Abstract
Objectives
This study reports on the feasibility of the SitLess with MS trial, an intervention targeting sedentary behavior in individuals with multiple sclerosis (MS).
Design
Single group, pre-post intervention design.
Setting
Community.
Participants
Participants (N=41) with mild to moderate disability from MS.
Intervention
The intervention was 15 weeks, with a 7-week follow-up, and included 2 stages: SitLess and MoveMore. During the SitLess stage, participants were encouraged to break up prolonged sitting bouts over a 7-week period, whereas the MoveMore stage promoted increased steps per day and interrupting sitting over a 7-week period. The intervention was delivered through weekly one-on-one coaching sessions via telerehabilitation and an accompanying newsletter based on social-cognitive theory. Activity was monitored throughout the program using a Fitbit.
Main Outcome Measures
Process (eg, recruitment) and resource and management (eg, personnel requirements) metrics were assessed, along with efficacy outcomes (eg, effect). Progression criteria were set a priori and were related to safety, fatigue, satisfaction, and attrition. Sedentary behavior, measured using the ActivPal, was reported pre- and postintervention, as well as 7 weeks postintervention. Effect sizes (pre to post, pre to 7 weeks post) were calculated for the sedentary behavior outcomes (eg, time sitting, transitions from sitting to standing, number of long sitting bouts). Experiences with the intervention were explored through an online survey.
Results
Forty-one participants enrolled, 39 of whom completed the intervention. All participants but 1 were satisfied with the experience. Pre-post intervention effect sizes for change in total sedentary time, number of transitions from sit to stand, and number of long (>30 min) sedentary bouts were 0.34, 0.02, and 0.39 respectively. All a priori progression criteria were met.
Conclusions
The SitLess with MS program, a novel intervention that emphasized and facilitated sitting less and moving more, was feasible and resulted in small changes in sedentary behavior in individuals with MS.
Keywords: Feasibility studies, Multiple sclerosis, Rehabilitation, Telerehabilitation
List of abbreviations: EDSS, expanded disability status scale; FSS, Fatigue Severity Scale; MS, multiple sclerosis
Multiple sclerosis (MS) occurs in nearly 2.5 million adults worldwide and results in a broad range of symptoms such as fatigue, pain, and depression, as well as balance and walking disabilities. Physical activity is one of the primary, nonpharmaceutical approaches for managing symptoms.1,2 Exercise training in particular slows the progression of the disease and improves fatigue, depression, mobility, and quality of life.3, 4, 5, 6, 7, 8 Despite the evidence, individuals with MS are less physically active than their nondisabled peers.9, 10, 11 They also demonstrate greater amounts of sedentary behavior,11 which is defined by postural (sitting or lying) and energy expenditure (energy expenditure equivalent to that at rest) parameters,12 and has independent health risks.13 Research on older adults and those with disability show that more sitting is associated with frailty14 and reduced mobility.15,16 Taken together, consistently low levels of physical activity participation combined with the problem of too much sitting support the consideration of a new approach to activity promotion in individuals with MS.
We designed an intervention focused on reducing sedentary behavior by breaking up sitting time and encouraging light intensity activity.17 The intervention starts with an emphasis on the sedentary end of the activity continuum, which is the opposite of more traditional exercise or physical activity programs that focus on increasing moderate-to-vigorous physical activity. This approach has been tested in individuals with stroke,18,19 who may have similar functional profiles as those with MS. Significant reductions in sitting time were achieved after the interventions.18,19 One study reported preliminary improvements in function.19 Interventions that target the reduction of sedentary behavior and inherently incorporate the functional action of moving from sitting to standing may be particularly applicable and beneficial for those with mobility disability.16
The primary objectives of the study were to test the feasibility (ie, process, management, resources, progression criteria) of the “SitLess with MS” intervention and to provide preliminary information regarding the efficacy of the intervention (ie, effect size) for changing sedentary behavior.
Methods
Design and ethics
The SitLess with MS study was administered using a pre-post, single-group, repeated measures intervention design. The protocol has been published.17 The current report reports feasibility outcomes (process and management, and progression criteria) and efficacy results related to the primary outcome (sedentary behavior). A subsequent report will detail the efficacy of changes in all symptom and physical performance outcomes. The study was approved by the Institutional Health Research Ethics Board at the University of Alberta (Pro000667657), the Northern Alberta Clinical Trials and Research Centre, and the Alberta Health Services Edmonton Zone (operational approval for recruitment through the Northern Alberta MS Clinic).
