Abstract
Background and Aims
Circadian misalignment (CM) leads to metabolic disorder. Metabolic (dysfunction) associated fatty liver disease (MAFLD) is a novel definition for fatty liver disease that requires the presence of metabolic dysfunction. As the association between CM and MAFLD remains unclear, this study is designed to explore whether there is an association between CM and MAFLD.
Methods
NHANES 2017–2018 database was used in this study. Liver steatosis and fibrosis were diagnosed by Fibroscan®. CM was defined by the presence of mistimed sleep, late sleep or irregular chronotype. Propensity score matching (PSM) was used to match subjects for their age and gender.
Results
A total of 4552 participants were included in the study, with 2089 (45.89%) identified as MAFLD and 894 (19.64%) as CM. Participants with CM were significantly younger than those without (46.06 ± 18.06 vs 50.93 ± 17.78, p<0.001). PSM for age and gender resulted in 894 participants with CM and 892 with non-CM. CM group had higher body mass index, liver enzymes, glucose and lipid levels. The prevalence of MAFLD was higher in the CM group than the non-CM group (45.41% vs 28.48%, p<0.001). The presence of CM increased the risk of MAFLD by more than twofold. Short sleep duration (<6 hours) was not independently associated with MAFLD or fibrosis if additionally adjusting for CM.
Conclusion
CM is independently associated with MAFLD, while short sleep duration (<6 hours) is not an independent risk factor for MAFLD or liver fibrosis after adjusting for CM.
Keywords: fatty liver disease, MAFLD, circadian, sleep, fibrosis
Introduction
Circadian system is the most important physiological regulator of mammals. It is affected by various environmental signals, especially the changes in light. In response to the light–dark cycle caused by the Earth rotation, humans have evolved an approximate 24-hour endogenous circadian clock. The circadian clock is controlled by the clock genes whose proteins are necessary for the generation and regulation of circadian rhythms.1 Circadian clock proteins also control the transcription of various genes of metabolic system. Circadian misalignment (CM) might lead to dysregulation of metabolic genes and eventually result in metabolic diseases.2,3
Sleep is the major physiological process for humans to regulate circadian rhythm. When the sleep time is not synchronized with the body’s natural circadian rhythm, it may cause CM. Given the socio-economic realities of modern societies, it is not possible to avoid excessive artificial light exposure.4 Epidemiological studies have shown that insufficient and mistimed sleep causes obesity, type-2 diabetes, cardiovascular disease and cancers.3 Shorter sleep duration was positively associated with liver fibrosis/stiffness.5 However, most studies have only focused on the sleep duration, neglecting the sleep chronotype.
Metabolic (dysfunction) associated fatty liver disease (MAFLD) is a new concept coming out in 2020.6 The diagnosis of MAFLD does not require the exclusion of other chronic liver diseases, while the presence of metabolic disorder is necessary, which helps to identify more cases at risk.7–9 The impact of CM on MAFLD is unclear. As CM increases the risk of metabolic disorders,10 we hypothesize that the change in circadian rhythm, including the sleep duration and chronotype, may have a substantial impact on the risk of MAFLD and liver fibrosis. To answer this question, we used a national survey database to match participants with or without CM, and compare the prevalence of MAFLD and significant fibrosis between the two groups, aiming to evaluate the impact of CM on MAFLD.
Methods
Study Population
The study dataset was obtained from the NHANES (National Health and Nutrition Examination Surveys) 2017–2018 database. NHANES is a national-based survey conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention of the United States and has been frequently used for the study of liver disease.11–13 NHANES 2017–2018 is the only publically available survey database with liver fibrosis assessment via Fibroscan® examination and has been used for the study of MAFLD.14 The National Center for Health Statistics Research Ethics Review Board approved the NHANES protocol and informed consent was obtained from all participants. The dataset could be assessed online free of any costs (https://www.cdc.gov/nchs/nhanes/index.htm).
Definition of Sleep Habit and Circadian Dysregulation
The sleep data were obtained from the results of the adapted Munich chronotype questionnaire15 in the NHANES database. The definitions used to classify sleep patterns were based on the American Academy of Sleep Medicine and Sleep Research Society recommendations. According to this recommendation, 7–9 hours of sleep is recommended for young adults and adults, and 7–8 hours for older adults.16 In this study, the short sleep duration was defined as total sleep time of less than 6 hours on weekdays. The reported bedtime of most people is 11 PM and wake-up time is 7–8 AM.17 In this study, we defined the late sleeper as those who usually “fell asleep” after 0:00 AM, ie, midnight. Mistimed sleepers were defined as those who fell asleep in the daytime (after 6 AM) or those who slept before 8 PM and woke up before 3 AM. Irregular chronotype was defined as the difference in time of falling asleep >5 hours between weekday and weekend (eg, 8 PM on weekday and 2 AM on the weekend). CM was diagnosed if any of the mistimed sleep, late sleep or irregular chronotype was present.
