Abstract
Objective
To understand the patient-influenced activities and characteristics associated with return to a single postacute care transitional care clinic visit in a cohort of patients cared for at the test health system site of the larger Comprehensive Post-Acute Stroke Services (COMPASS) cluster randomized trial.
Design
Retrospective cohort.
Setting
A large health system.
Participants
Patients discharged directly home between June 2016 and June 2018 after sustaining a stroke who did not receive formal inpatient rehabilitation services while being cared for in a single comprehensive stroke center, defined as a center that meet standards to rapidly diagnose and treat the most complex stroke cases.
Interventions
Study participants had the opportunity to participate in a (1) 2-day call, (2) comprehensive care transitions clinic visit, and (3) individualized care plan.
Main Outcome Measures
Patient participation in a single postacute care comprehensive care transitions visit, ideally completed within 7-14 calendar days post discharge vs not attending this visit. Care transition visits are where the responsibility for preventive care, other services, and posthospital follow-up are transitioned to outpatient providers.
Results
Among 1300 eligible patients (mean age 64.8 years; 45% female; 25.4% nonwhite; 9.7% uninsured), 95.7% had follow-up clinic visits scheduled before discharge, 22.6% received home health referrals before discharge, 60.2% completed the 2-day call, and 63.2% attended the COMPASS visit. Among attendees, 33.2% attended by day 14, 71.3% attended within 30 days, and 28.7% attended after day 30. The median driving distance to the COMPASS visit was 45.9 miles or 73.9 km. Odds of visit attendance were higher if COMPASS 2-day follow up calls were completed, if follow-up clinic appointments were scheduled before discharge, if the patient had a primary care provider, and if the patients experienced a stroke vs a transient ischemic attack. Additionally, when we used the number of referrals at hospital discharge for different types of outpatient therapy as a surrogate marker of poststroke impairment, patients having no therapy referrals (milder to no impairments) had lower odds of attending the COMPASS visit than those with 1 therapy referral. Likewise, those with more than 1 referral were also less likely to attend the COMPASS visit.
Conclusions
This analysis highlights that scheduling visits at discharge and completing timely telephone follow-up shortly after discharge may lead to greater adherence to in-person clinic follow-up after stroke.
Keywords: Outcome assessment, health care; Patient transfer; Rehabilitation; Subacute care
List of abbreviations: COMPASS, Comprehensive Post-Acute Stroke Services; ED, emergency department; NIHSS, National Institutes of Health Stroke Scale; PCP, primary care provider; TIA, transient ischemic attack; US, United States
Individuals who experience strokes, and those who support them, can have their lives significantly changed as they navigate the sequelae, including long-term disabilities, chronic comorbidities, and mental, social, and physical challenges. With prompt, high-quality care and support that starts during inpatient hospitalizations and continues as patients transition back into their communities, outcomes can be improved.1 Because the average length of hospital stay for stroke in the United States (US) is just 4-5 days,2,3 patients, families, and providers have a short window of time for discharge planning, especially when considering the complex nature of stroke and diverse needs of individual survivors.4 Prompt engagement in care and secondary prevention is paramount for patients with stroke because their risks of experiencing recurrent stroke events are highest in the first 6 months post stroke.5,6
Engaging in health care appointments and self-management activities in the weeks after hospitalizations can be particularly challenging because patients often need to juggle visits to multiple different providers, adhere to new complex medication regimens, and manage these expectations in the context of having new deficits and needs. Unfortunately, upward of 17%-21% of those discharged after an index stroke are readmitted within 30 days,3,7,8 35% are readmitted within 90 days,8,9 and 43%-62% are readmitted within a year,7,8 often for indications deemed preventable.10 It is likely that many of these readmissions could be avoided if well-coordinated transitional care visits and services were available and effectively used.11
As part of the funding received from the Patient Centered Outcomes Institute to implement a pragmatic cluster randomized trial (named the Comprehensive Post-Acute Stroke Services trial [COMPASS]),12 we initially engaged a testing site, also called a vanguard site, to test and enhance implementation activities, data collection processes, and analytical methods in preparation for the larger trial. The vanguard site additionally served as the clinical coordinating site for the COMPASS trial.
