Abstract
Objective
To better understand the role of the presence or absence of motor-evoked potentials (MEPs) in predicting functional outcomes following a severe-moderate stroke.
Design
Retrospective exploratory analysis. We compared the effects of the stimulation condition (active or sham), MEP status (+ or −), and a combination of stimulation condition and MEP status on outcome. Within-group and between-group changes were assessed with longitudinal repeated measures analysis of variance and longitudinal repeated measures analysis of covariance, respectively. The proportions of participants who achieved minimal clinically important differences (MCIDs) for the main outcome measures were calculated.
Setting
University research laboratory within a rehabilitation hospital.
Participants
A total of 129 subjects with severe-moderate stroke-related motor impairments who participated in previous studies combining neuromodulation and motor training
Interventions
Neuromodulation (active or sham) and motor training.
Main Outcome Measures
Fugl-Meyer Assessment (FMA) and Action Research Arm Test (ARAT).
Results
When participants were grouped by stimulation condition or MEP status, all groups improved from baseline to immediate postintervention and follow-up evaluations (all P<.05). Analysis by stimulation condition and MEP status found that the MEP−/active group improved by 4.2 points on FMA (P<.0001) and 1.8 on ARAT (P=.003) post intervention. The MEP+/active group improved by 5.7 points on FMA (P<.0001) and 3.9 points on ARAT (P<.0001) post intervention. There were no between-group differences (P>.05). Regarding MCIDs, in the MEP−/active group, 14.5% of individuals reached MCID on FMA and 8.3% on ARAT post intervention. In the MEP+/active group, 33.3% of individuals reached MCID on FMA and 27.3% on ARAT post intervention.
Conclusion
As expected, the MEP+ group had the greatest improvement in motor function. However, it was shown that individuals without MEPs can also achieve meaningful changes, as reflected by MCID, when neuromodulation is paired with motor training. To our knowledge, this is the first study to differentiate the effects of neuromodulation by MEP status.
Keywords: Rehabilitation, Transcranial direct current stimulation, Transcranial magnetic stimulation
List of abbreviations: ARAT, Action Research Arm Test; BMI, brain-machine interface; FMA, Fugl-Meyer Assessment; MCID, minimal clinically important difference; MEP, motor-evoked potential; MSO, maximum stimulator output; PNS, peripheral nerve stimulation; tDCS, transcranial direct current stimulation; TMS, transcranial magnetic stimulation
Approximately 795,000 individuals in the United States have a stroke each year.1 More than half will be dependent and need help to complete activities of daily living because of upper limb impairments.2 Because strokes are occurring at younger ages3 and lifespans are increasing, there is an urgent need to identify interventions that can promote functional recovery of the upper limb.
In the hours and days following a stroke, the presence or absence of motor-evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) predicts the extent of an individual’s motor recovery.4, 5 MEPs are thought to be an indicator of a functional corticospinal tract.6 An individual’s prognosis is better if MEPs can be evoked during the early period after acute stroke,6 regardless of interventions. There is, however, little published data that includes long-term follow-up of patients without MEPs and even less in patients with a severe-moderate stroke. Because additive interventions such as peripheral nerve stimulation (PNS), transcranial direct current stimulation (tDCS), or other neuromodulatory techniques may enhance motor recovery after stroke,7, 8, 9, 10, 11, 12 there is a need to understand better whether individuals with severe-moderate stroke, who are commonly considered to have little potential for functional improvement, may benefit from these procedures. Additionally, a better understanding of the mechanisms underlying the biological effects of these techniques could help guide a personalized approach to treatment. We hypothesized that individuals with severe-moderate stroke-related motor impairments without MEPs can still achieve meaningful functional improvements, defined as the minimal clinically important difference (MCID), when receiving intensive motor training, either with or without additive neuromodulation.
We conducted an exploratory analysis of data from participants in our previous 7 studies that combined neuromodulatory stimulation and upper extremity motor training who were at least 6 months post stroke and had severe-moderate motor impairments at the time of enrollment.7, 8, 9, 13, 14, 15 The purpose was to better understand the role of MEPs in predicting functional outcome with the long-term goal of identifying differences between individuals with severe-moderate stroke who do and do not respond to motor rehabilitation.
