Abstract
Background:
Parents of childhood cancer patients experience changes in relationships with their health-care team as the child transitions from treatment to long-term survivorship (LTS). These changes may affect parent receptivity of survivorship-health–related programs, yet little is known about the experience of changing clinical relationships for parents as treatment ends and children transition into LTS.
Methods:
In-depth, semistructured interviews were conducted with 20 English-speaking parents of childhood cancer survivors less than 13 years old who were greater than 1-year posttreatment. Audiotaped, transcribed interview content was analyzed using emergent themes grouped and refined in a process of multistaged constant comparison.
Results:
There was a consensus among parents regarding the emotional stressors of the period immediately after the end of treatment. Regardless of positive or negative recollection of treatment, parents commonly viewed their health-care team with affection and this period as one of stability and security. Transitioning off treatment was viewed as a severe disruption of the security of weekly, treatment-related contact with nurses, nurse practitioners, physician's assistants, and physicians. LTS was generally viewed as presenting lower levels, but new types of stress as new, psychosocial late effects were seen to emerge. Clinical needs shifted to prevention and late-effect management.
Conclusion:
Parents of young childhood cancer survivors experience a similar emotional trajectory from treatment to transitioning off treatment and into LTS. This period is seen by parents as uniquely distressing because it represents a disruption of the hard-won safety represented by regular clinical relationships.
Keywords: childhood cancer, parent distress, psychosocial late effects, survivorship
1. BACKGROUND
Childhood cancer survivors (CCS) are at risk for experiencing late effects from cancer treatments1–3 and are at higher risk for chronic diseases and early death compared with noncancer siblings and peers.4–7 Parents of CCS play a significant role in their child's adherence to survivorship screening schedules, management of late effects, and practice of preventive health behaviors.8–13 Several survivorship-related programs have been developed to help parents more effectively engage in different aspects of their child's survivorship care and thus improve long-term survivor outcomes.12,14–16 However, little is known about how such programs may interact with the different phases in parents' experience of the survivorship journey. As parents move from diagnosis through treatment, into follow-up care, and on into long-term survivorship (LTS), they are faced with different challenges in diverse contexts.17–20 The varying experiences of parents throughout this continuum may affect parents' relationship with their clinical team, as well as the receptivity of parents to engage with survivorship-related health programs. To date, the parent experience of progressing through this continuum and transitioning between its phases remains understudied.
The period around the end of treatment (EOT) has been shown to be characterized by parental emotional distress,21–23 even if in the context of other positive emotions.24,25 The transition off of treatment and into survivorship marks a dramatic change in the relationship between parents and the clinical care team. The regular routine of treatments and lab appointments abruptly ends, followed by increasingly extended intervals between follow-up appointments that eventually transition into even larger intervals between survivorship clinic appointments.
Much of the focus on parent experience after childhood cancer treatment has been placed on the period immediately after treatment. Cross-sectional studies show that parent emotional distress is substantially elevated at EOT and may remain higher than population norms long after.26–28 However, a longitudinal study by Maurice-Stam and colleagues21 showed a common trajectory of steady decline in parent distress after transient increase around EOT. Together, these findings suggest a common experiential trajectory among parents of CCS, but the survey data leave its detailed contours unexplored. A qualitative analysis and thematic modelling of subjective experience of this trajectory may provide important insight for the development of late-effect management and preventive health interventions for families of young CCS.
The present study adds to prior research on the parent experience of transitioning off treatment and into LTS—defined in this study by parents as a new phase beginning around two years off treatment—by focusing on parent perceptions of transitions in clinical support. While the personal, emotional dimensions around EOT have been described previously,23–25 little qualitative data has been published on how shifting clinical relationships are perceived and how these perceptions relate to parents' engagement in follow-up care and articulation of unmet information and resource needs. The primary aim of this study was to identify parent perceptions of clinical survivorship care relationships and support and to develop a thematic model that characterizes patterns of parent experience after EOT and into LTS.
