Abstract
Objective
To compare the efficacy of high- and low-frequency noninvasive translingual neurostimulation (TLNS) plus targeted physical therapy (PT) for treating chronic balance and gait deficits due to mild-to-moderate traumatic brain injury (mmTBI).
Design
Participants were randomized 1:1 in a 26-week double-blind phase 1/2 study (NCT02158494) with 3 consecutive treatment stages: in-clinic, at-home, and no treatment. Arms were high-frequency pulse (HFP) and low-frequency pulse (LFP) TLNS.
Setting
TLNS plus PT training was initiated in-clinic and then continued at home.
Participants
Participants (N=44; 18-65y) from across the United States were randomized into the HFP and LFP (each plus PT) arms. Forty-three participants (28 women, 15 men) completed at least 1 stage of the study. Enrollment requirements included an mmTBI ≥1 year prior to screening, balance disorder due to mmTBI, a plateau in recovery with current PT, and a Sensory Organization Test (SOT) score ≥16 points below normal.
Interventions
Participants received TLNS (HFP or LFP) plus PT for a total of 14 weeks (2 in-clinic and 12 at home), twice daily, followed by 12 weeks without treatment.
Main Outcome Measures
The primary endpoint was change in SOT composite score from baseline to week 14. Secondary variables (eg, Dynamic Gait Index [DGI], 6-minute walk test [6MWT]) were also collected.
Results
Both arms had a significant (P<.0001) improvement in SOT scores from baseline at weeks 2, 5, 14 (primary endpoint), and 26. DGI scores had significant improvement (P<.001-.01) from baseline at the same test points; 6MWT evaluations after 2 weeks were significant. The SOT, DGI, and 6MWT scores did not significantly differ between arms at any test point. There were no treatment-related serious adverse events.
Conclusions
Both the HFP+PT and LFP+PT groups had significantly improved balance scores, and outcomes were sustained for 12 weeks after discontinuing TLNS treatment. Results between arms did not significantly differ from each other. Whether the 2 dosages are equally effective or whether improvements are because of provision of PT cannot be conclusively established at this time.
Keywords: Balance, Facial nerve, Gait, Neurostimulation, Rehabilitation, Trigeminal nerve
List of abbreviations: 6MWT, 6-minute walk test; AE, adverse event; ANOVA, analysis of variance; DGI, Dynamic Gait Index; HFP, high-frequency pulse; ITP, in-clinic training program; LFP, low-frequency pulse; mmTBI, mild-to-moderate traumatic brain injury; PoNS, portable neuromodulation stimulator; PSQI, Pittsburgh Sleep Quality Index; PT, physical therapy; SOT, Sensory Organization Test; TBI, traumatic brain injury; TLNS, translingual neurostimulation
Traumatic brain injury (TBI) is a leading cause of injury-induced death and physical disability. Millions of people experience TBI every year,1,2 and an estimated 5.3 million people are living with TBI-related disabilities,3 with up to 57% of patients with TBI experiencing balance disorders.4 Mild-to-moderate traumatic brain injury (mmTBI) encompasses most of TBI cases (83%).5
For many people, the signs and symptoms of mmTBI resolve with time, allowing return to normal daily activities; however, 25%-50% of patients experience chronic symptoms.6, 7, 8, 9, 10 Instability or imbalance can persist after mild TBI,11 which has a significant negative effect on functional status, capacity to return to work, and quality of life7,12, 13, 14, 15, 16 and can increase the risk of falling and repeat injury.17 Rehabilitation techniques consist of basic gait and balance training, but may also include specialized therapies, such as vestibular rehabilitation therapy, vision therapy, motor control retraining, graded exercise, and others.18, 19, 20, 21, 22, 23, 24 Whereas some patients improve with these treatments, others do not.18,25,26
Neurostimulation combined with physical therapy (PT) can potentially affect rehabilitation outcomes,27, 28, 29 and noninvasive brain stimulation can affect neural excitability and may facilitate motor skill learning.30 Cranial nerves V and VII in the tongue and associated neural projections in the brain can be stimulated through noninvasive translingual neurostimulation (TLNS).31 Clinical studies by our group and others indicate that TLNS with targeted PT, combined, can significantly improve outcomes in those with degenerative neurologic disease, spinal cord injury, or stroke.32, 33, 34, 35 In a separate study, we treated 20 persons with multiple sclerosis and an identified gait disturbance with TLNS plus targeted PT.32 Over 14 weeks of treatment, Dynamic Gait Index (DGI) significantly improved from baseline.32 One group reported results from 2 people with chronic incomplete spinal cord injury who completed 12 weeks of TLNS plus balance or gait PT that indicated improvements in both walking speed and skilled walking function.34 Results from a separate randomized controlled trial demonstrated significant improvement in the Mini-Balance Evaluation Test after 2 weeks of TLNS plus targeted PT in 5 subacute stroke survivors.33 These results, as well as similarities in neural dysfunction mechanisms of stroke and TBI,35 support the possibility that TLNS plus targeted PT may be effective for treating chronic balance and gait deficits due to mmTBI.
This 26-week, randomized trial (Clinicaltrials.gov, NCT02158494) was developed to investigate high-frequency pulse (HFP) TLNS plus PT, as treatment for individuals with persistent balance deficit due to mmTBI, compared with low-frequency pulse (LFP) TLNS plus PT as a control. Since trial registration, notable difficulties in establishing controls in neurostimulation studies have become more prominent in the field, particularly focusing on how a low, minimally perceived stimulus serving as a sham can trigger neural activity and produce a response.36, 37, 38, 39, 40 This determination of optimal stimulation parameters has proven challenging across the neurostimulation field, including studies with transcutaneous electrical nerve stimulation,36,37,41, 42, 43 noninvasive trigeminal nerve stimulation,38,39,44 and TLNS.32 Because of these difficulties, the focus of this study shifted from using the LFP as a control to one of a comparison between the treatment arms (PT plus either HFP or LFP); balance assessment after 14 weeks of treatment was the primary outcome measure.
Methods
Study design
This 26-week, randomized, double-blind phase 1/2 study (NCT02158494) was performed at a single site in the United States from April 29, 2014, to October 31, 2017, and included 3 stages: twice-daily in-clinic training program (ITP) for 2 weeks (with at-home training during the intervening weekend); (2) a 12-week home training program; and (3) 12 weeks with no treatment and a return to normal activities. The University of Wisconsin Institutional Review Board approved the protocol. After initial approval, on discovery that minimally perceived stimulus in neurostimulation studies can trigger neural activity and produce a response,36,37,39,40 the focus of this study was shifted from using the LFP plus PT arm as a control to one of a dosage comparison between PT plus either HFP or LFP stimulation (details are described in supplemental appendix S1, available online only at http://www.archives-pmr.org/). This study was performed in accordance with the Declaration of Helsinki and in agreement with the International Conference on Harmonisation Guidelines on Good Clinical Practice. All participants provided written informed consent prior to study participation.