Methods
Participants
This study used data collected in 7 previous studies conducted by our group in our university research laboratory located within a rehabilitation hospital (6 published7, 8, 9, 13, 14, 15 and 1 unpublished pilot study). All studies were approved by the Institutional Review Board. Although the studies differed in methodology and intervention, all participants included in this analysis were at least 6 months post stroke and had a Fugl-Meyer Assessment upper extremity (FMA) score of ≤34 at baseline. This level of impairment is considered to be in the severe-moderate to severe range.16 Any participant who had TMS risk factors, and therefore did not undergo MEP assessments, was excluded. Participants in these studies received active or sham neuromodulatory stimulation (PNS and/or tDCS), followed by upper extremity motor therapy.
Intervention component 1: neuromodulatory stimulation
Participants received PNS or tDCS in each of the included studies.
Peripheral nerve stimulation
PNSa was delivered to 3 nerves. Target nerves varied by participant based on their individual impairments. They were chosen from 4 predetermined targets for stimulation: Erb’s point, posterior interosseous, radial, and median nerves. For active PNS, stimulation intensity was adjusted so that small compound muscle action potentials between 50-100μV were elicited in the absence of visible muscle contraction,17 which was generally below sensory threshold. Sham PNS intensity was set to 0V. PNS was delivered for 2 hours while the participants sat quietly, typically reading or watching a movie. Participants, therapists, and evaluators of motor function were masked to the treatment condition. Further details can be found in studies by Carrico7, 8, 9 and Salyers15 and colleagues.
Transcranial direct current stimulation
In the studies using tDCS,b participants received anodal, cathodal, dual, or sham stimulation. In excitatory anodal and inhibitory cathodal stimulation, the active electrode was placed over M1 of the targeted hemisphere, and the reference electrode was placed over the contralateral supraorbital area. In dual stimulation, both electrodes were active. For active stimulation (anodal, cathodal, dual), intensities from 1.4-2 mA were used, depending on the study. Some participants were able to feel tingling under the electrodes at these intensities; however, within 2-5 minutes, sensation of stimulation faded. The anodal technique was used for sham stimulation and was designed to mimic the sensations of active stimulation, thus maintaining masking.18 Therapists and evaluators of motor function were also masked to the condition of tDCS. Further details can be found in studies by Chelette13 and Powell14 and colleagues.
Intervention component 2: intensive motor training
Intensive motor training occurred immediately after neuromodulation was complete. Intensive task-oriented training was either delivered by an occupational therapist (5 studies) or robot-assisted training closely supervised by an occupational therapist (2 studies). In all studies, therapists were masked to the condition of neuromodulatory intervention. The training protocol followed the same principles of the intensive task-oriented therapy established with the EXCITE trial.19, 20, 21 Because of our participants’ low function, however, we did not constrain the unaffected side. Therapists selected tasks from a predetermined battery of tasks. Tasks in this battery were repeatable and had a functional goal such as pinching, grasping, reaching, releasing, and/or rotating. Therapy was delivered in a 1:1 therapist-to-participant ratio and involved repetitive attempts. The difficulty level was adjusted for each participant in a given session. The specific tasks chosen and the time practicing each was recorded. Robot-assisted training primarily consisted of reaching and grasp/release movements. Participants were secured in a height-adjustable chair with an over-the-shoulder harness that buckled at the lap and chest. The affected arm rested in an arm trough with the hand grasping a handle inside the trough. The arm trough connected to the robotic interface, which included a computer monitor that displayed visual cues in motor training sessions. It also included a robotic frame that provided active assistance to the paretic upper extremity. During training, a monitor displayed an image resembling a pie with 8 triangular pieces. A target appeared in successive fashion at the center of the pie and at 8 locations evenly spaced around the edge of the pie. The first 16 repetitions required unassisted participant performance, but the robot assisted with subsequent movements if a participant did not demonstrate necessary skill to complete the task. The task sequence included the initial 16 repetitions followed by 12 sets of 80 movements per set, totaling 960 potentially assisted movements. Training proceeded according to participants’ reported tolerable levels of comfort and fatigue. For both training with a therapist or with a robot, participants were constantly challenged by increasing the difficulty of tasks as improvements were made, a concept referred to as shaping.22 The shaping technique is a behavioral approach to motor therapy in which trainers (1) elicit performance requiring a skill level just beyond that already demonstrated and (2) provide verbal guidance concerning the sequence of movement. Rest breaks were given as needed. For further details, see Carrico,7, 8, 9 Powell,14 and Salyers15 and colleagues.
Outcome measures
Outcome measures were evaluated at baseline (≤7 days before first intervention) and immediately after the intervention period in all studies. Five studies had 1 follow-up evaluation at 1 month or 3 months post-intervention (follow-up 1).
