Abstract
Background
There is great variability in clinical presentation of COVID-19 worldwide. The current study evaluated the impact of obesity and its related complications on the course of COVID-19 in Egyptian patients.
Methods
We included 230 COVID-19 Egyptian patients from Tanta City. According to their body-mass index (BMI), patient were divided into three groups: normal weight (BMI <25 kg/m2), overweight (BMI >25–<30 kg/m2), and obese (BMI ≥30 kg/m2). Patients’ glycemic status, lipid profile, and serum levels of acute-phase reactants were assessed. The number of patients receiving intensive care and the number of deaths in each group were counted.
Results
Mean values of random blood sugar, serum cholesterol, triglycerides, serum ferritin, erythrocyte-sedimentation rate, LDH, CRP, D-dimer levels, and blood pressure were significantly higher in obese patients (165.6, 129.5, 105, 1,873, 26, 403, 56.45, 977.16 and 142/87, respectively) than in normal-weight (97.2, 103.5, 70.4, 479, 17.4, 252, 23.2, 612.4, and 118.6/76.8, respectively) and overweight patients (111.4, 106.3, 78.13, 491.3, 19.8, 269.27, 25.42, 618.4, and 120.3/79.3, respectively). Lymphopenia was also significantly predominant in the obese group. Multivariate logistic regression analysis revealed that elevated serum triglycerides, total cholesterol, low density–lipoprotein cholesterol, blood pressure, ferritin, CRP, and low relative lymphocyte count were significant risk factors in obese COVID-19 patients.
Conclusion
Obesity and its related complications increase the risk of presenting a more severe form of COVID-19 in Egyptian patients.
Keywords: obesity, COVID-19, Egyptian
Introduction
On March 11, 2020, the World Health Organization declaredCOVID-19 a global pandemic. COVID-19 represents a great challenge to health-care systems, due to its rapid spread, serious complications, and high fatality rate.1 The presentation of COVID-19 patients varies widely, andmany studies have evaluated risk factors (age, sex, comorbidities) that may be related to COVID-19 complications and deaths. Studies to date have shown a worldwide predominance of elderly males, as well as those with cardiovascular diseases, in adverse COVID-19 outcomes.
With its rising prevalence, obesity is a major risk factor in many noncommunicable diseases.4 People with obesity in general are more likely to develop serious illness and need more hospitalization or ICU admission.5
A recent meta-analysis provided evidence that obesity-associated complications are important associated risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection.6
Among obese patients, excess adipocytes may enhance immunological and inflammatory alterations.7 Increased mass of adipose tissue is a well known contributing factor in proinflammatory cascades.8
SARS-CoV2, which causes the current COVID-19 global pandemic, has high affinity for human ACE2.9–11 ACE2 receptors show increased gene expression in adipose tissue.1 As such, excess body fat consequently increases ACE2 receptors and may increase the risk of SARS-CoV2 infection and developing a severe form of COVID-19.12,13
The aim of this study was to assess the effect of increased body weight and its consequences on blood pressure, blood glucose, triglycerides, and cholesterol in COVID-19 complications and case-fatality rates (deaths divided by confirmed cases) in Egyptian patients from Tanta, Gharbia Governorate, Egypt.
Methods
Sample
This study was conducted on COVID-19 patients who were home-isolated. Procedures were performed after prior approval by the Ethics Review Committee of Al Azhar University Faculty of Medicine and in accordance to the principles expressed in the Declaration of Helsinki. Informed consent was obtained from all patients before participating in the study. All patients were recruited from Tanta during the COVID-19 pandemic. The study was performed during from June 2020 to July 2020. A total of 230 COVID-19 positive patients were included. Diagnosis of COVID-19 was established by positive PCR, and further assessment was done by chest CT. Blood samples were collected from patients for laboratory investigations at 5–7 days after the appearance of symptoms. According to their BMI, patients were divided into three groups:normal weight (30 patients with BMI <25 kg/m2), overweight (58 patients with BMI >–25 <30 kg/m2), and obese (142 patients with BMI ≥30 kg/m2). Patient were aged 20–52 years.
No statistically significant differences were recorded among the studied groups in mean age (44.2±13.8 years in normal-weight group, 44.6±17.2 in overweight group, and 46.8±11.2 in obese group). Sex distribution showed no statistically significant differences either (19 males [63%] and eleven females [37%] in normal-weight group, 32 males [55%] and 26 females [45%] in overweight group, and 75 males [53%] males and 49 females [47%] in obese group). We followed up with patients during the study. All patients were in the early stage of the disease, approximately the first week after appearance of symptoms, when enrolled in the study. Patients were receiving the same treatment protocol (oseltamivir, azithromycin, hydroxychloroquine, and paracetamol), in addition to regular treatment for diabetes and hypertension.
