Abstract
Background:
Solid organ transplant (SOT) recipients are considered to be “vulnerable” to COVID-19 infection due to immunosuppression. To date, there are no studies that compared the disease severity of COVID-19 in SOT recipients with nontransplant patients.
Methods:
In this case-control study, we compared the outcomes of COVID-19 between SOT recipients and their matched nontransplant controls. The cases were all adult SOT recipients (N=41) from our academic health center who were diagnosed with COVID-19 between 3/10/20 and 5/15/20 using positive reverse transcriptase polymerase chain reaction for SARS-CoV2. The controls(N=121) were matched on age(±5years), race, and admission status (hospital or outpatient). The primary outcome was death and secondary outcomes were severe disease, intubation and renal replacement therapy(RRT).
Results:
Median age of SOT recipients(9-heart, 3-lung, 16-kidney, 8-liver, 5-dual-organ) was 60 years, 80% were male and 67% were Black. Severe disease adjusted risk of death was similar in both the groups(HR=0.84[0.32–2.20]). Severity of COVID-19 and intubation were similar but the RRT use was higher in SOT (OR=5.32[1.26, 22.42]) compared to non-SOT COVID-19 patients. Among SOT recipients, COVID-19-related treatment with hydroxychloroquine (HCQ) was associated with ten-fold higher hazard of death compared to without HCQ (HR=10.62[1.24–91.09]).
Conclusions:
Although African-Americans constituted one-tenth of all SOT in our center, they represented two-thirds of COVID-19 cases. Despite high RRT use in SOT recipients, the severe disease and short-term death were similar in both groups. HCQ for the treatment of COVID-19 among SOT recipients was associated with high mortality and therefore, its role as a treatment modality requires further scrutiny.
Introduction:
Severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) that causes the clinical syndrome known as COVID-19 is a public health emergency and was declared a pandemic in March 2020.1 COVID-19 is highly contagious and the clinical presentation ranges from asymptomatic carriers to severe disease resulting in respiratory failure, shock and multi-organ failure. As of June 17, 2019, there are 8.4 million confirmed cases and 452,067 deaths worldwide, of which one-fourth of the cases and deaths occurred in the United States with a case fatality rate of 5.8%. 2 New York City was the first epicenter for COVID-19 in the United States followed by the Detroit metro and surrounding counties in Michigan. During the early phase of COVID-19 pandemic, there were significant decreases in total waitlist additions and transplant surgeries with increases in waitlist deaths in majority of US transplant domains. This impact was more prevalent in areas with high burden of COVID-19 e.g., New York City.3
Solid organ transplant (SOT) recipients are potentially at a higher risk of contracting or developing severe COVID-19 due to underlying immunosuppression, frequent contact with healthcare providers, and high prevalence of comorbidities such as obesity, hypertension, diabetes, chronic kidney disease, and heart disease, which are known risk factors for severe disease related to COVID-19. 4–6 Given the strong pathophysiological role of cytokine storm in many of the manifestations of severe disease in COVID-19, it is possible that SOT recipients with COVID-19 may not mount as severe an inflammatory response because of immunosuppression, which may be protective against severe disease.7,8 To date, the studies describing the clinical course of COVID-19 among SOT recipients, risk factors, and outcomes have been case series or case reports.4,6,9–11 In this study, we characterized COVID-19 illness and its clinical course among SOT recipients. Furthermore, we compared the outcomes between SOT recipients with COVID-19 and matched non-SOT controls with COVID-19 using a case-control study design.
Methods and Materials:
Study population and data:
Within the Michigan Institute for Clinical and Health Research, we developed a COVID-19 Rapid Response Registry for clinical characterization of persons with SARS-CoV-2 infection. The Registry includes core items from the International Severe Acute Respiratory and Emerging Infection Consortium Clinical Characterization Protocol.12
This study was performed as a case-control study. The cases consisted of all adult SOT recipients diagnosed with COVID-19, based on a positive confirmatory test for SARS-CoV-2 by reverse transcriptase polymerase chain reaction (PCR), between 3/10/20 to 5/15/20. Controls were likewise adult patients diagnosed with COVID-19 diagnosis using PCR, but no history of being an SOT recipient or candidate and sought care at Michigan Medicine during the study period. Up to 3 controls were individually matched from a cohort of 680 COVID-19 patients to each case based on age (±5 years), race (White, Black or Others), and admission status (admitted to the hospital or treated as outpatient) to circumvent the selection bias. Our small SOT sample size restricted the use of propensity matching or the number of variables we could match on, and because differences cannot be explored for matching variables since by definition the distributions are similar. Moreover, matching on comorbidities will not allow to examine the association between COVID-19 and comorbidities. We selected age and race with the intent that they would also balance co-morbidities. SOT recipients with COVID-19 were matched on admission status around the same time to account for the evolving management during the early phase of pandemic. Patients were followed up until death or June 10, 2020. This study was approved by the University of Michigan Institutional Review Board and completed in accordance with the Declaration of Helsinki.