Evaluation of motor function
The FMA was used as a motor function outcome measure for all studies. This assessment evaluates motor function and is based on the theory that stroke recovery occurs in a standard progression.23 It has been used extensively in populations with stroke.23 Five of the studies also used the Action Research Arm Test (ARAT) to measure upper extremity motor capacity. It is particularly responsive to recovery in populations with chronic stroke.24
Evaluation of cortical excitability
Cortical excitability was measured with TMS.c., d. Extensor digitorum communis was the target muscle in all studies because it is used in finger extension, a movement that is often difficult for individuals with severe-moderate motor impairments after stroke. The motor strip and surrounding areas of the ipsilesional hemisphere, located using a neuronavigation system,d were stimulated to determine whether an MEP in the contralateral extensor digitorum communis could be elicited. Stimulator intensity was increased in increments of 20% of maximum stimulator output (MSO) if an MEP could not be elicited at the previous intensity. When necessary, the stimulator intensity was increased up to 100% MSO, if tolerated by the participant. Participants for whom an MEP could be elicited at or below 100% MSO at the baseline evaluation were considered MEP positive; those who did not have an MEP at intensities up to 100% MSO at baseline were considered MEP negative. Additional information regarding complete TMS procedures can be found in the study by Powell et al.14
Data analysis
The primary outcomes of interest of these analyses were the within-group changes in FMA and ARAT from baseline to immediate postintervention and to follow-up. Changes by group were explored with participants first grouped by stimulation condition (active or sham) and then by MEP status (MEP positive or MEP negative). An additional question of interest was whether adding neuromodulatory stimulation differentially affects recovery in participants who were MEP positive or MEP negative. To address this question, participants were further grouped by both MEP status and stimulation condition.
All analyses were performed using SPSS Statistics 24e and SAS version 9.4.f A P value <.05 was predetermined to be significant. Baseline differences were assessed for FMA, ARAT, age, and months since stroke.
Because interest is in change over time with respect to FMA and ARAT, longitudinal repeated measures analysis of variance models with unstructured working covariance matrices were fit separately for each outcome, allowing for the assessment of changes to postintervention and follow-up within the framework of a single model. Importantly, such models allow the estimation and testing of within-group mean changes, which is the primary focus of these analyses, with comparison of groups with respect to changes being of secondary importance. However, between-group differences in changes were tested. Specifically, we conducted analyses based on longitudinal repeated measures analysis of covariance models, adjusting for baseline values, to ensure group comparisons were not influenced by baseline differences.
Additionally, the proportions of participants with available data who obtained an MCID for FMA and ARAT at each time point were calculated. A stringent MCID of 9 was used for FMA,25 and 5.7 was used for ARAT.26, 27 No statistical analysis was performed on these proportions.
Results
Data from 129 participants in the 7 studies were included in this analysis. Enrollment and follow-up took place from September 2008 to September 2015. More data were available for FMA than ARAT because all studies included FMA but not all included ARAT. Baseline characteristics and demographics are shown in table 1. There were no differences at baseline between the active and sham neurostimulation groups for FMA, ARAT, age, or months since stroke. Comparison of MEP-negative/sham, MEP-positive/sham, MEP-negative/active, and MEP-positive/active groups revealed baseline differences on FMA, ARAT, and age. The MEP-positive/active group had higher baseline scores on FMA and ARAT than the MEP-negative/sham (FMA P=.015, ARAT P=.004) and MEP-negative/active groups (FMA P=.032, ARAT P=.002). The MEP-positive/active group was also younger than the MEP-negative/active group (P=.017). No baseline group differences were found for months since the index stroke.
Table 1.
