Abstract
Purpose
This study investigated the clinical significance of postoperative neutrophil-to-lymphocyte ratio (NLR) changes in bladder cancer recurrence.
Patients and Methods
For evaluating the predictive value of postoperative dynamic change of NLR, a retrospective cohort study was performed to analyze 213 patients with bladder cancer who underwent surgical treatment from January 2013 to December 2019 at the Affiliated Tumor Hospital of Guangxi Medical University. Baseline characteristics and recurrence-free survival (RFS) were statistically compared, and a multivariate analysis was used to identify prognostic factors.
Results
Compared with preoperative NLR levels, postoperative decreased NLR in 130 patients and postoperative increased NLR in 83 patients were detected. The 1-, 3- and 5-year RFS rates were 88.0%, 75.4% and 75.4% in the decreased postoperative NLR group, respectively, and 51.2%, 25.8% and 16.1% in the increased postoperative NLR group, respectively (P < 0.05). Kaplan–Meier curves showed that the cumulative DFS rate in the increased group was significantly lower than that in the decreased group (P < 0.05). The preoperative NLR showed significant difference with postoperative NLR in the total cohort, high-grade non-muscle-invasive bladder cancer (HG-NMIBC) and muscle-invasive bladder cancer (MIBC) group, while there was no significant difference between postoperative NLR and NLR of recurrence or last follow-up. Multivariate analysis suggested that postoperative-preoperative NLR was an independent predictor for RFS (HR=6.206, 95% CI: 3.826–10.067, P < 0.001) in the total cohort, RFS (HR=9.373, 95% CI: 2.724–32.245, P < 0.001) in the LG-NMIBC group, RFS rates (HR=6.873, 95% CI: 2.486–18.999, P < 0.001) in the HG-NMIBC group and RFS rates (HR=6.109, 95% CI: 2.847–13.109, P < 0.001) in the MIBC group.
Conclusion
The dynamic change of postoperative NLR is a potential marker for the early detection of bladder cancer recurrence. Patients with increased NLR after surgery tend to have higher risk of recurrence.
Keywords: bladder cancer, NLR dynamic change, prognosis, recurrence
Introduction
Bladder cancer is a common malignant tumor in the urinary system.1 While 75% of newly diagnosed bladder tumors are non-muscle-invasive bladder cancers (NMIBC), muscle-invasive bladder cancers (MIBC) are about 25%. For NMIBC, the postoperative recurrence rate was over 70%, and for MIBC, after invasive radical cystectomy (RC) procedure, the rate of postoperative recurrence was still more than 50% and patients lost their lives because of this disease.2,3 Although there is a good deal of research on, and many developments in, new molecular markers for the early diagnosis and postoperative follow-up for bladder tumor,4,5 there is currently no ideal detection method that can replace cystoscopy, due to factors such as sensitivity and specificity. For patients with total bladder resection, postoperative follow-up can only rely on imaging examinations,6,7 and early detection of some small lesions is difficult. Therefore, there is an urgent need for a relatively objective and non-invasive high-sensitivity biomarker for postoperative follow-up monitoring of bladder tumor recurrence.
As a new integrated inflammatory marker, an elevated pretreatment neutrophil-to-lymphocyte ratio (NLR) value has been shown to be associated with advanced stages and a poor prognosis in many human tumors.8–11 This has also been shown in bladder tumor studies, whether NMIBC or MIBC, with high NLR associated with poor prognosis.12–14 However, the specific values of preoperative NLR in the predictive evaluation cannot be unified. Furthermore, the lack of standard reference values makes it less reliable as a valid prognostic factor. We believe that an accurate cut-off value of preoperative NLR that satisfies all the medical centers does not exist. Rather than formulating a rigid value, it is better to use preoperative NLR as a control, and it is more reliable to observe the dynamic change process, because the dynamic changes of NLR after surgery can directly reflect the effects of surgical treatment and the changes in the host’s systemic inflammatory response (SIR) and immune status after surgery. Because most current studies focus only on the pretreatment values of inflammatory markers, the clinical significance of the dynamic change of postoperative NLR in bladder cancer is largely unclear.15 Thus, our study was primarily designed to evaluate the clinical significance of postoperative NLR change in patients with bladder cancer surgery, whether it is a bladder-preserving operation or a total bladder resection operation. We also study the predictive value of NLR dynamic change in predicting postoperative recurrence of bladder cancer.
Methods
Patients and Data Collection
This is a retrospective study of 322 cases of bladder cancer without evidence of distant metastasis undergoing TURBT or RC from January 2013 to December 2019 at the Department of Urology, Affiliated Tumor Hospital of Guangxi Medical University. For this study, inclusion criteria were: 1) primary urothelial carcinoma of bladder without evidence of distant metastasis; 2) complete medical records and follow-up data; 3) no clinical symptom or signs of sepsis; and 4) none received radiotherapy, systemic chemotherapy or immunotherapy before surgery. Exclusion criteria included: 1) known active severe infection at the time of blood sampling; 2) patients with poor data integrity; 3) patients with coexistent hematological or autoimmune diseases; 4) tumors of non-urinary epithelial origin or other uncontrolled malignancies; and 5) severe complications during perioperative period. Overall, 72 cases were excluded due to lack of complete follow-up data, and another 37 cases were excluded because of severe preoperative infection or other uncontrolled malignancies. Of the patients included in the study, five cases have a history of other malignancies, but they are stable, and no recurrence is apparent upon review. In all, 213 cases were analyzed in this study, including 137 NMIBC cases that underwent TURBT and 76 MIBC cases that underwent RC.
