
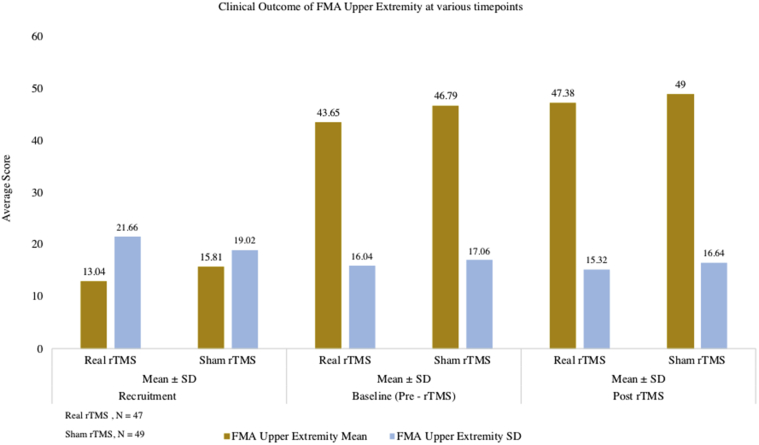
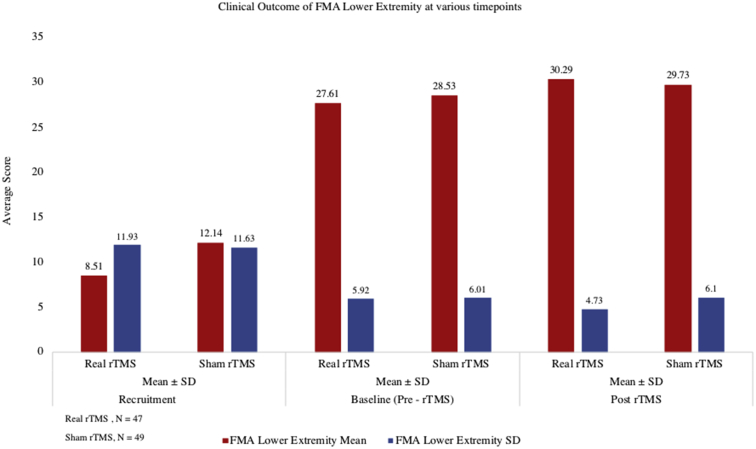
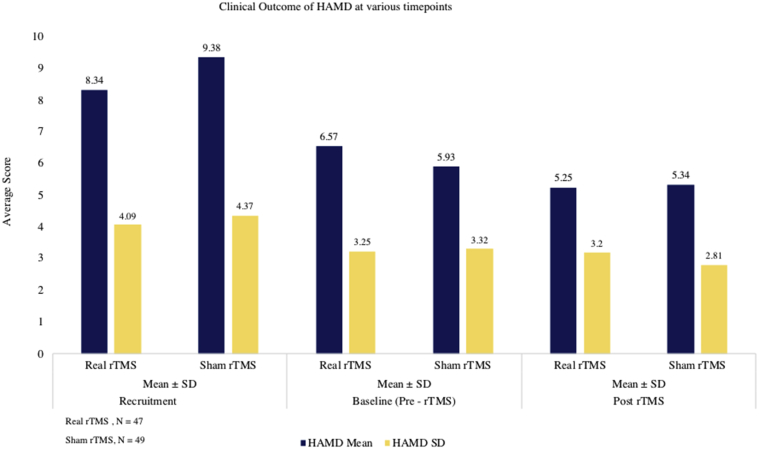
Statistically significant downward shift in the distribution of mRS scores in the real group (P<.001) compared to sham group (P=.07) was seen. In the sham group, 31% of patients (15/49) initially had a mRS score of 3 which reduced to 24.5% (12/49) after 3 months. In the real rTMS group, the initial proportion of cases with mRS score of 3 came down from 51.1% (24/47) to 23.4% (11/47). Only 14.3% (7/49) had lower mRS scores at 3 months compared to their baseline scores (indicating improvement) in the sham group as against 48.9% (23/47) showing improvement in the real rTMS group (P<.001) (figs 4 and 5). There was decrease in the NIHSS score (pre- to post-rTMS) in real rTMS (1.51±1.18) compared to sham rTMS (0.49±0.71) with P<.001 (fig 6). Fugl-Meyer Assessment score in both upper and lower extremity did not show any significant difference. Even though the change in the FMA scores from pre- to post-rTMS showed statistically significant improvement in real rTMS group (P<.001), they were less than the established minimal clinically important difference (MCID) of FMA-UE and FMA-LE (figs 7 and 8). Similarly, clinically no meaningful change in the depression scale (HAMD) was seen (fig 9) (see table 2).