Definition of MAFLD and Fibrosis
Liver steatosis and liver fibrosis were defined by the controlled attenuation parameter (CAP) and liver stiffness measurements (LSM) respectively, obtained via transient elastography (FibroScan®). CAP and LSM are validated tools to measure the steatosis (S) and liver fibrosis (F) in participants with fatty liver disease.18,19 The severity of steatosis was stratified as S0-3, and the thresholds of CAP for steatosis grade S1, S2 and S3 were 248, 268 and 280, respectively.20 Significant steatosis was defined as steatosis grade greater than S1. The fibrosis was stratified as F0-4 and the thresholds of LSM for F1, F2, F3 and F4 were 6.3, 8.3, 10.5 and 12.5, respectively.19 Significant liver fibrosis was diagnosed as fibrosis grade greater than F1. Cases with a fasting time of <3 hours, less than 10 complete LSM readings, or a liver stiffness interquartile (IQR) range/median LSM of more than 30% were deemed as failed FibroScan® measurement and were excluded.
MAFLD was diagnosed based on the evidence of hepatic steatosis and any of the following three conditions: overweight/obesity, diabetes mellitus or metabolic dysfunction. According to the definition of MAFLD, the metabolic dysfunction was defined as the presence of at least two of the following conditions: 1) waist circumference greater than 102 cm in male and 88 cm in female, 2) hypertension (arterial blood pressure ≥130/85 mmHg or under anti-hypertension therapy), 3) hyperlipidemia (triglyceride (TG) ≥1.70 mmol/L or with specific drug treatment), 4) low high-density lipoprotein cholesterol (HDL-C) level (<1.0 mmol/L for male and <1.3 mmol/L for female), 5) prediabetes and 6) hypersensitive C-reactive protein level >2 mg/L.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation. Kolmogorov–Smirnov test was used to examine the normality of continuous variables. The differences between continuous variables that were non-normally distributed were analyzed by the Mann–Whitney U-test, while normally distributed variables were analyzed by Student’s t-test. The categorical variables were expressed as percentages and analyzed by the Chi-squared test. Multivariate logistic regression was used to explore the independent factors for MAFLD or fibrosis. Propensity score matching (PSM) was used to match the participants with and without CM with a ratio of 1:1 and a clipper of 0.0001. All tests were two-tailed, and results with a p value less than 0.05 were considered statistically significant. All analysis was conducted by R 3.6.2 (https://www.r-project.org/).
Results
Baseline Characteristics of Overall Participants
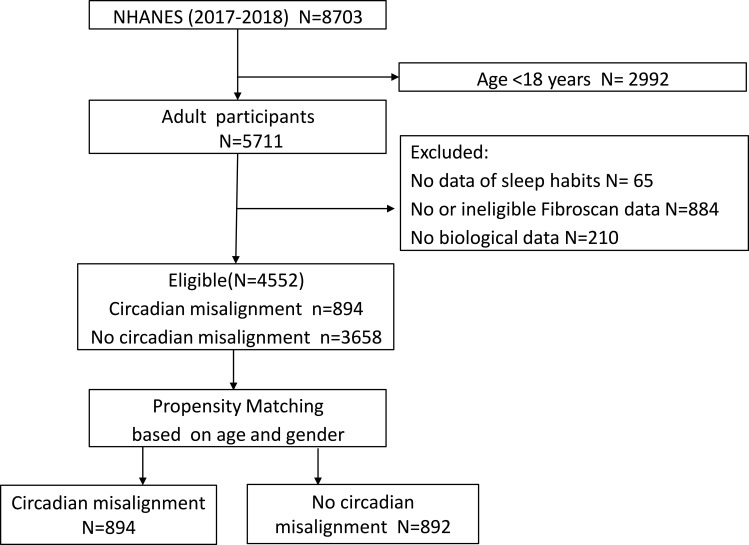
There were 8703 cases in the NHANES 2017–2018 database. After excluding 2992 participants who were less than 18 years old and 1159 with missing data, a total of 4552 cases were eligible for final analysis (Figure 1: consort diagram). The mean age of this population was 49.97 ± 17.94 years and 49.32% of them were male. The mean BMI was 29.49 ± 7.11 kg/m2. MAFLD was identified in 2089 (45.89%) participants. A total of 894 (19.64%) participants had CM and 3658 (80.36%) did not.
Figure 1.
Case selection flow.
Table 1 demonstrates the baseline difference between participants with or without CM, before and after propensity matching (Table 1). Compared to those without CM, participants with CM were significantly younger (46.06 ± 18.06 vs 50.93 ± 17.78, p<0.001), more likely to be male (55.82% vs 47.73%, p<0.001), less likely to have diabetes (17.34% vs 20.67%, p=0.029). The prevalence of MAFLD was similar between the two groups (45.41% vs 46.01%, p=0.778). The results of most biochemical tests, BMI and waist-to-hip ratio were not significantly different between these two groups.