Participants and recruitment
Participants were included based on the following criteria: (1) diagnosis of MS confirmed by the patient's neurologist, (2) MS duration of at least 1 year, (3) mild or moderate neurologic disability (Expanded Disability Status Scale [EDSS] score of 1-6.5), (4) relapse-free over the previous 3 months, (5) stable use of disease modifying drugs and rehabilitation over the previous 6 months, (6) physically inactive (defined as insufficiently active by a Godin-Shephard Leisure-Time Physical Activity Questionnaire score of <14),20 (7) able to walk with or without a walking aid for at least 10 meters, and (8) owned and used a smartphone.
The assistance of staff neurologists was enlisted to help with recruitment. Staff identified potential participants who met the inclusion criteria and asked whether they gave permission to be contacted by the research team. Those who agreed were contacted directly by a member of the research team who confirmed eligibility. Participants signed the informed consent document before baseline testing.
Intervention and procedures
The SitLess with MS intervention focuses on interrupting prolonged sitting and replacing it with light physical activity. The procedures are described in detail in the protocol report,17 but will be described briefly here. All participants attended an in-person assessment session before, immediately after, and 7 weeks after intervention (fig 1). The assessment sessions were the only in-person contact during the intervention, with all other study activities conducted remotely.
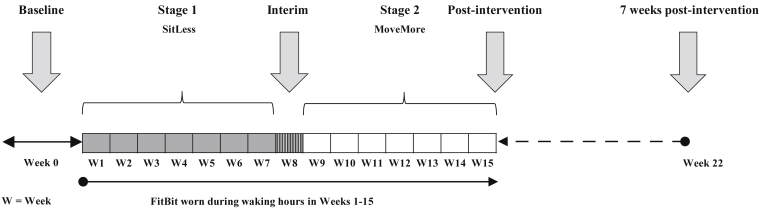
Fig 1.
Timeline of the SitLess with MS intervention program. Each stage was 7 weeks in length, with 1 week at the interim to allow transition to the MoveMore stage and interim physical activity measurement (not reported, as discussed in the Methods section). Participants were seen in person only at baseline, immediately postintervention, and 7 weeks postintervention. Measurement of symptoms and physical performance were completed during the in-person sessions. Weekly contact with the intervention coach (weeks 1-15) was conducted remotely using videoconferencing or phone.
The intervention was 15 weeks in length with 2 stages: SitLess and MoveMore. During weekly coaching sessions, conducted either through telerehabilitation (videoconferencing or phone), participants were encouraged to break up prolonged sitting bouts (focus of the SitLess stage) or promote increased steps per day, in addition to interrupting sitting (focus of the MoveMore stage). Weekly newsletters based on the core determinants of social cognitive theory21 (ie, knowledge to support behavior change, self-efficacy, goal setting, facilitators, and barriers) provided the foundation for behavior change. The goal of the weekly sessions was to facilitate the translation of knowledge and strategies for activity behavior change and support accountability and compliance with the intervention. A Fitbit One,a a valid tool to measure activity,22 was worn throughout the intervention as a self-monitoring tool. Both the participant and the intervention coach were able to view the activity information (ie, steps per day or gaps in activity analogous to sitting) and the results from the Fitbit were used to discuss and support weekly goal setting.
There were 2 primary intervention coaches who were both licensed physical therapists. Intervention coaches were trained regarding the main messages of the intervention, including use of scripts and general tips around goal setting. Scripts guided the coaching sessions and ensured that the same key messages were communicated to each participant. Coaches made notes after every session regarding general impressions, goals, and recorded falls or other events that may have affected participation (ie, medical or social support changes, planned vacations). After the 15-week intervention was complete, the participant kept the Fitbit. There was no contact or support for behavior change in between the postintervention data collection session and the 7-weeks post data collection session. Intervention fidelity was monitored through regular discussion and debrief with one of the principal investigators (RM).
Feasibility outcomes
The feasibility of recruitment and retention throughout the 15-week intervention and 7-week follow-up period were assessed from project coordinator records. Reasons for decisions to withdraw were recorded. The program itself was Internet-based and, therefore, most communications (ie, requiring staff resources) related to scheduling of coaching sessions were conducted by e-mail or phone. Coaches recorded preparation and direct contact time related to sessions. Coaches recorded the timing and completion of weekly chats, which allowed reporting of the length of time (d) to complete the intervention. The participant’s experience of the program was assessed using an online feedback survey that focused on perceptions of specific components of the program (coaching sessions, use of Fitbit, newsletters), as well as how the intervention might be modified for future trials.