2. METHODS
2.1. Participants
Study procedures were approved by the institutional review board of the University of California, Irvine (HS# 2016–3169). Participants elected to be contacted for interviews through a previously completed online survey of parents of CCS recruited through US-based childhood cancer parent support organizations. Participants provided separate informed consent for the interview. The foci of the survey were survivorship care and social support. Eligibility criteria for survey participation were (a) parent of a survivor of pediatric solid tumor or blood cancer, (b) at least 18 years old, (c) child is at least 1 year off treatment, (d) child is under 13 years old, and (e) English speaking. Parents who elected to be contacted were purposively sampled to achieve diverse representation in self-reporting of ethnicity, education level, cancer type, and social media use. A sample size of 20 was determined adequate using the “information power” criteria for qualitative sample size.29 These criteria, which take into account the aim of the study, sample specificity, use of theory, quality of data, and analytical strategy, can be used to determine in advance an adequate sample size. This is an alternative to the “saturation” method for determining sample size during the data collection and analysis process.
2.2. Procedure
The interviews were conducted by the lead researcher (J.W.) who is trained in qualitative interview techniques and is also a parent of a childhood cancer survivor. The semistructured interviews followed an interview guide consisting of open-ended questions focused on three broad themes: clinical follow-up care and survivorship resources, preventive health resources, and posttreatment social support. Survivorship-care–related questions are available in Data S1. Probing follow-up questions were asked for clarification or elaboration. All interviews were audio-recorded and transcribed verbatim.
2.3. Analysis
Transcribed interviews were analyzed by two coders (J.W. and S.H.) using an inductive grounded theory approach.30,31 According to this approach, data coding and theory selection inform each other in a multistage iterative process. An initial round of coding was completed in ATLAS.ti on the entire sample to generate all possible inductive codes.32 Memos and summaries were generated alongside this initial coding and used to achieve consensus on naming and meaning of themes. A second round of coding produced a matrix by which case data could be compared with each other by code in a constant comparison analysis.31 A full thematic model is produced by generating and integrating diagrams throughout iterative process of connecting emergent themes, subthemes, and related concepts, as described in Charmaz.31 To check for coding and thematic validity, each author and three parent participants reviewed the thematically organized coding matrix and deidentified raw data alongside the emergent thematic model. Suggested edits were made and resubmitted to authors and participants until consensus on coding and theory building was reached.
3. RESULTS
One-hundred and twelve parents completed the online survey, and 106 (95%) agreed to be contacted for an interview between August 2017 and April 2018. Forty parents were contacted, and 20 responded and completed telephone interviews. Respondents were from the United States and Canada and reported residence in 16 different counties or provinces. Participant characteristics are presented in Table 1. Response bias analyses indicated no significant differences in parent age (t(38) = −0.45, P = 0.65), ethnicity (χ2(5) = 2.23, P = 0.82), parent education (χ2(3) = 4.73, P = 0.19), child's diagnosis (χ2(6) = 5.39, P = 0.49), marital status (χ2(2) = 3.27, P = 0.19), or time since treatment (χ2(3) = 3.32, P = 0.34) between parents who responded to interview requests and those who did not. Participant quotes are presented in a Data S1 available online, referenced in text as Q with a corresponding number in the table.
TABLE 1.
