Abstract
Objective
To investigate feasibility and effects of table tennis training on balance control and physical function in individuals with Parkinson disease.
Design
Single group, observational, before-after trial.
Setting
Table tennis training in a gymnasium.
Participants
Community-dwelling individuals with Parkinson disease (N=9; 5 men, 4 women) with an average age of 66.9 years, average time since diagnosis of 8.6 years, and a modified Hoehn and Yahr score between 2 and 2.5 participated in this study. Participants were recruited via newspaper advertisement, at the patient organization, and at the university hospital outpatient clinic. Eight participants completed the study. One participant withdrew for logistical reasons.
Interventions
Group training program consisting of 2 table tennis training sessions per week (120min each) for 10 weeks.
Main Outcome Measures
The primary outcome was feasibility, including attendance rate, drop-out rate, a final questionnaire assessing the participants’ experience during the intervention, and any adverse events. The primary effect outcome was the Mini Balance Evaluation Systems Test (Mini-BESTest). Secondary effect outcomes were Parkinson's disease questionnaire-8, European quality of life questionnaire, Montgomery Åsberg Depression Rating Scale (MADRS), Unified Parkinson’s Disease Rating Scale, 10-meter walk test, generic walking scale, activities-specific balance confidence scale, and physical activity measured with an accelerometer and the Frändin-Grimby scale.
Results
The average attendance rate was 84%. There were no adverse events reported. The participants reported that the training improved well-being. The mean total score on the Mini-BESTest before and after intervention was 21.2 versus 23.3 (P=.093). Statistically significant positive effects without adjustment for multiple comparisons were found for MADRS and the Frändin-Grimby scale.
Conclusions
This study demonstrates that table tennis training is safe and feasible, and may have the potential to improve balance control, mental well-being, and self-reported physical activity level. Further studies are required before table tennis can be considered an evidence-based recommendation for individuals with Parkinson disease.
Keywords: Exercise, Parkinson disease, Postural balance, Rehabilitation
List of abbreviations: EQ-5D-3L, European quality of life questionnaire; MADRS, Montgomery Åsberg Depression Rating Scale; MiniBESTest, Mini Balance Evaluation Systems Test; PD, Parkinson disease; PDQ-8, Parkinson's disease questionnaire-8
Highlights
-
•
Previous publications regarding the effects of table tennis training in Parkinson disease are lacking.
-
•
Table tennis training in Parkinson disease is safe and feasible, and it may have beneficial effects.
Parkinson disease (PD) is a common neurodegenerative disorder traditionally described by the 4 cardinal symptoms: rest tremor, bradykinesia, rigidity, and postural instability. All symptoms except tremor are related to impaired balance control.1 As the disease progresses, balance impairment becomes more prevalent. Impaired balance predisposes to falls and injuries, reduces physical function, and reduces the ability to perform daily activities, which in turn has negative psychological effects.1,2 For individuals with PD, impaired balance control is one of the greatest influences on quality of life.3,4
Overall, balance impairment responds poorly to PD medication, and non-pharmacologic approaches need to be implemented.5 An increasing number of studies on varied training programs have shown positive effects on balance.2 For example, a highly challenging balance training in a group setting has been shown to be effective in improving balance in PD patients.6
Exercise that is enjoyable, motivating, and easily accessible has the potential for higher adherence,7, 8, 9 and group training has been shown to improve participants’ psychological health and quality of life.9 Table tennis is a popular sport with more than 300 million registered practitioners worldwide. It requires complex visuospatial perception and movements, including balance control.10 Table tennis players have been demonstrated to have faster visual reaction times,11 better executive control,12 and better visuospatial working memory than healthy control individuals.13 Playing table tennis has also been shown to improve motor skills and executive function in children with attention deficit hyperactivity disorder or autism, as well as both visual perception and executive function in children with mild intellectual disabilities.14, 15, 16 Furthermore, recreational table tennis among older men has been correlated with improved bone health, physical function, and muscle strength.17 Positive cognitive effects of table tennis training have also been demonstrated in the elderly.18,19 However, to our knowledge, no scientific study to date has evaluated the effects of playing table tennis in individuals with PD. The aim of the present pilot study was to investigate whether table tennis group training is feasible and beneficial for individuals with mild to moderate PD for improving balance control and physical function.