The following data were collected: 1) patient’s age, gender, smoking history, hypertension history, diabetes history; 2) pretreatment laboratory parameters were taken within 1 week before operation: absolute number of neutrophils, lymphocytes, platelets, hemoglobin; and 3) tumor size, number, pathological stage and grade (pathological information was confirmed by the pathology department of our hospital).
Follow-Up
At our institution, follow-up is performed according to the requirements of the National Comprehensive Cancer Network Guidelines.6 Postoperative follow-up performed every 3 months for the first 2 years, every 6 months for the patients three to 5 years after surgery, and annually thereafter. Oncological evaluation includes medical history, physical examination, blood routine, imaging of the chest, abdominal ultrasound or computerized tomography (CT), and cystoscopy in patients with bladder preservation. Due to the retrospective study, postoperative follow-up was not standardized. Since the absolute number of neutrophils in the early postoperative period is affected by the surgery, the first follow-up data start from 1 month after the surgery. The last follow-up data were defined as the data at the time of recurrence or the last follow-up without recurrence. We calculated NLR from the differential count by dividing the absolute neutrophil count by the absolute lymphocyte count. In addition, the NLR change is the ratio of the first postoperative follow-up NLR value to the preoperative NLR value. If the result is ≥1, then postoperative NLR change was defined as increased; otherwise, it was defined as decreased. The endpoint of the study is to obtain recurrence-free survival (RFS), which refers to no recurrence happening from the date of surgery to the date of disease recurrence or to the date of last follow-up. This study was approved by the Medical Ethics Committee of Guangxi Medical University Affiliated Tumor Hospital.
Statistical Analysis
We divided patients into three groups according to pathologic stage and grade: LG-NMIBC (Ta and T1 with low grade), HG-NMIBC (Ta and T1 with high-grade/G3), and MIBC.16,17 The median follow-ups are presented as the median and interquartile range (IQR). We used SPSS software version 22.0 to perform statistical analysis. Categorical variables were compared using the Χ2 test or Fisher’s exact test when appropriate, and continuous variables were compared using the Independent Samples t-test. The RFCs were calculated using the Kaplan–Meier method. The survival curves were constructed by the Kaplan–Meier method and compared by the Log rank test. The prognostic varieties in predicting RFS were assessed by multivariate Cox proportional hazards regression analysis. All statistical tests were two-sided, and a significant difference was considered when P < 0.05.
Results
Baseline Characteristics
In the present research, 213 patients with complete medical record and follow-up information were enrolled, including 172 males (80.8%) and 41 females (19.2%), with median age of 62 (range 25–85) years. The baseline characteristics of patients are shown in Table 1. In this cohort, 62 patients, or about 29.1% of the total, had a smoking history; 48 patients (22.5%) had hypertension, and 26 (12.2%) patients had diabetes. According to the pathologic stage, 137 patients were diagnosed as non-muscle-invasive bladder cancer (NMIBC), including 74 patients with LG-NMIBC and 63 patients with HG-NMIBC, and 76 patients were confirmed as muscle-invasive bladder cancer (MIBC), including 51 patients with T2 stage, 17 patients with T3 stage and eight patients with T4 stage. There were 97 patients with multifocal bladder cancer, and HG-NMIBC and MIBC patients had a higher percentage of multifocality than that of LG-NMIBC patients. Also, 94 patients had an average tumor size ≥3 cm, and a higher ratio of MIBC patients had large tumors. Of the 213 patients, 87 had recurrence and 1 patient died. The median preoperative NLR, median postoperative NLR and median NLR of recurrence (or last follow-up) were 2.3 (range 0.7–8.6), 2.0 (range 0.5–27.4), and 2.0 (range 0.49–27.4), respectively, as shown in Table 2. As shown in Figure 1A, the 3- and 5-year RFS rates were 50.6% and 45.75%, respectively, for all 213 patients.
Table 1.
Clinicopathological Characteristics of 213 Patients
| Clinical Factors | All Patients | LG-NMIBC | HG-NMIBC | MIBC |
|---|---|---|---|---|
| Total, n (%) | 213 (100) | 74 (34.7) | 63 (29.6) | 76 (35.7) |
| Age (years); median (range) | 62 (25–85) | 62 (25–82) | 64 (26–85) | 60 (34–85) |
| Gender, n (%) | ||||
| Female | 41 (19.2) | 18 (24.3) | 13 (20.6) | 9 (11.8) |
| Male | 172 (80.8) | 56 (75.7) | 50 (79.4) | 67 (87.2) |
| Hypertension, n (%) | ||||
| None | 165 (77.5) | 54 (73) | 51 (81) | 60 (78.9) |
| Yes | 48 (22.5) | 20 (27) | 12 (19) | 16 (21.1) |
| Smoking history, n (%) | ||||
| None | 151 (70.9) | 56 (75.7) | 39 (61.9) | 57 (75) |
| Yes | 62 (29.1) | 18 (24.3) | 24 (38.1) | 19 (25) |
| Diabetes, n (%) | ||||
| None | 187 (87.8) | 63 (84.9) | 56 (88.9) | 68 (89.5) |
| Yes | 26 (12.2) | 11 (15.1) | 7 (11.1) | 8 (10.5) |
| Pathologic stage, n (%) | ||||
| Ta | 50 (23.5) | 41 (55.4) | 9 (14.3) | - |
| T1 | 87 (40.8) | 33 (44.6) | 54 (85.7) | - |
| T2 | 51 (23.9) | - | - | 51 (67.1) |
| T3 | 17 (8) | - | - | 17 (22.4) |
| T4 | 8 (3.8) | - | - | 8 (10.5) |
| Pathologic grade, n (%) | ||||
| Low (G1,G2) | 84 (38.9) | 74 (100) | - | 10 (13.2) |
| High (G3) | 129 (61.1) | - | 63 (100) | 66 (86.8) |
| Multifocality, n (%) | ||||
| Single | 116 (54.5) | 51 (68.9) | 29 (46) | 36 (47.4) |
| Multiple | 97 (45.5) | 23 (31.1) | 34 (54) | 40 (52.6) |
| Tumor size, n (%) | ||||
| <3cm | 119 (55.9) | 63 (85.1) | 41 (65.1) | 15 (19.7) |
| ≥3cm | 94 (44.1) | 11 (14.9) | 22 (34.9) | 61 (80.3) |
Table 2.