Parent demographics and child’s clinical characteristics (N = 20)
| Variable | Parents | Children |
|---|---|---|
| Age: mean (SD) | 38.7 (6.7) | 8.2 (2.4) |
| Gender (%) | ||
| Female | 17 (85%) | |
| Male | 3 (15%) | |
| Ethnicity (%) | ||
| Non-Hispanic white | 15 (75%) | |
| Non-Hispanic black | 0 (0%) | |
| Hispanic or Latino | 1 (5%) | |
| Asian/Pacific Islander | 1 (5%) | |
| Other | 3 (15%) | |
| Diagnosis (%) | ||
| Brain tumor | 3 (15%) | |
| Leukemia | 9 (45%) | |
| Non-Hodgkin Lymphoma | 2 (10%) | |
| Neuroblastoma | 2 (10%) | |
| Sarcoma | 3 (15%) | |
| Ovarian Germ Cell Tumor | 1 (5%) | |
| Treatments Received (%) | ||
| Surgery (brain) | 3 (15%) | |
| Radiation | 7 (35%) | |
| Chemotherapy | 19 (95%) | |
| Time off treatment (%) | ||
| Between 1 and 2 years | 8 (40%) | |
| Between 2 and 5 years | 9 (45%) | |
| Between 5 and 10 years | 3 (15%) | |
| Marriage Status (%) | ||
| Married | 15 (75%) | |
| Separated | 0 (0%) | |
| Divorced | 3 (15%) | |
| Never married | 2 (10%) | |
| Education | ||
| <High school diploma | 0 (0%) | |
| High school diploma | 1 (5%) | |
| Some College | 6 (30%) | |
| College degree | 13 (65%) |
3.1. Parent clinical experiences during treatment
Parents recalled their child's treatment as difficult and challenging. In response to the open-ended question, “What was treatment like for you, your family, and your child?” parents recounted child, sibling, and parent emotional distress (Q1–5). Although the physical demands on parents, such as lack of sleep, eased over the course of treatment, parents' emotional distress continued as they reported coping with difficult adverse effects of their child's treatment, a loss of peer connection for their child during treatment, and adjusting to significant changes in daily lifestyle.
Because of the physical and emotional distress surrounding treatment, parents recalled looking forward to EOT (Q6–9). These expectations were unfulfilled, and upon reflection, parents commonly looked back on treatment as a time when clinical relationships provided a source of safety and security they did not recognize as such at the time. Regardless of how difficult treatment was and how anticipated was its end, from the vantage point of being off treatment, those difficult days of treatment were remembered as safe and secure.
3.2. End-of-treatment parent experiences
Despite the perceived difficulties of the treatment period, the juxtaposition of the “safety” and “security” of regular clinic visits, lab testing, and personal contact with physicians and nurses heightened parents' sense of absence when treatment ended. Some parents experienced EOT as even more difficult than treatment (Q10–11). Other parents experienced EOT as bringing with it new but not necessarily worse anxieties (Q12–14). Regardless of how parents compared anxiety at EOT with the challenges of treatment, parents unanimously perceived EOT to be marked by a distressing sense of absence. They saw the security of regular clinical relationships dissolve (Q15–18). They also saw the security of clinical roadmaps disappear (Q19–21). Finally, some parents experienced an absence of social support around EOT (Q22–24).
Parents' sense of absence at EOT led them to reflect on the lack of preparation for the distress of transitioning off treatment (Q25–26). In contrast, one parent reported having a farsighted oncology team that prepared her for the emotional challenges of transition (Q27). Others felt that sufficient preparation was not possible (Q28). Parents commonly reflected on the need for repairing emotional damage from treatment and EOT (Q29–30).
3.3. Transition into long-term survivorship
For many parents, the acute sense of distress experienced around EOT gave way to a lower level chronic concern about the long-term late effects of treatment and preventive health behaviors that might mitigate them. After the distress of losing the security of clinical treatment and associated relationships, parents reported finding “a new normal.” The immediate posttreatment late effects with which parents coped were largely physical and included fine and gross motor deficits, hearing loss, neuropathy, stunted linear growth, fatigue, excessive weight gain, and endocrine dysfunction. Even when there was no apparent posttreatment physical dysfunction, some parents and children had to learn to cope with new physical realities of treatment such as the two parents of survivors who reported difficulty coping with the loss of the child's eye, managing a new prosthetic eye, and the emotional and social changes that followed. This immediate posttreatment transition period was seen by parents to last between 6 months and 2 years and was not concluded by sudden changes in the child's health status or parent's distress levels. Instead, parents reported a slow shift from coping with immediate late effects and the fear of cancer recurrence to anticipating the challenges of LTS (Q31–32). This transition into a concern for LTS health was not unanimous, however (Q33). One parent's child was 3 years removed from treatment and continued to have significant limitations in motor function. He reported a desire to focus on the present.