The number of patients admitted to ICUs and the number of deaths in each group were counted. Random blood sugar, lipid profile, and systolic and diastolic blood pressure were measured for all patients. In addition, acute-phase reactants (serum ferritin, erythrocyte-sedimentation rate [ESR], LDH, CRP, D-dimer test, and relative lymphocyte count) were evaluated in all patients to assess the severity of the disease.
Laboratory Analysis
Serum glucose, total cholesterol, triglycerides, high density–lipoprotein cholesterol, and low density–lipoprotein cholesterol were determined by enzymatic methods using commercially available kits according to instructions. Serum CRP was measured with an autoanalyzer (Toshiba, Tokyo, Japan) and immunoturbidimetric analysis (CRP II Latex X2; Denka Seiken, Tokyo, Japan). Serum-ferritin levels were evaluated using an ELISA kit (RCD012R, BioVendor). Estimation of serum LDH was performed using an LDH-activity assay kit, (7D69-20, Abbott Diagnostics). Plasma D-dimer was measured with an ELISA kit (NB 110–8376, Novus Biologicals). Lymphocyte count were performed with an autohematology analyzer (Sysmex BC 3000).
Statistical Analysis
Pooled data and results are presented as means ± SD and were analyzed for statistical significance with SPSS 22. One way ANOVA with post hoc tests were used for calculation of significance between quantitative parameters, and ?2 tests to compare proportions between qualitative parameters. Results were considered significant at P<0.05. Alogistic regression analysis model was used for studied parameters to analyze their relation to obesity.
Results
Weight-Disaggregated Data of Studied Patients
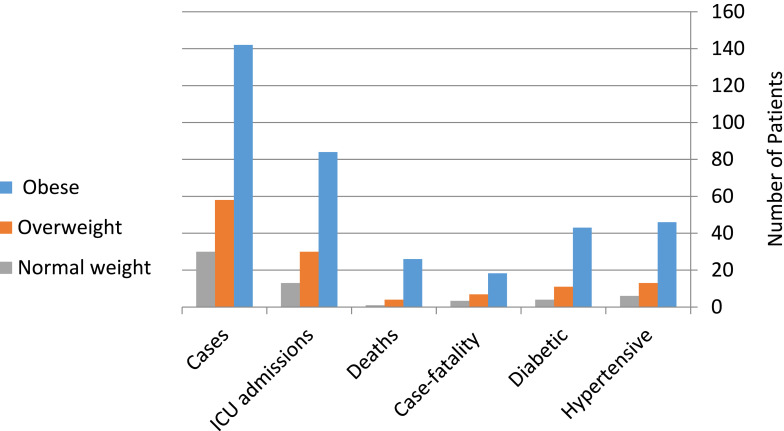
Figure 1 shows that of the 230 COVID-19 patients, 142 (61.7%) were obese, 58 (25.2%) overweight, and 30 (13%) of normal weight. The number of patients with diabetes was 43 (30.28%) in the obese group and eleven (18.9%) and four (13.3%) in the overweight and normal-weight groups, respectively. Regarding blood pressure, there were 46 hypertensive patients (32.4%) in the obese group and 13 (22.4%) and 6 (20%) in the overweight and normal-weight groups, respectively. Of the 142 COVID-19 obese patients, 84 (59.15%) were admitted to ICUs, where 26 (18.3%) died and 116 (81.7%) were discharged, while of the 58 overweight and 30 normal-weight COVID-19 patients, 30 (51.7%) and 13 (43.3%) were admitted to ICUs, where four (6.89%) and 1 (3.33%) died and 54 (93%) and 29 (96.6%) were discharged in the overweight and normal-weight groups, respectively. All patients that died were diabetic and hypertensive. Hospitalization and ICU admission due to COVID-19 was after an average of 2–3 weeks from the beginning of symptoms. All patients received mechanical ventilation and O2. All the aforementioned parameters were significantly higher in the obese group than the normal-weight and overweight groups.
Figure 1.
Weight-disaggregated data of patients.