Progression criteria
Criteria that supported moving to the next phase (ie, conduct a trial with a control group) were declared a priori as follows:
-
(1)
Safety, defined as no falls specifically attributable to the intervention. The incidence of falls was self-reported by participants on log sheets, and reported and discussed with intervention coaches weekly.
-
(2)
Minimal reported fatigue (pre- to postintervention), defined as at least 80% of participants reporting no increase in self-reported fatigue on the Fatigue Severity Scale (FSS).23
-
(3)
Participant satisfaction, as determined by responses for 2 questions on the postintervention survey regarding satisfaction and whether they would recommend the program to others. The criterion was that 80% of participants agree or strongly agree.
-
(4)
Completion rates, with participant attrition of 20% or lower.
Participant characteristics
Demographic and anthropometric information, including age, sex, highest level of education, smoking history, medications, use of walking aids, walking distance, type of MS, date of MS diagnosis, weight, and height, were collected. The EDSS score was used to characterize impairment.24
Sedentary behavior outcomes
Sedentary behavior (ie, total sitting time per day, number of transitions from sit to stand per day, and mean number of long [>30min] sedentary bouts per day) was measured using ActivPAL325,26,b at baseline, postintervention, and follow-up. The monitor was positioned and affixed by a research assistant on the participant’s stronger thigh with 3M Tegaderm.c Participants wore the monitor for 7 consecutive days, after which it was returned via mail.
Data analysis
Feasibility metrics, including recruitment and retention (eg, number of participants approached, number of participants enrolled, number of participants retained) and communication (eg, frequency), were reported. Descriptive statistics (mean ± SD) were used to characterize the sample. The analysis of the survey was descriptive, reporting frequency of responses. Outcomes related to sedentary behavior from ActivPAL3 were generated using the R package (PAactivpald).27 An unstructured linear effects model was used and provided information about means and variances, as well as changes in sedentary behavior outcomes. Effect sizes (Cohen’s d) specific to changes between baseline, postintervention, and 7 weeks postintervention were calculated for each of the sedentary behavior outcomes. SPSSe was used for all quantitative analysis.
Results
Participants
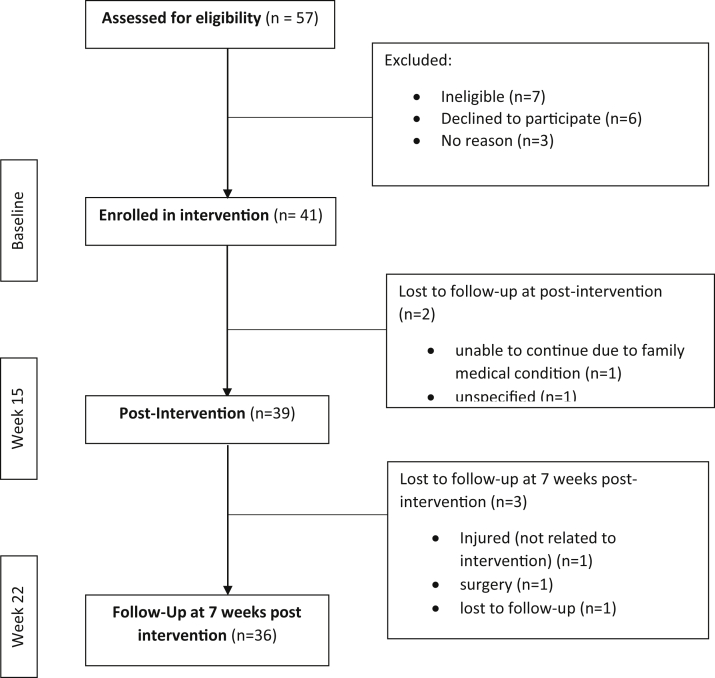
Forty-one participants were enrolled in the SitLess with MS study, 39 (95%) of whom completed both the baseline and immediate postintervention assessments (fig 2). Thirty-six participants (88% of those enrolled at baseline) were retained for the follow-up data collection session 7 weeks after intervention completion. The flow of participants, including reasons for loss to follow-up, are displayed in figure 2. Participant characteristics are reported in table 1. Participants were primarily women (90%) with varying levels of impairment demonstrated by EDSS scores that ranged between 1 and 6.5, reflecting mild to moderate disability status.
