Abstract
Objective
The objective of this study was to determine the feasibility of a rehabilitation approach focusing on cardiovascular, strength, and gait training intensity in the inpatient rehabilitation setting after a new onset of stroke. We additionally aimed to determine the efficacy of this intensity-based program on rehabilitation outcomes compared with usual care.
Design
Participants were pseudo-randomized to an intensity-based program focusing on gait, cardiovascular, and strength training or to usual care. Outcomes included FIM, 10-meter walk, 2-minute walk, timed Up and Go test, 5-time sit-to-stand test, and Tinetti balance assessment.
Intervention
The intervention consisted of 6 20-minute sessions per week dedicated to intensity of activity: 2 each for walking, cardiovascular training, and strength training.
Participants
Patients (N=49) with new onset stroke admitted to inpatient rehabilitation over the course of 1 year.
Setting
Four inpatient rehabilitation facilities with comprehensive neurologic rehabilitation teams.
Results
Thirty-five individuals (16 intervention, 19 controls) completed all testing. Subject compliance to the intensity intervention demonstrated completion of approximately half the prescribed sessions. All outcomes improved significantly from admission to discharge, and a significant interaction between treatment group and time was observed for the 2-minute walk and the Tinetti balance assessment. The 2-minute walk, Tinetti balance assessment, 10-meter walk, and FIM demonstrated between-group effect sizes greater than 0.60 in favor of the intervention group.
Conclusions
The intensity-based protocol was safe, and several measures demonstrated efficacy when compared with usual care. Results may have been limited by poor program compliance, showing a need to identify and ameliorate obstacles to integration of comprehensive intensity-based programs addressing endurance, strength, and gait training. Applying physiological principles of exercise to acute stroke rehabilitation demonstrates great promise for improving independent physical function.
Keywords: Exercise, Rehabilitation, Stroke, Walking
List of abbreviations: 2MWT, 2-minute walk test; 10-mw, 10-meter walk test; 5xSTS, 5 times sit to stand; CFIR, Consolidated Framework for Implementation Research; SSWS, self-selected walking speed
Stroke is the most common disabling condition in the United States today, with approximately two-thirds of the 795,000 individuals experiencing stroke each year requiring comprehensive, interdisciplinary therapy to promote functional independence. Individuals poststroke often experience severe deconditioning secondary to direct impairments from the stroke, as well as subsequent sedentary lifestyle.1 Initiating and progressing capacity-building exercise programs to combat the acute and long-term effects of stroke should be imperative during poststroke rehabilitation. Intensity of interventions, which we define as dosing and progressing according to prescribed standards associated with maximum heart rate, 1-repetition maximum resistance, and self-selected walking speed (SSWS),2 is critical if improved physical function is to be achieved. Intensity of training, a primary principle of neuromuscular plasticity,3 is consistently a major factor associated with success in improving function and driving neurologic recovery.4,5
Although intensity is recognized as an important factor in poststroke recovery research, it is rarely practiced in real-world environments. Translating new interventions, such as high intensity rehabilitation, to the clinical setting can be challenging, but it is critical to the advancement of best practice in neurologic rehabilitation. Surveys indicate that although 88% of physical therapists in the United States responded that intense aerobic exercise should be incorporated into stroke rehabilitation, 84% identified at least 1 barrier inhibiting implementation,6 and heart rates reach aerobic training zones for less than 5% of total physical therapy treatment time.7 Knowledge of sedentary activity reduction being critical to neurologic recovery has not changed the fact that patients with stroke in inpatient rehabilitation only spend 10 hours a day out of bed, with 87% of that time being sedentary (including 61.6% of their time participating in physical therapy).8 An urgent need exists to translate laboratory-tested interventions to real-world environments and to identify and ameliorate obstacles to comprehensive intensity-based programs capable of promoting walking recovery.