Obesity-Related Characteristics in COVID-19 Patients
Table 1 shows obesity-associated characteristics in studied groups, where the results revealed normal levels of random blood sugar, serum total cholesterol, triglycerides, and blood pressure in the normal-weight and overweight groups. A significant increase was observed in levels of these among the obese group when compared to the overweight and normal-weight groups (P<0.001). There were significant differences between the normal-weight and overweight groups regarding mean values of random blood glucose, triglycerides, and BMI, while the other parameters showed no significant difference.
Table 1.
Demographic and obesity-associated characteristics in studied groups
| Normal Weight (BMI <25 kg/m2), n=30 |
Overweight (BMI >25–<30 kg/m2), n=58 |
Obese (BMI ≥ 30 kg/m2), n=142 |
ANOVA | ||
|---|---|---|---|---|---|
| F | P | ||||
| Age (years) | 44.2±13.8 | 44.6±17.2 | 46.8±11.2 | 0.849 | 0.429 |
| Sex (male/female) | 19/11 63%/37% |
32/26 55%/45% |
75/49 53%/47% |
?2=0.683 | 0.711 |
| Random BG (mg/dL) | 97.2±6.3 | 111.4±8.1a | 165.6±13a,b | 764.209 | <0.001* |
| Serum triglycerides (mmol/L) | 70.4±4.2 | 78.13±4.5a | 105.4±14.3a,b | 185.218 | <0.001* |
| Serum cholesterol (mmol/L) | 103.5±5.7 | 106.3±6.3 | 129±5.5a,b | 464.268 | <0.001* |
| HDL cholesterol (mmol/L) | 49.4±3.1 | 47.9±3.2 | 41.9±4.2a,b | 79.560 | <0.001* |
| LDL cholesterol (mmol/L) | 57.6±8.3 | 59.7±8.4 | 87.11±2.6a,b | 701.003 | <0.001* |
| Systolic BP (mmHg) | 118.6±9.4 | 120.3±10.1 | 142±14.6a,b | 79.813 | <0.001* |
| Diastolic BP (mmHg) | 76.8±6.2 | 79.3±7.4 | 87±9.8a,b | 30.189 | <0.001* |
| BMI (kg/m2) | 21.4±3.2 | 27.8±4.3a | 34.1±5.1a,b | 106.719 | <0.001* |
Notes: Data expressed as means ± SD, One-way ANOVA with post hoc test used for calculation of significance. P<0.05 was considered significant. aSignificantly different from normal-weight group; bSignificantly different from overweight group. *Statistically significant difference.
Abbreviations: BMI, body-mass index; BG, blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BP, blood pressure.
Impact of Obesity on Severity of COVID-19
Table 2 shows levels of acute-phase reactants in the groups, where the results revealed increased levels of serum ferritin, ESR, LDH, CRP, and D-dimer in COVID-19 patients, with significant elevation observed in the obese group compared to the overweight and normal-weight groups (P<0.001). In addition, lymphocytes were reduced in all groups due to SARS-CoV2 infection, with a significant difference in the obese group when compared to the overweight and normal-weight groups, while there was no statistically significant difference when we compared the overweight and normal-weight groups.
Table 2.
Laboratory characteristics associated with COVID-19 severity in studied groups
| Normal weight (BMI <25 kg/m2), n=30 |
Overweight (BMI >25–<30 kg/m2), n=58 |
Obese (BMI ≥30 kg/m2), n=142 |
ANOVA | ||
|---|---|---|---|---|---|
| F | P | ||||
| Ferritin (ng/mL) | 479.4±59 | 491.3±61 | 1,873±138a,b | 3,949.63 | <0.001* |
| ESR in first hour (mm) | 17.5±7.4 | 19.8±7.1 | 26±4.26a,b | 44.537 | <0.001* |
| Lymphocytes (%) | 16.8±2.15 | 18±2.35 | 9±3.09a,b | 255.327 | <0.001* |
| LDH (U/L) | 252.31±33.11 | 269.27±30.21 | 403.4±54.16a,b | 243.978 | <0.001* |
| CRP (mg/L) | 23.2±3.01 | 25.42±3.13 | 56.45±5.28a,b | 1,313.485 | <0.001* |
| D-dimer (ng/mL) | 612.4±24.6 | 618.4±25.9 | 977.16±33a,b | 3,835.195 | <0.001* |
Notes: Data expressed as means ± SD. One-way ANOVA with post hoc test used for calculation of significance. P<0.05 was considered significant. aSignificantly different from normal-weight group; bsignificantly different from overweight group. *Statistically significant difference.
Abbreviation: ESR, erythrocyte-sedimentation rate.