Fig 2.
Participant flow diagram.
Table 1.
Baseline characteristics of participants enrolled in the SitLess with MS trial
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Age, y | 50.5±10.3 |
| Sex, women | 37 (90.2) |
| Education level | |
| High school and less | 10 (24.4) |
| College/diploma | 15 (36.6) |
| Bachelor degree | 11 (26.8) |
| Master degree | 5 (12.2) |
| Type of MS | |
| Relapsing remitting | 26 (63.4) |
| Primary progressive | 4 (9.8) |
| Secondary progressive | 11 (26.8) |
| Duration of diagnosis, y | 14.3±11.3 |
| Walking aid | |
| None | 18 (43.9) |
| Single cane | 6 (14.6) |
| Double cane | 6 (14.6) |
| Walker | 9 (22.0) |
| Quad cane | 2 (4.9) |
| BMI, kg/m2 | 28.4±6.2 |
| EDSS | |
| Median (IQR) | 5.5 (3.7) |
| Range | 1.5-6 |
Abbreviations: BMI, body mass index; IQR, interquartile range.
Feasibility and participant experience
Intervention coaches worked with a maximum of 8 participants per week. Intervention chat (ie, coaching sessions) length ranged between 10 and 35 minutes, with an average of 21 minutes (SD, 6min). Preparation time for chats was approximately 5 minutes per chat, involving review of Fitbit data from the previous week, reading intervention coach notes from the previous week, and reviewing the topic for the current week. The time to complete the intervention was, on average, 100 days (range, 91-118d), reflecting the planned weekly contact with the intervention coaches over the 15-week intervention period, with variation mainly owing to participant vacation schedules.
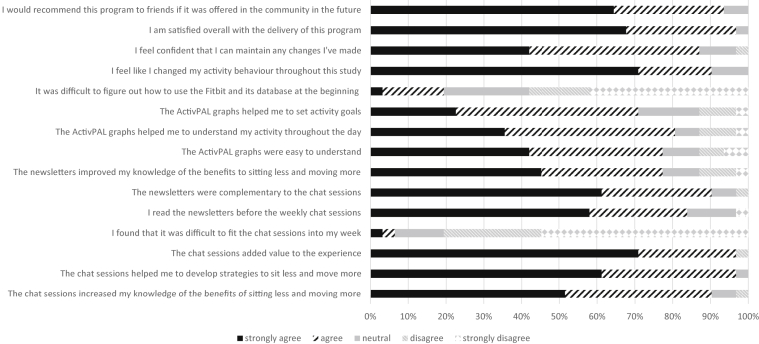
On the postintervention survey, participants agreed or strongly agreed with the majority of items (fig 3). Regarding self-monitoring, 76% reported checking step counts on their phone daily during the MoveMore stage, and 48% checked the activity graphs daily during the SitLess stage. Login was required during the SitLess stage as sedentary time and breaks in sedentary time can only be interpreted from the graphs on the Fitbit website (ie, not from the real-time output on a phone). Ninety percent of participants agreed or strongly agreed that they made activity behavior changes during the intervention. Eighty percent disagreed or strongly disagreed with the negatively worded question asking whether fitting the weekly sessions in was problematic.
Fig 3.
Participant responses to feedback survey. Thirty-one out of 39 participants responded to the postintervention survey.
Progression criteria
Intervention coaches and participants tracked adverse events, including falls. Eight participants fell 1 time, 1 person fell twice, and 1 person fell 5 times (total of 15 falls among 10 participants). From the participants’ perspective, falls were not associated with the intervention itself. There were no injuries, and no adverse events, aside from falls, occurred.
FSS scores from pre- to immediate postintervention indicated that 87% of participants (34 of 39) had a reduction in self-reported fatigue. On average, scores on the FSS decreased from preintervention (5.4±1.2) to immediate postintervention (4.6±1.2). This change does not exceed the reported minimal detectable change of 1.9 points.28
Participant responses to 2 questions on the survey were identified as criteria to continue to a future trial. Thirty out of 31 participants (97%) agreed or strongly agreed with the statements “I am satisfied overall with the delivery of this program” and “I would recommend this program to friends if it was offered in the community.”