Previous rehabilitation trials focused on the intensity of interventions have primarily examined a single intervention, such as aerobic training,9,10 walking interventions,11,12 or strengthening.13, 14, 15 In a review of strength training after chronic stroke, appropriately intense resistance training (at 75% of the 1-repetition maximum)13 produced improvements in strength, gait speed, functional outcomes, and quality of life. Importantly, there were no reports of increased spasticity or synergistic movement patterns.16 Intense cardiovascular training, defined as reaching a target heart rate or target rating of perceived exertion for a total of at least 20 minutes, produced significant gains in walking speed, distance walked, balance, and cognition compared with a non-targeted exercise control intervention.10 High intensity walking (training faster than SSWS) yielded significant improvements in SSWS, fastest comfortable walking speed, and 6-minute walk test distance.17,18
In spite of mounting evidence supporting dosing and progressing intensity-based interventions by clinically available metrics (age-based maximum heart rate, strength gauged as percentage of the 1-repetition maximum, SSWS),2 to date, no study has combined these approaches as part of a comprehensive inpatient rehabilitation treatment regimen. Combining all 3 areas into a comprehensive intervention offers the possibility of coalescing their individual effects into a therapy capable of maximizing mobility. In addition, therapists may be more likely to implement an intervention that not only meets the comprehensive needs of those in inpatient rehabilitation facilities but that also demonstrates the high probability of a clinical effect. The purpose of this study was to determine the feasibility of implementing an evidence-based rehabilitation approach focusing on cardiovascular, strength, and gait training intensity in the inpatient rehabilitation setting. We also aimed to determine the efficacy of an intensity-based program on inpatient rehabilitation outcomes compared with usual care.
Methods
Study design
The Systematic Collection of Objective and Progressive Exercise (SCOPE) trial was a multicenter, pseudo-randomized pragmatic trial that included patients admitted to inpatient rehabilitation during 2017 for management of new stroke. All participants participated in the informed consent process and completed an informed consent form approved by the institutional review board at the Medical University of South Carolina, which functioned as the institutional review board for all sites. Study participants were considered for enrollment after admission to 1 of 4 different inpatient rehabilitation facilities: (1) HealthSouth Rehabilitation Hospital, Charleston, SC (currently Encompass Health Rehabilitation Hospital of Charleston); (2) AnMed Health Rehabilitation Hospital, Anderson, SC; (3) HealthSouth Rehabilitation Hospital, Rock Hill, SC (currently Encompass Health Rehabilitation Hospital of Rock Hill); and (4) HealthSouth Rehabilitation Hospital, York, PA (currently Encompass Health Rehabilitation Hospital of York).
Inclusion criteria included (1) admission to inpatient rehabilitation for new stroke, even if there was an incidence of previous stroke; (2) stroke weighted FIM motor score between 26.15 and 51.05 (FIM group 3-9); and (3) ability to provide informed consent. Exclusion criteria included: (1) neurologic comorbidities other than stroke; (2) severe dementia (unable to follow motor commands or provide a rating of perceived exertion); (3) cardiovascular diagnosis other than stroke preventing intensity of rehabilitation; and (4) severe hypertension with systolic blood pressure greater than 200 mmHg and diastolic blood pressure greater than 110 mmHg at rest. Patient eligibility and group assignment was determined by the Director of Therapy Operations at each facility. Successive admissions were assigned to either the intervention or control group in an alternating pattern. This pseudo-randomization occurred according to clinic needs. Enrollment was completed by the treating physical therapists. Assessments were performed by the treating physical therapist within 3 days of admission and were repeated before discharge (within 24h of discharge). The study design was to be part of clinical practice and reflect a more pragmatic approach rather than serve as a true randomized controlled trial.
Patients assigned to the intervention group underwent a standardized intensity-based physical therapy rehabilitation program. The program was guided by a customized REDCapa survey tool developed by investigators at the Medical University of South Carolina. The REDCap tool provided therapists guidance on the intensity-based interventions by using patient assessment findings to specify individualized intensity dosing and progression targets. However, the REDCap tool was not prescriptive for the specific exercises or activities to be delivered during treatment sessions. The goal was for participants to receive 2 20-minute intensity-based intervention sessions per week to address each of the following: gait training, strength training, and cardiovascular training. Intensity of gait was prescribed as training performed between 110% and 125% of the patients’ SSWS, assessed at the beginning of each gait training bout, and could be completed either over ground or on a treadmill. Walking activities progressed as walking speed increased to consistently maintain 110% to 125% of SSWS. Strength training intensity prescription was based on 75% of the 1-repetition maximum, which is the equivalent of the 10-repetition maximum, and exercises progressed when the patient was able to complete more than 10 repetitions without fatigue. Specific strengthening activities were not prescribed by the protocol. Clinical decision-making regarding strengthening modalities was left to the treating therapist. Cardiovascular training was prescribed as maintaining heart rate within a target zone set at 60% to 80% of the age-predicted maximum heart rate (220 beats/min – age). For those participants demonstrating a compromised cardiovascular response to activity or exercise (eg, those on a beta-blocker or experiencing sympathetic nervous system dysfunction), an alternative intensity target of 11 to 15 on the Borg rating of perceived exertion scale was used.19 In the postacute stoke population, gait and strength training often produce increases in heart rate or rating of perceived exertion into the target zone. If target values were sustained for 20 minutes during gait or strength training sessions, these sessions were also counted as cardiovascular training.