Logistic Regression Analysis
Univariate and multivariate logistic regression analysis was performed to study the parameters included in the current research in relation to obesity. Table 3 showed that random blood glucose, serum triglycerides, total cholesterol, high density–lipoprotein cholesterol, low density–lipoprotein cholesterol, blood pressure, ferritin, ESR, lymphocytes, LDH, CRP, and D-dimer had a significant effect on predicting outcome of COVID-19 in obese patients on univariate analysis, while serum triglycerides, total cholesterol, low density–lipoproteincholesterol, blood pressure, ferritin, lymphocytes, and CRP were significant risk factors in obese COVID-19 patients on multivariate analysis.
Table 3.
Univariate and multivariate logistic regression analyses
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Age | 1.244 | 1.129 | 1.371 | 0.947 | — | |||
| Sex | 1.306 | 1.185 | 1.439 | 0.995 | — | |||
| Random BG (mg/dL) | 1.528 | 1.387 | 1.684 | 0.039* | — | |||
| Serum triglycerides (mmol/L) | 1.359 | 1.218 | 1.516 | 0.035* | 1.203 | 1.078 | 1.342 | 0.048* |
| Serum cholesterol (mmol/L) | 1.570 | 1.217 | 2.024 | 0.023* | 1.389 | 1.077 | 1.791 | 0.031* |
| HDL cholesterol (mmol/L) | 1.605 | 1.456 | 1.768 | 0.043* | — | |||
| LDL cholesterol (mmol/L) | 1.285 | 1.224 | 1.349 | 0.029* | 1.137 | 1.083 | 1.194 | 0.040* |
| Systolic BP (mmHg) | 0.414 | 0.167 | 1.027 | 0.016* | 0.366 | 0.148 | 0.909 | 0.022* |
| Diastolic BP (mmHg) | 3.066 | 1.911 | 6.869 | 0.009* | 2.713 | 1.691 | 6.079 | 0.013* |
| Ferritin (ng/mL) | 1.250 | 1.219 | 1.280 | 0.021* | 1.106 | 1.079 | 1.133 | 0.029* |
| ESR first hour (mm) | 1.214 | 1.047 | 1.407 | 0.032* | — | |||
| Lymphocytes (%) | 2.348 | 1.376 | 4.004 | 0.012* | 2.078 | 1.218 | 3.543 | 0.017* |
| LDH (U/L) | 0.739 | 0.487 | 1.122 | 0.044* | — | |||
| CRP (mg/L) | 0.105 | 0.019 | 0.549 | 0.003* | 0.093 | 0.017 | 0.486 | 0.004* |
| D-dimer (ng/mL) | 1.133 | 1.076 | 1.193 | 0.035* | — | |||
Notes: Univariate and multivariate logistic regression analysis used for studied parameters in obese COVID-19 patients. P <0.05 was considered significant. *Significant difference.
Abbreviations: BG, blood glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BP, blood pressure; ESR, erythrocyte-sedimentation rate.
Discussion
The pandemic of acute respiratory distress due to COVID-19 caused by SARS-CoV2 showed great variation regarding factors related to the severity and fatality of this disease.14,15
Of 230 patients included in the study, there were 142 obese, 58, overweight and 30 normal-weight patients (obese group ORO1.61; 95% CI 1.17–2.23).)
The number of obese COVID-19 patients admitted to ICUs was double that of both overweight and normal-weight groups (84 versus 43, respectively). The number of deaths and case-fatality ratio among obese COVID-19 patients were also significantly higher (26, 18.3%) than the overweight (4, 6.89%) and normal-weight groups (1, 3.33%). These differences show a higher susceptibility of obese patients to develop complications and a greater need for acute care and ICU admission. Many studies have reported similar results to ours regarding susceptibility of obese COVID-19 patients to requiring special care and admission to the ICU.16–21
Our results of poor outcomes in obese COVID-19 patients are in agreement meta-analyses conducted by Hussain et al and Pranata et al.12,13
Mean values of risk factors for COVID-19 initiation and progression, ie, prevalence of diabetes, hypertension, high blood glucose, dyslipidemia, and high blood pressure, were significantly higher in the obese group than the overweight and normal-weight groups. In accordance with our results, Caussy et al reported that obese COVID-19 patients were more susceptible to developing severe complications.22 These parameters are well known factors involved in alteration of the immune system in COVID-19 patients. Increased expression of ACE2 in adipose tissue may aggravate the presentation and condition of COVID-19 patients, because ACE2 receptors are clearly recognized as the main SARS-CoV2 receptors.23–26
Acute-phase reactants related to severity of inflammation, ie, CRP, serum ferritin, D-dimer, ESR, and LDH, showed increased levels in the obese group compared to the overweight and normal-weight groups. These findings demonstrate an important outcome of obesity in terms of severity of inflammatory response in obese patients infected with COVID-19. We performed logistic regression analysis to determine significant risk factors in relation toobesity. Univariate logistic regression analysis showed that all studied laboratory parameters were significant (P<0.05) in the obese group. Multivariate logistic regression analysis demonstrated that high levels of serum triglycerides, total cholesterol, low density–lipoprotein cholesterol, blood pressure, ferritin, and CRP and lowlymphocyte count were significant risk factors in obese COVID-19 patients.