Demographics, laboratory results, treatment information, and outcomes were abstracted from the medical chart and data integrity of electronic health record was cross-checked by manual review of the medical records. Co-morbidities were ascertained from documentation at the time of admission or from the last clinic note. Baseline renal function was assessed via estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula based on serum creatinine at the time of admission or last available serum creatinine prior to admission 13. Patients were classified into stages of CKD as per Kidney Disease Improving Global Outcomes guidelines 14. Most patients admitted to the hospital with COVID-19 had baseline and serial assessment of markers of inflammation including high-sensitivity C-reactive protein (CRP), serum ferritin, procalcitonin, lactate dehydrogenase, d-dimers and high sensitivity troponin.
Our institution has a live document that provides the written guidance for COVID-19 management. The criteria for intubation and transfer to ICU remained the same throughout the study independent of SOT status. An infectious diseases consult was recommended prior to the initiation of medical therapy for COVID-19 illness. Initially per local guidance, hydroxychloroquine (HCQ) was used with or without azithromycin for patients with COVID-19 who required supplemental oxygen. The dosing regimen was 600 mg twice a day on day 1, followed by 200 mg twice a day for 5 days. The local use of HCQ was abandoned by April 4th, 2020 because of data suggesting lack of benefit, although regional hospitals continued to use HCQ.15 Baseline electrocardiogram was taken on all the patients who were started on treatment with HCQ.
Tocilizumab was considered for COVID-19 in patients with rapid respiratory deterioration and evidence of hyperinflammation (described in more detail elsewhere) 16, and when enrollment into a randomized controlled trial of IL-6 inhibition was not an option.
Outcomes:
The primary outcome was death and the secondary outcomes were severe disease defined as transfer to the intensive care unit and requiring at least humidified high flow oxygen, need for intubation and use of renal replacement therapy (RRT) during hospitalization. Criteria for intubation and starting RRT was determined by the intensivist and nephrology consultation team.
Statistical Analyses:
The continuous variables were expressed as median and interquartile range and the categorical variables were expressed as counts and percentage. We used Kruskall-Wallis and chi-square or Fisher exact tests to compare continuous and categorical variables between cases and controls.
We used Kaplan-Meier survival analysis to examine the cumulative probability of death among cases and controls. We used Cox regression analysis to assess the factors associated with the risk of death. We fitted 3 models and adjusted for the covariates: 1) SOT status (cases vs. controls) 2) severe disease and SOT status and 3) sex and comorbidities (CKD, hypertension, diabetes, chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD)). The rationale behind choosing sex, CKD and COPD was based on the following observation(s): 1) significantly higher proportion of males than females in the SOT group (Table 1); 2) significantly higher prevalence of CKD and hypertension in SOT compared to non-SOT group (Table 2 and Table 3) significant association of diabetes, COPD and CVD with death in univariate analysis (Table S1).
Table 1.
Comparison of baseline characteristics and peak inflammatory markers between cases and controls.
| Characteristic | Cases Median(IQR) or n(%) | Controls Median(IQR) or n(%) | p-value |
|---|---|---|---|
| Demographics | |||
| Age (years), Median (IQR) | 60.0 (54.0 to 69.0) | 60.0 (52.0 to 67.0) | 0.67 |
| Female, n(%) | 7 (17) | 61 (50.4%) | <0.001 |
| Males n (%) | 34 (83%) | 60 (49.6%) | |