Inflammation Factors Characteristics of 213 Patients
| Group | Inflammation Factors | Preoperation Median (Range) | Postoperation Median (Range) | P value | Time of Recurrence (or Last Follow-Up) Median (Range) |
|---|---|---|---|---|---|
| All patients | Neutrophils (×109/L) | 4.0 (1.4–10.3) | 3.67 (1.15–44) | 0.729 | 3.8 (1.15–44) |
| Platelets (×109/L) | 254 (77–596) | 255 (51–796) | 0.115 | 247 (51–796) | |
| Lymphocytes (×109/L) | 1.7 (0.6–5.3) | 1.79 (0.58–4.04) | 0.887 | 1.8 (0.58–5.17) | |
| Hemoglobin (g/L) | 130 (43–289) | - | - | - | |
| NLR | 2.3 (0.7–8.6) | 2.0 (0.5–27.4) | 0.556 | 2.0 (0.5–27.4) | |
| PLR | 140.3 (51.5–515) | 135.5 (32.3–705.2) | 0.101 | 131.0 (32.3–705.2) | |
| LG-NMIBC | Neutrophils (×109/L) | 3.95 (1.43–10.08) | 3.46 (1.8–7.11) | 0.019 | 3.5 (1.81–8.23) |
| Platelets (×109/L) | 252.5 (77–454) | 240.5 (51–450) | 0.817 | 238 (51–450) | |
| Lymphocytes (×109/L) | 1.91 (0.67–5.31) | 2.0 (0.63–4.04) | 0.49 | 2.0 (0.86–5.17) | |
| Hemoglobin (g/L) | 134 (54–172) | - | - | - | |
| NLR | 2.00 (0.87–8.56) | 1.75 (0.71–6.08) | 0.025 | 1.67 (0.71–6.08) | |
| PLR | 125.3 (52.4–417.9) | 112.6 (32.3–523.3) | 0.546 | 114.8 (32.3–523.3) | |
| HG-NMIBC | Neutrophils (×109/L) | 3.61 (1.36–9.33) | 3.39 (1.87–11.08) | 0.620 | 3.49 (1.59–11.08) |
| Platelets (×109/L) | 244 (104–423) | 245.5 (120–415) | 0.785 | 238 (107–529) | |
| Lymphocytes (×109/L) | 1.7 (0.66–3.33) | 1.79 (0.6–2.96) | 0.819 | 1.82 (0.6–3.12) | |
| Hemoglobin (g/L) | 131 (43–289) | - | - | - | |
| NLR | 2.10 (0.71–7.46) | 1.99 (0.83–11.91) | 0.652 | 2.00 (0.83–11.91) | |
| PLR | 143.4 (51.5–411.4) | 142.9 (53.2–539.0) | 0.357 | 135.8 (44.6–539.0) | |
| MIBC | Neutrophils (×109/L) | 4.77 (1.79–10.3) | 4.39 (1.15–44) | 0.560 | 4.42 (1.15–44) |
| Platelets (×109/L) | 265.5 (108–596) | 291.5 (99–796) | 0.039 | 280 (99–796) | |
| Lymphocytes (×109/L) | 1.66 (0.6–3.16) | 1.64 (0.58–3.51) | 0.685 | 1.60 (0.58–3.51) | |
| Hemoglobin (g/L) | 122 (47–166) | - | - | - | |
| NLR | 2.95 (0.79–7.13) | 2.74 (0.5–27.4) | 0.202 | 3.14 (0.5–27.4) | |
| PLR | 147.2 (59.8–515) | 169.9 (72.5–705.2) | 0.045 | 167.6 (50.8–705.2) |
Figure 1.
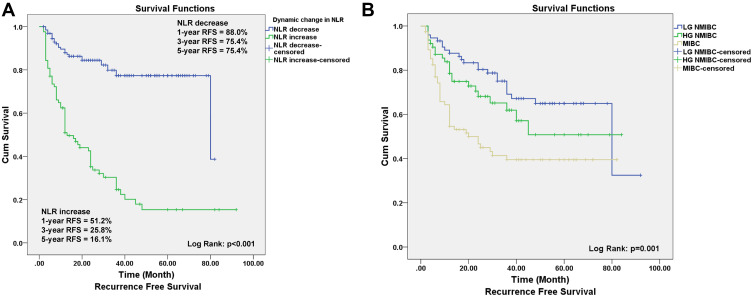
Survival percentages of patients with different NLR change. (A) The graph shows the RFS curves of patients with increased and decreased NLR. The difference between two groups were statistically significant (Log rank test, P < 0.001). (B) The RFS curves of patients with LG-NMIBC, HG-NMIBC, and MIBC (Log rank test, P < 0.001).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; LG-NMIBC, low-grade non-muscle-invasive bladder cancers; HG-NMIBC, high-grade non-muscle-invasive bladder cancers; MIBC, muscle-invasive bladder cancers.