Table 1.
Baseline Characteristics of Patients with or without Circadian Dysregulation
| Variables | Before PSM | After PSM | P value | |||
|---|---|---|---|---|---|---|
| Non-CM (n = 3658) | CM (n = 894) | P value | Non-CM (n = 892) | CM (n = 894) | ||
| Age | 50.93 ± 17.78 | 46.06 ± 18.06 | <0.001 | 46.1 ± 18.06 | 46.06 ± 18.06 | 0.96 |
| Gender, n (%) | <0.001 | 1 | ||||
| male | 1746 (47.73) | 499 (55.82) | 497 (55.72) | 499 (55.82) | ||
| female | 1912 (52.27) | 395 (44.18) | 395 (44.28) | 395 (44.18) | ||
| Race, n (%) | <0.001 | 0.239 | ||||
| Mexican American | 544 (14.87) | 99 (11.07) | 98 (10.99) | 99 (11.07) | ||
| Other Hispanic | 362 (9.9) | 84 (9.4) | 89 (9.98) | 84 (9.4) | ||
| Non-Hispanic White | 1282 (35.05) | 276 (30.87) | 275 (30.83) | 276 (30.87) | ||
| Non-Hispanic Black | 764 (20.89) | 251 (28.08) | 258 (28.92) | 251 (28.08) | ||
| Non-Hispanic Asian | 532 (14.54) | 118 (13.2) | 131 (14.69) | 118 (13.2) | ||
| Other race | 174 (4.76) | 66 (7.38) | 41 (4.6) | 66 (7.38) | ||
| Diabetes, n (%) | 0.029 | 0.349 | ||||
| No | 2902 (79.33) | 739 (82.66) | 753 (84.42) | 739 (82.66) | ||
| Yes | 756 (20.67) | 155 (17.34) | 139 (15.58) | 155 (17.34) | ||
| Hypertension, n (%) | 0.396 | 0.003 | ||||
| No | 2002 (54.73) | 504 (56.38) | 565 (63.34) | 504 (56.38) | ||
| Yes | 1656 (45.27) | 390 (43.62) | 327 (36.66) | 390 (43.62) | ||
| BMI (kg/m2) | 29.52 ± 6.96 | 29.36 ± 7.67 | 0.573 | 27.72 ± 6.81 | 29.36 ± 7.67 | <0.001 |
| Waist-to-hip ratio | 0.94 ± 0.08 | 0.93 ± 0.08 | 0.237 | 0.92 ± 0.08 | 0.93 ± 0.08 | <0.001 |
| FPG (mmol/L) | 5.68 ± 1.97 | 5.64 ± 1.95 | 0.645 | 5.46 ± 1.88 | 5.64 ± 1.95 | 0.048 |
| HbA1c (%) | 5.85 ± 1.09 | 5.78 ± 1.07 | 0.077 | 5.68 ± 1.02 | 5.78 ± 1.07 | 0.062 |
| hs CRP (mg/L) | 3.91 ± 7.2 | 4.13 ± 8.43 | 0.485 | 3.74 ± 7.22 | 4.13 ± 8.43 | 0.298 |
| TC (mmol/L) | 4.87 ± 1.05 | 4.8 ± 1.06 | 0.101 | 4.76 ± 1.04 | 4.8 ± 1.06 | 0.383 |
| TG (mmol/L) | 1.64 ± 1.26 | 1.6 ± 1.18 | 0.407 | 1.37 ± 0.86 | 1.6 ± 1.18 | <0.001 |
| UA (µmol/L) | 323.73 ± 88.24 | 327.77 ± 84.74 | 0.218 | 316.14 ± 87.39 | 327.77 ± 84.74 | 0.005 |
| HDL-C (mmol/L) | 1.38 ± 0.4 | 1.35 ± 0.38 | 0.089 | 1.4 ± 0.39 | 1.35 ± 0.38 | 0.008 |
| TBIL(μmol/L) | 7.89 ± 4.64 | 7.96 ± 4.71 | 0.672 | 7.68 ± 4.5 | 7.96 ± 4.71 | 0.197 |
| ALB (g/L) | 40.64 ± 3.23 | 40.81 ± 3.42 | 0.186 | 40.71 ± 3.38 | 40.81 ± 3.42 | 0.542 |
| ALT (U/L) | 22.53 ± 16.62 | 23.06 ± 21.78 | 0.513 | 12.84 ± 5.71 | 23.06 ± 21.78 | <0.001 |
| AST (U/L) | 22.1 ± 12.41 | 22.45 ± 15.86 | 0.553 | 16.66 ± 4.16 | 22.45 ± 15.86 | <0.001 |
| GGT (U/L) | 31.47 ± 39.94 | 34.61 ± 62.44 | 0.165 | 20.08 ± 15.92 | 34.61 ± 62.44 | <0.001 |
| ALP (U/L) | 78.59 ± 24.64 | 79.58 ± 33 | 0.413 | 75.78 ± 22.31 | 79.58 ± 33 | 0.006 |
| Creatinine (µmol/L) | 78.76 ± 37.69 | 80.8 ± 43.04 | 0.208 | 83.71 ± 58.28 | 80.8 ± 43.04 | 0.235 |
| MAFLD, n (%) | 0.778 | <0.001 | ||||
| No | 1975 (53.99) | 488 (54.59) | 638 (71.52) | 488 (54.59) | ||
| Yes | 1683 (46.01) | 406 (45.41) | 254 (28.48) | 406 (45.41) | ||
| FLD, n (%) | 0.986 | <0.001 | ||||
| No | 1935 (52.9) | 472 (52.8) | 623 (69.84) | 472 (52.8) | ||
| Yes | 1723 (47.1) | 422 (47.2) | 269 (30.16) | 422 (47.2) |
Note: Categorical values are shown as n (%). Continuous variables are shown as mean ± standard deviation.