Participants
Participants were recruited through print and radio advertising and were required to have mmTBI that occurred ≥1 year before enrollment, reached a functional plateau in their recovery (as defined by a discharge note from their physical therapist), and a NeuroComa Sensory Organization Test (SOT) composite score ≥16 points below normal after adjustment for age. Mild and moderate TBI diagnoses were made based on guidelines established by Veterans Affairs/Department of Defense.45 All participants had a nonremarkable neuroradiographic report after their most recent TBI, meaning that the findings were not significant per the clinical judgement of the neuroradiologist. Reports were reviewed to rule out refractory subdural hematomas, evidence of tumors, anatomical anomalies, or evidence of loss of gray matter. Neuroradiographic reports and therapy discharge notes were obtained through a medical records request; magnetic resonance imaging prior to enrollment was required if a participant lacked a neuroradiographic report.
Potential participants were excluded if they had oral or other health problems that would preclude TLNS or, in the opinion of the investigators, were unable to successfully complete the stimulation intensity level setting procedure for the device. Additional inclusion and exclusion criteria are available in supplemental appendix S1. Rolling recruitment was used, and enrolled participants had a unique 3-digit identifier that was used for double blinding and 1:1 randomization by a clinical monitor.
Treatment
TLNS was delivered through the portable neuromodulation stimulator (PoNS).b As previously described,46 PoNS uses 143 electrodes on the tongue array to deliver 19-volt amplitude-controlled, pulse-width modulated, unbalanced biphasic pulses to the anterior, superior surface of the tongue; a zero net direct current minimizes the potential for tissue irritation. The 2 stimulation conditions evaluated in this study were an HFP (study-defined active arm) and an LFP (study-defined control arm); the HFP/LFP stimulation ratio is 1875:1 (supplemental table S1, available online only at http://www.archives-pmr.org). The experimental stimulus intensity used during study treatments was determined during ITP in both groups (see supplemental appendix S1).
Participants completed training sessions of TLNS treatment (TLNS plus PT) 3 times daily for 14 weeks (table 1), with program intensity tailored to each participant’s functional ability throughout the study. In stage 1 (ITP), participants completed 2 training sessions daily under physical therapist supervision and 1 session daily at home, independently. In stage 2, the training sessions were carried out at home 6 days weekly, and participants returned to the clinic once weekly for a training program update. The PoNS device logged stimulation level, time, and date to monitor protocol compliance. During stage 3, participants did not undergo TLNS treatment and returned to normal daily activity. The type, frequency, and duration of exercise were documented and tabulated.
Table 1.
Daily training∗ schedule
| Time of Day | Treatment Type | Duration |
|---|---|---|
| Morning session | Warm-up exercises without PoNS | 10 min |
| Balance training† with PoNS | 20 min | |
| Gait training‡ with PoNS | 20 min | |
| BAT§ with PoNS | 20 min | |
| Break | 3-4 h | |
| Afternoon session | Balance training with PoNS | 20 min |
| Movement control‖ exercises w/o PoNS | 20 min | |
| Gait training with PoNS | 20 min | |
| Break | ||
| Evening session | BAT§ with PoNS | 20 min |
Abbreviations: BAT, breathing awareness training; w/o, without.
Exercises were progressed in difficulty as participants demonstrated mastery.
Balance training focused on developing stable balance while standing in progressively challenging conditions during PoNS treatment. The goal of balance training was to create body awareness, correct postural alignment, and improve stability by recalibrating proprioceptive, tactile, and vestibular inputs. Each balance training session required that the clinician work with participants to determine an appropriately challenging position based on their ability, progressing them during the study as they improved.
Gait training, in which participants walked on a treadmill and over ground at progressive speeds and were challenged to re-establish appropriate dynamic balance and gait patterns during PoNS treatment.
Breathing and awareness training aimed at developing relaxed and mindful respiration and body awareness during PoNS treatment.
Movement control training aimed at helping the participant develop the proper movement patterns and synergies. Emphasis was placed on the quality of movements performed with accurate control. Exercises included lower extremity isolation, core strengthening, and/or upper extremity movement to improve arm swing.
Assessments and endpoints
Endpoints and assessment time points are summarized in table 2. The primary endpoint was the change in composite SOT score (see supplemental appendix S1) from baseline to week 14. The SOT score was also determined at the end of each stage and every 3 weeks during stages 2 and 3. Key secondary endpoints were the 6-minute walk test (6MWT) and DGI. For the primary and key secondary endpoints, outcomes in each treatment arm and at each time point were compared to help determine any differences in efficacy between the 2 dosages.
Table 2.
Endpoints and assessment timing
| Endpoint | Timing of Assessment |
|---|---|
| Primary endpoint | |
| SOT | Baseline, end of each stage, and every 3 wk during stages 2 and 3 |
| Secondary endpoint | |
| 6MWT | Baseline, end of stage 1, every 3 wk during stages 2 and 3 |
| DGI | Baseline, end of stage 1, every 3 wk during stages 2 and 3 |
| Exploratory endpoint | |
| NSI | Baseline, end of each stage |
| BSI 18 | Baseline, end of each stage |
| PSQI | Baseline, end of each stage |
| HDI | Baseline, end of each stage |
Abbreviations: BSI, Brief Symptom Inventory; HDI, Headache Disability Index; NSI, Neurobehavioral Symptom Inventory.
Other exploratory endpoints included the Neurobehavioral Symptom Inventory, Brief Symptom Inventory 18, Pittsburgh Sleep Quality Index (PSQI), and Headache Disability Index. Videonystagmography was performed at baseline and at weeks 14 and 26 to observe potential changes in oculomotor function. Adverse events (AEs) were recorded throughout the study.