Impact of Postoperative NLR Changes on RFS
The median follow-up period for entire cohort was 24 months (IQR 11–47). After 24 months of follow-up, 87 patients suffered from tumor recurrence. Compared with preoperative NLR levels, postoperative NLR decreased in 130 patients and increased in 83 patients after surgery. The 1-, 3- and 5-year RFS rates were 88.0%, 75.4%, and 75.4%, respectively, for the decreased postoperative NLR group, and 51.2%, 25.8%, and 16.1%, respectively, for the increased postoperative NLR group, respectively (P < 0.05). Kaplan–Meier curves showed that the cumulative RFS rate in the postoperative increased group was significantly lower than that in the decreased group (Log rank test, P < 0.001, Figure 1A). Furthermore, compared with LG-NMIBC, HG-NMIBC had a relatively poor RFS (Figure 1B).
Clinical Significance of Postoperative NLR Dynamic Changes
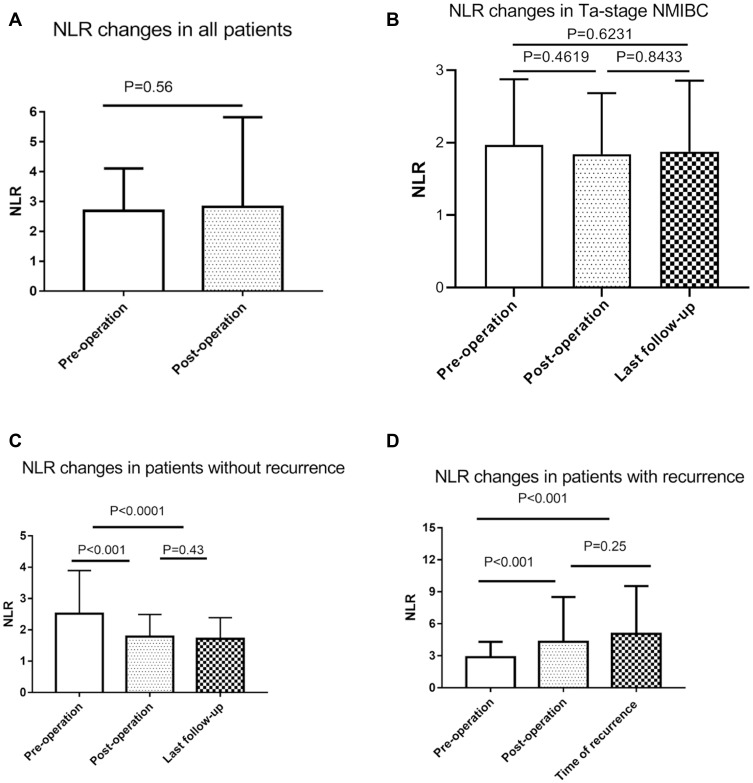
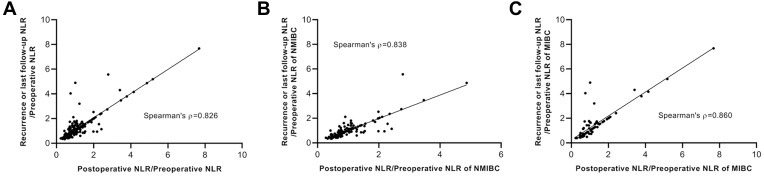
In all cases and Ta-stage NMIBC patients, there is no statistical significance in preoperative and postoperative NLR changes (Table 2, Figure 2A and B). Compared with the preoperative NLR, the postoperative NLR was decreased in 130 patients (61%) and increased in 83 patients (39%). When we analyzed the recurrence rate in the groups with increased and decreased postoperative NLR, we noticed that the recurrence rate in the increased Post-/Pre-NLR group was 73.5%, which was much higher than the 20% recurrence rate in the decreased NLR group (Table 3). However, MIBC patients had a higher recurrence rate (55.3%) in our cohort, compared with LG-NMIBC and HG-NMIBC groups. Next, the ratio of NLR of recurrence or last follow-up to preoperative NLR was measured and 124 patients had decreased NLR. Interestingly, a recurrence rate of 82% was seen in increased Recur- or final-/Pre-NLR patients, compared with 11.3% in the decreased group. Furthermore, while the preoperative NLR showed significant differences in both postoperative NLR and NLR of recurrence or last follow-up, there was no significant difference between postoperative NLR and NLR of recurrence or last follow-up (Table 4, Figure 2C and D). Also, the ratio of postoperative NLR to preoperative NLR showed a high correlation with the ratio of NLR of recurrence or last follow-up to preoperative NLR in total cohort, NMIBC and MIBC groups (Figure 3). These data indicated that postoperative NLR of a short time after surgery could be a prognostic factor for the recurrence of bladder cancer.
Figure 2.
NLR changes in patients with/without bladder cancer recurrence. (A) Preoperative NLR and changes in NLR after surgery for all 213 patients. (B) NLR in Ta-stage NMIBC patients at preoperation, postoperation, and last follow-up. (C) In nonrecurrence cases (N=126). The preoperative NLR decreased postoperatively and remained stable after surgery. (D) In recurrence cases (N=87), the NLR increased postoperatively and continued to the diagnosis of recurrence.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; NMIBC, non-muscle-invasive bladder cancers.
Table 3.