Those individuals randomized into the control group received usual care, defined as the physical therapy treatment normally provided at each individual facility. Treating physical therapists of patients in the control group were given no knowledge of the intervention group design, and scheduled treatment time was equivalent to the intervention group.
Outcomes
Participants in both groups underwent a standardized battery of common physical therapy outcome measures used with the cerebrovascular accident population in the inpatient rehabilitation setting. The battery was assessed at admission and discharge by the treating physical therapist and included the following measures: 10-meter walk test (10-mw),20 2-minute walk test (2MWT),21 timed Up and Go,22 5-times sit-to-stand (5xSTS),23 the Tinetti Performance Oriented Mobility Assessment,24 and the FIM.25 A 2018 clinical practice guideline published by the Academy of Neurologic Physical Therapy recommends outcome measures in the following domains: balance (standing, walking, and confidence), walking speed, walking distance, and transfers,26 with included measures reflecting all of the above recommendations. More specific measures for aerobic capacity (ie, exercise tolerance testing) and for strength training (ie, dynamometer testing) were beyond the clinical scope of this study. Patients who were unable to complete any of the standardized outcome measures at time of admission were assessed as soon as they could complete the measure without modification. For the 10-mw, 2MWT, Tinetti, and FIM, baseline scores were considered zero if they could not be completed. For the 5xSTS and timed Up and Go (where zero would reflect an impossible score), we replaced zeros at baseline with the time from the lowest performing individual on that outcome measure for statistical purposes.
Semi-structured follow-up phone interviews
We conducted follow-up phone interviews with the study physical therapists (n=5) to further understand barriers to implementation. The interviews were performed 3 months after study completion by an individual with experience collecting qualitative data who was not involved in previous study activities. A semi-structured interview guide was developed using the Consolidated Framework for Implementation Research (CFIR).27 The CFIR is a comprehensive framework of constructs related to implementation organized into 5 domains: Intervention Characteristics, Outer Setting, Inner Setting, Characteristics of Individuals, and Process.27 The interviews were audio-recorded and transcribed verbatim. Transcripts were analyzed using a content analysis approach.28 Coded data was categorized by CFIR construct and characterized as a barrier or facilitator. Because of the nature of the study and the set sample size, we report barriers and facilitators identified by at least 3 of the 5 participants. Survey questions were rated on a 4-point Likert scale (Strongly Agree, Agree, Disagree, Strongly Disagree). We calculated frequencies of responses on survey questions.
Statistical analysis
All data are reported as mean ± SD unless otherwise noted. Between-group effect sizes were calculated for all variables using the formula:
ESbetween=Δintervention – Δcontrol/standard deviationpooled.
A 2 × 2 analysis of variance was performed for each of the 6 outcome measures to evaluate main effect of group, time, and the group × time interaction. Sphericity of the models was tested using Mauchly’s test of sphericity. In the case of significant findings, Greenhouse-Geiser corrected P values are reported; otherwise, sphericity was assumed. Post hoc analyses were conducted using a Bonferroni adjustment. Normality was assessed using a Shapiro-Wilk test, and all non-normally distributed data were log-transformed. Alpha was preset to 0.05. All statistical analysis was performed using IBM SPSS Statistics for Windows, Version 24.b
Results
Sixteen individuals completed the intervention (10 men, 6 women; 2 black, 14 white) with an average age of 67.2±17.4 years and average chronicity of 36.8±72.9 days poststroke (2 patients were readmitted 251 and 186 days poststroke). Nineteen individuals were in the usual care control group (9 men, 10 women; 3 black, 1 Hispanic, 15 white) with an average age of 70.3±14.2 years and 8.2±5.6 days poststroke. Length of stay was 14.8±5.1 days for the intervention group and 13.8±4.0 days for the control group, which were not significantly different (P=.635 with the Mann-Whitney U test).