At the vanguard site, we developed, implemented, and evaluated a comprehensive, evidenced-based, postacute care model for patients who experienced a stroke or transient ischemic attack (TIA). Trial participants were those who were discharged directly to their homes after their hospitalizations. Postacute care coordinators reached out to patients within 2 business days of their discharge date to complete the COMPASS 2-Day Call. Patients were expected to attend a single transitional care visit within 7-14 calendar days post discharge.
In this current analysis of the vanguard site, we evaluated patients’ visit attendance at the transitional care follow-up clinic visit on a patient cohort. We explored the patient and patient-influenced characteristics associated with attending vs not attending the transitional care visit, henceforth called the COMPASS visit.
Understanding the characteristics of patients who attend transitional care visits and the activities that are associated with enhanced attendance is of interest to many stakeholders including health systems, clinicians, administrators, community-based organizations, payers, and others. Identifying characteristics associated with greater or lesser visit attendance can help guide targeted outreach activities to enhance engagement in transitional services and assist with program planning for current and future transitional care models.
Methods
Sample and data sources
A tertiary care center served as the vanguard test site. By focusing on just 1 clinical site, we minimized the effect of health care system factors on visit attendance and thus can more easily disentangle the patient-influenced factors associated with attending the clinic visit. We analyzed data on 1300 patients enrolled at the WFBMC site between June 27, 2016, and June 30, 2018. Patients were eligible for the COMPASS trial if they were 18 years or older; spoke English or Spanish; were diagnosed as having an acute ischemic stroke, hemorrhagic stroke (excluding subdural or aneurysmal hemorrhage), or TIA; and were discharged directly home.12 All patients discharged home were eligible, regardless of stroke severity. Data were collected from each patient’s hospitalization and via a phone call made ideally within 2 business days of hospital discharge (the COMPASS 2-day phone call) by a postacute care coordinator. The phone-call interview data were collected from either the patients or their caregivers. These data included patient demographics, medical history, stroke severity, number and types of therapy referrals made at hospital discharge (physical, occupational, or speech/language therapy), noted barriers to transportation, and whether home health care services were arranged. Additionally, patients were asked if they had scheduled appointments to see their primary care provider and if they had scheduled their 7-14 day COMPASS visit. Full descriptions of our methods, processes, and the overall COMPASS care model guiding the parent trial have been previously published.12,13 Institutional review board approval was granted through the prime institution’s institutional review board. At 90 days postindex hospital discharge, patients provided verbal informed consent for collection of outcomes.14 The data that support the findings of this study are available by request from the corresponding author.
Conceptual model
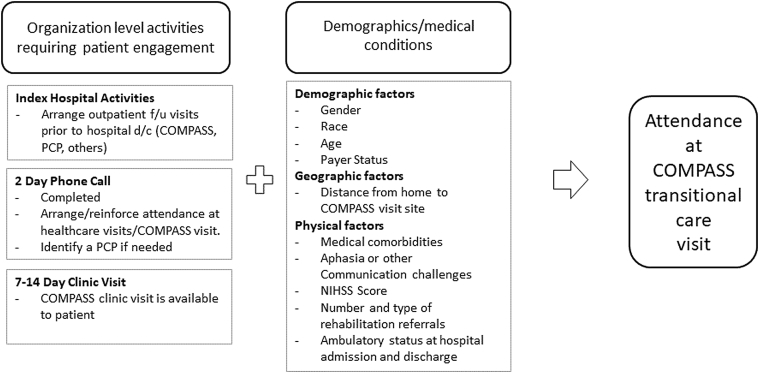
We developed a conceptual model that describes key variables captured during the COMPASS study that may influence a patient’s decision making and ability regarding attending the COMPASS visit (fig 1). These include stroke severity, presence of a caregiver, payer status, whether a postacute visit was scheduled before hospital discharge, whether the patient or caregiver completed a 2-day post discharge phone call with a postacute care coordinator, and whether they were referred for rehabilitation services at hospital discharge. Other prespecified factors include having a primary care provider (PCP), the distance in miles or km between their residence and the COMPASS visit site, and other patient medical comorbidities and characteristics.