Sample size
R (version 3.4.1)c was used for power analysis and sample size calculation. This study was powered for the primary outcome measure (SOT) only. Based on pilot study results of a mean improvement of 26.3 points in the SOT composite score after 2 weeks of treatment, it was assumed that the LFP group would achieve half of this improvement (13.2 points).47 Based on this assumption, a sample size of 17 participants in each group would have 80% power to detect a significant difference between the HFP and LFP groups using an independent t test with a 2-sided significance level of <.05. A previous controlled pilot study32 experienced a dropout rate of ≤25%, so the sample size for this study was increased from 34 to 44 participants, with 22 participants in each arm.47
Statistical analysis
The intent-to-treat population, the primary population for efficacy analyses, was analyzed using VassarStatsd and included all participants who were randomized to either the HFP or LFP treatment group. The per-protocol population included all randomized participants who had no significant protocol violation. All participants receiving at least 1 TLNS treatment (HFP or LFP) were included in the safety analysis.
Statistical comparisons between groups used t tests and chi-squared procedures for interval and categorical data, respectively. There was no imputation for missing data; a data analysis was performed on the existing data set as is. Changes from baseline at weeks 2, 14, and 26 (using paired t tests) and post hoc for week 5 were determined. The P value accepted for significance was .0125 (Bonferroni correction) for all secondary measures.
Multivariate repeated measures analysis of variance (ANOVA) testing was performed for the results of the SOT, DGI, and 6MWT assessments using Statistical Analysis System 2019.e For all 3 assessments, the independent variables consisted of 2 levels of treatment (HFP and LFP, combined) and 5 time points (baseline and weeks 2, 5, 14, 26). Descriptive statistics were provided for demographic variables and exploratory endpoints.
Results
Participants
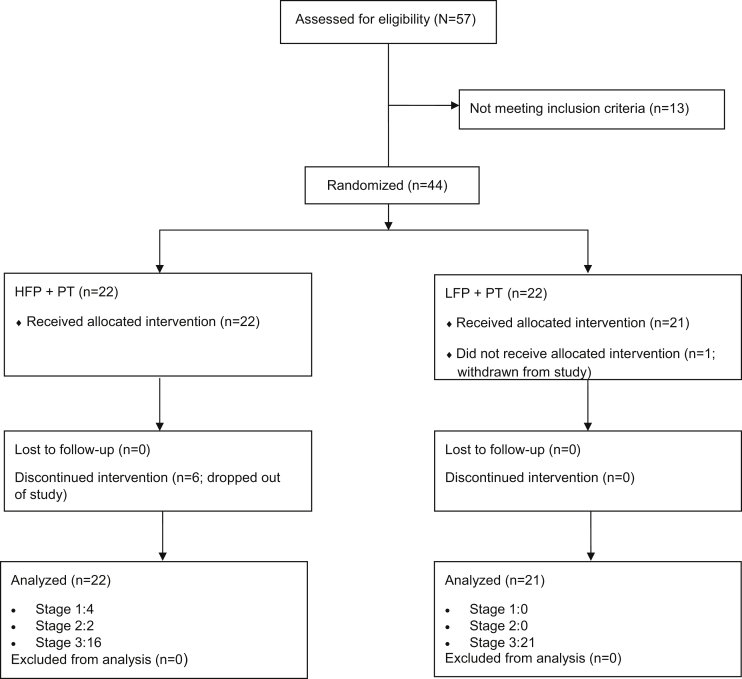
Fifty-seven candidates were screened and 44 were randomized (16 men, 28 women) to receive TLNS treatment (TLNS+PT) at an HFP or an LFP (fig 1). Forty-three participants completed at least 1 stage of the study (intent-to-treat population: 22 HFP, 21 LFP), and 39 and 37 participants completed 2 and 3 stages, respectively. Participants in the treatment arms were well balanced for all demographic and clinical variables (table 3), except for the 6MWT, which was significantly higher in the LFP group than in the HFP group (P=.031). The mean time from the qualifying injury to enrollment was 6.5 years (range, 1-33y), and the mean age was 55.0 years (standard deviation, 8.4y). After initial injury, participants underwent a mean ± SD of 5.4±7.6 months of outpatient PT for balance and other injury-related issues. Most therapies were provided in individual treatment sessions scheduled from 1 to 5 times weekly.
Fig 1.
CONSORT diagram.
Table 3.
Baseline demographic and clinical characteristics
| Variable | HFP+PT | LFP+PT | P Value |
|---|---|---|---|
| N | 22 | 21 | |
| Age | 54.05±5.91 | 53.24±10.55 | .757 |
| Sex (%) | >.99 | ||
| Female | 14±63.6 | 14±66.7 | |
| Male | 8±36.4 | 7±33.3 | |
| Race (%) | .261 | ||
| African American | 2±9.1 | 0±0.0 | |
| American Indian | 0±0.0 | 1±4.8 | |
| Interracial | 1±4.5 | 0±0.0 | |
| White | 19±86.4 | 20±95.2 | |
| Ethnicity (%) | >.99 | ||
| Non-Hispanic | 20±90.0 | 20±95.2 | |
| Unknown | 2±9.1 | 1±4.8 | |
| Education (y) | 14.86±3.26 | 15.90±2.51 | .248 |
| Age at most recent traumatic brain injury (y) | 47.73±10.20 | 46.76±11.09 | .768 |
| SOT∗ | 42.77±17.54 | 36.24±16.09 | .211 |
| 6MWT (m) | 358.10±78.55 | 407.80±66.83 | .031 |
| DGI | 17.95±5.29 | 19.29±3.72 | .173 |
| NSI | 38.59±18.23 | 36.62±15.98 | .709 |
| BSI-18 | 60.77±10.90 | 59.90±12.33 | .808 |
| PSQI | 9.32±4.87 | 9.18±4.93 | .926 |
| HDI | 40.91±27.39 | 40.76±29.32 | .987 |
NOTE. Values are mean ± SD unless otherwise indicated.
Abbreviations: BSI, Brief Symptom Inventory; HDI, Headache Disability Index; NSI, Neurobehavioral Symptom Inventory.
Imbalance is considered with a SOT score ≤69.
SOT composite score
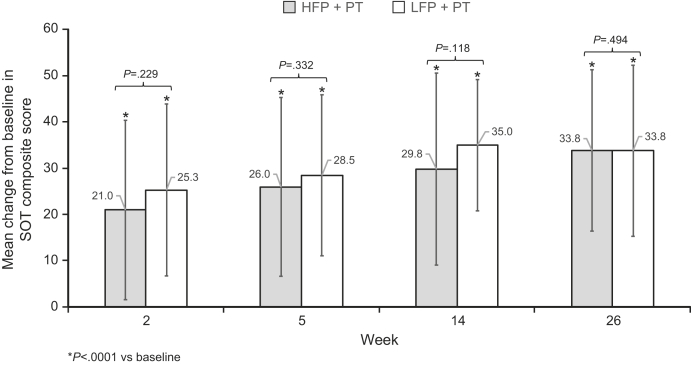
Significant improvements in the SOT composite scores from baseline to each postbaseline assessment (P<.0001) were demonstrated for both treatment groups (fig 2). No significant difference in SOT score was observed between the 2 treatment groups at baseline or at weeks 2, 5, 14, or 26.