Methods
This study was approved by the local ethical board. All participants provided written informed consent. Participants were recruited from the investigators’ outpatient clinic, via the local patient association’s e-mail list, and by advertising in local newspapers. Data was collected from August 2018 to November 2018. The inclusion criteria were individuals with mild to moderate idiopathic PD, modified Hoehn and Yahr score of 2 or 2.5, and individuals who could independently ambulate indoors and outdoors without walking aid. Individuals were excluded if they had impaired cognitive function affecting the ability to follow instructions or participate in training, or other medical conditions that would substantially influence participation in table tennis group training. Eleven participants showed interest and were invited for screening, 2 of whom did not meet the inclusion criteria. The average age of the remaining 9 participants (4 women, 5 men), was 66.9 years, and the average time since diagnosis was 8.6 years. The baseline demographic and clinical characteristics of the participants are shown in table 1. The levodopa equivalency daily dose was calculated in accordance with Tomlinson et al.20
Table 1.
Participant characteristics
| Characteristics | N=9 |
|---|---|
| Age, y | 66.9±5.5 |
| Sex, male/female | 5 (55)/4 (45) |
| Time since diagnosis, y | 8.6±4.9 |
| UPDRS motor score | 23±11 |
| H&Y, 2/2.5 | 8 (88)/1 (12) |
| Levodopa equivalent daily dose, mg | 566±266 |
NOTE. Data are mean ± SD or as otherwise indicated. Levodopa equivalency daily dose is calculated in accordance with Tomlinson et al.13
Abbreviation: H&Y, Hoehn and Yahr.
The intervention program consisted of 10 weeks of table tennis with 2 sessions per week lasting 120 minutes each (16:00-18:00 on Fridays and Sundays). Sessions included time for warm-up, instruction, and a 10-minute break. The participants played on regular-sized tables against each other, a table tennis robot, or an experienced trainer from a local table tennis club. The training was demanding, individually adapted, and included exercises of stance and footwork (ie, “side to side” and “in and out”), basic table tennis stroke techniques, and ball control exercises, such as controlling the direction and force of the strokes or playing with the non-dominant hand. Baseline table tennis skills varied from complete beginner to some experience of leisure-time playing. Attendance was registered. The trainer observed the most difficulties in exercises involving high speed and in ball control exercises that included the additional task of walking in a circle, which caused dizziness. Although not formally assessed, all participants became better table tennis players during the study. Evaluation was carried out within 2 weeks after the training period was completed. The participants were tested in ON-phase. There was no control group.
The primary outcome was feasibility, including attendance rate, drop-out rate, a final questionnaire assessing the participants’ experience during the intervention, and any adverse events. The primary effect outcome was balance performance assessed with the Mini Balance Evaluation Systems Test (Mini-BESTest) before and after the intervention. The Mini-BESTest is a clinical performance-based test of dynamic balance control. It is a 14-item test, subdivided in 4 components (anticipatory postural adjustments, postural responses, sensory orientation, and stability in gait). Each item is scored from 0 (unable or requiring help) to 2 (normal). The maximum score is 28 points.21 The test has been shown to adequately measure dynamic balance among individuals with PD,22 with good reliability for inter-rater and test-retest reproducibility.23 The Mini-BESTest was performed by the same experienced physiotherapist on both occasions.