| Race, n(%) | 0.72 | ||
| Black n(%) | 27 (66) | 81 (67) | |
| White n(%) | 13 (32) | 39 (32) | |
| Unknown n(%) | 1 (2) | 1 (1) | |
| Admitted, n(%) | 35 (85) | 104 (86) | 0.93 |
| Comorbidities | |||
| BMI, Median (IQR) (n=157) | 27.0 (24.2 −32.1) (n=41) | 31.7 (26.7 – 38.0) (n=116) | <0.001 |
| Underweight n(%) | 3 (7) | 2 (2) | |
| Healthy weight n(%) | 14 (34) | 16 (14) | |
| Overweight n(%) | 10 (24) | 28 (24) | |
| Obese n(%) | 14 (34) | 70 (60) | |
| Heart disease, n(%) | 14 (34) | 31 (26) | 0.29 |
| CKD, n(%) | 33 (80) | 36 (30) | <0.001 |
| Obstructive sleep apnea, n(%) | 8 (20) | 29 (24) | 0.56 |
| Cerebrovascular disease, n(%) | 6 (15) | 12 (10) | 0.41 |
| Dementia, n(%) | 2 (5) | 8 (7) | 0.69 |
| Diabetes, n(%) | 23 (56) | 55 (45) | 0.24 |
| Liver disease, n(%) | 7 (17) | 11 (9) | 0.16 |
| Malignancy, n(%) | 7 (17) | 14 (12) | 0.36 |
| HTN, n(%) | 39 (95) | 73 (60) | <0.001 |
| CKD stage, n(%) (n=155) | <0.001 | ||
| eGFR >60 ml/min/m 2 | 8 (20) | 83 (75) | |
| eGFR 30–60 ml/min/m 2 | 23 (56) | 20 (18) | |
| eGFR <30 ml/min/m 2 | 10 (24) | 8 (7) | |
| Missing, n | 0 | 10 | |
| COPD, n(%) | 5 (12) | 19 (16) | 0.58 |
| Asthma, n(%) | 2 (5) | 12 (10) | 0.32 |
| AIDS, n(%) | 0 (0) | 3 (2) | 0.31 |
| Baseline laboratory Values | |||
| Absolute lymphocyte count, (n=144) | 0.9 (0.6 – 1.2) (n=28) | 1.0 (0.7 – 1.5) (n=116) | 0.22 |
| Absolute Neutrophil count, (n=144) | 4.9 (2.9 – 7.3) (n=28) | 4.7 (3.3 – 7.1) (n=116) | 0.57 |
| HGB, Median (IQR) (n=145) | 11.5 (9.6 – 13.0) (n=28) | 13.0 (11.5 – 14.0) (n=117) | 0.01 |
| Lymphocyte, (n=144) | 12.7 (7.6 −16.4) (n=28) | 16.4 (10.0 – 21.3) (n=116) | 0.03 |
| Neutrophils, (n=144) | 78.8 (63.5 – 83.7) (n=28) | 75.5 (68.0 – 80.2) (n=116) | 0.38 |
| Platelets, (n=145) | 152 (106.5 – 205.5) (n=28) | 207 (155.0 – 263.0) (n=117) | <0.001 |
| WBC (n=145) | 6.8 (5.4 – 9.4) (n=28) | 6.3 (4.8 – 8.7) (n=117) | 0.46 |
| Albumin (n=142) | 3.8 (3.3 – 4.2) (n=32) | 3.9 (3.6 – 4.3) (n=110) | 0.05 |
| Alkaline phosphate (n=142) | 79.0 (53.0 – 108.0) (n=33) | 76.0 (63.0 −104.0) (n=109) | 0.69 |
| ALT (n=141) | 26.0 (20.0 – 39.0) (n=33) | 33.0 (22.5 – 51.5) (n=108) | 0.13 |
| AST (n=142) | 37.0 (27.0 – 69.0) (n=33) | 41.0 (29.0 – 65.0) (n=109) | 0.37 |
| Total bilirubin (n=141) | 0.6 (0.4 – 0.8) (n=32) | 0.5 (0.4 – 0.8) (n=109) | 0.53 |
| BUN (n=145) | 40.0 (28.5 – 64.5) (n=28) | 17.0 (12.0 – 26.0) (n=117) | <0.001 |
| CO2 (n=145) | 20.5 (18.0 −25.5) (n=28) | 26.0 (23.0 −28.0) (n=117) | <0.001 |
| CR (n=146) | 2.1 (1.6 – 4.2) (n=29) | 1.0 (0.8 – 1.4) (n=117) | <0.001 |
| TBILI (n=141) | 0.6 (0.4 – 0.8) (n=32) | 0.5 (0.4 – 0.8) (n=109) | 0.53 |
| CRP (n=132) | 9.6 (5.1 – 21.8) (n=29) | 7.4 (4.5 – 15.6) (n=103) | 0.20 |
| DDIMER (n=120) | 1.7 (1.0 – 4.2) (n=24) | 1.2 (0.6 – 2.0) (n=96) | 0.03 |
| FERRITIN (n=135) | 1330 (931 – 1850) (n=30) | 617 (224 – 1370) (n=105) | 0.001 |
| IL6 (n=28) | 78.3 (44.1 – 507.6) (n=4) | 38.5 (21.8 – 91.9) (n=24) | 0.39 |
| LDH (n=131) | 307 (230 – 564) (n=32) | 390 (271 – 500) (n=99) | 0.32 |
| Peak Inflammatory Markers | |||
| Peak Serum Ferritin (n=145) | 1531 (1100 – 2776) (n=27) | 798 (281 – 1580) (n=105) | 0.002 |
| Peak DDimer (n=132) | 2.5 (1.7 – 7.4) (n=25) | 1.5 (1.0 – 4.9) (n=97) | 0.04 |
| Peak CRP (n=134) | 18.5 (5.0 to 30.7) (n=30) | 10.8 (5.8 – 19.1) (n=104) | 0.21 |
| Peak LDH (n=126) | 488 (234 – 627) (n=27) | 420 (321 – 595) (n=99) | 0.98 |
| Peak Creatinine (n=147) | 2.8 (2.1 – 5.9) (n=30) | 1.1 (0.9 – 1.7) (n=117) | <0.001 |
| Peak Procalcitonin (n=126) | 1.8 (0.5 – 16.1) (n=26) | 0.2 (0.1 – 1.2) (n=100) | <0.001 |
| Peak hsTroponin (n=111) | 63.0 (32.0 – 203.0) (n=21) | 18.5 (10.0 – 46.0) (n=90) | <0.001 |
| Peak SOFA (n=127) | 7.5 (4.5 – 13.5) (n=24) | 4.0 (3.0 – 7.0) (n=103) | 0.01 |
| Treatment | |||
| HCQ treatment, n(%) | 12 (29) | 34 (28) | 0.89 |
| IL6inhibitors, n(%) | 11 (27) | 11 (9) | 0.01 |
| Pressors, n(%) | 6 (17) | 8 (8) | 0.15 |
Table 2.