Fig 2.
Mean changes ± SD from baseline to weeks 2, 5, 14, and 26 in SOT composite score for the HFP and LFP groups. Mean SOT composite score change from baseline to each assessment point was calculated for both the HFP (dark gray) and LFP (light gray) treatment arms. P values for comparison between the 2 arms are shown on the graph; an * denotes P<.0001 for changes from baseline at each assessment time.
DGI and 6MWT
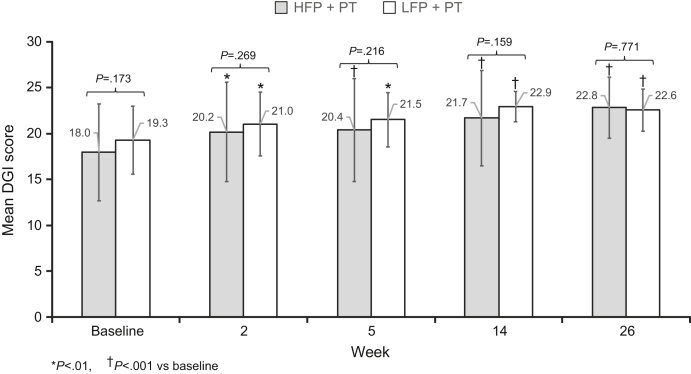
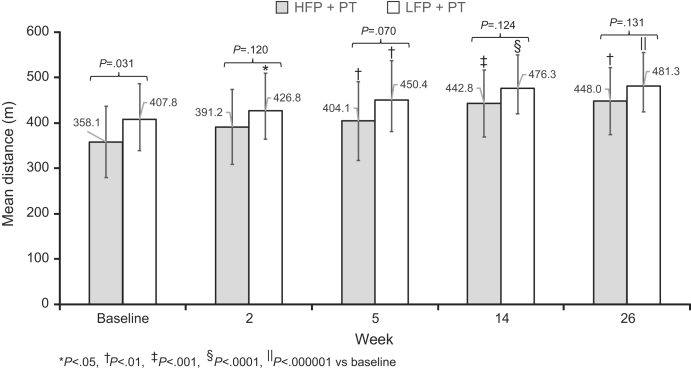
Both treatment groups showed significant improvement in DGI from baseline to weeks 2, 5, 14, and 26, and there was no significant difference between the 2 treatment groups at these times (fig 3). A significant improvement in 6MWT distance from baseline was observed for each treatment group at weeks 5, 14, and 26 and for the LFP group at week 2 (fig 4). There was no significant difference in 6MWT distance between the treatment groups at any of the assessment time points.
Fig 3.
Mean DGI score ± SD by HFP and LFP treatment arm. The mean DGI score at baseline and at each assessment time is shown for both the HFP (dark gray) and LFP (light gray) treatment arms. P values for comparison between the 2 arms are shown on the graph. *Denotes a P value <.01 and †P<.001.
Fig 4.
Mean 6MWT score ± SD for 6MWT by HFP and LFP treatment arm. The mean DGI score at baseline and at each assessment time is shown for both the HFP (dark gray) and LFP (light gray) treatment arms. P values for comparison between the 2 arms are shown on the graph. *Denotes a P value <.05, †<.01, ‡<.001, §<.0001, and ‖<.000001.
Multivariate ANOVA
ANOVA testing of scores from the SOT assessments calculated a significant improvement in SOT score from baseline to week 2 (P<.0001) and from week 2 to week 14 (P=.0178). In this analysis, the difference in SOT score was not significant between weeks 2 and 5 (P=.2368) or weeks 5 and 14 (P=.2234).
For the DGI assessment, statistically significant improvements were observed from baseline to week 2 (P<.0001) and between weeks 2 and 14 (P=.0126) and weeks 5 and 14 (P=.0479). For the 6MWT, statistically significant improvements were observed from baseline to week 2 (P<.0001), week 2 to week 14 (P=.0009), week 5 to week 14 (P=.0472).
Efficacy−exploratory endpoints
Results for the exploratory endpoints showing improvements from baseline for both the HFP and LFP groups are shown in table 4. Although these results were not assessed with inferential statistics, there appeared to be improvements from baseline for both groups.
Table 4.
Mean measure for exploratory assessments at baseline and wk 2, 14, and 26
| Measure | HFP+PT |
LFP+PT |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Wk 2 | Wk 14 | Wk 26 | Baseline | Wk 2 | Wk 14 | Wk 26 | |
| NSI∗ | 38.59 | 26.38 | 30.47 | 31.33 | 36.62 | 29.10 | 28.25 | 26.95 |
| BSI∗ | 60.77 | 56.91 | 58.74 | 55.67 | 59.90 | 57.86 | 56.55 | 57.00 |
| PSQI∗ | 9.32 | 8.68 | 8.61 | 8.29 | 9.18 | 7.81 | 8.71 | 8.24 |
| HDI∗ | 40.91 | 24.91 | 33.26 | 30.67 | 40.76 | 36.68 | 28.40 | 25.80 |
NOTE. All values are means.
Abbreviations: BSI, Brief Symptom Inventory; HDI, Headache Disability Index; NSI, Neurobehavioral Symptom Inventory.
Lower score represents improvement.
Safety
There were no deaths during the study. A total of 91 AEs were reported, 87 mild or moderate in severity. Most of AEs were considered related to musculoskeletal injuries, headaches, or illnesses that normally occur in this population; the most common AEs are summarized in table 5. The 4 severe AEs reported during this study were general disorder/other (nausea, high fever, impaired balance that required hospital admission), a gallbladder obstruction, a urinary tract obstruction, and a neoplasm. None of these severe AEs were considered related to TLNS treatment. There were 2 mild and 6 moderate AEs related to treatment: 3 were considered possibly related, 2 probably, and 3 definitely (vertigo, pain, or headache). There were 4 device-related AEs and all were considered mild.
Table 5.