Fig 1.
Conceptual model: patient influenced factors associated with attendance at the COMPASS visit. Abbreviations: d/c, discharge; f/u, follow-up.
Statistical analysis
We performed descriptive univariate analyses and multivariable logistic regression analysis to identify which characteristics were associated with attending the COMPASS after hospital discharge (tables 1 and 2). We used backward stepwise selection in our multivariable logistic regression model to identify predictors of COMPASS visit attendance. We estimated the odds ratios and 95% confidence intervals for attendance at the COMPASS visit associated with each variable in this adjusted model.
Table 1.
Baseline characteristics according to completion status of the COMPASS transitional care clinic visit
| Characteristic | Overall (N=1300) | Visit Completed (n=822) | Visit Not Completed (n=478) | P Value∗ |
|---|---|---|---|---|
| Age (y), mean ± SD | 64.8±14.5 | 65.0±14.3 | 64.5±14.8 | .5887 |
| Female, n (%) | 585 (45.0) | 384 (46.7) | 201 (42.1) | .1030 |
| Race, n (%) | ||||
| White | 964 (74.6) | 621 (76.1) | 343 (71.9) | .0947 |
| Nonwhite | 329 (25.4) | 195 (23.9) | 134 (28.1) | |
| Missing, n | 7 | 6 | 1 | |
| Hispanic ethnicity, n (%) | 38 (2.9) | 23 (2.8) | 15 (3.1) | .7292 |
| Missing, n | 4 | 3 | 1 | |
| Uninsured, n (%) | 124 (9.7) | 67 (8.3) | 57 (12.2) | .0211 |
| Missing, n | 23 | 11 | 12 | |
| Diagnosis, n (%) | ||||
| Stroke | 1028 (79.1) | 666 (81.0) | 362 (75.7) | .0238 |
| TIA | 272 (20.9) | 156 (19.0) | 116 (24.3) | |
| NIHSS, n (%) | ||||
| =0 | 337 (29.8) | 215 (29.5) | 122 (30.3) | .1428 |
| =1-4 | 565 (50.0) | 377 (51.8) | 188 (46.7) | |
| =5-42 | 229 (20.2) | 136 (18.7) | 93 (23.1) | |
| Missing, n | 169 | 94 | 75 | |
| Aphasia at admission, n (%) | 283 (21.8) | 180 (21.9) | 103 (21.5) | .8829 |
| Caregiver present at discharge, n (%) | 1147 (88.2) | 727 (88.4) | 420 (87.9) | .7557 |
| Primary care physician on record, n (%) | 1009 (77.6) | 674 (82.0) | 335 (70.1) | <.0001 |
| Medical history & comorbidity, n (%) | ||||
| Hypertension | 942 (72.5) | 600 (73.0) | 342 (71.5) | .5740 |
| Diabetes mellitus | 421 (32.4) | 259 (31.5) | 162 (33.9) | .3760 |
| Prior stroke | 259 (19.9) | 156 (19.0) | 103 (21.5) | .2633 |
| Prior TIA | 88 (6.8) | 56 (6.8) | 32 (6.7) | .9349 |
| Atrial fibrillation | 179 (13.8) | 109 (13.3) | 70 (14.6) | .4850 |
| Heart failure | 97 (7.5) | 56 (6.8) | 41 (8.6) | .2430 |
| Coronary artery disease | 268 (20.6) | 172 (20.9) | 96 (20.1) | .7178 |
| Depression | 86 (6.6) | 57 (6.9) | 29 (6.1) | .5441 |
| BMI, mean ± SD | 29.2±7.0 | 29.2±6.6 | 29.2±7.6 | .9281 |
| Missing, n | 323 | 209 | 114 | |
| Postacute follow-up, n (%) | ||||
| Follow-up visit scheduled prior to discharge | 1244 (95.7) | 795 (96.7) | 449 (93.9) | .0172 |
| COMPASS 2-day call completed | 783 (60.2) | 544 (66.2) | 239 (50.0) | <.0001 |
| COMPASS clinic visit completed | 822 (63.2) | |||
| Home health referral at discharge, n (%) | 279 (22.6) | 172 (21.8) | 107 (23.9) | .3942 |
| Missing, n | 65 | 34 | 31 | |
| No. of therapy referrals at discharge, n (%)† | ||||
| 0 | 767 (62.1) | 490 (62.2) | 277 (62.0) | .0441 |
| 1 | 191 (15.5) | 136 (17.3) | 55 (12.3) | |
| 2 | 182 (14.7) | 108 (13.7) | 74 (16.6) | |
| 3 | 95 (7.7) | 54 (6.9) | 41 (9.2) | |
| Missing, n | 65 | 34 | 31 | |
| Distance to COMPASS clinic location, n (%) | ||||
| 0-30 miles (0-48.3km) | 727 (55.9) | 472 (57.4) | 255 (53.3) | .1040 |
| 30-60 miles (48.3-96.6km) | 306 (23.5) | 196 (23.8) | 110 (23.0) | |
| >60 miles (>96.6km) | 267 (20.5) | 154 (18.7) | 113 (23.6) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
P value based on a χ2 test for categorical variables and a t test for continuous variables.