Secondary effect outcomes were Parkinson's disease questionnaire-8 (PDQ-8), European quality of life questionnaire (EQ-5D-3L), Montgomery Åsberg Depression Rating Scale (MADRS), Unified Parkinson’s Disease Rating Scale, 10-meter walk test, generic walking scale, activities-specific balance confidence scale, and physical activity measured with an ActiGraph GT3x+a accelerometer and the Frändin-Grimby scale. The Unified Parkinson’s Disease Rating Scale was completed by an experienced PD specialist research nurse, and all questionnaires, except the activities-specific balance confidence scale and generic walking scale, were answered when visiting the neurologic clinic. Habitual physical activity was defined as daily steps and the vector magnitude (total activity counts) per day in ordinary daily life setting. Each participant wore an accelerometer attached to an elastic band around the waist for 7 consecutive days. The recorded data were processed using the ActiLife 6.a A minimum of 4 days with 9 hours of daily wear time was required for inclusion in the analysis. Daily wear time was determined using established procedures and the mean value of 4 to 7 days was used to obtain the daily step count.24
Statistical Analysis
Statistical analysis was conducted using R software, version 3.2.3.b Data were analyzed descriptively, and exploratory non-parametric statistics were performed with the Mann-Whitney U test or Wilcoxon rank-sum test. The significance level was set at a P value of .05 or less. No correction was performed for multiple comparisons.
Results
Nine participants were enrolled, but 1 participant dropped out after a few weeks for logistical reasons. The average attendance rate for the 8 participants who completed the study was 84%. No adverse events, injuries, or newly emerged pain were reported. During the test period, only 1 participant changed his or her medication, adding amantadine for dyskinesia. The final self-evaluation questionnaire revealed an overall positive and joyful experience, and all 8 participants completing the study reported an interest in continuing table tennis training. Five of the 8 participants self-reported positive effects on their physical function and improved well-being. However, 1 participant asked for more “intense” training and another wished for the training period to be longer. More than 1 participant remarked that the scheduled timepoints for training were suboptimal.
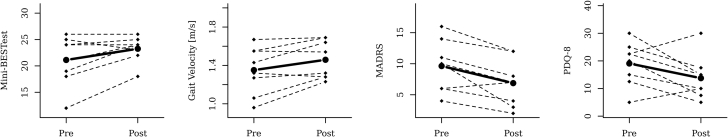
The Mini-BESTest scores increased or remained unaltered for 7 of the 8 participants. The mean difference was 2.1 points higher post-intervention (95% confidence interval, 0.2-4.1), but the difference was not significant (P=.093). For gait velocity, calculated using the 10-meter walk test, 5 of the 8 showed increased velocity, with a mean increase of 0.11 m/s (P=.055). All participants except 1 scored lower on the MADRS post-intervention, with the mean decreasing by 2.7 points from 9.6 to 6.9 points (P=.02). For physical activity measurements, the Frändin-Grimby activity scale showed increased physical activity for all participants except 1 (P=.031). No significant differences were demonstrated in the other exploratory outcome measures, but positive trends were seen for the questionnaires assessing quality of life (PDQ-8 and EQ-5D-3L). All results are listed in table 2, and individual results are shown in figure 1.
Table 2.