Nine individuals who signed informed consent withdrew from the intervention group, whereas only 5 withdrew from the control group. The difference was primarily because 3 individuals from the intervention group withdrew secondary to decreased activity tolerance (table 1). The amount of therapy per day was similar across both groups, with those in the intervention group participating in 150.1±15.8 minutes and control patients participating in 144.3±10.3 minutes.
Table 1.
Withdrawals
| Group | Enrolled | Completed | Withdraw | Reason for Withdrawal |
|---|---|---|---|---|
| Intervention | n=25 | n=16 | n=9 | Acute care transfer (n=3) Decreased activity tolerance/subjective (n=3) Unexpected early discharge (n=1) Family request (n=1) Unable to perform outcome measures (n=1) |
| Control | n=24 | n=19 | n=5 | Acute care transfer (n=2) Unexpected early discharge (n=1) Unreported reasons (n=2) |
The intervention target was 2 sessions each per week of cardiovascular training, strength training, and intensive gait training. The average intervention period from admission to discharge testing was 12.12 days. Therefore, full compliance with the intervention protocol, not accounting for weekend treatment, would have yielded 14.5 sessions (4.83 sessions of each type per stay). However, intervention participants failed to meet these goals with a per stay average of 1.86 sessions for cardiovascular training, 2.09 sessions for strength training, and 2.59 sessions for intensive gait training. The a priori goal was 90% intervention compliance, but the 6.54 completed sessions reflected a compliance rate of only 45.1%.
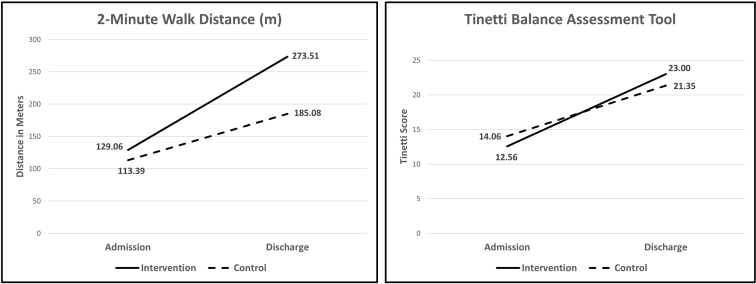
For all outcome measures, there was a significant main effect of time (F>18.5, P<.001). Post hoc analyses demonstrated that there were no significant differences between the groups for any variable at baseline (P>.56). Both the intervention and control groups improved significantly in each outcome measure (P<.03), but the change scores from admission to discharge were consistently larger in the intervention group for all variables except the 5xSTS (table 2). Both the 2MWT and Tinetti demonstrated a significant interaction when assessed with a 2-way analysis of variance (P<.05) (fig 1), and the FIM, 10-mw, 2MWT, and Tinetti all had between-group effect sizes that exceeded 0.60 (see table 2).
Table 2.
Change scores for each outcome
| Outcome Measure | Intervention Mean Δ | Control Mean Δ | Main Effect Time | Interaction Effect | Between Group Effect Size |
|---|---|---|---|---|---|
| FIM | 49.88±7.32 | 42.70±14.95 | P<.001 | P=.10 | 0.61 |
| 10-mw speed (m/s) | 0.40±0.27 | 0.24±0.23 | P<.001 | P=.25 | 0.62 |
| 2MWT distance (feet) | 144.44±118.22 | 71.69±94.12 | P<.001 | P=.02 | 0.68 |
| TUG time (s) | -22.07±23.72 | -16.71±19.80 | P<.001 | P=.36 | 0.24 |
| 5xSTS time (s) | -6.88±5.80 | -7.90±12.26 | P<.001 | P=.99 | -0.11 |
| Tinetti | 10.44±4.76 | 7.29±4.98 | P<.001 | P=.03 | 0.64 |
NOTE. Note that the intervention group had 3 individuals with decreased activity tolerance, whereas no one withdrew for that reason from the lower intensity control group.
Abbreviation: TUG, timed Up and Go.