Forty-one participants started the SitLess with MS program, 39 of whom completed the intervention. This represented an attrition rate of 12%, less than the identified threshold of 20%.
Sedentary behavior outcomes
The total sedentary time and number of long (>30min) bouts of sedentary time per day decreased significantly (P<.05) from pre- to postintervention (table 2). There were no other significant changes. Effect sizes for pre- to immediate postintervention change in total sedentary time, number of transitions, and number of long (>30min) sedentary bouts were 0.34, 0.06, and 0.39, respectively. From preintervention to 7 weeks postintervention, effect sizes were 0.21, 0.02, and 0.20 for total sedentary time, number of transitions, and number of long (>30min) sedentary bouts, respectively.
Table 2.
Baseline, immediate postintervention, and 7-week postintervention sedentary behavior outcomes as measured by ActivPal
| Variables | Baseline (n=40) |
Immediate Postintervention (n=37) |
7 Weeks Postintervention (n=30) |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Sedentary time in minutes (per d) | 626.4 (581.1-670.5) | 577.9 (544.5-630.8)∗ | 596.5 (545.3-645.3) |
| Number of breaks (per d) | 54.6 (48.6-60.7) | 55.8 (48.6-61.4) | 54.1 (47.2-61.1) |
| Number of sedentary bouts >30 minutes (per d) | 5.8 (5.1-6.5) | 5.0 (4.5-5.7)∗ | 5.4 (4.6-6.3) |
NOTE. Reduction in sample size across sessions resulted from participant attrition or other factors (eg, <4 valid days of output, equipment malfunction).
Abbreviation: CI, confidence interval.
Significantly different from baseline (P<.05).
Discussion
The SitLess with MS study tested a novel intervention that shifts away from a singular focus on moderate-to-vigorous intensity activity toward a broader, and perhaps more feasible, approach that encourages reductions in sedentary behavior and increases in light-intensity activity. The intervention was feasible, acceptable, and yielded changes in sedentary time. Participants were satisfied with the experience of the intervention and the one-on-one delivery through telerehabilitation. All progression criteria were met for advancing toward a study design that incorporates an appropriate control group. Future work will be informed by our reported effect sizes related to sedentary behavior.
The effect sizes were small for baseline to postintervention change in total sedentary time (0.34) and number of long (>30min) bouts (0.39). The number of transitions from sit to stand did not change, indicating that participants had longer bouts of standing when they did get up. Our reported effect sizes specific to the reduction of sitting time are similar to others. For example, interventions focused on reduction of sedentary behavior in those with chronic stroke (0.32)18 and chronic MS (0.41)29 also reported small effect sizes. A feasibility study testing an 8-week intervention among individuals with subacute stroke19 (ie, 3mo poststroke) found that participants reduced their sitting time by 54 minutes daily, a change that equates to a moderate effect size (0.59).19 These findings suggest that time since diagnosis or event influences the potential magnitude of change, and that the capacity for change is greater in the earlier stages of a condition. This may be especially true for those with stroke, which is a nonprogressive condition. For those with MS, a progressive condition, the effect of chronicity on capacity for activity behavior change is less clear but is most likely related to level of disability. Differing disability levels, program delivery (frequency, in person vs remotely), and use and incorporation of feedback from a monitoring device are elements that require more testing to identify the active ingredient and appropriate intervention features for maximizing behavior change.
The 3 studies discussed above,18,19,29 which generally report small effect sizes, along with the work reported herein provide some of the first evidence regarding sedentary behavior interventions in people with conditions causing disability. Although the changes are small, they represent a substitution of sedentary time for movement. Recent guidelines suggest that the greatest health benefits come from moving from inactive to taking part in some level of activity regularly.30 This is welcome news to those with mobility disability who may find changing activity behavior challenging. Successful behavior changes reducing sedentary behavior, such as those we saw, may provide success that could set the stage for sustained activity behavior changes.
Researchers working with individuals with chronic obstructive pulmonary disease have cautioned the messaging of SitLess as challenging for those who have good reasons for needing to sit (ie, to rest and enable other activities).31 A priori, we were cognizant that an intervention using messaging to break up sitting time frequently was opposite to the energy conservation message often delivered to individuals with MS.32 We report reductions in fatigue with this intervention, thus our study suggests that concerns of greater amounts of fatigue with an intervention that focused on sitting less and moving more were unwarranted. Nevertheless, clinicians must recognize and communicate their understanding of the reasons to sit, and help patients to set achievable goals related to sitting less. Clinicians can also help patients to recognize the link between the action of moving from sitting to standing (ie, breaking up sitting time) and meaningful opportunities moving may have for activities that promote quality of life (such as getting out of the house, visiting friends).