Results
| Outcome Measure | n | Mean (SE) |
Mean (95% CI) |
P value | |
|---|---|---|---|---|---|
| Pretest | Posttest | Difference | |||
| Mini-BESTest | 8 | 21.1 | 23.3 | 2.1 | .093 |
| (1.7) | (0.9) | (0.2-4.1) | |||
| PDQ-8 | 8 | 19.1 | 13.8 | -5.3 | .103 |
| (2.79) | (2.75) | (-10.9 to 0.23) | |||
| EQ-5D-3L | 8 | 0.73 | 0.79 | 0.06 | .361 |
| (0.02) | (0.05) | (-0.03 to 0.16) | |||
| VAS EQ-5D-3L | 8 | 73.1 | 79.4 | 6.3 | .457 |
| (4.3) | (4.5) | (-7.9 to 20.4) | |||
| MADRS | 8 | 9.6 | 6.9 | -2.7 | .02∗ |
| (1.5) | (1.3) | (-4.3 to -1.2) | |||
| UPDRS III | 8 | 21.4 | 26.8 | 5.4 | .150 |
| (3.6) | (4.1) | (-0.55 to 11.3) | |||
| UPDRS total | 8 | 34.4 | 39.4 | 5.0 | .205 |
| (5.0) | (4.3) | (-1.4 to 11.4) | |||
| Gait velocity [m/s] | 8 | 1.35 | 1.46 | 0.11 | .055 |
| (0.088) | (0.071) | (0.02-0.19) | |||
| Walk-12G | 7 | 10.9 | 8.3 | -2.6 | .462 |
| (2.3) | (3.3) | (-9.3 to 4.1) | |||
| Average vector magnitude counts | 7 | 379229 | 359213 | -20015 | .688 |
| (65445) | (59565) | (-71669 to 31639) | |||
| Steps/d | 7 | 4934 | 4855 | -79.2 | >.99 |
| (677) | (863) | (-1186 to 1027) | |||
| ABC | 7 | 1268 | 1403 | 134 | .297 |
| (112) | (209) | (-171 to 439) | |||
| Frändin-Grimby | 8 | 3.25 | 4.5 | 1.25 | .031∗ |
| (0.16) | (0.33) | (0.62-1.88) | |||
Abbreviations: ABC, activities-specific balance confidence; CI, confidence interval; MADRS, Montgomery Åsberg Depression Rating Scale; VAS, visual analog scale; Walk-12G, psychometric performance of generic walking scale.
Statistically significant (P≤.05).
Fig 1.
Individual results and mean pre- and postintervention for (A) Mini-BESTest, (B) gait velocity, (C) MADRS, and (D) PDQ-8.
Discussion
To our knowledge this is the first scientific study on the feasibility and effects of table tennis training in individuals with mild to moderate PD. This pilot study indicates that table tennis group training, led by an experienced table tennis trainer, is safe and feasible. The attendance rate was high, the drop-out rate was low, and there were no adverse events or injuries during the training sessions. The participants reported a joyful experience with positive effects on mental well-being and quality of life, which were also indicated by the questionnaires assessing quality of life and depressive symptoms. This corresponds well with the recognized positive relationship between physical activity and depressive symptoms in patients with depression or chronic disease. 25,26 The depressive symptoms were mild both at baseline and at follow-up, but the mean decrease in MADRS by 2.7 points is larger than the minimally clinically important difference in MADRS, which has been estimated to 1.6 to 1.9 points.27
Although we were not able to show a significant training effect on balance performance in this pilot study, the improvements were comparable to other balance interventions in individuals with PD.6,28 Furthermore, the average increase in balance performance exceeded the level of measurement error at group level previously reported for the Mini-BESTest.23 Also, the improvement of 0.11 m/s seen in gait velocity can be regarded as a clinically meaningful change, high above the clinically meaningful difference of 0.06 m/s defined by Hass et al.29 In line with these results, all but 1 individual reported that their physical activity level was higher after the intervention period, although the activity monitors could not assess this change.
Study limitations
Because this was a pilot study, it was not powered to demonstrate significant effects on the outcome measures. The lack of a control group and the relatively short intervention period make it difficult to draw firm conclusions regarding effects. Additionally, the baseline score on the Mini-BESTest was rather high, indicating that the group had rather mild balance deficits, which caused difficulties demonstrating improvement in this outcome measure owing to a celling effect. However, the results indicate a potentially positive effect on balance control, and an increase in gait velocity indicates an additional possible effect on physical function. For physical activity, the winter climate generates a general negative effect.30 Despite this, our accelerometer data shows that the participants were able to maintain their level of physical function, although the post assessments were done in winter climate.
Conclusions
This pilot study indicates that table tennis group training in PD, led by an experienced table tennis trainer, is safe and feasible, but additional studies of the efficacy and effectiveness are required before table tennis group training can be considered an evidence-based recommendation for individuals with PD. Table tennis is a popular sport played worldwide. Reasonably, table tennis training should be readily available. Implementation is realistic, and the basis for adherence is quite good. It could be a joyful and cost-effective form of training or leisure-time activity. Positive psychosocial effects could be an additional benefit. Further studies should therefore include cognitive effect outcomes and a more qualitative methodology of the participants’ experiences.