The long-term late effects in survivors that many parents reported were psychosocial as well as physical. Poor emotional regulation, anxiety, cognitive delay, and poor social functioning were reported as current health problems that parents saw as being potentially long term. The focus on long-term late effects led parents to turn their attention to preventive health behaviors (Q34–35). But parents did not see this interest in preventive health behavior to be matched in clinical survivorship care. In many cases, parents reported receiving no treatment summary or survivorship care plan (Q36–38). Even when such documentation was provided, parents perceived a lacuna in their child's survivorship care with regard to preventive health information (Q39). Other parents believed that survivorship programs must exist at the hospital, but they just have not been made aware of them (Q40–41).
While some parents desired more intensive resources such as cooking classes, child-focused exercise classes, formal health behavior education classes, and referrals to dietitians, health coaches, and counselors, other parents desired “just a conversation” or “a sit down” with their oncologist to discuss preventive health behaviors in survivorship (Q42). Some parents with older children expressed desire for the oncologist to discuss directly with the child the importance of preventive health behaviors “because it would reinforce what I'm saying” (Q43).
3.4. Gender roles
Both mothers and fathers reflected on the ways gendered roles determined how treatment and survivorship were experienced. Mothers and fathers experienced “a division of labor” in which parents “picked our roles.” This division was characterized by the mother taking the lead as caregiver for the child, disease expert, and primary liaison with doctors and therapists (Q44–45). This common pattern was seen to lead to a lack of social support for fathers (Q46–47). While fathers saw the division of labor as primarily concerning medical information and adherence rather than direct caregiving, some mothers saw the division of labor as fundamentally structuring the childhood cancer caregiving experience (Q48–49). These different experiences often led to the mother making final decisions on medical care and the father following her lead.
3.5. Model of experiential trajectory
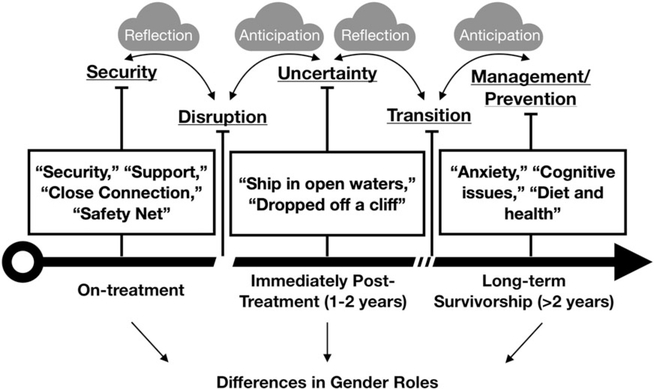
An emergent thematic model (Figure 1) was developed to represent patterns of parent caregiving experiences in the context of childhood cancer survivorship. The integrative themes that emerged from individual codes and intermediate themes revolved around narrative trajectories in which each experience related to childhood cancer was made sense of as a reflection on the immediate prior experience and an anticipation of the experiences that were expected to follow. Each new phase was interpreted through direct reflection on the previous stage and in anticipation of the stage that was expected to follow.
FIGURE 1.