Referrals include those to physical therapy, occupational therapy, and speech and language therapy.
Table 2.
Parameter estimates averaged over 50 imputed data sets: Multivariable logistic
| Parameter | Parameter Estimate |
Odds Ratio∗ | 95% Confidence Limits for Odds Ratio | P Value† | |
|---|---|---|---|---|---|
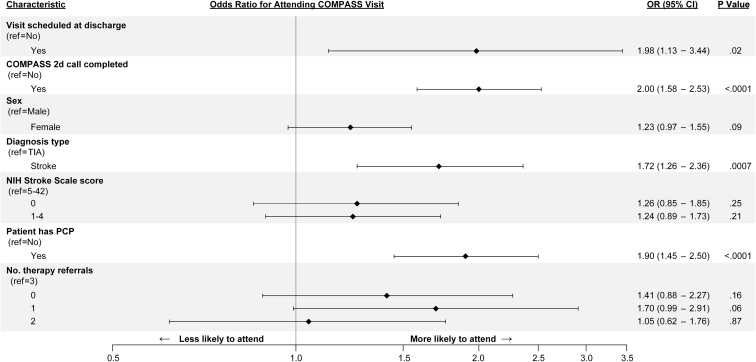
| Visit scheduled at discharge=yes | 0.6809 | 1.98 | 1.13 | 3.44 | .02 |
| 2-Day follow-up call completed=yes | 0.6931 | 2.00 | 1.58 | 2.53 | <.0001 |
| Sex=female | 0.2034 | 1.23 | 0.97 | 1.55 | .09 |
| Diagnosis=stroke | 0.5434 | 1.72 | 1.26 | 2.36 | .0007 |
| NIHSS score=0‡ | 0.2269 | 1.26 | 0.85 | 1.85 | .25 |
| NIHSS score=1-4 | 0.2140 | 1.24 | 0.89 | 1.73 | .21 |
| NIHSS score=5-42 | 0.0000 | ||||
| Has primary care provider=yes | 0.6433 | 1.90 | 1.45 | 2.50 | <.0001 |
| No. of discharge therapy referrals=0 | 0.3430 | 1.41 | 0.88 | 2.27 | .16 |
| No. of discharge therapy referrals=1 | 0.5278 | 1.70 | 0.99 | 2.91 | .06 |
| No. of discharge therapy referrals=2 | 0.0438 | 1.05 | 0.62 | 1.76 | .87 |
| No. of discharge therapy referrals=3 | 0.0000 | ||||
Odds ratio comparing to reference group. For example, the odds ratios for NIHSS score compared with the 5-42 group.
P values are based on maximum likelihood estimates averaged over 50 imputed data sets. The MI and MIANALYZE procedures in SAS were used for this analysis.
NIHSS score and stroke diagnosis were included in the model as variables of perceived a priori importance. All other variables were selected for inclusion in the model using the methods described above.