Time to death: results of Cox PH models (20 deaths; 6 among SOT cases, 14 among controls)
| HR [95% CI] | p-value | |
|---|---|---|
| Model 1: Unadjusted | ||
| Case vs. control | 1.11 [0.43, 2.89] | 0.83 |
| Model 2: Severe disease adjusted | ||
| Case vs. control | 0.84 [0.32, 2.20] | 0.73 |
| Severe disease (yes vs. no) | 8.41 [2.80, 25.30] | <0.001 |
| Model 3: Sex and comorbidity adjusted | ||
| Case vs. control | 0.90 [0.31, 2.63] | 0.84 |
| Female vs. male | 0.75 [0.25, 2.24] | 0.61 |
| CKD (yes vs. no) | 1.85 [0.54, 6.40] | 0.33 |
| COPD (yes vs. no) | 2.80 [1.09, 7.21] | 0.03 |
| HTN (yes vs. no) | 0.34 [0.07, 1.55] | 0.16 |
| DM (yes vs. no) | 2.94 [0.92, 9.39] | 0.07 |
| CVD (yes vs. no) | 3.14 [1.09, 9.07] | 0.03 |
Table 3.
Comparison of Outcomes between SOT recipients (Cases) and non-SOT (controls) with COVID-19
| Model | Matched set conditional Odds Ratio [95% CI] | p-value |
|---|---|---|
| Severe Disease Cases (N=18) vs. Controls (N=34) | ||
| Unadjusted | 2.01 [0.94, 4.28] | 0.07 |
| Sex, CKD, COPD, HTN, DM, and CVD adjusted | 1.94 [0.70, 5.34] | 0.20 |
| Sex, CKD, COPD, HTN, DM, CVD, and ferritin adjusted* | 1.46 [0.49, 4.32] | 0.50 |
| Intubation Cases (N=11) vs. Controls (N=21 | ||
| Unadjusted | 1.69 [0.73, 3.91] | 0.22 |
| Sex, CKD, COPD, HTN, DM, and CVD adjusted | 1.45 [0.47, 4.50] | 0.52 |
| Sex, CKD, COPD, HTN, DM, CVD, and ferritin adjusted* | 1.69 [0.50, 5.72] | 0.40 |
| RRT use Cases (N=11) vs. Controls (N=7) | ||
| Unadjusted | 6.94 [2.18, 22.12] | 0.001 |
| Sex, CKD, COPD, HTN, DM, and CVD adjusted | 5.08 [0.90, 28.88] | 0.07 |
| Sex, CKD, COPD, HTN, DM, CVD, and ferritin adjusted* | 5.01 [0.82, 30.72] | 0.08 |
Serum ferritin was available in 30 cases and 105 controls
We used conditional logistic regression analysis to compare the odds of severe disease, intubation, and RRT between cases and controls. The first unadjusted model included age, race, and admission status. The second was adjusted for sex and comorbidities adjusted, and the third model was adjusted for sex, CKD, COPD and serum ferritin level (at admission). These covariates were significantly different between cases and controls.
In a secondary post hoc analysis, we examined the association between HCQ and survival separately for cases and controls (after observing an association suggesting excess risk of death among cases but not controls) by testing the interaction of HCQ with SOT status on death using Cox regression. The first unadjusted model showed the interaction between HCQ and SOT status (cases vs. control). The second model was inverse probability treatment weighting (IPTW) propensity matched cases and control17–19. The propensity score of HCQ treatment was based on presence of heart disease, CKD, diabetes and chronic pulmonary disease. The third model tested for interaction and severe disease.
Lastly, we performed sensitivity analyses by excluding the cases and controls who were managed at home for primary and secondary outcomes. All analyses were performed in SAS 9.4.
Results:
Patient characteristics:
Forty-one SOT recipients tested positive for SARS-CoV-2 during the study period. The SOT recipients had the following transplants: 9 heart, 3 lung, 16 kidney, 8 liver and 5 dual organ transplant recipients (2 kidney-pancreas transplant, 1 combined heart-kidney, 1 simultaneous liver-kidney and 1 kidney after liver). Of those 41 SOT recipients, 6 were managed at home and the remaining 35 were admitted to the hospital. The median age of cases was 60 years (IQR: 54–69), and they were predominantly males (80%) and Black (67%) (Table 1). The median time from transplant to COVID-19 infection was 9 years (IQR: 5–16). None of the recipients were within the first year of transplant. The most common symptom was shortness of breath (43%) followed by cough (32%) and fever (32%). Fatigue and myalgia was seen in 27% and 12% and diarrhea in 15% of the cases.