A model of the experiential trajectory of the clinical transition off treatment and into long-term survivorship for parents of young childhood cancer survivors
The unexpected feelings of security and safety that parents reported when recalling their child's cancer treatment were commonly seen in the context of reflections on the jarring disruption of the EOT transition. The disruption of the transition was felt acutely because of the juxtaposition between the security of treatment and uncertainty of survivorship. The immediate posttreatment phase of survivorship was unanimously experienced as a time of uncertainty as parents struggled to find new sources of the routine, supportive, and secure relationships they felt during treatment. The uncertainty surrounding EOT was seen to fade for most parents as a “new normal” was established. However, this new normal was often marked by additional, emerging challenges with survivors' physical and mental health, school performance, and social functioning. The recognition of the persistence of these challenges, and a desire to reduce anxiety and uncertainty, initiated a transition from the ambiguity of the immediate posttreatment phase into a more stable LTS phase. In the latter, parents shifted their focus to late-effect management and long-term health-risk prevention. Concerns about cancer recurrence slowly faded in this period, replaced by concerns about what survivors' lives would look like decades in the future. In this latest phase, parents expressed disappointment that clinical late-effect management felt disjointed and pieced together between several different specialists, a primary care doctor who seemed unprepared to care for a CCS, and a long-term follow-up oncologist who was seen rarely and focused primarily on screening.
The gendered differences in caregiving experiences meant that mothers reported experiencing each phase and transition more acutely than fathers. The division of labor led fathers to take an ancillary role in treatment, which extended into survivorship care. However, fathers reported similar trajectories that began with the difficult but secure context of treatment, followed by the uncertainty of EOT, and concluded with a focus on long-term late-effect management and disease prevention.
4. DISCUSSION
This study described the perceptions and experiences of parents of young CCS with regard to the changes in clinical survivorship care relationships and support from treatment into survivorship care. The interview data were integrated into an emergent, thematic model that presents caregiving experiences in the context of survivorship as an experiential trajectory that begins with the difficult but secure experience of cancer treatment, punctuated by the jarring interruption in support marked by EOT. This disorientation was experienced as marking a transitional phase of deep uncertainty. No single event concluded this phase; parents instead reported a slow and steady transition into long-term late effect management and focus on longterm disease prevention. Both phases after treatment were characterized by parents' disappointment in clinical supportive care.
Parent anxiety around EOT has been identified previously. Wakefield and colleagues22 reviewed 15 studies on the psychosocial impact of treatment completion on parents of CCS and found that these caregivers were at increased risk for feelings of anxiety, uncertainty, helplessness, and loneliness compared with noncancer populations. They interpreted the qualitative literature as demonstrating that parental concern over recurrence was the key driver of emotional distress at EOT. Thus, Wakefield and colleagues suggest effective risk communication targeting fears of recurrence may alleviate parent emotional distress. However, our data suggest that recurrence is just one part of a complex set of concerns parents have during the transition. These concerns should be understood in the context of a common experiential trajectory, rather than as an acute or chronic miscalculation of risk.
Parents understood their distress as emerging out of a transition from a difficult-but-secure steady state of treatment in which, despite its obvious difficulties, parents felt “safe,” secure,“” supported,“” and “connected.” The common fear of recurrence was only a part of a larger structure of concern that included their child's physical, mental, and social functioning, as well as a more difficult-to-articulate sense of lost routine, security, and support. Even after fears of recurrence subsided, parents in the study expressed continued, yet low-level, concern about their child in part because they perceived they were facing present and future late effects without the same level of support they received during treatment. Effective risk communication may alleviate some anxiety, but such interventions would be unlikely to assuage concerns over a loss of regular routine, support, and caring or adequately meet parents desire for more integrated late-effect management and preventive health resources.
Our findings are congruent with studies that show, despite the difficulties of cancer treatment, parents feel better prepared to cope with it than they do with life after treatment. Greenzang and colleagues11 recently estimated that 87% of parents reported feeling well prepared for their child's cancer treatment, whereas 62% reported feeling well prepared for life after treatment. Importantly, they found that at EOT both parents and clinicians dramatically underestimated the long-term health challenges survivors would face. Our data suggest that as parents move into the third phase of management and prevention (Figure 1), parents begin to recognize the full extent of the survivorship health challenges their child faces. The question of whether these challenges are adequately acknowledged in LTS clinical care remains open.