Selection of variables for model
The following variables were considered for inclusion in the logistic regression model: age, race (white vs nonwhite), sex, ethnicity (Hispanic vs non-Hispanic), body mass index, aphasia, indicator for a follow-up visit scheduled at discharge, indicator for 2-day call completion, history of stroke, history of TIA, stroke diagnosis (stroke vs TIA) and National Institutes of Health Stroke Scale (NIHSS) score (categorized as 0, 1-4, 5-42; a categorization driven by having only 20% of values in the 5-42 range). We also included absolute number of referrals to physical therapy, speech therapy, or occupational therapy at discharge (0-3), an indicator of home health referral at discharge, documentation of a PCP, payer source, presence of a caregiver, and driving distance to the COMPASS clinic categorized as 0-30 (0-48.3), 30-60 (48.3-96.6) or >60 (96.6) miles (km) measured using geocodes.
Missing data
The following variables exhibited some level of missing data: race, NIHSS score, absolute number of referrals to rehabilitation, whether there were home health referrals or not, whether or not the patient had insurance coverage, body mass index, and ethnicity. Missing predictor variables were imputed using multiple imputation by chained equations.15,16 The equations used in the procedure were themselves derived using a backward selection process for each (generalized) linear model using the complete set of covariates and employing a cutoff of α=0.2. We constructed 50 pseudo-complete data sets that were each analyzed, and the results were then combined using standard techniques to estimate covariate effects and perform tests of association.16,17 We eliminated variables that were not significant at the α=0.1 level based on this combined inference, with the exception of NIHSS score and stroke diagnosis, which were not subject to model selection. Because of the amount of missing data of the NIHSS, we used number and types of referral post discharge as an indicator of stroke severity.
The final model results are shown in table 2.
Results
As of June 30, 2018, a total of 1300 patients were eligible and enrolled for the postacute COMPASS visit at the vanguard site (see table 1). Overall, 822 participants (63.2%) completed the COMPASS visit. Of the 822 patients who completed the visit, 33.2 % completed the visit within 14 days, an additional 38.1% attended by day 30, and 28.7 % were seen after 30 days. The median number of days from hospital discharge to attendance at the clinic visit was 20 (interquartile range, 12-34). In our univariate analysis, among those that completed the COMPASS visit, 66.2% received and completed a 2-day phone call, whereas 50% of those who did not attend the visit completed a 2-day phone call. Using the number of referrals at hospital discharge for different types of outpatient therapy as a surrogate marker of poststroke impairment, where more referrals indicate greater impairment, patients who did not have any therapy referrals (thus mild-to-no impairment) were less likely than those with a single referral to attend the COMPASS visit. Patients with more than 1 outpatient therapy referral, thus who likely had moderate-to-severe impairments, were also less likely to attend the COMPASS visit than those with 1 referral. Those referred specifically to receive home health care were less likely to attend the COMPASS visits. Those who experienced a stroke vs a TIA and those with a PCP vs no PCP were more likely to attend the COMPASS visit.
The multivariable logistic regression analysis (see table 2 and fig 2) identified statistically significant adjusted odds ratios (P<.05) for greater attendance of the COMPASS visit, with independent effects for having a follow-up visit scheduled at discharge, completing the 2-day call, having a PCP documented at study enrollment, and having had a stroke vs a TIA at the index hospitalization. Receipt of a single referral to outpatient therapy on discharge, suggesting milder impairment, was weakly associated with greater odds of attending the COMPASS visit compared with those with no referrals or more than 1. Driving distance, including distances of >60 miles or 96.6 km as measured from patients’ homes to the COMPASS visit site, was not associated with visit attendance rates. Other variables without independent effects on visit attendance included sex, history of stroke before the index hospitalization, race, receipt of a referral for home health services, and having medical insurance.
Fig 2.
Multivariable logistic model, odds ratios for attending the COMPASS visit.
Discussion
Our findings offer a unique contribution to the transitional care literature by highlighting characteristics of patients with recent strokes who are more likely to attend a structured and comprehensive transitional care visit. To our knowledge, this may be one of the first studies dedicated to describing these characteristics among survivors of stroke.