Suppliers
-
a.
ActiGraph.
-
b.
R software, version 3.2.3; The R Foundation for Statistical Computing.
Acknowledgments
We thank Sanna Asp (MSc, registered physiotherapist), Margareth Lundgren (MSc, registered nurse), and Lasse Naesström (BA [Educ], table tennis trainer).
Footnotes
No specific funding was received for this work. Dr Johansson received a grant for clinical Parkinson disease research from Ulf Davidson during the conduct of the study.
Disclosures: none.
References
- 1.Kim S.D., Allen N.E., Canning C.G., Fung V.S. Postural instability in patients with Parkinson's disease. Epidemiology, pathophysiology and management. CNS Drugs. 2013;27:97–112. doi: 10.1007/s40263-012-0012-3. [DOI] [PubMed] [Google Scholar]
- 2.Shen X., Wong-Yu I.S., Mak M.K. Effects of exercise on falls, balance, and gait ability in Parkinson's disease: a meta-analysis. Neurorehabil Neural Repair. 2016;30:512–527. doi: 10.1177/1545968315613447. [DOI] [PubMed] [Google Scholar]
- 3.de Boer A.G., Wijker W., Speelman J.D., de Haes J.C. Quality of life in patients with Parkinson's disease: development of a questionnaire. J Neurol Neurosurg Psychiatry. 1996;61:70–74. doi: 10.1136/jnnp.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrag A., Jahanshahi M., Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronte-Stewart H.M., Minn A.Y., Rodrigues K., Buckley E.L., Nashner L.M. Postural instability in idiopathic Parkinson's disease: the role of medication and unilateral pallidotomy. Brain. 2002;125:2100–2114. doi: 10.1093/brain/awf207. [DOI] [PubMed] [Google Scholar]
- 6.Conradsson D., Lofgren N., Nero H. The effects of highly challenging balance training in elderly with Parkinson's disease: a randomized controlled trial. Neurorehabil Neural Repair. 2015;29:827–836. doi: 10.1177/1545968314567150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzolini S., Mertens D.J., Oh P.I., Plyley M.J. Self-reported compliance to home-based resistance training in cardiac patients. Eur J Cardiovasc Prev Rehabil. 2010;17:35–41. doi: 10.1097/HJR.0b013e32832da020. quiz 42-9. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen G., Wikman J.M., Jensen C.J., Schmidt J.F., Gliemann L., Andersen T.R. Health promotion: the impact of beliefs of health benefits, social relations and enjoyment on exercise continuation. Scand J Med Sci Sports. 2014;24(Suppl 1):66–75. doi: 10.1111/sms.12275. [DOI] [PubMed] [Google Scholar]
- 9.Picorelli A.M., Pereira D.S., Felicio D.C. Adherence of older women with strength training and aerobic exercise. Clin Interv Aging. 2014;9:323–331. doi: 10.2147/CIA.S54644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Y., Yu C., Shao S., Baker J.S. Effects of table tennis multi-ball training on dynamic posture control. PeerJ. 2019;6 doi: 10.7717/peerj.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhabhor M.K., Vidja K., Bhanderi P., Dodhia S., Kathrotia R., Joshi V. A comparative study of visual reaction time in table tennis players and healthy controls. Indian J Physiol Pharmacol. 2013;57:439–442. [PubMed] [Google Scholar]
- 12.Meng F., Li A., You Y., Xie C. Motor expertise modulates unconscious rather than conscious executive control. PeerJ. 2019;7:e6387. doi: 10.7717/peerj.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W., Wang B., Lu Y., Zhu Q., Shi Z., Ren J. The relationship between different exercise modes and visuospatial working memory in older adults: a cross-sectional study. PeerJ. 2016;4:e2254. doi: 10.7717/peerj.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan C.Y., Tsai C.L., Chu C.H., Sung M.C., Huang C.Y., Ma W.Y. Effects of physical exercise intervention on motor skills and executive functions in children with ADHD: a pilot study. J Atten Disord. 2019;23:384–397. doi: 10.1177/1087054715569282. [DOI] [PubMed] [Google Scholar]