How generalizable is the thematic model produced from the coding process? Arnold33 found a similar psychosocial response among adult breast cancer survivors to the EOT transition. These survivors described their need for a continued “safety net of support” after treatment ended. As in Wakefield and colleagues' review of studies on parents of CCS,22 a central concern was disease recurrence. However, these adult survivors also expressed a loss of social support, routine, safety, and direction that treatment offered. Treatment was comforting because patients saw it as effectively treating their disease. But the social and institutional apparatus surrounding the provision of chemotherapy was just as important to their psychosocial well-being. Our findings suggest that this is a potentially common theme for the experience of the cancer continuum for both caregivers and patients.
5. CONCLUSION
This study described parent narratives of their experiences enduring cancer treatment for their child. Interpreting these parent narratives has contributed to the development of a thematic model based on emergent themes of parent experiences during and after cancer treatment. The experiential trajectory model highlights inflection points in the journey of parents of CCS. The identified points of transition were not only experienced by parents as turbulent transitions, but offer target time points that are ripe for interventions. The uncertainty and anxiety parents experience at EOT was not the result of objectively challenging circumstances but rather a response to the safe and secure routine of the otherwise difficult phase of cancer treatment. The final phase of survivorship for these parents was marked by a concern for better late-effect management and preventive health behaviors. This concern grew out of reflections on the uncertainty of being off treatment, a desire to reduce this uncertainty, and an anticipation of longterm quality of life for the survivor.
5.1. Study limitations
This cross-sectional study did not follow participants over time. The heterogeneity of our sample also limits our ability to draw conclusions relating to subgroups of caregivers such as those with specific diagnoses or treatment exposures. Nevertheless, the diversity of geography and diagnoses indicates that emergent patterns represented in the thematic model may not be strongly influenced by geography and cancer type. Additionally, the overrepresentation of white, highly educated mothers may have biased the results. The three fathers in our sample reported similar experiences across the cancer continuum, although from the perspective of an “assistant” to the lead caregiver. It is likely that had we recruited more fathers we would have recorded accounts of different experiences of fathering in survivorship. Finally, our qualitative sample was selected from a larger sample that was recruited through private social media support groups for parents of CCS. Thus, we did not recruit parents who did not use social media, and thus it is unknown how these parents' experiences may differ. Further, because recruitment relied partially upon posts in online support groups, we could not calculate a response rate for the online survey.
5.2. Clinical implications
These findings have several implications for supportive care after EOT. First, improved psychosocial preparation by the clinical care team may help parents anticipate the jarring transition to EOT by encouraging them to draw on social networks for support and engage in effective anxiety-reducing practices. Understanding that EOT is not only a time of joy but can also be stressful may provide parents with a sense of orientation in an otherwise disorienting period. Second, more accessible care during this transition may provide parents with extended feelings of security they felt during treatment. One solution that would avoid burdening clinical care teams would be the development of online and mobile support that could connect parents to more experienced peers at EOT. Peer health coach models have been developed and evaluated with success in other contexts34–38 and may be ideal for parents at EOT who not only seek reassurance about the future but also have many immediate caregiving concerns. Finally, a significant gap in preventive health behavior education and support could be addressed, again with minimal burden on survivorship clinic resources, through community-based programs. Currently, adult survivors are offered free or subsidized survivorship-focused exercise programs through LIVESTRONG at the YMCA.39,40 Although presently no specific CCS programs have been developed, survivorship clinics serving large metropolitan regions should consider developing relationships with these programs to develop appropriate exercise resources for child, adolescent, and young adult survivors. Additionally, there is a need for both clinicians and parents to advocate for increased research and programmatic support in preventive health for CCS.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Biobehavioral Shared Resource and the Biostatistical Shared Resource of the Chao Family Comprehensive Cancer Center, University of California, Irivine and the National Cancer Institue (P30 CA062203).