Baseline characteristics/demographics
| Characteristics/Demographics | MEP−/Sham | MEP+/Sham | MEP−/Active | MEP+/Active |
|---|---|---|---|---|
| FMA, mean ± SD (range)∗ | 17.3±7.5 (2-34) | 21.6±8.2 (8-33) | 18.8±7.2 (6-33) | 23.2±7.2 (9-34) |
| ARAT, mean ± SD (range)∗ | 4.7±3.4 (0-12) | 10.6±5.7 (4-19) | 5.7±3.7 (0-15) | 11.6±9.5 (3-35) |
| Age at enrollment, mean ± SD (range) (y)∗ | 66.8±7.0 (46-80) | 63.4±12.3 (37-77) | 60.0±13.0 (19-80) | 66.7±8.9 (41-80) |
| Time since stroke, mean ± SD (range) (mo) | 46.6±61.5 (6-219) | 34.7±37.4 (6-118) | 44.9±38.8 (7-182) | 51.0±54.4 (6-194) |
| Sex, n (M/F) | 14/7 | 6/3 | 35/34 | 16/14 |
| Stroke type, n (ischemic/hemorrhagic) | 15/6 | 7/2 | 47/22 | 24/6 |
| Stroke location, n (cortical/subcortical/cortical and subcortical/other) | 12/8/0/1 | 5/4/0/0 | 48/19/2/0 | 20/8/0/2 |
| Handedness before stroke, n (L/R) | 3/18 | 0/9 | 5/64 | 8/22 |
| Paretic upper extremity, n (L/R) | 9/12 | 5/4 | 38/31 | 11/19 |
Overall significant differences were found between groups.
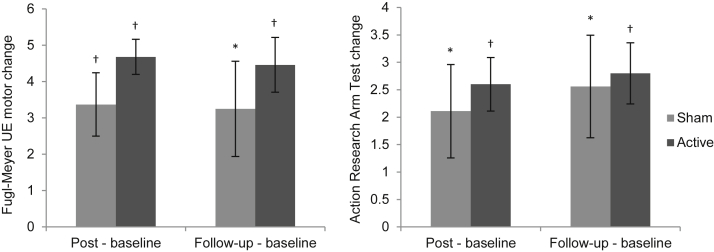
Analysis of within-group changes by sham and active stimulation showed that both groups had improvements on both FMA and ARAT immediately post intervention and at follow-up (all P<.05) (fig 1). The group receiving active stimulation had nonsignificantly greater improvements than the sham stimulation group on both outcome measures at both time points.
Fig 1.
Change from baseline in behavioral outcome measures by stimulation condition. Statistically significant improvements were observed for both sham and active stimulation groups at both time points on FMA and ARAT. Abbreviation: UE, upper extremity. *Statistical significance (P≤.05). †Statistical significance with the Bonferroni correction for multiple comparisons (P≤.0125).
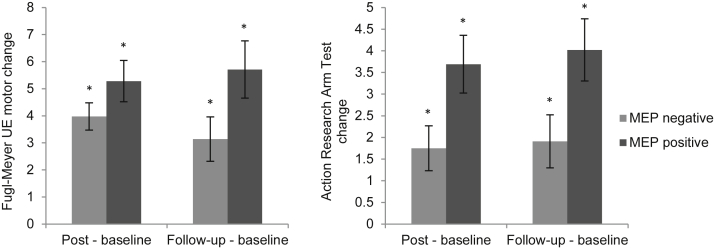
Within-group analyses of changes from baseline to immediately post intervention and follow-up with participants grouped by MEP-negative or MEP-positive status revealed improvements for both groups (all P<.05) (fig 2). For both outcome measures and at both time points, the MEP-positive group had nonsignificantly greater improvements than the MEP-negative group.
Fig 2.
Change from baseline in behavioral outcome measures by MEP status. MEP-negative and MEP-positive groups had statistically significant improvements on FMA and ARAT at both time points. The improvements were more pronounced for MEP positives than MEP negatives. Abbreviation: UE, upper extremity. *Statistical significance with the Bonferroni correction for multiple comparisons (P≤.0125).
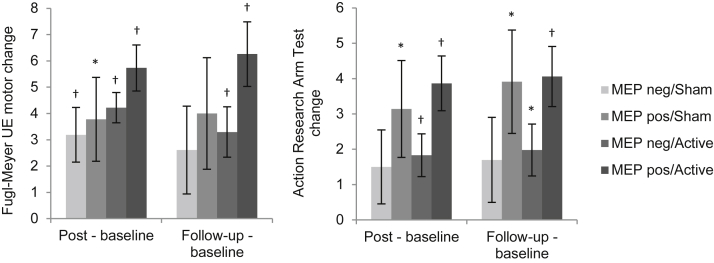
The results of the analysis with participants grouped by both stimulation condition and MEP status are shown in figure 3, with improvements on FMA immediately post intervention for all groups. Improvements were only found for MEP-negative/active and MEP-positive/active groups at follow-up. For ARAT, improvements were found for MEP-positive/sham, MEP-negative/active, and MEP-positive/active groups both at immediately post intervention and follow-up. In comparing the different groups, the MEP-negative/sham group always had the least improvement, whereas the MEP-positive/active group always had the greatest improvement. Both MEP-positive and MEP-negative groups had better outcomes when active stimulation was delivered.