Abbreviations: CM, circadian misalignment; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; hs CRP, hypersensitive C-reactive protein; TC, total cholesterol; TG, triglyceride; UA, uric acid; HDL-C, high-density lipoprotein cholesterol; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; MAFLD, Metabolic-associated fatty liver disease; FLD, Fatty liver disease.
As age and gender are known to be associated with the development of metabolic disorder and fatty liver disease,22,23 we chose propensity score-matched participants with and without CM by age and gender. After PSM, 894 cases with CM and 892 without were included in the analysis (Table 1). The age, gender and race were comparable after PSM. Compared to participants without CM, the age- and gender-matched CM participants had higher BMI (29.36 ± 7.67 vs 27.72 ± 6.81 kg/m2, p<0.001), waist-to-hip ratio (0.93 ± 0.08 vs 0.92 ± 0.08, p<0.001), fasting plasma glucose (5.64 ± 1.95 vs 5.46 ± 1.88 mmol/L, p=0.048), TG (1.60 ± 1.18 vs 1.37 ± 0.86 mmol/L, p<0.001), while lower HDL (1.35 ± 0.38 vs 1.40 ± 0.39 mmol/L, p=0.008). The liver enzymes were significantly increased in the CM group than the non-CM group (alanine transaminase (ALT): 23.06 ± 21.78 vs 12.84 ± 5.71 U/L, p<0.001; aspartate aminotransferase (AST): 22.45 ± 15.86 vs 16.66 ± 4.16 U/L, p<0.001). The prevalence of MAFLD was almost twofold higher in CM (45.41% vs 28.48%, p<0.001).
Sleeping Habit in Participants After PSM
Participants were divided into MAFLD group and non-MAFLD group. MAFLD participants had shorter sleep length (6.90 ± 1.82 vs 7.36 ± 1.80, p<0.001) and higher proportion of short sleep duration (24.54% vs 16.78%, p<0.001). MAFLD cases were more likely to have at least one type of CM (61.52% vs 43.34%, p<0.001). Late sleep was the most commonly seen in MAFLD (in 43.18% MAFLD cases). (Table 2).
Table 2.
The Demographic Characteristics and the Sleep Habit in Cases with MAFLD or Fibrosis
| MAFLD | Fibrosis | |||||
|---|---|---|---|---|---|---|
| Variables | Non-MAFLD (n = 1126) |
MAFLD (n = 660) |
p | Non-Fibrosis (n = 1402) |
Fibrosis (n = 384) |
p |
| Age(years) | 43.37 ± 18.26 | 50.71 ± 16.7 | <0.001 | 44.93 ± 17.95 | 50.27 ± 17.82 | <0.001 |
| Male, n (%) | 601 (53.37) | 395 (59.85) | 0.009 | 750 (53.5) | 246 (64.06) | <0.001 |
| Race, n (%) | <0.001 | 0.093 | ||||
| Mexican American | 94 (8.35) | 103 (15.61) | 149 (10.63) | 48 (12.5) | ||
| Other Hispanic | 110 (9.77) | 63 (9.55) | 135 (9.63) | 38 (9.9) | ||
| Non-Hispanic White | 336 (29.84) | 215 (32.58) | 440 (31.38) | 111 (28.91) | ||