In agreement with the results of our study, many studies have reported higher values for inflammatory markers in aggravated cases of pneumonia.7,8,27 The appropriate detection of patients susceptible to developing fulminant inflammatory responses plays a key role in the management of COVID-19 patients.
The presence of lymphopenia is a sensitive marker for the severity of COVID-19. The lymphopenia found in the obese group was significantly different from the overweight and normal-weight groups. These results were in agreement with results reported by Tan et al.28
We believe that modulation of inflammatory markers and inflammatory immunoresponse can be an important key in management of patients with obesity suffering from SARS-CoV2. The results of our study should guide preventive measures required for vulnerable groups against catching SARS-CoV2. A limitation of the present study was that treatment strategies were not included in the research. The study was conducted on a small number of patients, so we recommend further studies on large numbers of Egyptian patients with COVID-19 to explore the most suitable protocols for management of COVID-19 patients suffering from obesity.
In conclusion, this study demonstrated that obesity and its related comorbidities can increase the risk of presenting a more severe form of COVID-19 in Egyptian patients. Therefore, the study recommends the importance of raising awareness of obesity-prevention strategies and recommendations, such as limiting unhealthy foods, increasing physical activity, limiting television time, screen time, and other “sitting times” to increase resistance to SARS-COV2 complications.
Acknowledgment
The authors would like to extend their sincere thanks for the financial support of Taif Univesrsity Researchers Supporting Project number (TURSP-2020/82), Taif University, Taif, Saudi Arabia.
Data-Sharing Statement
Data are available upon request.
Ethics Approval and Consent to Participate
Ethics approval was obtained from the Ethics Review Committee of Al Azhar University Faculty of Medicine (270-6/2020). Informed consent was obtained from all patients before participation in the study.
Disclosure
All authors declare that they have no conflicts of interest for this work.
References
- 1.Bello-Chavolla O, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):2752–2761. doi: 10.1210/clinem/dgaa346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, et al. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11. doi: 10.1186/s13293-020-00304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508 [DOI] [PubMed] [Google Scholar]
- 5.Frühbeck G, Toplak H, Woodward E, Yumuk V, Maislos M, Oppert JM; Executive Committee of the European Association for the Study of Obesity. Obesity: the gateway to ill health – an EASO position statement on a rising public health, clinical and scientific challenge in Europe. Obes Facts. 2013;6(2):117–120. doi: 10.1159/000350627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12(7):6049–6057. doi: 10.18632/aging.103000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10(3):207–215. doi: 10.1159/000471488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obesity Med. 2020;19:100283. doi: 10.1016/j.obmed.2020.100283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6):e13034). doi: 10.1111/obr.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Obesity Res Clin Pract. 2020;14(4):295–300. doi: 10.1016/j.orcp.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Pranata R, Lim MA, Yonas E, et al. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab. 2020;S1262-3636(20)30097–5. doi: 10.1016/j.diabet.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 16.Cariou B, Hadjadj S, Wargny M, et al. CORONADO investigators. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busetto L, Bettini S, Fabris R, et al. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring). 2020;28:1600–1605. doi: 10.1002/oby.22918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28(7):1195–1199. doi: 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Intensive Care National Audit & Research Centre. Audit I. ICNARC Report on COVID-19 in Critical Care April 10 2020. London, UK: Intensive Care National Audit and Research Centre; 2020. [Google Scholar]
- 20.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caussy C, Pattou F, Wallet F, et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562–564. doi: 10.1016/S2213-8587(20)30160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wernstedt Asterholm I, Tao C, Morley TS, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103–118. doi: 10.1016/j.cmet.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan L, Kang X, Ji X, et al. Validation of predictors of disease severity and outcomes in COVID-19 patients: a descriptive and retrospective study. Med (NY). 2020. doi: 10.1016/j.medj.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]