Recurrence of Bladder Cancer and NLR Change
| Status | Total No. | Recurrence No. | Percentage of Recurrence | |
|---|---|---|---|---|
| NLR change | (Post-/pre-) Decreased | 130 | 26 | 20 |
| (Recur- or final follow-up/pre-) Decreased | 124 | 14 | 11.3 | |
| (Post-/pre-) Increased | 83 | 61 | 73.5 | |
| (Recur- or final follow-up/pre-) Increased | 89 | 73 | 82 | |
| Category | LG-NMIBC | 74 | 23 | 31.1 |
| HG-NMIBC | 63 | 22 | 34.9 | |
| MIBC | 76 | 42 | 55.3 | |
Table 4.
Comparison of NLR Characteristics in Recurrence and Non-Recurrence Patients
| Inflammation Factors | Recurrence (n=87) | Non-Recurrence (n=126) | ||
|---|---|---|---|---|
| Median (Range) | P value | Median (Range) | P value | |
| Preoperative NLR | 2.6 (1.0–7.1) | 2.0 (0.7–8.6) | ||
| Postoperative NLR | 3.1 (0.8–27.4) | 0.0034 | 1.6 (0.5–4.9) | < 0.001 |
| NLR (Time of recurrence) |
3.8 (1.0–27.4) | < 0.001 | - | - |
| NLR (last follow-up) | - | - | 1.6 (0.5–3.8) | < 0.0001 |
Figure 3.
Correlation analysis of the ratio of postoperative NLR to preoperative NLR and the ratio of NLR of recurrence or last follow-up to preoperative NLR. (A) Entire cohort. (B) NMIBC patients. (C) MIBC patients.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; NMIBC, non-muscle-invasive bladder cancers; MIBC, muscle-invasive bladder cancers.
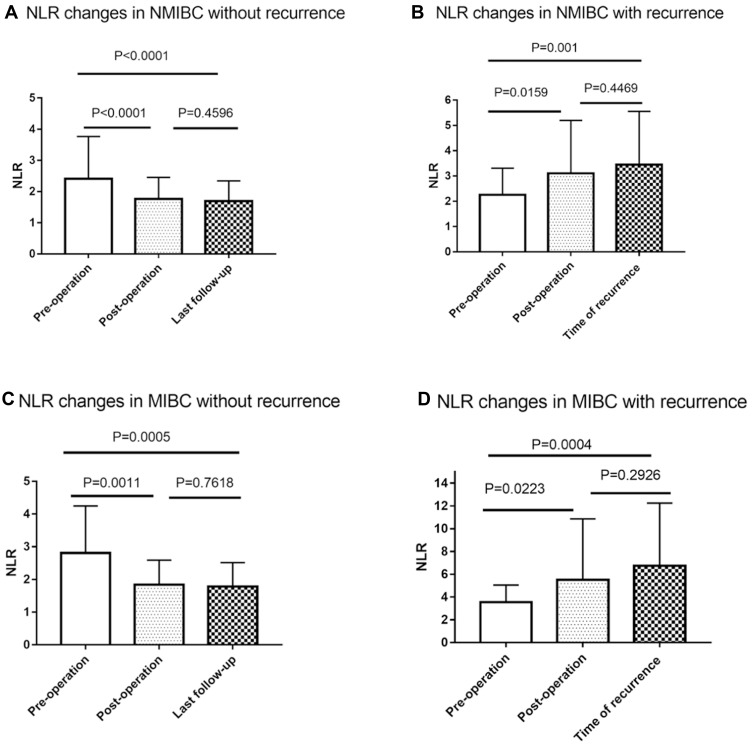
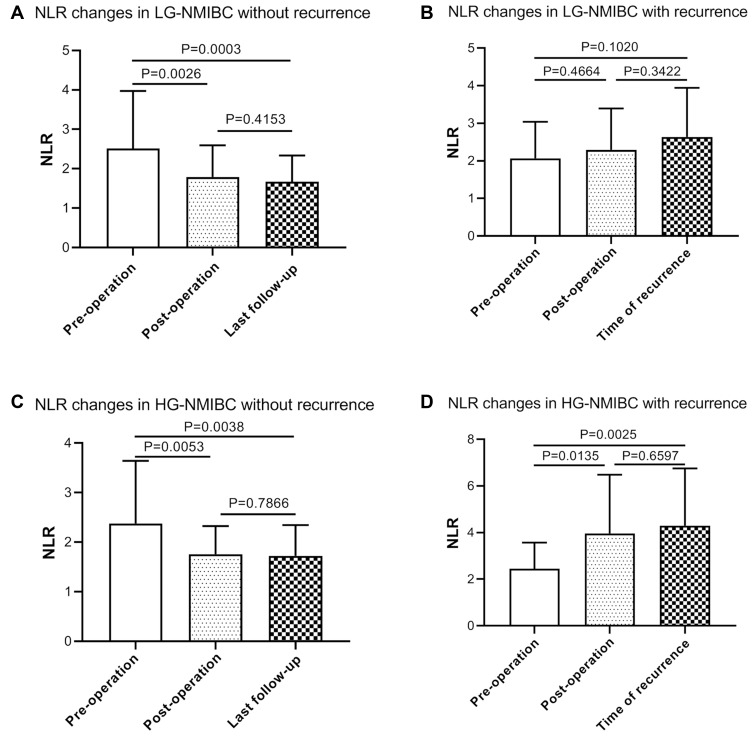
After further stratification of the total number of patients, it is found that both in NMIBC and MIBC, the preoperative NLR decreased postoperatively and remained stable after surgery in patients without recurrence, but the preoperative NLR increased postoperatively, and this continued to the diagnosis in patients with recurrence (Table 5, Figure 4). HG-NMIBC (Ta and T1 with high-grade/G3) was considered to have a strong correlation with recurrence.18 After studying our data, we found that the NLR change in HG-NMIBC patients could significantly predict the recurrence status (Figure 5, Table 5). However, in LG-NMIBC patients with recurrence, there was no significant change in postoperative NLR. These data suggested that for NMIBC patients, the high-grade category could achieve a more accurate predictive result by NLR change for recurrence status.