| Non-Hispanic Black | 356 (31.62) | 153 (23.18) | 384 (27.39) | 125 (32.55) | ||
| Non-Hispanic Asian | 155 (13.77) | 94 (14.24) | 210 (14.98) | 39 (10.16) | ||
| Other race | 75 (6.66) | 32 (4.85) | 84 (5.99) | 23 (5.99) | ||
| Sleep time (hours) | 7.36 ± 1.8 | 6.9 ± 1.82 | <0.001 | 7.23 ± 1.8 | 7.04 ± 1.88 | 0.071 |
| Sleep duration< 6 hours, n (%) | 189(16.78) | 162(24.54) | <0.001 | 263(18.76) | 88(22.92) | 0.069 |
| Circadian misalignment, n (%) | 488 (43.34) | 406 (61.52) | <0.001 | 665 (47.43) | 229 (59.64) | <0.001 |
| Number of circadian misalignments, n (%) | <0.001 | <0.001 | ||||
| 0 | 638 (56.66) | 254 (38.48) | 737 (52.57) | 155 (40.36) | ||
| 1 | 422 (37.48) | 347 (52.58) | 572 (40.8) | 197 (51.3) | ||
| 2 | 65 (5.77) | 58 (8.79) | 92 (6.56) | 31 (8.07) | ||
| 3 | 1 (0.09) | 1 (0.15) | 1 (0.07) | 1 (0.26) | ||
| Mistimed sleep, n (%) | 140 (12.43) | 128 (19.39) | <0.001 | 196 (13.98) | 72 (18.75) | 0.025 |
| Late sleep, n (%) | 350 (31.08) | 285 (43.18) | <0.001 | 479 (34.17) | 156 (40.62) | 0.022 |
| Irregular chronotype, n (%) | 65 (5.77) | 53 (8.03) | 0.079 | 84 (5.99) | 34 (8.85) | 0.059 |
Regardless the presence of MAFLD, the comparison between fibrosis and non-fibrosis groups showed that the length of sleep time, as well as the proportion of short sleep duration, did not differ between participants with or without fibrosis (both p>0.05). The comparison of CM and its subtypes demonstrated that participants with fibrosis were more likely to have at least one subtype of CM. Late sleep is the most common subtype of CM in the fibrotic population (Table 2).
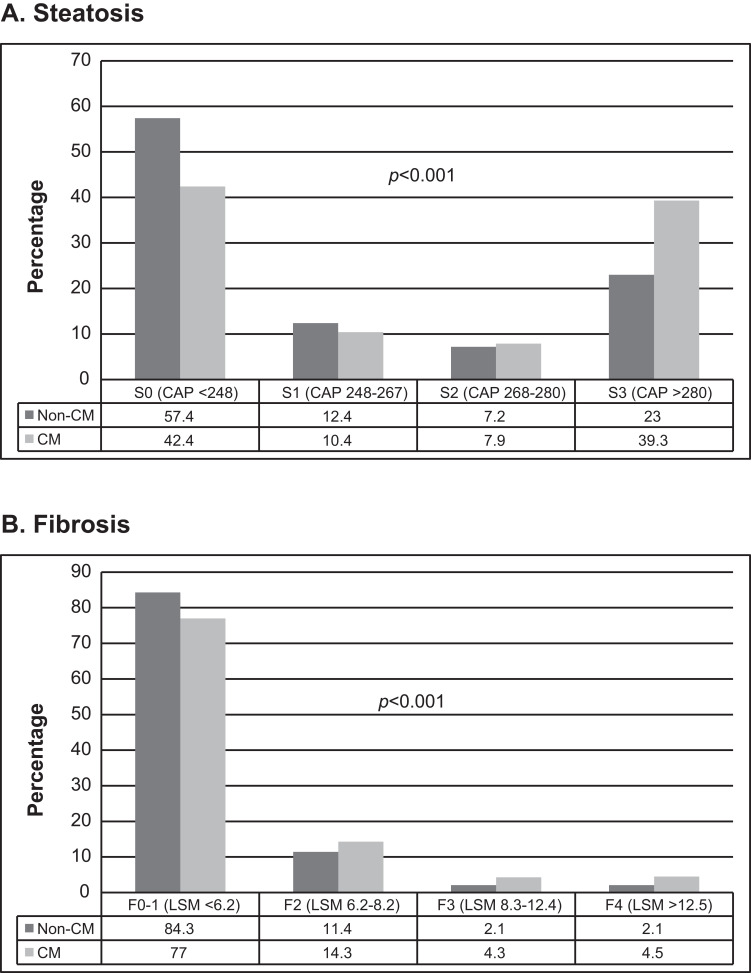
Figure 2 exhibits the proportion of different degrees of hepatic steatosis and fibrosis in the CM and non-CM groups. Compared to the non-CM group, the CM participants had significantly higher proportions of S3-4 (Figure 2A) and F3-4 (Figure 2B).
Figure 2.
The distribution of different degrees of hepatic steatosis (A) and fibrosis (B) in the CM and non-CM groups.
Multivariate Analysis for Sleeping Habit and the Risk of MAFLD
We performed a multivariate regression to evaluate the risk of CM and sleep duration for MAFLD. Three models were used to evaluate the odds ratio (OR) of aforementioned factors. Model 1 adjusted for age, gender and race. Model 2 adjusted for age, gender, race and the presence of metabolic profiles (diabetes, hypertension, waist circumstance, overweight, triglyceride, uric acid and high-density lipoprotein cholesterol). Model 3 adjusted for additional sleep duration and CM on the basis of Model 2.