Any-cause AEs with ≥1 occurrence in either treatment group
| AE | HFP+PT (n=22) | LFP+PT (n=21) |
|---|---|---|
| Any event | 54 | 37 |
| Ear pain | 2 | 0 |
| Falls | 9 | 3 |
| Cold | 3 | 4 |
| Headache | 5 | 5 |
| Muscle weakness | 3 | 3 |
| Musculoskeletal injury | 5 | 0 |
| Neck pain | 2 | 3 |
| Pain in extremity | 3 | 5 |
| Surgical and medical procedures | 2 | 0 |
| Urinary tract infection | 2 | 0 |
Discussion
Results from this double-blind, randomized, clinical trial demonstrated significant improvement in balance. Improvements in the SOT composite score from baseline to all time points evaluated for both TLNS treatment arms ([HFP or LFP]+PT) were statistically significant; however, there was not a significant difference between the HFP and LFP arms. The mean composite SOT scores for both groups reached the normal range (>69)48 by week 14 and improved by week 26 in the HFP group; the LFP group showed a small decline in score during the withdrawal phase. Improvements in the SOT score after combining the treatment groups ranged from >20 points at week 2 to >30 points at week 26. These increases greatly exceeded an 8.48-point increase in the SOT composite score considered clinically significant for individuals with concussion47 or who received vestibular rehabilitation therapy (8-13 points).26,49,50
Results for the key secondary endpoints related to gait and balance also suggest a clinically meaningful benefit of TLNS treatment. DGI scores <19 are indicative of an elevated risk of falls,46 and a change of 3 points is generally considered clinically significant.51, 52, 53 DGI scores improved to near-normal levels at weeks 14 and 26, changing 3 points from baseline. Similarly, by the end of the study, participants in both groups exhibited clinically meaningful increases in 6MWT distance, approaching normal values.54, 55, 56 Significant improvements in SOT, DGI, and 6MWT scores were all noted after multivariate repeated measures ANOVA testing. These findings support that the study was sufficiently powered to avoid a Type I error and confirms the key results of the primary analysis.
The benefit of TLNS treatment was also observed in additional assessments. In both treatment arms, Headache Disability Index scores were reduced by approximately 40%, driven primarily by lower headache severity and frequency. The Pittsburgh Sleep Quality Index also improved, primarily in the sleep-wake cycle. Both groups had baseline NSI values exceeding 24 at baseline (considered clinically elevated).57,58 After treatment, there was a 31% and a 23% decrease in mean NSI score for the HFP and LFP groups, respectively, suggesting a reduced effect of TBI symptoms on participants. Finally, BSI scores dropped from the upper limit of the normal range59 to within the normal range (<59) for both treatment groups. These trends demonstrate that TLNS treatment, targeted to improve balance and gait, has the potential to affect a multitude of mmTBI-related symptoms. Further analysis of secondary and exploratory endpoints is underway.
Importantly, the improvements achieved with TLNS treatment persisted for at least 12 weeks after the treatment was terminated. One possible explanation is that participants were less encumbered, leading to more activity, possibly influencing plasticity. Also, the intervention may have activated a currently unknown neurological mechanism that continued to function during the neurorehabilitation process. Other studies of electrical or magnetic nerve stimulation have demonstrated clinical benefits for 1 month60 to 12 months61 after the termination of neuromodulation.
In studies comparing high- and low-frequency stimulation, it is not uncommon to observe little or no difference in treatment effect. The results with HFP and LFP TLNS treatment are similar to those from transcutaneous electrical nerve stimulation studies, which have indicated significant benefits of both low- and high-frequency stimulation.36, 37, 38, 39,41,42,44 A study of vagus nerve stimulation for treatment-resistant depression also indicated that there was no significant difference between low-, medium-, and high-frequency stimulation.43 These reports prompted study design deviations (see supplemental appendix S1) to investigate outcomes from devices with 2 different stimulus levels as opposed to considering the LFP device a control. Regarding the potential for a placebo effect, high- and low- frequency stimulation does not differ in experimental animal studies,62, 63, 64 a setting in which participant expectations regarding potential treatment benefit are unlikely to influence study results.65
The underlying neurological mechanisms of the neurorehabilitation process are only now beginning to be understood. Recent animal studies help demonstrate the promising strategy of trigeminal nerve stimulation in TBI symptom management. In a rat model of stroke, infarction volume was decreased after trigeminal nerve stimulation via the forehead; changes comparable to a diving response, which can have a neuroprotective component and potential therapeutic benefit,66 were also elicited. In related animal research, direct stimulation of the trigeminal nerve induced a pressor response and improved cerebral blood flow by causing cerebrovasodilation through activation of the trigemino-cerebrovascular system and trigemino-parasympathetic reflex67; beneficial effects included increased cerebral perfusion and reduction in edema, blood–brain barrier disruption, and lesion volume.
That participants in both treatment arms responded robustly to TLNS plus PT, after plateauing on previous PT, suggests that TLNS treatment may activate a neural or glial network associated with the targeted activities, which then reaches a sensory threshold and activates a neural network. The significant increases from baseline in the SOT composite score suggest improvements in somatosensory, visual, and vestibular systems that contribute to postural control. There were also significant improvements in both the DGI and 6MWT and a positive trend in the ancillary measures of sleep, headache, neurobehavioral symptoms, and cognitive performance. The threshold of neural activation may be more important than how many pulses are generated, as LFP stimulation yielded only slightly lower assessment scores than HFP stimulation, with no significant difference between the HFP and LFP arms for any of the endpoints evaluated. These findings suggest that both dosages were above a currently unknown activation threshold for effecting functional neurorehabilitation.
Study limitations
One limitation of this study is the inherent variable presentation of TBI68; however, the indication of mmTBI helped to create a more homogeneous cohort. Differences in the nature of mmTBI, participant age, symptom number and severity, time since injury, age at time of injury, and degree of success with prior therapy could each have contributed to the variability observed with each assessment. Furthermore, even with matched representation between treatment arms, sex differences in physiologic and neurologic responses to both the initial brain injury and to physical activity could have affected the results.69 Although men experience approximately 1.4 times as many TBIs as women,70 the number of female participants in this study was 1.75 times more than males. This difference may be attributed to the recruitment method, the fact that women are more likely than men to seek medical care for symptoms, or simply participant availability. Also, the presence or absence of oculomotor deficits in the participant cohort was not controlled for in this study. Although most participants had normal or corrected vision and normal videonystagmography scores, 5 of the 44 participants exhibited significant oculomotor control abnormalities, which could contribute to postural and gait instability, difficulties with visual attention and reading, and headache severity.9,19
Although all participants had previously completed some form of balance or gait rehabilitation therapy, each had his/her own physical, cognitive, and emotional capacity for the training program. Study participation required a large commitment of time, energy, and resources (eg, material, financial, emotional support). Many of the participants were not local, creating challenges with respect to travel to the study site. Dedicating 2-3 hours to treatment 6 days weekly over 14 weeks was challenging and was the primary factor in participant attrition. Even so, 37 of the 43 participants (86%) completed the entire 26-week study. Absence of data for the participants who did not complete the study may have contributed to the variance in the results, because imputation for absent data was not employed. External factors likely had variable influence on participant level of exertion for the daily treatments and then monthly monitoring visits during the withdrawal period.