Study limitations
The design of this study did not include a control group. Therefore, future research is needed to test the effect of the intervention versus a group that does not receive the intervention. In terms of safety reporting, the measurement of falls was done weekly via self-report during the coaching session. Although no participant stated that a fall was related to the intervention, they were on their feet more (ie, sitting less) and it is thus impossible to rule out whether the intervention played a role in the fall. Anecdotally, the participants who fell told us that it was not unusual for them, but we did not record a fall history, which is a limitation.
Many participants suggested that future commercially available activity monitoring devices should record sit to stand transitions in real time. For some, particularly those who had more challenges following directions or remembering information between sessions, the Fitbit was challenging to synch with the phone and use effectively for self-monitoring. The baseline data collection session included time for set up and synching of the Fitbit. However, in some cases, this was not possible because the appropriate devices were not available during those sessions. In future trials, in-person set up and synching of devices is strongly recommended. Finally, some participants were reasonably active throughout the day at the outset of the study and reported that the SitLess stage was not challenging enough. Our inclusion criteria with respect to activity level were based on the Godin questionnaire, which focuses on leisure time exercise habits in bouts of activity 15 minutes in length. Future researchers may wish to include a screening question specific to movement throughout the day to more fully identify those who sit for significant periods during the day.
Conclusions
This study demonstrates the feasibility of a novel telerehabilitation intervention to encourage sitting less and moving more among individuals with MS. Preliminary information regarding magnitude of change will help to inform future work in this area.
Suppliers
-
a.
Fitbit One; Fitbit Corp.
-
b.
ActivPAL; PAL Technologies Ltd.
-
c.
Tegaderm; 3M Company.
-
d.
The R Project for Statistical Computing.
-
e.
SPSS Statistics for Windows, version 24.0; IBM Corp.
Acknowledgments
We thank Penny Smyth, MD, Jennifer McCombe, MD, and Margaret Prociuk, RN, from the Northern Alberta MS Clinic at the University of Alberta, for their invaluable help with recruitment in this project.
Footnotes
Clinical Trial Registration No.:NCT03136744.
Supported by Alberta Innovates, Alberta/Novartis Translational Research Fund.
Disclosures: none.
References
- 1.Motl R.W., Pilutti L.A. Is physical exercise a multiple sclerosis disease modifying treatment? Expert Rev Neurother. 2016;16:951–960. doi: 10.1080/14737175.2016.1193008. [DOI] [PubMed] [Google Scholar]
- 2.Dalgas U., Langeskov-Christensen M., Stenager E. Exercise as medicine in multiple sclerosis-time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep. 2019;19:88. doi: 10.1007/s11910-019-1002-3. [DOI] [PubMed] [Google Scholar]
- 3.Motl R.W. Benefits, safety, and prescription of exercise in persons with multiple sclerosis. Expert Rev Neurother. 2014;14:1429–1436. doi: 10.1586/14737175.2014.983904. [DOI] [PubMed] [Google Scholar]
- 4.Sandroff B.M., Dlugonski D., Weikert M. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand. 2012;126:256–262. doi: 10.1111/j.1600-0404.2011.01634.x. [DOI] [PubMed] [Google Scholar]
- 5.Dalgas U., Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5:81–95. doi: 10.1177/1756285611430719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latimer-Cheung A.E., Ginis K.A.M., Hicks A.L. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch Phys Med Rehabil. 2013;94:1829–1836.e7. doi: 10.1016/j.apmr.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Cederberg K.L., Motl R.W., McAuley E. Physical activity, sedentary behavior, and physical function in older adults with multiple sclerosis. J Aging Phys Act. 2018;26:177–182. doi: 10.1123/japa.2016-0358. [DOI] [PubMed] [Google Scholar]