The results of multivariable analysis (Table 3) showed MAFLD was independently associated with CM after adjusting for anthropometric and metabolic parameters (ORs were around 2 in both model 1 and 2, p<0.05). After adjusting for the sleep duration, the effect of CM was still significant (OR=1.893, 95% CI: 1.469–2.440, p<0.001). Short sleep duration (<6 hours) was independently associated with MAFLD if only adjusted for age, gender and race (Model 1, OR=1.554, 95% CI: 1.219–1.982, p<0.001) or metabolic profiles (Model 2, OR=1.653, 95% CI: 1.239–2.204, p=0.001). However, after additionally adjusting for CM, the association between sleep duration and MAFLD attenuated and was no longer statistically significant (Model 3, OR=1.267, 95% CI: 0.932–1.723, p=0.131).
Table 3.
Multivariate Analysis for the Relationship Between Circadian Misalignment/Sleep Duration and the Risk of MAFLD
| OR | Circadian Misalignment | P value | Sleep Duration <6 Hours | P value |
|---|---|---|---|---|
| Univariate | 2.090(1.718–2.543) | <0.001 | 1.613(1.273–2.043) | <0.001 |
| Model 1 | 2.198(1.795–2.691) | <0.001 | 1.554(1.219–1.982) | <0.001 |
| Model 2 | 2.024(1.594–2.569) | <0.001 | 1.653(1.239–2.204) | 0.001 |
| Model 3 | 1.893(1.469–2.440) | <0.001 | 1.267(0.932–1.723) | 0.131 |
Notes: Model 1 adjusted for age, gender and race. Model 2 adjusted for age, gender, race and metabolic profiles (diabetes, hypertension, waist circumstance, overweight, triglyceride, uric acid and high-density lipoprotein cholesterol). Model 3 adjusted for additional sleep duration or circadian misalignment on the basis of Model 2.
We analyzed the relationship between different circadian components and the prevalence of MAFLD by adjusting age, gender, race and sleep duration. In this multivariate analysis, the mistimed sleep (OR=2.035, 95% CI: 1.477–2.804, p<0.001) and late sleep (OR=1.891, 95% CI: 1.523–2.347, p<0.001) independently increased the risk for MAFLD, while the irregular chronotype (OR=0.988, 95% CI: 0.641–1.523 p=0.957) was not significantly associated with MAFLD.
Multivariate Analysis for Sleeping Habit and the Risk of Fibrosis in MAFLD Population
In this multivariable analysis, three models were adjusted for the same variables as the MAFLD (Table 4). The correlation between CM and advanced fibrosis persisted in each model (OR 1.307–1.637, p<0.05), while the short sleep duration was not associated with advanced fibrosis in both univariate and multivariate analysis (all p>0.05).
Table 4.
Multivariate Analysis for the Relationship Between Circadian Misalignment/Sleep Duration and the Risk of Fibrosis in MAFLD Population
| OR | Circadian Misalignment | P value | Sleep Duration <6 Hours | P value |
|---|---|---|---|---|
| Univariate | 1.637(1.302–2.059) | <0.001 | 1.288(0.980–1.692) | 0.070 |
| Model 1 | 1.450(1.142–1.840) | 0.002 | 1.140(0.858–1.513) | 0.366 |
| Model 2 | 1.321(1.027–1.699) | 0.030 | 1.157(0.857–1.562) | 0.342 |
| Model 3 | 1.307(1.002–1.706) | 0.049 | 1.040(0.757–1.429) | 0.808 |
Notes: Model 1 adjusted for age, gender and race. Model 2 adjusted for age, gender, race and metabolic profiles (diabetes, hypertension, waist circumstance, overweight, triglyceride, uric acid and high-density lipoprotein cholesterol). Model 3 adjusted for additional sleep duration or circadian misalignment on the basis of Model 2.
In the analysis of the components of CM, mistimed sleep (OR=1.458, 95% CI: 1.012–2.102, p=0.043), and late sleep (OR=1.434; 95% CI: 1.116–1.843, p=0.005) were significantly associated with fibrosis while irregular chronotype was not (OR=1.369, 95% CI: 0.786–2.048, p=0.330).
Discussion
The main and novel finding of our study is that, compared to short sleep (<6 hours), CM is more important for the development of MAFLD. These findings have important implications in the overall management of participants with MAFLD.