Factors that may affect the response to an intervention include the placebo effect, Hawthorne effect, and nonspecific attention and care.71,72 There may be an elevated placebo effect when using a medical device, but the evidence is inconclusive.73 For example, studies using transcutaneous electrical nerve stimulation demonstrated that it is no more effective than treatment with a placebo.74, 75, 76 The personal view of one’s own disease condition may also result in the placebo effect; by believing that TBI-related symptoms are transient or improving, study participants may increase their effort in the therapeutic intervention or may develop higher expectations based on experiences with previous treatments.71 Many, if not all, of the participants in this study agreed to join because their symptoms had stopped improving and had not fully resolved in response to previous PT. This point leaves open the possibility that participants could have had the expectation that this was a better type of PT, which could contribute to a placebo effect.
Conclusions
The results of this clinical trial demonstrate that there were statistically significant improvements from baseline for balance and gait assessments in both treatment arms. There was no significant difference in outcomes of TLNS treatment between the HFP and LFP groups. Importantly, the observed benefits produced sustained improvements for another 12 weeks after treatment discontinuation. Whether these improvements can be associated with an equal effectiveness of the 2 dosages or whether they result from the provision of PT to both groups cannot be conclusively established at this time. Future research is needed to assess the dosing parameters of TLNS, as well as additional and longer-term benefits of this treatment.
Suppliers
-
a.
NeuroCom Sensory Organization Test; Natus Medical.
-
b.
PoNS; Simplex Scientific.
-
c.
R (version 3.4.1); R Foundation for Statistical Computing.
-
d.
VassarStats; VassarStats.
-
e.
Statistical Analysis System; SAS Institute.
Footnotes
Supported by the TCNL Fund at the University of Wisconsin Foundation. Supported by the Department of Defense (contract no. W81XWH-14-C-0056). Helius did not provide financial support for the conduct of this study, only support for editorial development of the manuscript.
Disclosures: M.T., K.K., and Y.D. are inventors of the PoNS, an investigational device that is part of an ongoing FDA-approved investigational protocol for the treatment of balance deficit due to mild-to-moderate traumatic brain injury.
M.T., K.K., and Y.D. are founders and owners of Advanced Neurorehabilitation, LLC (Madison, WI), which holds the intellectual property rights to the PoNS technology; these rights are exclusively licensed to Helius Medical Technologies.
M.T., K.K., and Y.D. are founders of NeuroHabilitation Corporation, and M.T. is a board member of Helius Medical Technologies (Newtown, PA), and each owns stock in Helius. K.S. is now an employee of Helius Medical Technologies (Newtown, PA) and owns stock in Helius.
Clinical Trials Registration No.: NCT02158494.
Supplementary Data
References
- 1.Dewan M.C., Rattani A., Gupta S. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018 Apr 1 doi: 10.3171/2017.10.JNS17352. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Taylor C.A., Bell J.M., Breiding M.J., Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) CDC grand rounds: reducing severe traumatic brain injury in the United States. MMWR Morb Mortal Wkly Rep. 2013;62:549–552. [PMC free article] [PubMed] [Google Scholar]
- 4.Akin F.W., Murnane O.D., Hall C.D., Riska K.M. Vestibular consequences of mild traumatic brain injury and blast exposure: a review. Brain Inj. 2017;31:1188–1194. doi: 10.1080/02699052.2017.1288928. [DOI] [PubMed] [Google Scholar]
- 5.Li M., Zhao Z., Yu G., Zhang J. Epidemiology of traumatic brain injury over the world: a systematic review. Austin Neurol Neurosci. 2016;1:1007. [Google Scholar]
- 6.Bagalman E. Congressional Research Service; Washington, DC: 2015. Health care for veterans: traumatic brain injury.https://digitalcommons.ilr.cornell.edu/key_workplace/1401/ Available at: [Google Scholar]
- 7.Peterson M., Greenwald B.D. Balance problems after traumatic brain injury. Arch Phys Med Rehabil. 2015;96:379–380. doi: 10.1016/j.apmr.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Thurman D., Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 9.Kontos A.P., Elbin R.J., Lau B. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. Am J Sports Med. 2013;41:1497–1504. doi: 10.1177/0363546513488751. [DOI] [PubMed] [Google Scholar]
- 10.Smith C., Gentleman S.M., Leclercq P.D. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol. 2013;39:654–666. doi: 10.1111/nan.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogoda T.K., Hendricks A.M., Iverson K.M. Multisensory impairment reported by veterans with and without mild traumatic brain injury history. J Rehabil Res Dev. 2012;49:971–984. doi: 10.1682/jrrd.2011.06.0099. [DOI] [PubMed] [Google Scholar]
- 12.Hillier S.L., Sharpe M.H., Metzer J. Outcomes 5 years post-traumatic brain injury (with further reference to neurophysical impairment and disability) Brain Inj. 1997;11:661–675. doi: 10.1080/026990597123214. [DOI] [PubMed] [Google Scholar]
- 13.Chamelian L., Feinstein A. Outcome after mild to moderate traumatic brain injury: the role of dizziness. Arch Phys Med Rehabil. 2004;85:1662–1666. doi: 10.1016/j.apmr.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Thornton M., Marshall S., McComas J., Finestone H., McCormick A., Sveistrup H. Benefits of activity and virtual reality based balance exercise programmes for adults with traumatic brain injury: perceptions of participants and their caregivers. Brain Inj. 2005;19:989–1000. doi: 10.1080/02699050500109944. [DOI] [PubMed] [Google Scholar]
- 15.Benson B.W., Meeuwisse W.H., Rizos J., Kang J., Burke C.J. A prospective study of concussions among National Hockey League players during regular season games: the NHL-NHLPA Concussion Program. CMAJ. 2011;183:905–911. doi: 10.1503/cmaj.092190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr Z.Y., Zuckerman S.L., Wasserman E.B., Covassin T., Djoko A., Dompier T.P. Concussion symptoms and return to play time in youth, high school, and college American football athletes. JAMA Pediatr. 2016;170:647–653. doi: 10.1001/jamapediatrics.2016.0073. [DOI] [PubMed] [Google Scholar]
- 17.Peterson M., Greenwald B.D. in collaboration with the University of Washington Model Systems Knowledge Translation Center (MSKTC). Balance problems after traumatic brain injury. https://msktc.org/lib/docs/Factsheets/TBI_Balance_Problems_and_TBI.pdf Available at:
- 18.Alsalaheen B.A., Mucha A., Morris L.O. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34:87–93. doi: 10.1097/NPT.0b013e3181dde568. [DOI] [PubMed] [Google Scholar]
- 19.Wallace B., Lifshitz J. Traumatic brain injury and vestibulo-ocular function: current challenges and future prospects. Eye Brain. 2016;8:153–164. doi: 10.2147/EB.S82670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quatman-Yates C., Cupp A., Gunsch C., Haley T., Vaculik S., Kujawa D. Physical rehabilitation interventions for post-mTBI symptoms lasting greater than 2 weeks: systematic review. Phys Ther. 2016;96:1753–1763. doi: 10.2522/ptj.20150557. [DOI] [PubMed] [Google Scholar]
- 21.Dobkin B.H. Behavioral self-management strategies for practice and exercise should be included in neurologic rehabilitation trials and care. Curr Opin Neurol. 2016;29:693–699. doi: 10.1097/WCO.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall S., Bayley M., McCullagh S., Velikonja D., Berrigan L. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257–267. [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis M.J., Leddy J.J., Willer B. Physiological, vestibulo-ocular and cervicogenic post-concussion disorders: an evidence-based classification system with directions for treatment. Brain Inj. 2015;29:238–248. doi: 10.3109/02699052.2014.965207. [DOI] [PubMed] [Google Scholar]
- 24.Barbeau H., Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil. 2003;84:1458–1465. doi: 10.1016/s0003-9993(03)00361-7. [DOI] [PubMed] [Google Scholar]
- 25.Bland D.C., Zampieri C., Damiano D.L. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: a systematic review. Brain Inj. 2011;25:664–679. doi: 10.3109/02699052.2011.576306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badke M.B., Shea T.A., Miedaner J.A., Grove C.R. Outcomes after rehabilitation for adults with balance dysfunction. Arch Phys Med Rehabil. 2004;85:227–233. doi: 10.1016/j.apmr.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Bolognini N., Pascual-Leone A., Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemler M.A. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13–18. [Google Scholar]
- 29.Vaziri P.M. Low frequency repetitive transcranial magnetic stimulation to improve motor function and grip force of upper limbs of patients with hemiplegia. Iran Red Crescent Med J. 2014;16:e13579. doi: 10.5812/ircmj.13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S., Zaninotto A.L., Neville I.S., Paiva W.S., Nunn D., Fregni F. Clinical utility of brain stimulation modalities following traumatic brain injury: current evidence. Neuropsychiatr Dis Treat. 2015;11:1573–1586. doi: 10.2147/NDT.S65816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danilov Y., Paltin D. Translingual Neurostimulation (TLNS): a novel approach to neurorehabilitation. Phys Med Rehabil Int. 2017;4:1117. [Google Scholar]
- 32.Tyler M.E., Kaczmarek K.A., Rust K.L., Subbotin A.M., Skinner K.L., Danilov Y.P. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: a randomized double blind controlled pilot trial. J Neuroeng Rehabil. 2014;11:79. doi: 10.1186/1743-0003-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galea M.P., Cofré Lizama L.E., Bastani A., Panisset M.G., Khan F. Cranial nerve non-invasive neuromodulation improves gait and balance in stroke survivors: a pilot randomised controlled trial. Brain Stimul. 2017;10:1133–1135. doi: 10.1016/j.brs.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Chisholm A.E., Malik R.N., Blouin J.S., Borisoff J., Forwell S., Lam T. Feasibility of sensory tongue stimulation combined with task-specific therapy in people with spinal cord injury: a case study. J Neuroeng Rehabil. 2014;11:96. doi: 10.1186/1743-0003-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramlett H.M., Dietrich W.D. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015;32:1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Tommaso M., Fiore P., Camporeale A. High and low frequency transcutaneous electrical nerve stimulation inhibits nociceptive responses induced by CO2 laser stimulation in humans. Neurosci Lett. 2003;342:17–20. doi: 10.1016/s0304-3940(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 37.Desantana J.M., Sluka K.A., Lauretti G.R. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: a randomized controlled trial. Clin J Pain. 2009;25:12–19. doi: 10.1097/AJP.0b013e31817d1070. [DOI] [PubMed] [Google Scholar]
- 38.Silberstein S.D., Mechtler L.L., Kudrow D.B., ACT1 Study Group Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56:1317–1332. doi: 10.1111/head.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeGiorgio C.M., Soss J., Cook I.A. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology. 2013;80:786–791. doi: 10.1212/WNL.0b013e318285c11a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tassorelli C., Grazzi L., de Tommaso M., for the PRESTO Study Group Noninvasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology. 2018;91:e364–e373. doi: 10.1212/WNL.0000000000005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamza M.A., White P.F., Ahmed H.E., Ghoname E.A. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology. 1999;91:1232–1238. doi: 10.1097/00000542-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Hansson P., Ekblom A. Transcutaneous electrical nerve stimulation (TENS) as compared to placebo TENS for the relief of acute oro-facial pain. Pain. 1983;15:157–165. doi: 10.1016/0304-3959(83)90015-5. [DOI] [PubMed] [Google Scholar]
- 43.Aaronson S.T., Carpenter L.L., Conway C.R. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6:631–640. doi: 10.1016/j.brs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Goadsby P.J., de Coo I.F., Silver N. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38:959–969. doi: 10.1177/0333102417744362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veterans Affairs/Department of Defense (VA/DoD) VA/DoD clinical practice guideline management of concussion/mild traumatic brain injury. Washington (DC): VA/DoD. 2009 https://www.healthquality.va.gov/ Available at: [Google Scholar]
- 46.Kaczmarek K.A. The portable neuromodulation stimulator (PoNS) for neurorehabilitation. Sci Iran Transac D. 2017;24:3171–3180. [Google Scholar]
- 47.Tyler ME, Braun JG, Danilov YP. Spatial mapping of electrotactile sensation threshold and intensity range on the human tongue: initial results. Paper presented at: 31st Annual IEEE International Conference on Engineering in Medicine and Biology Society. September 2-6, 2009; Minneapolis, MN. [DOI] [PubMed]