Table 5.
NLR Changes in NMIBC and MIBC Patients with or without Recurrence
| Status | Preoperation Median (Range) | Postoperation Median (Range) | P value | Time of Recurrence (or Last Follow-Up) | |
|---|---|---|---|---|---|
| LG-NMIBC | Recurrence | ||||
| Yes | 1.8 (1.0–4.4) | 2.0 (0.8–6.1) | 0.4664 | 2.4 (1.0–6.1) | |
| No | 2.0 (0.9–8.6) | 1.6 (0.7–4.9) | 0.0026 | 1.5 (0.7–3.8) | |
| HG-NMIBC | Recurrence | ||||
| Yes | 2.2 (1.0–6.0) | 3.2 (1.3–11.9) | 0.0135 | 4.1 (1.5–11.9) | |
| No | 2.1 (0.7–7.5) | 1.6 (0.8–3.6) | 0.0053 | 1.6 (0.8–3.6) | |
| MIBC | Recurrence | ||||
| Yes | 2.9 (0.8–7.1) | 1.7 (0.5–3.6) | 0.0005 | 1.6 (0.5–3.6) | |
| No | 3.2 (1.0–7.1) | 3.5 (1.8–27.4) | 0.0004 | 5.1 (2.0–27.4) | |
Figure 4.
NLR changes in NMIBC and MIBC patients with or without recurrence. (A) NLR changes in NMIBC without recurrence (N=92). NLR decreased postoperatively and remained stable after surgery. (B) NLR changes in NMIBC with recurrence (N=45). NLR increased postoperatively and continued to the diagnosis of recurrence. (C) NLR changes in MIBC without recurrence (N=34). NLR decreased postoperatively and remained stable after surgery. (D) NLR changes in MIBC with recurrence (N=42). NLR increased postoperatively and continued to the diagnosis of recurrence. (P values were calculated using the Log rank test).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; NMIBC, non-muscle-invasive bladder cancers; MIBC, muscle-invasive bladder cancers.
Figure 5.
NLR changes in LG-NMIBC and HG-NMIBC patients with or without recurrence. (A) NLR changes in LG-NMIBC without recurrence (N=51). (B) NLR changes in LG-NMIBC with recurrence (23). (C) NLR changes in HG-NMIBC without recurrence (N=41). (D) NLR changes in HG-NMIBC with recurrence (N=22).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; LG-NMIBC, low-grade non-muscle-invasive bladder cancers; HG-NMIBC, high-grade non-muscle-invasive bladder cancers.
Univariate and Multivariate Analysis of Prognostic Factors for RFS
As shown in Table 6, univariate analysis demonstrated that postoperative NLR change, smoking, number of tumors, tumor size, pathologic stage, and pathological grade were significantly related to RFS. Multivariate analysis revealed that smoking (HR=0.539, 95% CI: 0.298–0.977, P < 0.05), pathological stage (T1: HR=1.427, 95% CI: 0.763–2.669, P > 0.05; T2: HR=1.985, 95% CI: 1.036–3.801, P < 0.05; T3: HR=4.230, 95% CI: 1.83–9.776, P < 0.05; T4: HR=4.004, 95% CI: 1.374–11.663, P < 0.05; Ref=Ta), and NLR changes (HR=6.206, 95% CI: 3.826–10.067, P < 0.05) were prognostic factors for RFS of bladder tumor patients after surgery.
Table 6.
Univariate and Multivariate Analysis of Prognostic Factors for RFS in 213 Bladder Tumor Patients After Surgery
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.006 | 0.989–1.02 | 0.486 | |||
| Pre-NLR | 1.219 | 1.057–1.40 | 0.006 | |||
| Post-/pre-NLR | ||||||
| Increased vs Decreased | 5.253 | 3.325–8.29 | <0.001 | 6.206 | 3.826–10.067 | <0.001 |
| Gender | ||||||
| Male vs Female | 0.985 | 0.579–1.67 | 0.957 | |||
| Hypertension | ||||||
| Yes vs No | 1.116 | 0.683–1.82 | 0.660 | |||
| Smoking history | ||||||
| Yes vs No | 0.496 | 0.288–0.85 | 0.011 | 0.539 | 0.298–0.977 | 0.042 |
| Diabetes | ||||||
| Yes vs No | 0.659 | 0.317–1.36 | 0.262 | |||
| Pathologic stage | ||||||
| Ta | Ref | |||||
| T1 | 1.238 | 0.672–2.28 | 0.493 | 1.427 | 0.763–2.669 | 0.266 |
| T2 | 2.027 | 1.087–3.78 | 0.026 | 1.985 | 1.036–3.801 | 0.039 |
| T3 | 3.776 | 1.738–8.20 | 0.001 | 4.230 | 1.83–9.776 | 0.001 |
| T4 | 4.664 | 1.692–12.8 | 0.003 | 4.004 | 1.374–11.663 | 0.011 |
| Pathologic grade | ||||||
| High vs Low | 1.667 | 1.065–2.61 | 0.025 | |||
| Multifocality | ||||||
| Multiple vs Single | 1.622 | 1.064–2.47 | 0.025 | |||
| Tumor size | ||||||
| ≥3cm vs <3cm | 2.232 | 1.448–3.44 | <0.001 | |||
Furthermore, we performed multivariate analysis for LG-NMIBC, HG-NMIBC and MIBC patients, as shown in Table 7. The data showed that the ratio of postoperative NLR to preoperative NLR was the only significant factor for all three categories.