Based upon the experiences of 1300 patients with stroke discharged directly home after an index stroke or TIA at a single institution, our results suggest that those on the opposite spectrums of stroke severity (those with very mild and those with severe limitations) may need additional outreach efforts to optimize their engagement with transitional services. Our results also highlight the importance of having outpatient visits scheduled before hospital discharge and close follow-up via phone communications shortly after discharge to enhance attendance at care transition visits.
Our results reflect similar conclusions reached by authors evaluating posthospital follow-up among patient populations beyond survivors of stroke.18, 19, 20, 21, 22, 23 Thus, although we were unable to find other stroke-specific studies evaluating factors associated with attending transition visits, other investigators have evaluated variables associated with greater or lesser attendance soon after hospital or emergency department (ED) visits among populations not specifically restricted to survivors of stroke. Their work adds insights into our findings and may help guide our future work in the population with stroke.
For instance, Lam et al performed a retrospective cohort study of 214 patients discharged from hospitals in Toronto, where they identified that having a primary care appointment booked before hospital discharge increased the odds of patients attending follow-up appointments within 14 days post discharge.23 Similar to our study, patient sex was not associated with visit attendance at the 0.05 level. In their study, they were able to differentiate whether or not patients were given postdischarge appointments with specialists or PCPs and noted that referrals to specialists reduced the odds of attending visits with PCPs within the 2-week time period. Their study also noted enhanced odds of attending a visit with a PCP if the patient had established familiarity with a PCP vs when patients were requesting new PCPs.23 We cannot ascertain whether or not such an association exists with our data because we did not specifically ask about the strength or length of subjects’ relationships with PCPs.
Among a large US urban general medicine population discharged from both EDs and inpatient stays from the New York-Presbyterian Hospital/Weill Cornell Internal Medicine Center, Sinha et al noted that each additional year of age was associated with 1% higher odds of completing a follow-up appointment within 14 days.24 Additionally, for both groups, greater numbers of comorbid conditions were associated with greater odds of attendance. Women had 37% greater odds of attending the outpatient visits than men, but only for the inpatient stay group. For the inpatient cohort, longer lengths of stay were also associated with greater compliance with outpatient visits. Like our study, insurance status was not associated with attendance at outpatient visits.
In a retrospective analysis by Messina et al, 1174 patients being discharged from a safety-net ED in Indianapolis, Indiana, were offered a dedicated phone line and 24-hour-a-day ED staff member assistance with scheduling follow-up visits with various medical specialists.25 In their study, the follow-up visits were free of charge. Unlike our study, they noted greater follow-up visit attendance among older patients. They also analyzed whether the type of outpatient medical specialty provider referral was associated with greater or lesser attendance at the respective follow-up visits and found greater attendance rates with ophthalmology follow-up appointments, with lesser attendance (in decreasing order) with urology, neurology, and other specialist appointments. Race, similar to our findings, was not associated with visit attendance. The unusually high compliance rates with follow-up care in their study (80% vs a reported benchmark of 65% in ED follow-up studies) may indicate particularly promising strategies to include in current or future transitions programs, including using dedicated phone lines for patients to make their own appointments before discharge, offering lower-cost alternatives for follow-up care, and implementing standard scheduling processes and templates.
Lastly, in an analysis using medical claims on more than 78,000 patients who experienced ischemic strokes between January and November 2012 throughout the United States, Terman et al found that 61% and 16% visited a PCP or neurologist, respectively, within 30 days of their hospitalization. Greater odds of attendance at primary care visits was associated with older age, female sex, presence of uncomplicated diabetes mellitus, and having home health services.26 Black patients were less likely to attend primary care and neurology visits than white patients. Interestingly being female and having home health visits were associated with lower odds of attending the neurology visits.