Baseline immunosuppression consisted of calcineurin inhibitor (tacrolimus or cyclosporine) in 36 SOT recipients (88%), with tacrolimus being most common (87%). Twenty-six patients (63%) were on chronic prednisone therapy. The dose of prednisone was 5 mg once a day in all of them. Twenty-two patients (54%) were on mycophenolate. Calcineurin inhibitors and steroids were continued in all the patients where as mycophenolate was held in all but 2 SOT recipients after the COVID-19 diagnosis.
There were 121 non-SOT matched controls with a median age of 60.5 years (IQR: 52–67). In the non-SOT group, 50% were male, and 67% were Black. Sixteen (13%) of 121 were managed at home. Table 1 shows the comparison of baseline characteristics between SOT and non-SOT. While they were well-matched on age, race and admission status, SOT recipients were more likely to be males (83% vs. 50%; p<0.001), to be at a healthy weight (34% vs. 14%; p<0.001), to have CKD stage 3 and higher (80% vs. 25%; p<0.001), and to have hypertension (95% vs. 60%; p=0.004). There was no difference in white blood cell count and absolute lymphocyte count between the 2 groups but the platelet counts were lower in SOT recipients compared to non-SOT COVID-19 patients (Table 1). There were no differences in the liver chemistries or baseline and peak values of C-reactive protein between the groups. The SOT recipients had higher levels of d-dimer and ferritin on admission compared to non-SOT patients with COVID-19. The peak sequential organ failure assessment score was higher for SOT compared to non-SOT recipients with COVID-19 suggesting higher severity of organ dysfunction among SOT recipients than non-SOT patients with COVID-19 (Table 1).
A third of patients were treated with HCQ for COVID-19 and its use was similar among SOT and non-SOT matched controls with COVID-19. However, a significantly higher proportion of SOT recipients were treated with tocilizumab compared to non-SOT matched controls (27% vs. 9%; p=0.01). These differences were due to lack of eligibility of SOT recipients for placebo controlled clinical trial (e.g., remdesivir, sarilumab) and were offset by increased use of tocilizumab. Fourteen patients (2 SOT: 1-sarilimab, 1 convalescent serum and 12 non-SOT: 5 remdesivir, 5 sarilumab, 1 ORCHID, 1 convalescent serum) were enrolled in various placebo controlled clinical trials for COVID-19 therapy (remdesivir and IL-6 inhibitors). None of the patients received remdesivir for compassionate care.
The overall follow up time was 51 days (IQR: 23–65). There was no difference in the median follow up time between cases and controls (SOT recipients: 48 days [IQR: 35–56], non-SOT matched control: 53 days [IQR:16–66]; P=0.64).
Primary and secondary outcomes:
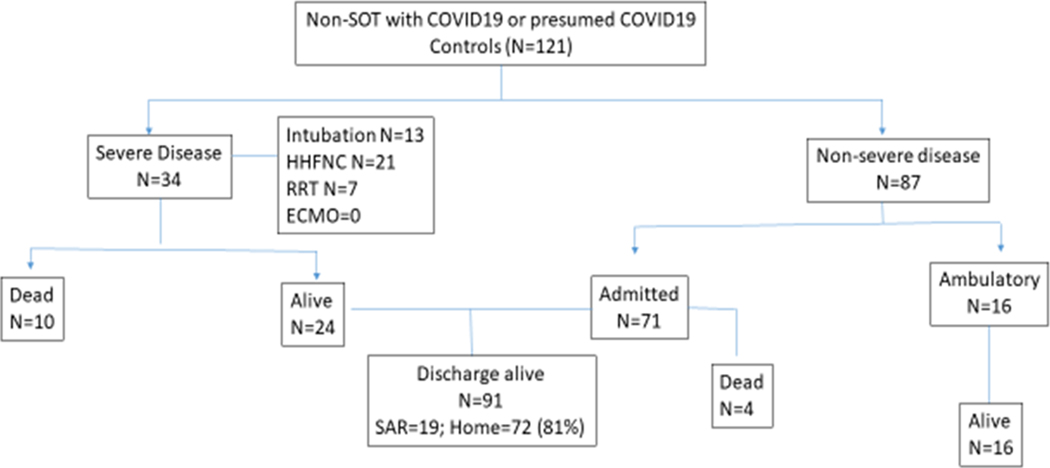
Of 41 cases, 36 were admitted to the hospital and 18 had severe disease, 11 required intubation, 11 required RRT and 2 required extracorporeal membrane oxygenation (Figure 1). Of 121 controls, 104 were admitted to the hospital and 34 had severe disease, 21 needed intubation and 7 needed RRT (Figure 2). There was no significant difference in the median length of stay between SOT (8 days [IQR: 5–22]) and non-SOT matched controls (IQR: 6 days [3–14]) with COVID-19. The time spent in the intensive care unit was also similar in both the groups (SOT: 12 days [IQR: 6–16]) and non-SOT matched controls (8 days [IQR: 4–14]).