Fig 3.
Change from baseline in behavioral outcome measures by stimulation condition and MEP status. All groups had improvements on FMA and ARAT at immediately postintervention and follow-up evaluations. MEP-negative/sham consistently showed the least improvement whereas MEP-positive/active consistently had the most improvement. Statistically significant improvements were consistently found for MEP-negative/active and MEP-positive/active groups. Abbreviation: UE, upper extremity. *Statistical significance (P≤.05). †Statistical significance with the Bonferroni correction for multiple comparisons (P≤.00625).
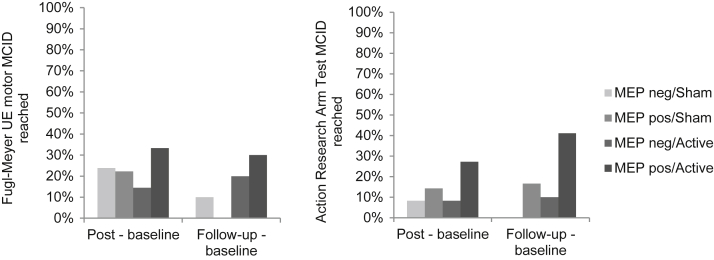
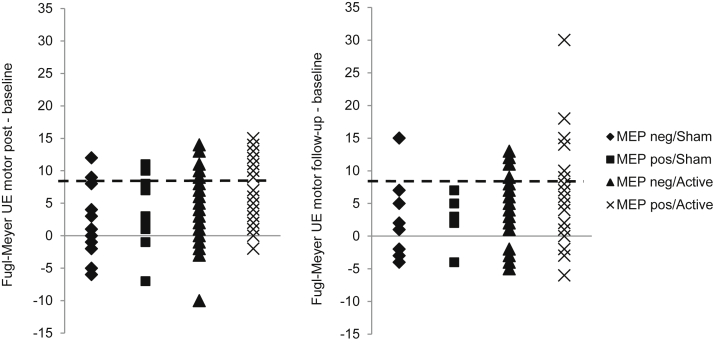
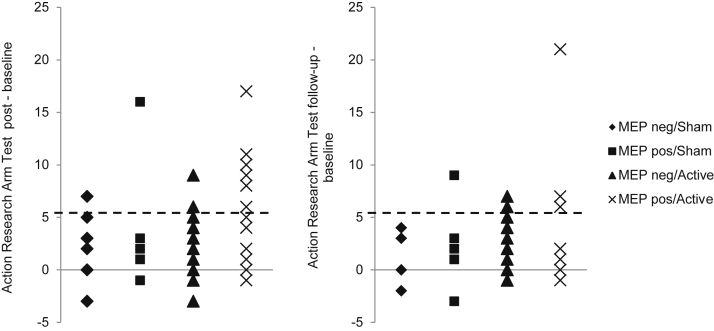
Figure 4 shows the proportions of participants in each of the 4 groups who reached or exceeded the MCID on FMA and ARAT. The MEP-positive/active group had the numerically greatest proportion of participants who achieved an MCID. For FMA, 33% reached MCID at immediately post intervention, and 30% reached it at follow-up; for ARAT, 27% reached MCID at immediately post intervention, and 41% reached it at follow-up. Small proportions of participants in the MEP-negative groups were also able to achieve an MCID. In the MEP-negative/sham group, 24% and 10% reached MCID for FMA at immediately post intervention and follow-up, respectively, and 8% and 0% reached it for ARAT. In the MEP-negative/active group, 14% and 20% reached MCID for FMA at immediately post intervention and follow-up, respectively, and 8% and 10% reached it for ARAT, respectively. The distribution of changes from baseline for all participants is shown for FMA (fig 5) and ARAT (fig 6). Again, the greatest improvements were in participants in the MEP-positive/active group. The majority of participants in all groups, however, improved.
Fig 4.
Proportion of participants who achieved MCID by stimulation condition and MEP status. The MEP-positive/active group had the greatest proportion of participants who achieved MCID on FMA and ARAT at both time points. All groups had ≥1 participant achieve MCID at the immediately postintervention assessment on FMA and ARAT. Both MEP-negative/active and MEP-positive/active groups had participants reach MCID on FMA and ARAT immediately post intervention and at follow-up. Abbreviation: UE, upper extremity.
Fig 5.