The prevalence of MAFLD was similar between participants with or without CM in overall population. However, the CM group was significantly younger than the non-CM group. As age and gender are known risk factors for the development of metabolic syndrome,24 not surprisingly that after matching for age and gender, the proportions of participants with deranged metabolic profiles and MAFLD were significantly higher in CM than the non-CM group. Further multivariate analysis revealed that CM was independently associated with nearly twofold higher risk of MAFLD independent of other metabolic disorders. The results of our study are in line with previously published epidemiology studies showing that night shift work is associated with higher odds of developing non-alcoholic fatty liver disease25 and deranged liver enzymes.26 Thus, the management of sleep should be another important part of lifestyle management for the prevention and treatment of MAFLD.
Shorter sleep duration is known to be associated with obesity and metabolic syndrome.27–29 The results of this study show that rather than sleep duration, it is actually the CM which is the predominant risk factor for steatosis and significant fibrosis. We firstly showed the short sleep duration (<6 hours/day) was independently associated with MAFLD before adjusting for CM. This result is consistent with the previous meta-analysis.30 However, once adjusted for CM, this relationship was no longer significant. This observation can be explained by the fact that sleep time is controlled by circadian genes, and some people physiologically tend to have short sleep.15,31 Sleep duration also changes with advancing age.32,33 However, CM due to the artificial light–dark cycle is not a normal physiological process. Continuous and prolonged CM may result in significant changes in circadian clock and downstream metabolic gene which may eventually lead to a pathological change.34
In this population-based study, we also established a significant correlation between CM and fibrosis. Circadian rhythm is frequently studied in animal experiment models because it is easier to control light–dark cycle in laboratory. Evidences from animal studies show that the loss of circadian gene Per2 (period 2) increases the risk of fibrosis by increasing hepatic stellate cell activation and inhibiting its apoptosis,35 therefore exacerbates cholestatic fibrosis in mice.36 The secretory product of the pineal gland, melatonin has also been shown to up-regulate clock genes expression and attenuate CCl4-induced fibrosis in mice and human hepatic stellate cells.37 These studies may explain the fibrogenesis mechanism of CM.
This study is the first population-based study using Fibroscan®, a recommended tool for detecting hepatic steatosis and fibrosis in clinical practice, to explore the association between CM and MAFLD/fibrosis. Propensity matching methods make the results even more robust. However, our study should be interpreted in light of some limitations. First, the type of work and proportion of participants involved in night shift work are not available in this dataset. Population with rotating work or night shift work only accounts for a small proportion of the general population and thus is neither representative nor the results are applicable to wider population at risk. This study directly analyzed the sleep habit in general population and the results are more representative. Second, data regarding sleep apnea, clinical diagnosis of insomnia and the use of sleeping pills were not available, which may have a bearing on the study results.
In conclusion, CM is independently associated with MAFLD whereas short sleep duration (<6 hours) is not an independent risk factor after adjusting for CM. Efforts should be directed to advice patients on the management of sleep in addition to diet, exercise and pharmacologic therapy.
Acknowledgments
Rahul Kumar and Su Lin are joint senior authors for this study.
Funding Statement
This research is supported by Qingzhong Medical Science Research Fund (B17344).
Abbreviations
NHANES, National Health and Nutrition Examination Surveys; MAFLD, metabolic-associated fatty liver disease; CM, circadian misalignment; CAP, controlled attenuation parameter; LSM, liver stiffness measurement; IQR; liver stiffness interquartile; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; PSM, propensity score matching.
Data Sharing Statement
The data of this study are from a public database which is at https://www.cdc.gov/nchs/nhanes/index.htm.
Author Contributions
Study concept and design: Zhiyuan Weng and Su Lin; acquisition, cleaning of data: Jiaofeng Huang and Su Lin; drafting of the manuscript: Su Lin, Zhiyuan Weng, Medha Singh and Rahul Kumar; critical revision: Mingfang Wang, Yueyong Zhu, Medha Singh and Rahul Kumar; statistical analysis: Su Lin and Weijie Ou. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti K, Stern N, et al. The circadian syndrome: is the metabolic syndrome and much more! J Intern Med. 2019;286:181–191. doi: 10.1111/joim.12924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SN, Oster H. How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res. 2015;24:476–493. doi: 10.1111/jsr.12307 [DOI] [PubMed] [Google Scholar]
- 4.Jniene A, Errguig L, El Hangouche AJ, et al. Perception of sleep disturbances due to bedtime use of blue light-emitting devices and its impact on habits and sleep quality among young medical students. Biomed Res Int. 2019;2019:7012350. doi: 10.1155/2019/7012350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin-Alejandre BA, Abete I, Cantero I, et al. Association between sleep disturbances and liver status in obese subjects with nonalcoholic fatty liver disease: a comparison with healthy controls. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 7.Lin S, Huang J, Wang M, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082–2089. doi: 10.1111/liv.14548 [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 9.Sun DQ, Jin Y, Wang TY, et al. MAFL D and risk of CKD. Metabolism. 2020;115:154433. doi: 10.1016/j.metabol.2020.154433 [DOI] [PubMed] [Google Scholar]