Figure 1.
Outcomes of solid organ transplant recipients with COVID-19
Figure 2.
Outcomes of nonsolid organ transplant matched controls with COVID-19
There were 6 deaths among SOT (3 heart, 2 kidney and 1 liver) and 14 deaths among non-SOT COVID-19 matched controls, respectively. All but 1 among SOT recipients received HCQ and azithromycin, 1 received hydrocortisone for 7 days and 1 received dexamethasone for COVID-19 treatment. All 6 of them developed multi-organ failure: 2 had myocarditis, 1 developed progressive right heart failure and limb ischemia and 1 with severe encephalopathy. Bacterial superinfection was present in 3 of the 6 SOT-recipients who died during their hospitalization.
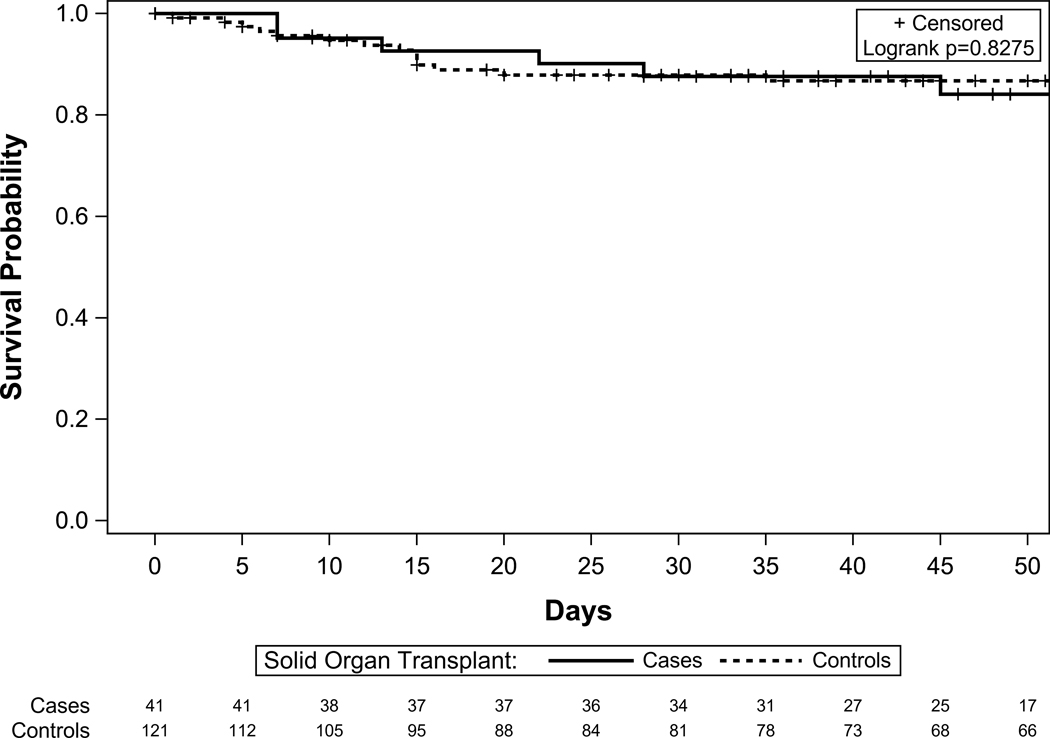
Figure 3 shows the similar cumulative survival in both the groups. The overall case fatality rates were similar for SOT (14.6%) and non-SOT (11.4%) COVID-19 patients. For those who were admitted, case fatality rate was 17% for SOT and 13% for non-SOT matched controls (non-significant difference); for those with severe disease case fatality rate was 33% for SOT-recipients compared to 29% for non-SOT matched controls with COVID-19 (nonsignificant difference). In an unadjusted analysis, age, heart disease, dementia, diabetes, and COPD were associated with increased risk of death in addition to the sequential organ failure assessment score. Lymphocyte count, serum blood urea nitrogen, and baseline serum potassium were also significantly associated with increased risk of death (Table S1). We also examined the effect of the month of admission and SOT status on death. The month of admission (March vs. May: p=0.58; April vs. May: p=0.08) and SOT status (p=0.46) were not associated with the hazard of death suggesting that the changes in protocol made during the course of the study period had no impact on outcomes in the overall cohort of patients with COVID-19 in our center.
Figure 3.
Cumulative survival in COVID19 infected patients stratified by cases and control
Table 2 shows the various covariate adjusted models. Severe disease was associated with high risk of death independent of SOT recipient status (Table 2). In a separate model adjusted for sex, significantly different comorbidities, and SOT status, COPD and CVD were independently associated with high risk of death independent of SOT status (Table 2). There was a trend towards high mortality risk and diabetes, it did not reach statistical significant.