Change in individual scores on FMA at immediately postintervention and follow-up assessments by stimulation condition and MEP status. The majority of participants across all groups showed some amount of improvement (>0), whereas a smaller proportion exceeded the MCID of 9 (dashed line). Abbreviation: UE, upper extremity.
Fig 6.
Change in individual scores on ARAT at immediately postintervention and follow-up assessments by stimulation condition and MEP status. Most participants demonstrated improvements with some participants exceeding the MCID of 5.7 (dashed line).
Discussion
The results of this exploratory analysis suggest that a subset of individuals with severe-moderate stroke-related motor impairments and without MEPs in response to TMS can nonetheless achieve clinically meaningful improvements, defined as reaching or exceeding the MCID on FMA. Furthermore, neuromodulation such as tDCS and PNS can augment the effects of motor training in these individuals. This preliminary finding is crucial because of the belief that individuals with severe-moderate poststroke impairments, particularly those without MEPs, have limited or no capacity for neuroplasticity.
To better understand the clinical implications of these improvements, MCIDs must be evaluated in those with severe-moderate deficits who are at least 6 months post stroke. Various MCIDs for different populations with stroke have been estimated for outcome measures, including FMA and ARAT. The FMA MCID estimates of 4.25-7.2528 and 6.624 have been proposed for individuals who are more than 1 year post stroke and have a mild to moderate impairment. These MCIDs were not applied in our study because individuals with a severe-moderate impairment were not included in the prior work. A previous study in individuals with chronic, severe impairment used an MCID of 3, with the theory that smaller changes may be more impactful in patients with long-term severe impairments than in those with mild impairments.29, 30 The more stringent MCID of 9, which was used in our analysis, was determined in individuals between 1 and 6 months post stroke and with varied levels of impairment, including those with severe-moderate deficits.25 The MCID of 5.7 for ARAT,24, 27 which also was used in our analysis, was calculated from data on individuals with mild to moderate impairments who were at least 1 year post stroke. An alternative MCID of 12 on the dominant side and 17 on the nondominant side, which was calculated in individuals an average of 9 days post stroke with an average ARAT of 22 at baseline,31 has also been proposed. Although this population includes individuals with severe-moderate impairments, they are also in the stage in which spontaneous recovery commonly occurs, and hence this MCID is not appropriate to use in individuals beyond the spontaneous recovery phase. As these strikingly varied MCIDs indicate, the definition of “clinically important” depends on both the amount of time that has passed since stroke and the level of impairment. Therefore, it is important that MCIDs be established for individuals at least 6 months post stroke with severe-moderate impairments to better understand the effect of interventions in this population.
Comparison with changes in FMA scores from other studies of individuals with severe impairments at least 6 months post stroke can help elucidate the effectiveness of our neuromodulatory interventions paired with motor training, though no other study in this population has accounted for MEP status. A study of 127 individuals at least 6 months post stroke with baseline FMA scores ranging from 7-38 compared intensive robotic therapy and intensive comparison therapy, with 3 sessions per week over 12 weeks.29 FMA scores improved by an average of 3.87 with robotic therapy and 4.01 points with intensive comparison therapy. A separate study of 26 individuals with a median baseline FMA of 17.5 and maximum of 35, tested a brain-machine interface (BMI) for triggering movement of the arm and fingers with an orthosis as well as muscle stimulation to enable individuals to pick up pegs.32 Ten days of 40-minute BMI sessions and 40 minutes of standard occupational therapy resulted in a median FMA increase of 2 points. A study of 31 more severely impaired individuals, with baseline FMA of 19 or less, compared 45-minute sessions of mirror therapy with passive mobilization of the affected upper extremity.33 After 24 sessions over 8 weeks, the mirror therapy and passive mobilization groups increased only 0.1 and 0.5 points on FMA, respectively. Finally, 18 individuals with a mean baseline FMA of 19.2 participated in a 2-phase inpatient study that included 10 days of BMI training and occupational therapy followed by 3 weeks of hybrid assistive neuromuscular dynamic stimulation for 8 hours per day, which included 90 minutes of occupational therapy 5 days per week.34 Following the BMI training, FMA scores had increased by 3.3 points and increased an additional 5.9 points after the hybrid assistive neuromuscular dynamic stimulation, for an increase of 9.2 points from baseline. Our studies yield greater improvements than other studies of similar populations, with the exception of the last mentioned study that required participants to stay in the hospital and wear an assistive device for 8 hours every day. The study by Lo et al,29 which had improvements most similar to ours, achieved these results over a period of 12 weeks, whereas ours were achieved in intervention periods of 6 weeks or less. Therefore, these results suggest that neuromodulatory stimulation may enhance or allow for faster recovery from impairment in this particular population than conventional or other experimental interventions. Again, the aforementioned studies do not report the MEP status of the participants.