Fig 4.
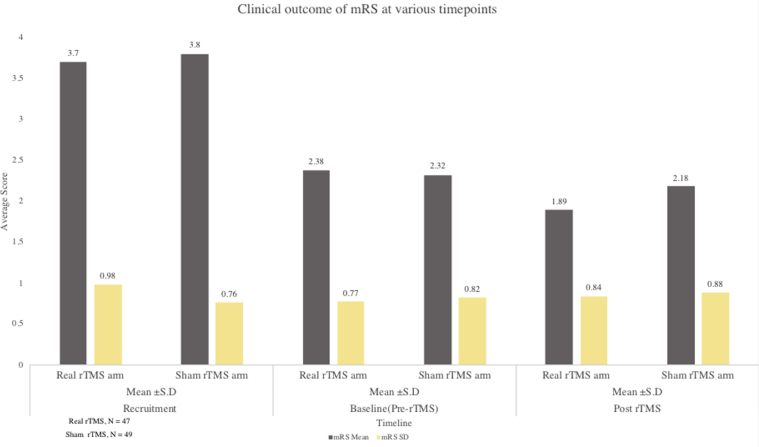
Shift in the mRS score from pre-rTMS to post-rTMS between the real rTMS, n=47 and sham rTMS group, n=49.
Fig 5.
Shift in the distribution stroke disability mRS score from pre- to post-rTMS between real rTMS, n=47 and sham rTMS group, n=49.
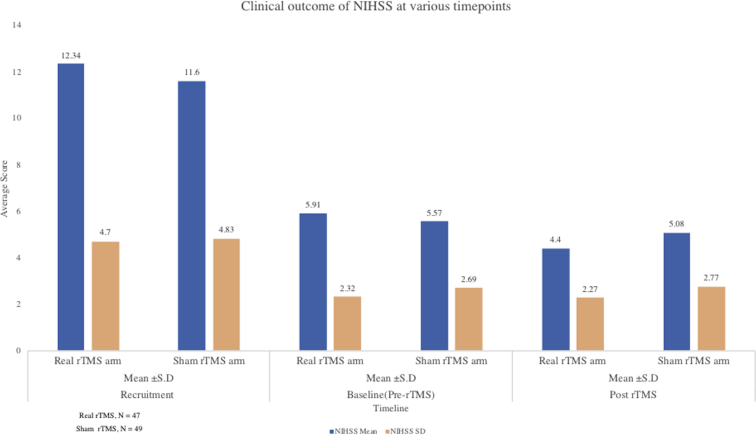
Fig 6.
Change in the stroke severity score from pre- to post-rTMS between real rTMS, n=47 and sham rTMS group, n=49.
Fig 7.
Change in the motor outcome lower extremity score from pre- to post-rTMS between real rTMS, n=47 and sham rTMS group, n=49.
Fig 8.
Change in the motor outcome upper extremity score from pre- to post-rTMS between real rTMS, n=47 and sham rTMS group, n=49.
Fig 9.
Change in the depression score from pre- to post-rTMS between real rTMS, n=47 and sham rTMS group, n=49.
Adverse events
One participant in the real TMS arm developed seizure 18 hours after the fourth session and about 1 hour after waking from sleep in the morning. The episode of seizure was characterized by generalized tonic-clonic movement of the body with deviation of head, clenching of teeth, and bleeding from the mouth lasting for about 40 seconds; no fresh changes in the noncontrast computerized tomography brain scan were observed refuting any new structural cause for seizures. The EEG of the patient revealed background activity consisting of 8-9 Hz, 30-50 μV posterior dominant alpha activity, and the presence of rhythmic slowing delta waves of 2-3 Hz and 15-20 μV in the frontocentral region of the left hemisphere, reflecting abnormal EEG findings with left frontocentral slowing. No family history of seizure could be elicited, and the metabolic parameters were reported to be in the normal range including blood glucose levels, excluding the possibility of hypoglycemia-induced seizure, because the patient had diabetes mellitus. He was treated with phenytoin. The event was reported to the institute ethics committee and later published.6,7 The participant did not receive any more TMS sessions. No other adverse events were reported in the participants.