Study limitations
Our study has some limitations, which include missing data and potential for measurement error in, for example, the presence of a PCP. We do not know which patients had prior relationships with PCPs before their index hospitalizations. We learned from many of our clinical team members that patients were often set up with PCPs after their stroke as part of hospital discharge planning or as part of the 2-day call activities. Thus, it is challenging to know how to interpret associations between having a PCP and attending the COMPASS visits, but if the estimate of the effect of having a relationship with a PCP is diluted by the mere naming of a PCP in the database for some cases, the bias in our estimate would be toward the null. Our team intends to explore this using administrative claims data where we will evaluate associations between having established PCPs vs new PCPs and transitional care visit attendance among the insured population. Second, despite our assumption that hospital-level factors would be held constant in this context, we do not know if there may have been some variation in availability of COMPASS visits and outreach efforts by staff members making the 2- day calls on any given day; thus, our results may be affected by unmeasured site-level factors. However, we cannot predict the direction of influence this may have had on our odds ratio estimates. Lastly, it is known that a complete-case analysis can lead to biased effect estimates. To address this, our analyses used multiple imputation by chained equations to account for missing covariates in our regression analyses.
Conclusions
We found that activities that require patient engagement with health system processes, such as participation in 2-day calls and working with hospital staff to arrange postdischarge care visits, were associated with enhanced attendance at a transitional stroke care visit. Our results reinforce the importance of coordinating care in and around the time of hospital discharge to improve the attendance at these visits such that all patients are given the opportunity to engage with providers and care teams to assess patients’ needs post stroke and ensure pathways to optimal secondary prevention and recovery.
Acknowledgments
We thank Gayle Wooten, RN, COMPASS postacute care coordinator at Wake Forest Baptist Health; Dr. Allison Brashear, Chair of the Department of Neurology at Wake Forest Baptist Health; and all of our patients and stakeholders involved in COMPASS study. Additionally, we thank team members Mark Gwynne, DO; Anna Johnson, PhD, MSPH; Mysha Sissine, MSPH; Sabina Gesell, PhD; Gladys Lundy-Lamm, MA; Barbara Lutz, PhD, RN, FAHA; Wayne Rosamond, PhD, MS, FAHA; and Pamela Duncan, PhD, PT, FAPTA, FAHA, who all contributed significantly to this research.
Footnotes
Supported by the Patient-Centered Outcomes Research Institute (PCORI) Award (PCS-1403-14532). All statements are solely those of the presenters and do not necessarily represent the views of PCORI or its Board of Governors or Methodology Committee.
Clinical Trial Registration No.: NCT02588664.
Disclosures: J.R. Halladay: Research Grant, PCORI, NIH, NHLBI, AHRQ, NIDA, and NCATS; C.D. Bushnell: Research Grant, PCORI, NINDS and Ownership Interest, Care Directions (Dr Bushnell is founder of Care Directions, a start-up shell of a company that is currently looking for a pathway to commercialize the COMPASS-CP or eCare application that was used in the COMPASS Study. This is in the early stages of a start-up; M.A. Psioda: Research Grant, PCORI; S.B. Jones: Research Grant, PCORI; S.L. Lycan: Research Grant, PCORI; C.N. Condon: Research Grant, PCORI; J.G. Xenakis: Research Grant, PCORI; M.D.J. Prvu-Bettger: Research Grant, PCORI.
References
- 1.Kalanithi L., Tai W., Conley J., Platchek T., Zulman D., Milstein A. Better health, less spending: delivery innovation for ischemic cerebrovascular disease. Stroke. 2014;45:3105–3111. doi: 10.1161/STROKEAHA.114.006236. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Mozaffarian D., Roger V.L. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kind A.J., Smith M.A., Frytak J.R., Finch M.D. Bouncing back: patterns and predictors of complicated transitions 30 days after hospitalization for acute ischemic stroke. J Am Geriatr Soc. 2007;55:365–373. doi: 10.1111/j.1532-5415.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prvu Bettger J., McCoy L., Smith E.E., Fonarow G.C., Schwamm L.H., Peterson E.D. Contemporary trends and predictors of postacute service use and routine discharge home after stroke. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankey G.J., Jamrozik K., Broadhurst R.J. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29:2491–2500. doi: 10.1161/01.str.29.12.2491. [DOI] [PubMed] [Google Scholar]