- 48.Gera G., Freeman D.L., Blackinton M.T., Horak F.B., King L. Identification of balance deficits in people with Parkinson disease; is the Sensory Organization Test enough? Int J Phys Med Rehabil. 2016;4:1. doi: 10.4172/2329-9096.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broglio S.P., Ferrara M.S., Sopiarz K., Kelly M.S. Reliable change of the sensory organization test. Clin J Sport Med. 2008;18:148–154. doi: 10.1097/JSM.0b013e318164f42a. [DOI] [PubMed] [Google Scholar]
- 50.Brown K.E., Whitney S.L., Wrisley D.M., Furman J.M. Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope. 2001;111:1812–1817. doi: 10.1097/00005537-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 51.Shumway-Cook A., Baldwin M., Polissar N.L., Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77:812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 52.Romero S., Bishop M.D., Velozo C.A., Light K. Minimum detectable change of the Berg Balance Scale and Dynamic Gait Index in older persons at risk for falling. J Geriatr Phys Ther. 2011;34:131–137. doi: 10.1519/JPT.0b013e3182048006. [DOI] [PubMed] [Google Scholar]
- 53.Whitney S.L., Marchetti G.F., Schade A., Wrisley D.M. The sensitivity and specificity of the timed “Up & Go” and the Dynamic Gait Index for self-reported falls in persons with vestibular disorders. J Vestib Res. 2004;14:397–409. [PubMed] [Google Scholar]
- 54.Mossberg K.A. Reliability of a timed walk test in persons with acquired brain injury. Am J Phys Med Rehabil. 2003;82:385–390. doi: 10.1097/01.PHM.0000052589.96202.BE. [DOI] [PubMed] [Google Scholar]
- 55.Merritta C., Cherian B., Macaden A.S., John J.A. Measurement of physical performance and objective fatigability in people with mild-to-moderate traumatic brain injury. Int J Rehabil Res. 2010;33:109–114. doi: 10.1097/MRR.0b013e32832e6b37. [DOI] [PubMed] [Google Scholar]
- 56.Peters D.M., Jain S., Liuzzo D.M. Individuals with chronic traumatic brain injury improve walking speed and mobility with intensive mobility training. Arch Phys Med Rehabil. 2014;95:1454–1460. doi: 10.1016/j.apmr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soble J.R., Silva M.A., Vanderploeg R.D. Normative data for the Neurobehavioral Symptom Inventory (NSI) and post-concussion symptom profiles among TBI, PTSD, and nonclinical samples. Clin Neuropsychol. 2014;28:614–632. doi: 10.1080/13854046.2014.894576. [DOI] [PubMed] [Google Scholar]
- 58.Lange R.T., Brickell T.A., Lippa S.M., French L.M. Clinical utility of the Neurobehavioral Symptom Inventory validity scales to screen for symptom exaggeration following traumatic brain injury. J Clin Exp Neuropsychol. 2015;37:853–862. doi: 10.1080/13803395.2015.1064864. [DOI] [PubMed] [Google Scholar]
- 59.Derogatis L.R. NCS Pearson; Minneapolis, MN: 2000. BSI-18: Brief Symptom Inventory 18 - administration, scoring, and procedures manual. [Google Scholar]
- 60.Khedr E.M., Elbeh K.A., Abdel Baky A., Abo-Elfetoh N., El-Hammady D.H., Korashy F. A double-blind randomized clinical trial on the efficacy of magnetic sacral root stimulation for the treatment of monosymptomatic nocturnal enuresis. Restor Neurol Neurosci. 2015;33:435–445. doi: 10.3233/RNN-150507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodson B.T., Gillespie M.B., Soose R.J. STAR Trial Investigators. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151:880–887. doi: 10.1177/0194599814544445. [DOI] [PubMed] [Google Scholar]
- 62.King E.W., Sluka K.A. The effect of varying frequency and intensity of transcutaneous electrical nerve stimulation on secondary mechanical hyperalgesia in an animal model of inflammation. J Pain. 2001;2:128–133. doi: 10.1054/jpai.2001.19963. [DOI] [PubMed] [Google Scholar]
- 63.Ainsworth L., Budelier K., Clinesmith M. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120:182–187. doi: 10.1016/j.pain.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 64.Vance C.G., Radhakrishnan R., Skyba D.A., Sluka K.A. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys Ther. 2007;87:44–51. doi: 10.2522/ptj.20060032. [DOI] [PubMed] [Google Scholar]
- 65.Benedetti F., Carlino E., Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology. 2011;36:339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiflett J.M., Parent A.D., Britz G.W., Golanov E.V. Forehead stimulation decreases volume of the infarction triggered by permanent occlusion of middle cerebral artery in rats. J Neurol Stroke. 2015;2 [Google Scholar]
- 67.Chiluwal A., Narayan R.K., Chaung W. Neuroprotective effects of trigeminal nerve stimulation in severe traumatic brain injury. Sci Rep. 2017;7:6792. doi: 10.1038/s41598-017-07219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Si B., Dumkrieger G., Wu T. Sub-classifying patients with mild traumatic brain injury: a clustering approach based on baseline clinical characteristics and 90-day and 180-day outcomes. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mollayeva T., El-Khechen-Richandi G., Colantonio A. Sex and gender considerations in concussion research. Concussion. 2018;3:CNC51. doi: 10.2217/cnc-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faul M., Xu L., Wald M.M., Coronado V.G. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and death 2002-2006.https://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf Available at: [Google Scholar]
- 71.Oken B.S. Placebo effects: clinical aspects and neurobiology. Brain. 2008;131:2812–2823. doi: 10.1093/brain/awn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polich G., Iaccarino M.A., Kaptchuk T.J., Morales-Quezada L., Zafonte R. Placebo effects in traumatic brain injury. J Neurotrauma. 2018;35:1205–1212. doi: 10.1089/neu.2017.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaptchuk T.J., Goldman P., Stone D.A., Stason W.B. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–792. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 74.Langley G.B., Sheppeard H., Johnson M., Wigley R.D. The analgesic effects of transcutaneous electrical nerve stimulation and placebo in chronic pain patients. A double-blind non-crossover comparison. Rheumatol Int. 1984;4:119–123. doi: 10.1007/BF00541180. [DOI] [PubMed] [Google Scholar]
- 75.Deyo R.A., Walsh N.E., Martin D.C., Schoenfeld L.S., Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. 1990;322:1627–1634. doi: 10.1056/NEJM199006073222303. [DOI] [PubMed] [Google Scholar]
- 76.Hughes S.C., Dailey P.A., Patridge C. Transcutaneous electrical nerve stimulation for labor analgesia. Anesth Anal. 1998;67:S1–S266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.