The odds of severe disease and intubation were similar between the 2 groups (Table 3). The odds of RRT were significantly higher in SOT recipients compared to non-SOT matched controls with COVID-19 in unadjusted analyses (Model 1: SOT vs. non-SOT matched upon age, race and admission status). There was a trend towards high odds of RRT use in comorbidity adjusted and comorbidity and ferritin adjusted models (Table 3). Of the 11 SOT-recipients who required RRT (4 kidney, 5 heart and 1 each were lung and liver), 4 died, 2 were transitioned to chronic dialysis and 5 had renal recovery. Of the 7 non-SOT matched controls with COVID-19 who required RRT, 3 died, 3 were transitioned to chronic dialysis and 1 had renal recovery.
Post hoc secondary analysis: Association with HCQ and death
Treatment of COVID-19 with HCQ was associated with increased risk of death among SOT recipients (HR=13.24, p=0.02) but not among non-SOT matched controls (HR=0.59, p=0.41) (Figure 3). In an unadjusted model examining the interaction between HCQ and SOT status on death, we found a significant interaction where SOT recipients treated with HCQ had a significantly higher hazard of death compared to those who were not treated with HCQ (Table 4), but this relationship was not seen in controls.
Table 4.
HCQ treated associated with increased mortality among SOT cases. Results of Cox PH regression models.
| Model | HR [95% CI] | P |
|---|---|---|
| Model 1: Unadjusted interaction (n=162; n=20 events) | ||
| HCQ treated vs. untreated (SOT) | 13.16 [1.54, 112.63] | 0.02 |
| HCQ treated vs. untreated (NonSOT) | 0.58 [0.16, 2.07] | 0.40 |
| Model 2: IPTW interaction* (n=162; n=20 events) | ||
| HCQ treated vs. untreated (SOT) | 9.37 [1.82, 48.10] | 0.01 |
| HCQ treated vs. untreated (NonSOT) | 0.72 [0.35, 1.49] | 0.37 |
| Model 3: Severe disease adjusted (n=162; n=20 events) | ||
| HCQ treated vs. untreated (SOT) | 10.62 [1.24, 91.09] | 0.03 |
| HCQ treated vs. untreated (NonSOT) | 0.44 [0.12, 1.58] | 0.21 |
| Severe disease (yes vs. no) | 8.54 [2.81, 25.96] | <0.001 |
Propensity scores of HCQ treatment based on CAD, CKD, diabetes, and COPD
Among SOT cases: 5/12 deaths among HCQ treated; 1/29 deaths among untreated
Among matched controls: 3/34 deaths among HCQ treated; 11/87 among untreated
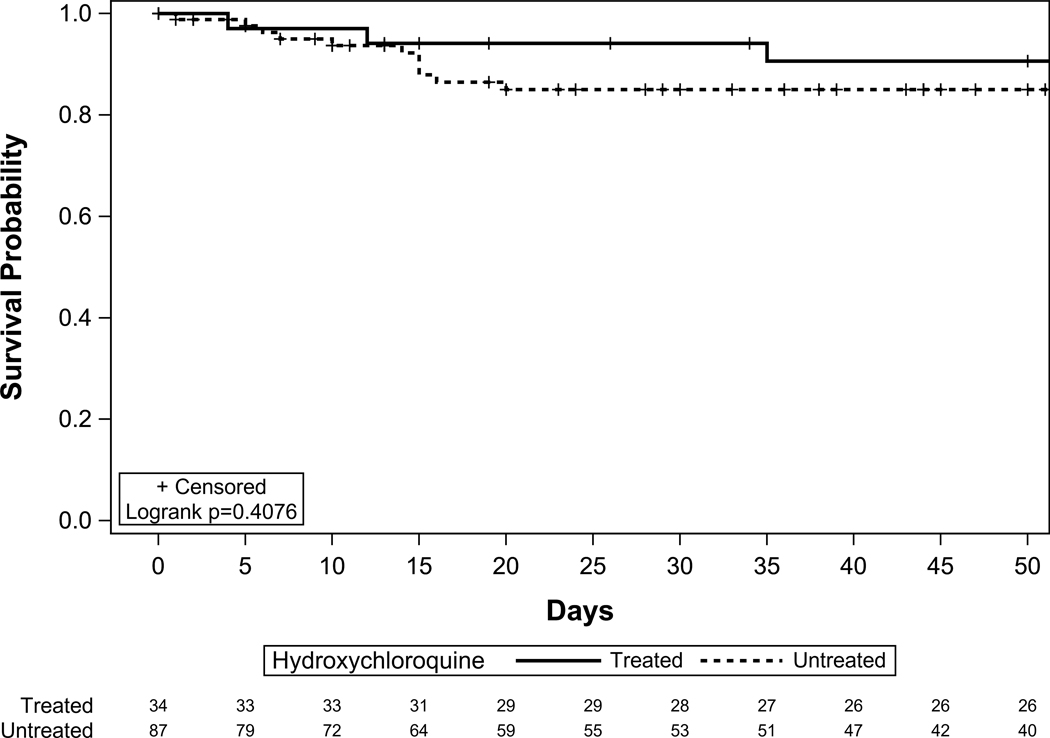
In an IPTW propensity matching on heart disease, CKD, diabetes and COPD (covariates based on differences between cases and control and previous studies), the interaction between SOT recipient status and treatment with HCQ was still significant. This association was not significant for the controls. Figure 4 shows the adjusted survival among cases by HCQ treatment. Similarly, in the model adjusted for interaction term and severe disease, the interaction between HCQ and SOT recipients and severe disease were both significant.