Discussion
In this randomized controlled trial, low-frequency (1 Hz) rTMS along with concurrent standard physiotherapy was found to be superior to standard physiotherapy and sham stimulation in improving functional independence in patients with subacute ischemic stroke. The difference in the Barthel Index (BI) scores were more than the MCID of the BI in stroke participants (1.85).8 Even though there was significant difference in change in NIHSS, FMA, and HAMD scores (pre- to post-rTMS), their clinical significance is debatable because these changes were less than clinically important difference in these scores. The current available evidence of TMS in stroke is of low quality. The sample size was small in most trials without adequate power. Many trials used different motor and physiological parameters, but very few have used functional outcome measures.9, 10, 11, 12 Most of the trials have not given consideration for spontaneous recovery poststroke. We tried to address most of these issues in our study. Our study is the first and the largest randomized controlled trail (RCT) in subacute ischemic stroke and the second largest study in the field of TMS and stroke to study overall functional outcome. The primary outcome used in our study was activity of daily living score (mBI), with a run-in period of 75±7 days after recruitment prior to randomization with conventional physiotherapy to account for spontaneous recovery after stroke.
Five RCTs had activity of daily living (mBI) as primary outcome but each used different stimulation parameters and recorded clinical outcomes at different periods. Meta-analysis of 2 of these studies where data were available for BI showed that rTMS was not associated with any change in BI and there was significant heterogeneity between the trials. Du et al13 used low-frequency rTMS (0.5 Hz) on 60 participants with poststroke depression with cognitive impairment to stimulate both frontal lobes and BI was measured at 8 weeks. Jin et al14 used transcranial magnetic stimulation (TMS) on acute ischemic stroke participants (4h-10d), and BI was measured after 2 weeks of intervention. The inclusion criteria and parameters studied were widely different from our study and so comparison of our results with that of these studies may not be ideal.
Studies conducted at subacute stage by Theilig,15 Dafotakis,16 Grefkes,17 and Nowak18 and colleagues in very small sample sizes (<15 patients) with a mean stroke onset of 1.8 months using 1-Hz rTMS have shown a trend of improvement in the index finger tapping, grip force, and motor performance in the paretic hand.
There are 2 window periods before chronic phase during which neuromodulation may amplify the brain reorganization poststroke: (1) acute stage: plastic changes last for weeks, up to 45 days poststroke onset; and (2) restorative phase: ongoing restorative mechanism lasts until 90 days.19,20 Very few RCTs have targeted this subacute stage.21,22 We had chosen this second window period for neuromodulation because spontaneous biologic recovery augmented by standard of care is mostly attained by 6-10 weeks and they will not act as confounders. Even if baseline motor deficits are balanced, initial deficit cannot predict recovery to intervention. Hence, apparently balanced groups may still have biological differences which can confound the recovery process. We have measured the primary outcome at 3 months poststroke which coincides with immediate post-rTMS period. This might help in finding out the clinically meaningful difference within a process of spontaneous recovery. If treatment is delivered in early stage and outcome is measured after 3 months, there may not be any difference since control group might have also caught up by then. If outcome was measured earlier, the effects on rate of recovery can be found even though there may be no difference in final outcome.
An important aspect while enrolling participants in subacute phase is the confounding role of endogenous plasticity. Moreover, if the participant was not using the paretic limb, enrolling into the trial itself will cause improvement because the participant will be stimulated more by using the paretic arm. This improvement that occurs even in control arm could have been a serious confounder. In order to account for this, we had incorporated a run-in period, so that all individuals have already experienced specific activities and attained plateau in motor functions. By timing the intervention in the subacute stage and measuring the primary outcome immediately after intervention which coincides with 3 months poststroke, we tried to circumvent these issues.
This trial has shown that rTMS delivered in the subacute change can cause meaningful improvement in functional independence. The most common stroke subtype in our study was others or undermined. The persistence of benefit beyond 3 months of stroke onset is still not known. The failure to accomplish clinical significance in the secondary outcomes might have been due to the trial having less rTMS intervention duration. The control group in this study had received standard physiotherapy during the run-in period and even during 2 weeks of TMS sessions they received supervised physiotherapy. This may have contributed to good response in control group and caused a ceiling effect in clinical response. See supplemental appendix S1 (available online only at http://www.archives-pmr.org/).
Study limitations
Four patients had to be excluded after randomization since the diagnosis was wrong. These patients had in fact posterior circulation stroke which was an exclusion criterion. Hence, we had to do modified intention to treat analysis. The type of lesion in our study was not measured. Therefore, the effect of rTMS on the outcome cannot be correlate with the nature of stroke. For the localization, neuronavigation technique was not used as the concerned department did not have that facility; hence, 10-20 EEG method was used. The primary outcome was immediately after 2 weeks of TMS at subacute stage; therefore, regular follow-up would have revealed the sustainability of effects of rTMS. Also, future trails should employ larger sample size to find out MCID.
Implications for future
(1) Future trials should employ larger sample size to find out MCID in the secondary outcomes of this trial; (2) Monthly rTMS sessions along with conventional physiotherapy should be given to rule out the clinical functional outcome. (3) Long-term follow-up should be done to assess the clinical outcome in stroke patients of this trial.