Table 7.
Multivariate Analysis of Prognostic Factors for RFS in LG-NMIBC HG-NMIBC and MIBC
| Factors | LG-NMIBC | HG-NMIBC | MIBC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.000 | 0.956–1.045 | 0.986 | 0.962 | 0.924–1.002 | 0.061 | 1.044 | 1.009–1.080 | 0.013 |
| Post-/pre- NLR | |||||||||
| Increased vs Decreased | 9.373 | 2.724–32.245 | <0.001 | 6.873 | 2.486–18.999 | <0.001 | 6.109 | 2.847–13.109 | <0.001 |
| Gender | |||||||||
| Male vs Female | 0.914 | 0.269–3.109 | 0.886 | 0.438 | 0.156–1.231 | 0.117 | 0.797 | 0.293–2.164 | 0.655 |
| Smoking history | |||||||||
| Yes vs No | 0.988 | 0.291–3.359 | 0.984 | 0.404 | 0.098–1.673 | 0.212 | 0.669 | 0.280–1.597 | 0.365 |
| Multifocality | |||||||||
| Multiple vs Single | 0.773 | 0.293–2.040 | 0.603 | 0.537 | 0.202–1.426 | 0.212 | 3.542 | 1.704–7.358 | 0.001 |
| Tumor size | |||||||||
| ≥3cm vs <3cm | 2.359 | 0.858–6.481 | 0.096 | 1.962 | 0.773–4.979 | 0.156 | 1.197 | 0.532–2.693 | 0.665 |
Discussion
This is the first study to show that the dynamic change of postoperative NLR is an independent prognostic factor for the early detection of bladder tumor recurrence in NMIBC patients treated with TURBT as well as MIBC patients treated with RC. In addition, the study showed that patients with increased NLR after surgery correlate with higher risk of recurrence, and that postoperative NLR dynamic change can be used as a reliable biomarker to predict bladder tumor recurrence.
The host’s SIR and immune system are considered to be significantly associated with the development and progression of many malignancies.19 As a convenient biomarker, NLR represents the balance between host inflammatory response and immune response, and has been shown to be associated with clinical outcomes in many tumors, including bladder tumors.20,21 Mano et al22 found higher recurrence rates in patients with preoperative NLR > 2.43 in NMIBC patients; Ceylan23 mentioned that if an NMIBC patient’s preoperative NLR > 3.96, it should be followed up closely and considered as high-risk tumors; Viers et al24 showed that a patient with preoperative NLR > 2.7 who is undergoing RC is associated with significantly increased risk for locally advanced disease as well as subsequent disease recurrence. However, some researchers have reached different conclusions, including Demirates, who reported that there was no correlation between preoperative NLR and prognosis.15 In our results, univariate analysis suggested that preoperative NLR could be used as a prognostic factor for RFS in bladder tumors, but it was not statistically significant in multivariate analysis. Although most studies show elevated pretreatment NLR values being associated with an inferior outcome, there is no consistency in the cut-off value ranging from 2 to 5 being used in the reported studies, making it less reliable as a valid prognostic factor.
The reasons for the inconsistency in cut-off values include not only different races, living environments, tumor types, tumor heterogeneity, and treatment methods but also the patient’s own factors such as age, SIR, and immune status. Therefore, we believe that an accurate cut-off value of preoperative NLR that satisfies all of the medical centers does not exist. Rather than formulating a rigid value, it is better to use preoperative NLR as a control, and it is more reliable to observe the dynamic changes of postoperative NLR, which was not influenced by objective factors and can directly reflect the dynamic change of the SIR and immune system from preoperative to postoperative. Postoperative NLR was expected to decrease after the surgical removal of tumor, indicating the host’s reduced SIR level after treatment, while anti-tumor immunity was enhanced, and the balance was tipped in favor of an anti-tumor immune response. Otherwise, the failure of postoperative NLR to decline indicated two factors: that inducing SIR has not been completely eliminated, or else there are still remnants of tumor and anti-tumor immunity is weakened. This results in the balance being tipped in favor of a pro-tumor inflammatory response and predicts poor oncologic outcomes. At present, the clinical significance of the dynamic changes of postoperative NLR in bladder tumor is unclear. There have been no studies addressing postoperative NLR changes as a prognostic marker of tumor recurrence after TURBT in NMIBC patients. Our results proved this hypothesis, and multivariate analysis revealed that smoking, tumor staging, and postoperative NLR change were independent prognostic factors for RFS. More importantly, our data showed that the ratio of postoperative NLR to preoperative NLR also could be a predictive factor of recurrence for HG-NMIBC and MIBC patients. This result suggests that the dynamic changes of postoperative NLR can be used as a reliable biomarker for predicting bladder tumor recurrence as significant as tumor stage.
The present study shows that the proportion of patients with recurrence in the increased NLR group is 73.5%, which is far higher than the 20% in the decreased NLR group. The 1-, 3- and 5-year RFS was 88.0%, 75.4%, and 75.4% for the decreased postoperative NLR group, respectively, and 51.2%, 25.8%, and 16.1% for the increased postoperative NLR group, respectively (P<0.05). Kaplan–Meier curves showed that the cumulative RFS rate in the postoperative increased group was significantly lower than that in the decreased group. After further stratification of the total number of patients, it is found that in NMIBC as well as MIBC patients, NLR decreased postoperatively and remained stable after surgery in patients without recurrence, but it was also found that NLR increased postoperatively, and continued to the diagnosis of recurrence in patients (Figure 4). More importantly, postoperative NLR dynamic change is not affected by objective factors, and is more reliable than preoperative NLR in predicting recurrence of bladder tumors. During follow-up, in patients with increased NLR after surgery, we recommend that more reliable imaging examinations be performed to exclude tumor recurrence. If necessary, appropriate adjuvant therapy would be performed to improve the patient’s prognosis.