- 10.Koren D, Taveras EM. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism. 2018;84:67–75. doi: 10.1016/j.metabol.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 11.Sirota JC, McFann K, Targher G, Chonchol M, Jalal DI. Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988-1994. Am J Nephrol. 2012;36:466–471. doi: 10.1159/000343885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Liu Y, Wan B, et al. Association between Vitamin D status and non-alcoholic fatty liver disease: a population-based study. J Nutr Sci Vitaminol (Tokyo). 2019;65:303–308. doi: 10.3177/jnsv.65.303 [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Lin S, Wang MF, et al. Association between NAFLD and risk of prevalent chronic kidney disease: why there is a difference between east and west? BMC Gastroenterol. 2020;20:139. doi: 10.1186/s12876-020-01278-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciardullo S, Monti T, Perseghin G. Prevalence of liver steatosis and fibrosis detected by transient elastography in adolescents in the national health and nutrition examination survey 2017-2018. Clin Gastroenterol Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 15.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679 [DOI] [PubMed] [Google Scholar]
- 16.Watson NF, Badr MS, Belenky G, et al. Joint Consensus Statement of the American Academy of Sleep Medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38:1161–1183. doi: 10.5665/sleep.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walch OJ, Cochran A, Forger DB. A global quantification of “normal” sleep schedules using smartphone data. Sci Advan. 2016;2:e1501705. doi: 10.1126/sciadv.1501705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25:6053–6062. doi: 10.3748/wjg.v25.i40.6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassinotto C, Boursier J, de Lédinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–1827. doi: 10.1002/hep.28394 [DOI] [PubMed] [Google Scholar]
- 20.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 21.Eslam M, Newsome PN, Rinella M, et al. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 22.Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594:2061–2073. doi: 10.1113/JP270538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papatheodoridi AM, Chrysavgis L, Koutsilieris M, Chatzigeorgiou A. The role of senescence in the development of nonalcoholic fatty liver disease and progression to nonalcoholic steatohepatitis. Hepatology. 2020;71:363–374. doi: 10.1002/hep.30834 [DOI] [PubMed] [Google Scholar]
- 24.Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34–42. doi: 10.1016/j.phrs.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Wang Y, Wang Z, et al. Rotating night shift work and non-alcoholic fatty liver disease among steelworkers in China: a cross-sectional survey. Occup Environ Med. 2020;77:333–339. doi: 10.1136/oemed-2019-106220 [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Zhang L, Wu S, et al. Night shift work and abnormal liver function: is non-alcohol fatty liver a necessary mediator? Occup Environ Med. 2019;76:83–89. doi: 10.1136/oemed-2018-105273 [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among U.S adults. Obesity (Silver Spring, Md). 2014;22:598–607. doi: 10.1002/oby.20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elder BL, Ammar EM, Pile D. Sleep duration, activity levels, and measures of obesity in adults. Public Health Nursing (Boston, Mass) 2016, 33: 200–205. [DOI] [PubMed] [Google Scholar]
- 29.Hung HC, Yang YC, Ou HY, Wu JS, Lu FH, Chang CJ. The association between self-reported sleep quality and overweight in a Chinese population. Obesity (Silver Spring, Md). 2013;21:486–492. [DOI] [PubMed] [Google Scholar]
- 30.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Short sleep duration and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:1802–1807. doi: 10.1111/jgh.13391 [DOI] [PubMed] [Google Scholar]
- 31.Ashbrook LH, Krystal AD, Fu YH, Ptáček LJ. Genetics of the human circadian clock and sleep homeostat. Neuropsychopharmacology. 2020;45:45–54. doi: 10.1038/s41386-019-0476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaput JP, Dutil C, Sampasa-Kanyinga H. Sleeping hours: what is the ideal number and how does age impact this? Nat Sci Sleep. 2018;10:421–430. doi: 10.2147/NSS.S163071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11. doi: 10.1016/j.jsmc.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rijo-Ferreira F, Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Med. 2019;11(1):82. doi: 10.1186/s13073-019-0704-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen P, Han Z, Yang P, Zhu L, Hua Z, Zhang J. Loss of clock gene mPer2 promotes liver fibrosis induced by carbon tetrachloride. Hepatol Res. 2010;40(11):1117–1127. doi: 10.1111/j.1872-034X.2010.00695.x [DOI] [PubMed] [Google Scholar]
- 36.Chen P, Kakan X, Wang S, et al. Deletion of clock gene Per2 exacerbates cholestatic liver injury and fibrosis in mice. Exp Toxicol Pathol. 2013;65(4):427–432. doi: 10.1016/j.etp.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 37.González-Fernández B, Sánchez DI, Crespo I, et al. Melatonin attenuates dysregulation of the circadian clock pathway in mice with CCl(4)-induced fibrosis and human hepatic stellate cells. Front Pharmacol. 2018;9:556. doi: 10.3389/fphar.2018.00556 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]