Our study also shows that there is a spectrum of effects in response to rehabilitative interventions within MEP-positive and MEP-negative groups. Although the different neuromodulatory interventions and motor training paradigms used in the studies in our retrospective analysis may partially explain interindividual variability, the spectrum of responses is not a novel observation. The majority of studies in stroke rehabilitation, including our work, only report group results and neglect the range of individual responses. There is a need to further our understanding of the interplay of factors that can affect individual responses to neurorehabilitation to help guide personalized neurorehabilitation.
Study limitations
Our exploratory analysis has several limitations. The protocols of the included studies are heterogeneous. Although each involved neuromodulatory stimulation followed by motor therapy, there was variability in the type of neuromodulatory stimulation and therapy administered. Additionally, the participant sample was heterogeneous and included those with both ischemic and hemorrhagic strokes, various stroke locations, and a wide range of time elapsed since the stroke.
Conclusions
This analysis suggests that the absence of MEPs in individuals with chronic and severe-moderate poststroke motor impairments does not necessarily predict poor recovery. When receiving motor training, individuals without MEPs may be capable of improvement, though to a lesser degree than individuals who have MEPs. Recovery is enhanced in those with and without MEPs with neuromodulatory stimulation. Future studies should include participants with severe-moderate deficits.
Suppliers
-
a.
Peripheral nerve stimulation: S88 stimulator and SIU8T stimulus isolation unit; Grass Technologies.
-
b.
Transcranial direct current stimulation: Magstim; Whitland.
-
c.
Transcranial magnetic stimulation: 2002, Magstim; Whitland.
-
d.
Neuronavigation: Brainsight; Rogue Research Inc.
-
e.
SPSS Statistics 24; IBM.
-
f.
SAS version 9.4; SAS Institute.
Footnotes
Supported in part by the Cardinal Hill Stroke and Spinal Cord Injury Endowment (no. 0705129700). The funding body had no role in the collection, analysis, and interpretation of data or in writing the manuscript.
Disclosures: none.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics-2018 update: a report from the american heart association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Carandang R., Seshadri S., Beiser A. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 3.de los Rios F., Kleindorfer D.O., Khoury J. Trends in substance abuse preceding stroke among young adults: a population-based study. Stroke. 2012;43:3179–3183. doi: 10.1161/STROKEAHA.112.667808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapisarda G., Bastings E., de Noordhout A.M., Pennisi G., Delwaide P.J. Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation? Stroke. 1996;27:2191–2196. doi: 10.1161/01.str.27.12.2191. [DOI] [PubMed] [Google Scholar]
- 5.Pennisi G., Rapisarda G., Bella R., Calabrese V., Maertens De Noordhout A., Delwaide P.J. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999;30:2666–2670. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- 6.Smith M.C., Stinear C.M. Transcranial magnetic stimulation (TMS) in stroke: ready for clinical practice? J Clin Neurosci. 2016;31:10–14. doi: 10.1016/j.jocn.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 7.Carrico C., Chelette K.C., 2nd, Westgate P.M. Nerve stimulation enhances task-oriented training in chronic, severe motor deficit after stroke: a randomized trial. Stroke. 2016;47:1879–1884. doi: 10.1161/STROKEAHA.116.012671. [DOI] [PubMed] [Google Scholar]
- 8.Carrico C., Chelette K.C., 2nd, Westgate P.M., Salmon-Powell E., Nichols L., Sawaki L. Randomized trial of peripheral nerve stimulation to enhance modified constraint-induced therapy after stroke. Am J Phys Med Rehabil. 2016;95:397–406. doi: 10.1097/PHM.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrico C., Westgate P.M., Salmon Powell E. Nerve stimulation enhances task-oriented training for moderate-to-severe hemiparesis 3-12 months after stroke: a randomized trial. Am J Phys Med Rehabil. 2018;97:808–815. doi: 10.1097/PHM.0000000000000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikuno K., Kawaguchi S., Kitabeppu S. Effects of peripheral sensory nerve stimulation plus task-oriented training on upper extremity function in patients with subacute stroke: a pilot randomized crossover trial. Clin Rehabil. 2012;26:999–1009. doi: 10.1177/0269215512441476. [DOI] [PubMed] [Google Scholar]