Fig 1.
Interactions for 2-minute walk distance (left) and the Tinetti balance assessment tool (right). No significant differences were present at baseline, but a significant interaction (P<.05) is present for both variables.
Follow-up phone interviews
Survey questions
The study physical therapists’ (n=5) responses to survey questions are presented in table 3.
Table 3.
Physical therapists’ responses to survey questions
| Survey Question | Strongly Agree, n (%) | Agree, n (%) | Disagree, n (%) | Strongly Disagree, n (%) |
|---|---|---|---|---|
| It was difficult to integrate the intensity-based program into my clinical practice. | 0 (0) | 0 (0) | 5 (100) | 0 (0) |
| It was challenging to recruit patients to participate in the study. | 1 (20) | 3 (60) | 1 (20) | 0 (0) |
| Once enrolled, patients responded favorably to the intensity-based approach.∗ | 0 (0) | 5 (100) | 1 (20) | 0 (0) |
| I had sufficient resources, including time, to deliver the intensity-based program. | 0 (0) | 2 (40) | 2 (40) | 1 (20) |
| The REDCap survey tool was easy to use. | 2 (40) | 2 (40) | 1 (20) | 0 (0) |
| I consistently delivered the intensity dosage and progression generated by the REDCap tool. | 1 (20) | 4 (80) | 0 (0) | 0 (0) |
| I consistently administered the specified outcome measures to study participants at admission and discharge. | 3 (60) | 2 (40) | 0 (0) | 0 (0) |
| Upon reflection, the intensity-based approach was effective in my setting. | 3 (60) | 2 (40) | 0 (0) | 0 (0) |
| Involvement in the study positively impacted the way I practice now. | 3 (60) | 2 (40) | 0 (0) | 0 (0) |
NOTE. Four of the 6 outcome measures collected demonstrated between-group effect sizes greater than 0.60. The 2MWT and Tinetti demonstrated significant group × time interactions (P<.05).
One participant selected “Agree” and “Disagree” and stated that it depended on the patient.
Open-ended interview questions
Barriers and facilitators to implementation of the intensity-based program organized by CFIR domain and construct are presented in table 4. The reported barriers to implementation were complexity of participant selection criteria (n=4), lack of clinical utility of the REDCap data entry tool (n=4), lack of support from management in the enrollment process (n=3), scheduling issues (n=3), and lack of knowledge on use of the REDCap tool and delivery of the intensity-based program (n=3). The therapists’ belief that the intervention was beneficial for their patients (n=5) was a facilitator to implementation.
Table 4.
Barriers and facilitators organized by CFIR domains and constructs
| CFIR Domain and Construct29 | Therapists Reporting, n=5 | |
|---|---|---|
| Intervention characteristics | ||
| Complexity | Barrier: Complexity of participant selection criteria was a barrier to enrollment. | 4 |
| Design quality & packaging | Barrier: REDCap tool did not allow entry of sufficient treatment detail or allow longitudinal tracking of patient data. | 4 |
| Inner setting | ||
| Readiness for implementation, leadership engagement | Barrier: Lack of support from management in the enrollment process. | 3 |
| Readiness for implementation, available resources | Barrier: Scheduling issues were a barrier to adherence to the intensity protocol. | 3 |
| Readiness for implementation, access to knowledge and information | Barrier: Lack of knowledge on use of REDCap tool and delivery of intensity program. | 3 |
| Characteristics of individuals | ||
| Knowledge & beliefs about the intervention | Facilitator: Therapists view the intervention as being beneficial for their patients. | 5 |
Discussion
The purpose of this study was to determine the feasibility of using a rehabilitation framework focused on incorporating physiological principles of exercise for individuals poststroke in the inpatient rehabilitation setting. In addition, we aimed to determine the efficacy of this framework on inpatient rehabilitation outcomes compared with usual care. Although there were no serious adverse events associated with the intervention, treatment compliance was low, with patients only receiving approximately half of the prescribed intensive sessions over the course of their inpatient rehabilitation stay. In addition, 3 individuals in the intervention group withdrew secondary to decreased activity tolerance. None withdrew for tolerance reasons from the lower intensity control group. This intolerance for intensive activity highlights one of the challenges of encouraging exercise programs dosed on principles of exercise for those poststroke. Despite the low treatment compliance and the relatively short lengths of stay, individuals in the intervention group demonstrated gains in all outcome measures that exceeded those in the usual care control group with the exception of the 5xSTS. The lack of improved gains in the 5xSTS may reflect that sit-to-stand exercises are often part of usual care, whereas intensive cardiovascular conditioning and gait training are less common. Significant interactions were observed in both the 2MWT distance and the Tinetti balance assessment tool, demonstrating the efficacy of the intervention, even given the relatively small sample size. The FIM, 10-mw speed, 2MWT distance, and Tinetti all demonstrated between group effect sizes less than 0.60. Although treatment effects were medium to large, and efficacy was demonstrated with the 2MWT and Tinetti, the feasibility is unclear given the challenges discussed above.