There are some limitations in this study. First, this is a retrospective analysis from one single institution, and the case number is relatively small. Second, the follow-up period was relatively short for some cases. Thirdly, because there was no routine in our daily practice, we were lacking data related to the combination of certain inflammatory factors with NLR, such as C-reactive protein, which is why there is no carcinoma in situ information in our data.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding Statement
This study was supported by grant from the Project for Development, Promotion and Application of Feasible Medical Health Technologies of Guangxi (No. S2019048).
Ethics Statement
The study performed a retrospective analysis. All data were collected as part of routine diagnosis and treatment, and all patient data were kept completely confidential. The study was conducted in compliance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Affiliated Tumor Hospital of Guangxi Medical University.
Disclosure
All of the authors declare that they have no conflicts of interest for this work.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108. doi: 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–461. doi: 10.1016/j.eururo.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 3.Witjes J, Lebret T, Comperat E, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475. doi: 10.1016/j.eururo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 4.D’Costa JJ, Goldsmith JC, Wilson JS, et al. A systematic review of the diagnostic and prognostic value of urinary protein biomarkers in urothelial bladder cancer. Bladder Cancer. 2016;2:301–317. doi: 10.3233/BLC-160054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frantzi M, Van Kessel K, Zwarthoff E, et al. Development and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center study. Clin Cancer Res. 2016;22(16):4077–4086. doi: 10.1158/1078-0432.CCR-15-2715 [DOI] [PubMed] [Google Scholar]
- 6.Flaig TW, Spiess PE, Agarwal N, et al. NCCN guidelines insights: bladder cancer, version 5.2018. J Natl Compr Canc Netw. 2018;16(9):1041–1053. doi: 10.6004/jnccn.2018.0072 [DOI] [PubMed] [Google Scholar]
- 7.Prague M, Regensburg M, Nijmegen J, et al. European Association of Urology guidelines 2020. Eur Board Urol. 2020;1–19. [Google Scholar]
- 8.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. doi: 10.1038/bjc.2013.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vartolomei M, Mathieu R, Margulis V, et al. Promising role of preoperative neutrophil-to-lymphocyte ratio in patients treated with radical nephroureterectomy. World J Urol. 2017;35(1):121–130. doi: 10.1007/s00345-016-1848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One. 2013;8(3):e58184. doi: 10.1371/journal.pone.0058184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orditura M, Galizia G, Diana A, et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (dmfs) in early breast cancer: a propensity score-matched analysis. ESMO Open. 2016;2(1):e000038. doi: 10.1136/esmoopen-2016-000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morizawa Y, Miyake M, Shimada K, et al. Neutrophil-to-lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urol Oncol. 2016;6(34):e211–257. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol. 2015;11(56):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Can C, Baseskioglu B, Yilmaz M, Colak E, Ozen A, Yenilmez A. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int. 2012;89(4):468–472. doi: 10.1159/000343278 [DOI] [PubMed] [Google Scholar]
- 15.Demirtas A, Sabur V, Akinsal E, et al. Can neutrophil-lymphocyte ratio and lymph node density be used as prognostic factors in patients undergoing radical cystectomy? Sci World J. 2013;2013:703579. doi: 10.1155/2013/703579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantiello F, Russo GI, Vartolomei MD, et al. Systemic inflammatory markers and oncologic outcomes in patients with high-risk non-muscle-invasive urothelial bladder cancer. Eur Urol Oncol. 2018;1(5):403–410. doi: 10.1016/j.euo.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Vartolomei MD, Ferro M, Cantiello F, et al. Validation of neutrophil-to-lymphocyte ratio in a multi-institutional cohort of patients with T1G3 non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(6):445–452. doi: 10.1016/j.clgc.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 18.Ferro M, Di Lorenzo G, Buonerba C, et al. Predictors of residual T1 high grade on re-transurethral resection in a large multi-institutional cohort of patients with primary T1 high-grade/grade 3 bladder cancer. J Cancer. 2018;9(22):4250–4254. doi: 10.7150/jca.26129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grivennikov S, Greten F, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh J, Jung J, Jeong C, Kwak C, Kim H, Ku J. Clinical significance of pre-treated neutrophil-lymphocyte ratio in the management of urothelial carcinoma: a systemic review and meta-analysis. Front Oncol. 2019;9:1365. doi: 10.3389/fonc.2019.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Zhao X, Wang Y, et al. Pretreatment neutrophil-lymphocyte ratio as a predictor in bladder cancer and metastatic or unresectable urothelial carcinoma patients: a pooled analysis of comparative studies. Cell Physiol Biochem. 2018;46(4):1352–1364. doi: 10.1159/000489152 [DOI] [PubMed] [Google Scholar]
- 22.Mano R, Baniel J, Shoshany O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol. 2015;2(33):e61–67. [DOI] [PubMed] [Google Scholar]
- 23.Ceylan C, Doluoglu O, Keles I, et al. Importance of the neutrophil-to-lymphocyte ratio in muscle-invasive and non-muscle invasive bladder tumors. Urologia. 2014;81(2):120–124. doi: 10.5301/uro.5000031 [DOI] [PubMed] [Google Scholar]
- 24.Viers B, Boorjian S, Frank I, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66(6):1157–1164. doi: 10.1016/j.eururo.2014.02.042 [DOI] [PubMed] [Google Scholar]