- 11.Boggio P.S., Nunes A., Rigonatti S.P., Nitsche M.A., Pascual-Leone A., Fregni F. Repeated sessions of noninvasive brain dc stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- 12.Kang N., Summers J.J., Cauraugh J.H. Transcranial direct current stimulation facilitates motor learning post-stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:345–355. doi: 10.1136/jnnp-2015-311242. [DOI] [PubMed] [Google Scholar]
- 13.Chelette K., Carrico C., Nichols L., Salyers E., Sawaki L. Natal, Brazil; October 15-18, 2014. Effects of electrode configurations in transcranial direct current stimulation after stroke. Paper presented at: e-Health Networking, Applications and Services (Healthcom), 2014 IEEE 16th International Conference. [Google Scholar]
- 14.Powell E.S., Carrico C., Westgate P.M. Time configuration of combined neuromodulation and motor training after stroke: a proof-of-concept study. NeuroRehabilitation. 2016;39:439–449. doi: 10.3233/NRE-161375. [DOI] [PubMed] [Google Scholar]
- 15.Salyers E., Carrico C., Chelette K.C., Nichols L., Henzman C., Sawaki L. Natal, Brazil; October 15-18, 2014. Dose-response effects of peripheral nerve stimulation and motor training in stroke: preliminary data. Paper presented at: e-Health Networking, Applications and Services (Healthcom), 2014 IEEE 16th International Conference. [Google Scholar]
- 16.Woytowicz E.J., Rietschel J.C., Goodman R.N. Determining levels of upper extremity movement impairment by applying a cluster analysis to the fugl-meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil. 2017;98:456–462. doi: 10.1016/j.apmr.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaelin-Lang A., Luft A.R., Sawaki L., Burstein A.H., Sohn Y.H., Cohen L.G. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandiga P.C., Hummel F.C., Cohen L.G. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Wolf S.L., Thompson P.A., Morris D.M. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 20.Wolf S.L., Winstein C.J., Miller J.P. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 21.Wolf S.L., Winstein C.J., Miller J.P. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf S.L., Thompson P.A., Winstein C.J. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41:2309–2315. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladstone D.J., Danells C.J., Black S.E. The Fugl-Meyer Assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 24.van der Lee J.H., Beckerman H., Lankhorst G.J., Bouter L.M. The responsiveness of the Action Research Arm Test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med. 2001;33:110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- 25.Arya K.N., Verma R., Garg R.K. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil. 2011;18(Suppl 1):599–610. doi: 10.1310/tsr18s01-599. [DOI] [PubMed] [Google Scholar]
- 26.Pandian S., Arya K.N. Stroke-related motor outcome measures: do they quantify the neurophysiological aspects of upper extremity recovery? J Bodyw Mov Ther. 2014;18:412–423. doi: 10.1016/j.jbmt.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Van der Lee J.H., De Groot V., Beckerman H., Wagenaar R.C., Lankhorst G.J., Bouter L.M. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82:14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 28.Page S.J., Fulk G.D., Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92:791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 29.Lo A.C., Guarino P.D., Richards L.G. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo A.C., Guarino P., Krebs H.I. Multicenter randomized trial of robot-assisted rehabilitation for chronic stroke: methods and entry characteristics for va robotics. Neurorehabil Neural Repair. 2009;23:775–783. doi: 10.1177/1545968309338195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang C.E., Wagner J.M., Dromerick A.W., Edwards D.F. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil. 2006;87:1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Nishimoto A., Kawakami M., Fujiwara T. Feasibility of task-specific brain-machine interface training for upper-extremity paralysis in patients with chronic hemiparetic stroke. J Rehabil Med. 2018;50:52–58. doi: 10.2340/16501977-2275. [DOI] [PubMed] [Google Scholar]
- 33.Colomer C., Noé E., Llorens R. Mirror therapy in chronic stroke survivors with severely impaired upper limb function: a randomized controlled trial. Eur J Phys Rehabil Med. 2016;52:271–278. [PubMed] [Google Scholar]
- 34.Kawakami M., Fujiwara T., Ushiba J. A new therapeutic application of brain-machine interface (BMI) training followed by hybrid assistive neuromuscular dynamic stimulation (HANDS) therapy for patients with severe hemiparetic stroke: a proof of concept study. Restor Neurol Neurosci. 2016;34:789–797. doi: 10.3233/RNN-160652. [DOI] [PubMed] [Google Scholar]