Several factors served as barriers to compliance and feasibility with the protocol. In the facilities participating in the investigation, there were often coverage issues with inconsistency in therapists treating the intervention participants. These facilities also use a fair amount of group or concurrent treatments, making it more difficult to find the time to engage in the higher intensity treatments. Philosophically, many inpatient rehabilitation centers are very functional outcome biased because of dependency on the FIM as the primary outcome measure, and many therapists are reluctant to deviate from task-specific practice. Furthermore, in this study, the treating therapists were the only individuals in each facility involved in the investigation, and it proved too time consuming for them to consent individuals, collect all outcome measures, and perform the interventions without additional facility support. For example, only 49 individuals were consented into the study out of the approximately 700 persons admitted with stroke among the 4 participating sites. Lastly, for those individuals also receiving speech therapy, there were fewer opportunities for physical therapy sessions, as each patient received a total of 3 hours of therapy per day for all therapies, and the investigation was not funded for additional treatment time.
Semi-structured interviews yielded other interesting results from the treating therapists. Although there was initial resistance to deviating from task-specific treatment, all participating therapists disagreed with the statement that the program was difficult to integrate into an inpatient program. However, 4 out of 5 either agreed or strongly agreed that it was challenging to recruit patients. This was partly because of the lack of institutional support, but also because of the hesitancy for individuals poststroke to voluntarily participate in more physically challenging rehabilitation, regardless of the substantial evidence supporting higher intensity treatments. Interestingly, all therapists agreed that once enrolled, patients responded favorably to the intensity-based framework, perhaps suggesting that 1 obstacle may be psychological on the part of the patients. It is possible that the word “intensity,” which was used in the informed consent form, should be replaced by “exercise guidelines,” as the American College of Sports Medicine guidelines were the basis of the theoretical framework.29 Three out of 5 therapists stated that they did not have sufficient resources to carry out all aspects of the investigation, even though 4 felt that they consistently delivered the required elements. All therapists either agreed or strongly agreed that administering outcome measures was not an obstacle and that the program was “effective” for their patients. However, it must be considered that all therapists involved in the investigation were graduates of a neurologic residency program and certified as neurologic clinical specialists. Thus, they may not be representative of all rehabilitation therapists.
Study limitations
As this was designed as a pragmatic trial, there were several areas of control that were not attainable in this setting, including truly randomized group assignment, blinding of evaluation, evaluators conducting treatment, and concealment of group allocation. Although this adversely affected the internal validity of the study, it emphasized the real-world aspect of the attempted translation. An unexpected limitation was a relatively small sample size considering the numbers of individuals poststroke admitted annually to each of the participating rehabilitation centers. Patient recruitment and enrollment was limited, partly because of stringent exclusion criteria, such as the requirements for intact cognition (caregiver consent was not allowed in this protocol). As the treating therapists were also responsible for consenting participants, there may have been a selection bias toward higher functioning patients, as represented by baseline mean walking speeds (control=0.45 m/s, intervention=0.40). However, the groups were not significantly different at baseline, so any bias would have affected the 2 groups similarly. In addition, cognitive deficits are often correlated with poststroke functional impairments, and the current study only allowed for participation if the patient could provide informed consent. As patients could likely participate in this intervention if they were able to follow a 3-step motor command, this restriction may have artificially limited enrollment, a factor which should be considered in future trials. Lastly, an additional limitation was due to the acute nature of this patient population. Those acutely admitted to inpatient rehabilitation are often unable to participate in the required treatment elements, and several patients were dropped from enrollment because of transfers back to acute facilities, a not uncommon occurrence in this treatment setting.