- 6.Burn J., Dennis M., Bamford J., Sandercock P., Wade D., Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke. 1994;25:333–337. doi: 10.1161/01.str.25.2.333. [DOI] [PubMed] [Google Scholar]
- 7.Zhong W., Geng N., Wang P., Li Z., Cao L. Prevalence, causes and risk factors of hospital readmissions after acute stroke and transient ischemic attack: a systematic review and meta-analysis. Neurol Sci. 2016;37:1195–1202. doi: 10.1007/s10072-016-2570-5. [DOI] [PubMed] [Google Scholar]
- 8.Fehnel C.R., Lee Y., Wendell L.C., Thompson B.B., Potter N.S., Mor V. Post-acute care data for predicting readmission after ischemic stroke: a nationwide cohort analysis using the minimum data set. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002145. e002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonarow G.C., Smith E.E., Reeves M.J. Hospital-level variation in mortality and rehospitalization for medicare beneficiaries with acute ischemic stroke. Stroke. 2011;42:159–166. doi: 10.1161/STROKEAHA.110.601831. [DOI] [PubMed] [Google Scholar]
- 10.Nahab F., Takesaka J., Mailyan E. Avoidable 30-day readmissions among patients with stroke and other cerebrovascular disease. Neurohospitalist. 2012;2:7–11. doi: 10.1177/1941874411427733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condon C., Lycan S., Duncan P., Bushnell C. Reducing readmissions after stroke with a structured nurse practitioner/registered nurse transitional stroke program. Stroke. 2016;47:1599–1604. doi: 10.1161/STROKEAHA.115.012524. [DOI] [PubMed] [Google Scholar]
- 12.Duncan P.W., Bushnell C.D., Rosamond W.D. The Comprehensive Post-Acute Stroke Services (COMPASS) study: design and methods for a cluster-randomized pragmatic trial. BMC Neurol. 2017;17:133. doi: 10.1186/s12883-017-0907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bushnell C.D., Duncan P.W., Lycan S.L. A person-centered approach to poststroke care: the COMprehensive Post-Acute Stroke Services Model. J Am Geriatr Soc. 2018;66:1025–1030. doi: 10.1111/jgs.15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews J.E., Moore J.B., Weinberg R.B. Ensuring respect for persons in COMPASS: a cluster randomised pragmatic clinical trial. J Med Ethics. 2018;44:560–566. doi: 10.1136/medethics-2017-104478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 16.Rubin D.B. John Wiley & Sons; New York: 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 17.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 18.Costantino M.E., Frey B., Hall B., Painter P. The influence of a postdischarge intervention on reducing hospital readmissions in a Medicare population. Popul Health Manag. 2013;16:310–316. doi: 10.1089/pop.2012.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 20.Hansen L.O., Young R.S., Hinami K., Leung A., Williams M.V. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Int Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 21.Kyriacou D.N., Handel D., Stein A.C., Nelson R.R. Breif report: factors affecting outpatient follow-up compliance of emergency department patients. J Gen Intern Med. 2005;20:938–942. doi: 10.1111/j.1525-1497.2005.0216_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balaban R.B., Weissman J.S., Samuel P.A., Woolhandler S. Redefining and redesigning hospital discharge to enhance patient care: a randomized controlled study. J Gen Intern Med. 2008;23:1228–1233. doi: 10.1007/s11606-008-0618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam K., Abrams H.B., Matelski J., Okrainec K. Factors associated with attendance at primary care appointments after discharge from hospital: a retrospective cohort study. CMAJ Open. 2018;6:E587–E593. doi: 10.9778/cmajo.20180069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha S., Seirup J., Carmel A. Early primary care follow-up after ED and hospital discharge - does it affect readmissions? Hosp Pract (1995) 2017;45:51–57. doi: 10.1080/21548331.2017.1283935. [DOI] [PubMed] [Google Scholar]
- 25.Messina F.C., McDaniel M.A., Trammel A.C., Ervin D.R., Kozak M.A., Weaver C.S. Improving specialty care follow-up after an ED visit using a unique referral system. The Am J Emerg Med. 2013;31:1495–1500. doi: 10.1016/j.ajem.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Terman S.W., Reeves M.J., Skolarus L.E., Burke J.F. Association between early outpatient visits and readmissions after ischemic stroke. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.117.004024. e004024. [DOI] [PMC free article] [PubMed] [Google Scholar]