Funding information
Biobehavioral Shared Resource and the Biostatistical Shared Resource of the Chao Family Cancer Center, School of Medicine, University of California, Irvine, Grant/Award Number: N/A; National Cancer Institute, Grant/Award Number: P30 CA062203
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Cancer. 2005;104(8):1751–1760. [DOI] [PubMed] [Google Scholar]
- 2.Saha A, Gardner S. Late effects in survivors of childhood CNS tumors: review of results from the two largest survivorship cooperative groups. Curr Cancer Ther Rev. 2013;9(2):126–136. [Google Scholar]
- 3.Ljungman L, Cernvall M, Grönqvist H, Ljótsson B, Ljungman G, von Essen L. Long-term positive and negative psychological late effects for parents of childhood cancer survivors: a systematic review. PLoS ONE. 2014;9(7):e103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson TM, Ehrhardt MJ, Ness KK. Obesity and metabolic syndrome among adult survivors of childhood leukemia. Curr Treat Options Oncol. 2016;17(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakefield CE, Butow P, Fleming CA, Daniel G, Cohn RJ. Family information needs at childhood cancer treatment completion. Pediatr Blood Cancer. 2012;58(4):621–626. [DOI] [PubMed] [Google Scholar]
- 9.Doshi K, Kazak AE, Hocking MC, et al. Why mothers accompany adolescent and young adult childhood cancer survivors to follow-up clinic visits. J Pediatr Oncol Nurs. 2014;31(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vetsch J, Rueegg CS, Gianinazzi ME, Bergsträsser E, von der Weid NX, Michel G. Information needs in parents of long-term childhood cancer survivors. Pediatr Blood Cancer. 2015;62(5):859–866. [DOI] [PubMed] [Google Scholar]
- 11.Greenzang KA, Cronin AM, Mack JW. Parental preparedness for late effects and long-term quality of life in survivors of childhood cancer. Cancer. 2016;122(16):2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raber M, Swartz MC, Maria DS, et al. Parental involvement in exercise and diet interventions for childhood cancer survivors: a systematic review. Pediatr Res. 2016;80(3):338–346. [DOI] [PubMed] [Google Scholar]
- 13.Williams LK, Lamb KE, McCarthy MC. Parenting behaviors and nutrition in children with leukemia. J Clin Psychol Med Settings. 2015;22(4):279–290. [DOI] [PubMed] [Google Scholar]
- 14.Gramatges MM, de Nigris FB, King J, Horowitz ME, Fordis M, Poplack DG. Improving childhood cancer survivor care through web-based platforms. Oncology (Williston Park). 2018;32(1):e1–e10. [PubMed] [Google Scholar]
- 15.Schadler KL, Kleinerman ES, Chandra J. Diet and exercise interventions for pediatric cancer patients during therapy: tipping the scales for better outcomes. Pediatr Res. 2017;83:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mays D, Black JD, Mosher RB, Heinly A, Shad AT, Tercyak KP. Efficacy of the Survivor Health and Resilience Education (SHARE) program to improve bone health behaviors among adolescent survivors of childhood cancer. Ann Behav Med. 2011;42(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norberg AL. Burnout in mothers and fathers of children surviving brain tumour. J Clin Psychol Med Settings. 2007;14(2):130–137. [Google Scholar]
- 18.Norberg AL, Boman KK. Parent distress in childhood cancer: a comparative evaluation of posttraumatic stress symptoms, depression and anxiety. Acta Oncol. 2009;47:267–274. [DOI] [PubMed] [Google Scholar]
- 19.Leventhal-Belfer L, Bakker AM, Russo CL. Parents of childhood cancer survivors. J Psychosoc Oncol. 2008;11(2):19–41. [Google Scholar]
- 20.Tackett AP, Cushing CC, Suorsa KI, et al. Illness uncertainty, global psychological distress, and posttraumatic stress in pediatric cancer: a preliminary examination using a path analysis approach. J Pediatr Psychol. 2015;41:309–318. [DOI] [PubMed] [Google Scholar]