Future studies investigating the effectiveness of intensity based physical therapy treatment plans should be broadened to include other diagnoses within the inpatient rehabilitation setting. An explicit approach to measuring intensity of interventions should be used in both the intervention and control groups to truly identify differences and potentially determine causative factors. The results of this study indicate a need for a larger, well-controlled examination of using exercise principles as a framework for neurologic rehabilitation. Furthermore, long-term outcomes of this intervention need to be assessed to determine the effect on quality of life, continued physical activity, and potential reduction in poststroke secondary health conditions.
Conclusions
As our health care system evolves, presenting continued reductions for reimbursement in the inpatient rehabilitation setting and effectively shortening patients’ length of stay, it is imperative to identify highly effective and efficient physical therapy treatment strategies. An increased intervention intensity during the inpatient rehabilitation stay is a simple way to maximize patient function.
Suppliers
-
a.
REDCap; Vanderbilt University.
-
b.
SPSS; IBM Inc.
Acknowledgments
We thank the following therapists and facilities for their participation in this research study (employment at the time of the study): Megan Crawford, DPT, NCS, HealthSouth Rehabilitation Hospital, York, PA (currently Encompass Health Rehabilitation Hospital of York); Amanda Feller, DPT, NCS, HealthSouth Rehabilitation Hospital, Charleston, SC (currently Encompass Health Rehabilitation Hospital of Charleston); Laura Good, DPT, NCS, AnMed Health Rehabilitation Hospital, Anderson, SC; Tim Lesondak, DPT, NCS, HealthSouth Rehabilitation Hospital, Rock Hill, SC (currently Encompass Health Rehabilitation Hospital of Rock Hill); and Christine Duffy, DPT, NCS, HealthSouth Rehabilitation Hospital, York, PA (currently Encompass Health Rehabilitation Hospital of York).
Footnotes
Current affiliation for Powell, Roper St. Francis Healthcare, Charleston, SC.
AnMed Health Rehabilitation Hospital is now Encompass Rehabilitation Hospital, Columbia, SC, and HealthSouth Rehabilitation Hospital is now Encompass Rehabilitation Hospital, Charleston, SC.
Disclosures: none.
Supported by the HealthSouth Corporation and by the National Institutes of Health (grant nos. P20-GM109040 and P2C-HD086844). This work does not reflect the views of the United States Department of Veterans Affairs or the National Institutes of Health.
References
- 1.Hornnes N., Larsen K., Boysen G. Little change of modifiable risk factors 1 year after stroke: a pilot study. Int J Stroke. 2010;5:157–162. doi: 10.1111/j.1747-4949.2010.00424.x. [DOI] [PubMed] [Google Scholar]
- 2.Billinger S.A., Arena R., Bernhardt J. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 3.Kleim J.A., Jones T.A. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 4.Dickstein R. Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair. 2008;22:649–660. doi: 10.1177/1545968308315997. [DOI] [PubMed] [Google Scholar]
- 5.Chan B. Effect of increased intensity of physiotherapy on patient outcomes after stroke: an economic literature review and cost-effectiveness analysis. Ont Health Technol Assess Ser. 2015;15:1–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Boyne P., Billinger S., MacKay-Lyons M., Barney B., Khoury J., Dunning K. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of US physical therapists. J Neurol Phys Ther. 2017;41:119–128. doi: 10.1097/NPT.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacKay-Lyons M.J., Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Arch Phys Med Rehabil. 2002;83:1378–1383. doi: 10.1053/apmr.2002.35089. [DOI] [PubMed] [Google Scholar]
- 8.Barrett M., Snow J.C., Kirkland M.C. Excessive sedentary time during in-patient stroke rehabilitation. Top Stroke Rehabil. 2018;25:366–374. doi: 10.1080/10749357.2018.1458461. [DOI] [PubMed] [Google Scholar]
- 9.Bowden MG, Monsch E, Kraft SV, Hammond S. Systematic collection and objective progressive exercise. Paper presented at: American Physical Therapy Association Combined Sections Meeting. February 24, 2018; New Orleans, LA.