- 15.Pan C.Y., Chu C.H., Tsai C.L., Sung M.C., Huang C.Y., Ma W.Y. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190–202. doi: 10.1177/1362361316633562. [DOI] [PubMed] [Google Scholar]
- 16.Chen M.D., Tsai H.Y., Wang C.C., Wuang Y.P. The effectiveness of racket-sport intervention on visual perception and executive functions in children with mild intellectual disabilities and borderline intellectual functioning. Neuropsychiatr Dis Treat. 2015;11:2287–2297. doi: 10.2147/NDT.S89083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naderi A., Degens H., Rezvani M.H., Shaabani F. A retrospective comparison of physical health in regular recreational table tennis participants and sedentary elderly men. J Musculoskelet Neuronal Interact. 2018;18:200–207. [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai C.L., Pan C.Y., Chen F.C., Tseng Y.T. Open- and closed-skill exercise interventions produce different neurocognitive effects on executive functions in the elderly: a 6-month randomized, controlled trial. Front Aging Neurosci. 2017;9:294. doi: 10.3389/fnagi.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeoung B.J. Relationships of exercise with frailty, depression, and cognitive function in older women. J Exerc Rehabil. 2014;10:291–294. doi: 10.12965/jer.140128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 21.Franchignoni F., Horak F., Godi M., Nardone A., Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lofgren N., Benka Wallen M., Sorjonen K., Conradsson D., Franzen E. Investigating the Mini-BESTest's construct validity in elderly with Parkinson's disease. Acta Neurol Scand. 2017;135:614–621. doi: 10.1111/ane.12640. [DOI] [PubMed] [Google Scholar]
- 23.Lofgren N., Lenholm E., Conradsson D., Stahle A., Franzen E. The Mini-BESTest--a clinically reproducible tool for balance evaluations in mild to moderate Parkinson's disease? BMC Neurol. 2014;14:235. doi: 10.1186/s12883-014-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benka Wallen M., Franzen E., Nero H., Hagstromer M. Levels and patterns of physical activity and sedentary behavior in elderly people with mild to moderate Parkinson disease. Phys Ther. 2015;95:1135–1141. doi: 10.2522/ptj.20140374. [DOI] [PubMed] [Google Scholar]
- 25.Kvam S., Kleppe C.L., Nordhus I.H., Hovland A. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67–86. doi: 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 26.Herring M.P., Puetz T.W., O'Connor P.J., Dishman R.K. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:101–111. doi: 10.1001/archinternmed.2011.696. [DOI] [PubMed] [Google Scholar]
- 27.Duru G., Fantino B. The clinical relevance of changes in the Montgomery-Asberg Depression Rating Scale using the minimum clinically important difference approach. Curr Med Res Opin. 2008;24:1329–1335. doi: 10.1185/030079908x291958. [DOI] [PubMed] [Google Scholar]
- 28.Leavy B., Joseph C., Lofgren N., Johansson H., Hagstromer M., Franzen E. Outcome evaluation of highly challenging balance training for people with Parkinson disease: a multicenter effectiveness-implementation study. J Neurol Phys Ther. 2020;44:15–22. doi: 10.1097/NPT.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 29.Hass C.J., Bishop M., Moscovich M. Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J Neurol Phys Ther. 2014;38:233–238. doi: 10.1097/NPT.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y.T., Luben R., Wareham N., Griffin S., Jones A.P. Weather, day length and physical activity in older adults: cross-sectional results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Norfolk Cohort. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177767. [DOI] [PMC free article] [PubMed] [Google Scholar]