- 21.Maurice-Stam H, Oort FJ, Last BF, Grootenhuis MA. Emotional functioning of parents of children with cancer: the first five years of continuous remission after the end of treatment. Psychooncology. 2008;17(5):448–459. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield CE, McLoone JK, Butow P, Lenthen K, Cohn RJ. Parental adjustment to the completion of their child's cancer treatment. Pediatr Blood Cancer. 2010;56(4):524–531. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie SE, Curle C. ‘The end of treatment is not the end’: parents' experiences of their child's transition from treatment for childhood cancer. Psychooncology. 2012;21(6):647–654. [DOI] [PubMed] [Google Scholar]
- 24.Björk M, Nordström B, Wiebe T, Hallström I. Returning to a changed ordinary life--families' lived experience after completing a child's cancer treatment. Eur J Cancer Care (Engl). 2011;20(2):163–169. [DOI] [PubMed] [Google Scholar]
- 25.Muskat B, Jones H, Lucchetta S, Shama W, Zupanec S, Greenblatt A. The experiences of parents of pediatric patients with acute lymphoblastic leukemia, 2 months after completion of treatment. J Pediatr Oncol Nurs. 2017;34(5):358–366. [DOI] [PubMed] [Google Scholar]
- 26.Grootenhuis MA, Last BF. Parents' emotional reactions related to different prospects for the survival of their children with cancer. J Psychosoc Oncol. 1997;15(1):43–62. [Google Scholar]
- 27.Boman K, Lindahl A, Bjork O. Disease-related distress in parents of children with cancer at various stages after the time of diagnosis. Acta Oncol. 2003;42(2):137–146. [DOI] [PubMed] [Google Scholar]
- 28.Wijnberg-Williams BJ, Kamps WA, Klip EC, Hoekstra-Weebers JEHM. Psychological adjustment of parents of pediatric cancer patients revisited: five years later. Psychooncology. 2005;15(1):1–8. [DOI] [PubMed] [Google Scholar]
- 29.Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2015;26:1753–1760. [DOI] [PubMed] [Google Scholar]
- 30.Corbin J, Strauss A. Basics of qualitative research: techniques and procedures for developing grounded theory. Thousand Oaks, CA: SAGE Publications; 2014. [Google Scholar]
- 31.Charmaz K. Constructing grounded theory. Thousand Oaks: SAGE; 2014. [Google Scholar]
- 32.Morse JM. Data were saturated. Qual Health Res. 2015;25(5):587–588. [DOI] [PubMed] [Google Scholar]
- 33.Arnold EM. The cessation of cancer treatment as a crisis. Soc Work Health Care. 1999;29(2):21–38. [DOI] [PubMed] [Google Scholar]
- 34.Moskowitz D, Thom DH, Hessler D, Ghorob A, Bodenheimer T. Peer coaching to improve diabetes self-management: which patients benefit most. J Gen Intern Med. 2013;28(7):938–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013;11(2):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leahey T, Wing R. A Randomized controlled pilot study testing three types of health coaches for obesity treatment: professional, peer, and mentor. Obesity. 2012;21:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghorob A, Vivas MM, De Vore D, et al. The effectiveness of peer health coaching in improving glycemic control among low-income patients with diabetes: protocol for a randomized controlled trial. BMC Public Health. 2011;11(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. J Med Internet Res. 2011;13(1):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin ML, Cartmel B, Harrigan M, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer. 2017;123(7):1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heston AH, Schwartz AL, Justice-Gardiner H, Hohman KH. Addressing physical activity needs of survivors by developing a community-based exercise program: LIVESTRONG® at the YMCA. Clin J Oncol Nurs. 2015;19(2):213–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.