One patient had seizure 18 hours after the fourth session of rTMS. We had reported this case and discussed in detail the pathophysiological mechanisms and high possibility of it being a poststroke seizure and association with rTMS was merely coinicidental.6
Conclusions
In first ever subacute ischemic stroke participants, 1-Hz low-frequency rTMS on contralesional motor cortex along with conventional physical therapy caused significant change in mBI score. Hence, rTMS should be used as part of standard of care in stroke rehabilitation.
Suppliers
-
a.
Magstim Rapid2 stimulator; Magstim Co Ltd.
-
b.
Stata version 14.1; StataCorp
Footnotes
Supported by the Indian Council of Medical Research (grant no. CTRI/2016/02/006620).
Disclosures: none
Supplementary Data
References
- 1.Ward N.S. Restoring brain function after stroke—bridging the gap between animals and humans. Nat Rev Neurol. 2017;13:244–255. doi: 10.1038/nrneurol.2017.34. [DOI] [PubMed] [Google Scholar]
- 2.Hao Z., Wang D., Zeng Y., Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev. 2013;(5):CD008862. doi: 10.1002/14651858.CD008862.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu W.-Y., Cheng C.-H., Liao K.-K., Lee I.-H., Lin Y.-Y. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- 4.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khedr E.M., Ahmed M.A., Fathy N., Rothwell J.C. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- 6.Kumar N., Padma Srivastava M.V., Verma R., Sharma H., Modak T. Can low-frequency repetitive transcranial magnetic stimulation precipitate a late-onset seizure in a stroke patient? Clin Neurophysiol. 2016;127:1734–1736. doi: 10.1016/j.clinph.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Nitsche M.A. Co-incidence or causality? Seizures after slow rTMS in stroke patients. Clin Neurophysiol. 2016;127:1020–1021. doi: 10.1016/j.clinph.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh Y.W., Wang C.H., Wu S.C., Chen P.C., Sheu C.F., Hsieh C.L. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair. 2007;21:233–238. [Google Scholar]
- 9.Liepert J., Zittel S., Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in acute stroke: a double-blind placebo-controlled crossover trial. Restor Neurol Neurosci. 2007;25:461–465. [PubMed] [Google Scholar]
- 10.Sasaki N., Mizutani S., Kakuda W., Abo M. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2013;22:413–418. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Fregni F., Boggio P.S., Valle A.C. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 12.Pomeroy V.M., Cloud G., Tallis R.C., Donaldson C., Nayak V., Miller S. Transcranial magnetic stimulation and muscle contraction to enhance stroke recovery: a randomized proof-of-principle and feasibility investigation. Neurorehabil Neural Repair. 2007;21:509–517. doi: 10.1177/1545968307300418. [DOI] [PubMed] [Google Scholar]
- 13.Du J., Tian L., Liu W. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol. 2016;23:1666–1672. doi: 10.1111/ene.13105. [DOI] [PubMed] [Google Scholar]
- 14.Jin X., Wu X., Wang J. [Effect of transcranial magnetic stimulation on rehabilitation of motor function in patients with cerebral infarction] Zhonghua Yi Xue Za Zhi. 2002;82:534–537. [PubMed] [Google Scholar]
- 15.Theilig S., Podubecka J., Bösl K., Wiederer R., Nowak D.A. Functional neuromuscular stimulation to improve severe hand dysfunction after stroke: does inhibitory rTMS enhance therapeutic efficiency? Exp Neurol. 2011;230:149–155. doi: 10.1016/j.expneurol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Dafotakis M., Grefkes C., Eickhoff S.B., Karbe H., Fink G.R., Nowak D.A. Effects of rTMS on grip force control following subcortical stroke. Exp Neurol. 2008;211:407–412. doi: 10.1016/j.expneurol.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Grefkes C., Nowak D.A., Wang L.E., Dafotakis M., Eickhoff S.B., Fink G.R. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50:233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak D.A., Grefkes C., Ameli M., Fink G.R. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 19.Cramer S.C. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 20.Hermann D.M., Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11:369–380. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seniów J., Bilik M., Leśniak M., Waldowski K., Iwański S., Członkowska A. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: a randomized, double-blind, placebo-controlled study. Neurorehabil Neural Repair. 2012;26:1072–1079. doi: 10.1177/1545968312445635. [DOI] [PubMed] [Google Scholar]
- 22.Blesneag A.V., Slavoaca D.F., Popa L. Low-frequency rTMS in patients with subacute ischemic stroke: clinical evaluation of short and long-term outcomes and neurophysiological assessment of cortical excitability. J Med Life. 2015;8:378–387. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.