- 10.Globas C., Becker C., Cerny J. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabil Neural Repair. 2012;26:85–95. doi: 10.1177/1545968311418675. [DOI] [PubMed] [Google Scholar]
- 11.Hershberg J.A., Rose D.K., Tilson J.K. The interface of clinical decision-making with study protocols for knowledge translation from a walking recovery trial. J Neurol Phys Ther. 2017;41:59–67. doi: 10.1097/NPT.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornby T.G., Holleran C.L., Leddy A.L. Feasibility of focused stepping practice during inpatient rehabilitation poststroke and potential contributions to mobility outcomes. Neurorehabil Neural Repair. 2015;29:923–932. doi: 10.1177/1545968315572390. [DOI] [PubMed] [Google Scholar]
- 13.Flansbjer U.B., Miller M., Downham D., Lexell J. Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation. J Rehabil Med. 2008;40:42–48. doi: 10.2340/16501977-0129. [DOI] [PubMed] [Google Scholar]
- 14.Hunnicutt J.L., Aaron S.E., Embry A.E. The effects of POWER training in young and older adults after stroke. Stroke Res Treat. 2016;2016:7316250. doi: 10.1155/2016/7316250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aaron S.E., Hunnicutt J.L., Embry A.E., Bowden M.G., Gregory C.M. POWER training in chronic stroke individuals: differences between responders and nonresponders. Top Stroke Rehabil. 2017;24:496–502. doi: 10.1080/10749357.2017.1322249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pak S., Patten C. Strengthening to promote functional recovery poststroke: an evidence-based review. Top Stroke Rehabil. 2008;15:177–199. doi: 10.1310/tsr1503-177. [DOI] [PubMed] [Google Scholar]
- 17.Holleran C.L., Straube D.D., Kinnaird C.R., Leddy A.L., Hornby T.G. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabil Neural Repair. 2014;28:643–651. doi: 10.1177/1545968314521001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornby T.G., Holleran C.L., Hennessy P.W. Variable intensive early walking poststroke (VIEWS): a randomized controlled trial. Neurorehabil Neural Repair. 2016;30:440–450. doi: 10.1177/1545968315604396. [DOI] [PubMed] [Google Scholar]
- 19.Compagnat M., Salle J.Y., Mandigout S., Lacroix J., Vuillerme N., Daviet J.C. Rating of perceived exertion with Borg scale in stroke over two common activities of the daily living. Top Stroke Rehabil. 2018;25:145–149. doi: 10.1080/10749357.2017.1399229. [DOI] [PubMed] [Google Scholar]
- 20.Steffen T., Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88:733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- 21.Kosak M., Smith T. Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J Rehabil Res Dev. 2005;42:103–107. doi: 10.1682/jrrd.2003.11.0171. [DOI] [PubMed] [Google Scholar]
- 22.Chan P.P., Si Tou JI., Tse M.M., Ng S.S. Reliability and validity of the timed up and go test with a motor task in people with chronic stroke. Arch Phys Med Rehabil. 2017;98:2213–2220. doi: 10.1016/j.apmr.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Mong Y., Teo T.W., Ng S.S. 5-repetition sit-to-stand test in subjects with chronic stroke: reliability and validity. Arch Phys Med Rehabil. 2010;91:407–413. doi: 10.1016/j.apmr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Canbek J., Fulk G., Nof L., Echternach J. Test-retest reliability and construct validity of the tinetti performance-oriented mobility assessment in people with stroke. J Neurol Phys Ther. 2013;37:14–19. doi: 10.1097/NPT.0b013e318283ffcc. [DOI] [PubMed] [Google Scholar]
- 25.Dodds T.A., Martin D.P., Stolov W.C., Deyo R.A. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- 26.Moore J.L., Potter K., Blankshain K., Kaplan S.L., O'Dwyer L.C., Sullivan J.E. A core set of outcome measures for adults with neurologic conditions undergoing rehabilitation: a clinical practice guideline. J Neurol Phys Ther. 2018;42:174–220. doi: 10.1097/NPT.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaismoradi M., Turunen H., Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15:398–405. doi: 10.1111/nhs.12048. [DOI] [PubMed] [Google Scholar]
- 29.American College of Sports Medicine . 9th ed. Lippincott Wiliams & Wilkins; Philadelphia: 2014. ACSM's guidlines for exercise testing and prescription. [Google Scholar]