- 8.Pearson M., Dieberg G., Smart N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil. 2015;96:1339–1348.e7. doi: 10.1016/j.apmr.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Ezeugwu V., Klaren R.E., Hubbard E.A. Mobility disability and the pattern of accelerometer-derived sedentary and physical activity behaviors in people with multiple sclerosis. Prev Med Rep. 2015;2:241–246. doi: 10.1016/j.pmedr.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motl R.W., McAuley E., Snook E.M. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler J. 2005;11:459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki J., Motl R., Cutter G. National estimates of self-reported sitting time in adults with multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4 doi: 10.1177/2055217318754368. 2055217318754368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay M.S., Aubert S., Barnes J.D. Sedentary Behavior Research Network (SBRN)–Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healy G.N., Matthews C.E., Dunstan D.W. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011;32:590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Pozo-Cruz B., Mañas A., Martín-García M. Frailty is associated with objectively assessed sedentary behaviour patterns in older adults: evidence from the Toledo Study for Healthy Aging (TSHA) PLoS One. 2017;12 doi: 10.1371/journal.pone.0183911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mañas A., del Pozo-Cruz B., García-García F.J. Role of objectively measured sedentary behaviour in physical performance, frailty and mortality among older adults: a short systematic review. Eur J Sport Sci. 2017;17:940–953. doi: 10.1080/17461391.2017.1327983. [DOI] [PubMed] [Google Scholar]
- 16.Manns P.J., Dunstan D.W., Owen N. Addressing the nonexercise part of the activity continuum: a more realistic and achievable approach to activity programming for adults with mobility disability? Phys Ther. 2012;92:614–625. doi: 10.2522/ptj.20110284. [DOI] [PubMed] [Google Scholar]
- 17.Aminian S., Motl R.W., Rowley J. Management of multiple sclerosis symptoms through reductions in sedentary behaviour: protocol for a feasibility study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English C., Healy G.N., Olds T. Reducing sitting time after stroke: a phase ii safety and feasibility randomized controlled trial. Arch Phys Med Rehabil. 2016;97:273–280. doi: 10.1016/j.apmr.2015.10.094. [DOI] [PubMed] [Google Scholar]
- 19.Ezeugwu V.E., Manns P.J. The feasibility and longitudinal effects of a home-based sedentary behavior change intervention after stroke. Arch Phys Med Rehabil. 2018;99:2540–2547. doi: 10.1016/j.apmr.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Amireault S., Godin G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills. 2015;120:604–622. doi: 10.2466/03.27.PMS.120v19x7. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 22.Treacy D., Hassett L., Schurr K. Validity of different activity monitors to count steps in an inpatient rehabilitation setting. Phys Ther. 2017;97:581–588. doi: 10.1093/ptj/pzx010. [DOI] [PubMed] [Google Scholar]
- 23.Krupp L.B., LaRocca N.G., Muir-Nash J. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 24.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 25.Kozey-Keadle S., Libertine A., Lyden K. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43:1561–1567. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- 26.Lyden K., Kozey Keadle S.L., Staudenmayer J.W. Validity of two wearable monitors to estimate breaks from sedentary time. Med Sci Sport Exerc. 2012;44:2243–2252. doi: 10.1249/MSS.0b013e318260c477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyden K., Keadle S.K., Staudenmayer J. The activPALTM accurately classifies activity intensity categories in healthy adults. Med Sci Sport Exerc. 2016;49:1022–1028. doi: 10.1249/MSS.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Learmonth Y.C., Dlugonski D., Pilutti L.A. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci. 2013;331:102–107. doi: 10.1016/j.jns.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Ryan J.M., Fortune J., Stennett A. Safety, feasibility, acceptability and effects of a behaviour-change intervention to change physical activity behaviour among people with multiple sclerosis: results from the iStep-MS randomised controlled trial. Mult Scler. 2019 doi: 10.1177/1352458519886231. 1352458519886231. [DOI] [PubMed] [Google Scholar]
- 30.Piercy K.L., Troiano R.P., Ballard R.M. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weedon A.E., Saukko P.M., Downey J.W. Meanings of sitting in the context of chronic disease: a critical reflection on sedentary behaviour, health, choice and enjoyment. Qual Res Sport Exerc Health. 2020;12:363–376. [Google Scholar]
- 32.Blikman L.J., van Meeteren J., Twisk J.W. Effectiveness of energy conservation management on fatigue and participation in multiple sclerosis: a randomized controlled trial. Mult Scler. 2017;23:1527–1541. doi: 10.1177/1352458517702751. [DOI] [PubMed] [Google Scholar]