Figure 4a.
Covariate adjusted survival among HCQ treated SOT vs HCQ untreated SOT (Cases)
Sensitivity analysis:
Sensitivity analysis of excluding SOT and non-SOT matched controls who were not admitted to the hospital did not change the results for primary and secondary outcomes.
Discussion:
The COVID-19 pandemic has the potential to severely impact SOT recipients, who are extremely vulnerable to infection because of their weakened immune system. This has driven concern that SOT recipients will both be at increased risk to contract and to develop worse outcomes from COVID-19. Here, we compared the clinical course and outcomes of COVID-19 infection in SOT recipients with age-, race- and admission status-matched non-SOT controls. The case fatality rate was similar between SOT recipients and their matched non-SOT controls with COVID-19 despite high SOFA score among SOT cases. Although the SOT recipients had higher baseline and peak inflammatory response than controls, the odds of COVID-19 associated severe disease and intubation rates were similar in both the groups. However, the use of RRT was significantly higher among cases compared to the controls.
In contrast to early reports of high case fatality rate in SOT reported by transplant centers in NY up to 52.9%, several recent studies including recent study from Detroit20 and a systematic review of 24 studies, with 39 SOT recipients reported lower fatality rate of 23 and 25% respectively. This is in alignment with our findings of case fatality rate of 17%. 5.4 This could be due to differences in the baseline characteristics of SOT recipients such as the median time to COVID-19 from transplant, which was longer in our cohort or the use of emerging COVID-19 treatments in different communities and settings.
While the SOT recipients did not experience higher risk of death, the need for RRT support was significantly higher compared to non-SOT with COVID-19. This can be explained due to increased prevalence CKD stage 3 or higher in SOT versus non-SOT group due to use of CNI and presence of a solitary kidney in half of SOT recipients. Epidemiological data revealed the severe illness rate of COVID-19 is as high as 25%, and even though the lungs are the main organs affected, the kidney is also often affected in severe illness.9 In a study of 36 kidney transplant patients, the use of RRT was 21%.21 A meta-analysis reported that presence of CKD increased the odds of COVID-19 disease severity by more than two-fold.22 The use of RRT was higher among SOT recipients compared to non-SOT matched controls despite similar proportion of severe disease in both the groups in our study. CKD is a known co-morbidity among SOT recipients 23,24. Moreover, AKI is a common complication of COVID-19. In 1 study of 161 patients with severe COVID-19, AKI was seen in 28% of the patients and 35% of those had stage 3 CKD or higher. 25 One may argue that AKI in CKD created a perfect storm for the increased need for RRT in SOT recipients. However, this effect was independent of baseline CKD. Finally, the question arises whether the AKI in COVID-19 is a direct viral effect mediated through angiotensin converting enzyme-2 in kidney or related to systemic inflammatory response as a result of cytokine storm.
Data reported by the Michigan Department of Health and Human Services demonstrated that Blacks were overrepresented in COVID-19 cases and deaths. Black race represents 15% of the total population in Michigan, yet accounted for 35% of COVID-19 cases and 42% of COVID-19 related deaths. This is in striking contrast to 40% of COVID-19 cases and 26% of COVID-19 related deaths occurring in Whites who represent 75% of population in Michigan. We validated this finding among SOT recipients with COVID-19. Blacks or African-Americans constitute 9–10% of all SOT recipients in our center but accounted for two-thirds of all the SOT COVID-19 cases. Medical comorbidities like hypertension, CKD, CVD and diabetes associated with poor outcomes in COVID-19, also have a high prevalence among Black race. It is increasingly recognized that health inequities are often rooted in structural racism.26 Furthermore, data released by the City of Detroit showed that COVID-19 cases and deaths are not equally distributed by ZIP code.27 ZIP codes with lower percentages of Blacks had lower numbers of infections.27
Like others, we also found a higher preponderance of male sex with COVID-19 28. This may be explained, in part, due to hormonal differences. Testosterone has largely suppressive effects on immune function, which may explain the greater susceptibility to infectious diseases observed in men.29 Other postulated mechanisms include male hormone-regulated increased expression of genes encoding for the SARS-CoV-2 entry receptors angiotensin converting enzyme-2 receptor.30 Furthermore, the behavioral aspects that potentially differ between sexes, such as tobacco use or adherence to hand hygiene, might contribute to differences in risk of COVID-19. 9
We found an independent association between the risk of death and HCQ among SOT recipients. This association remained significant even after the IPTW propensity matching of cases with controls. QTc interval was similar in those who received HCQ in the SOT and non-SOT groups. The reason of increased death observed in SOT with use of HCQ likely multifactorial and may be related to drug-drug interactions. We examined the effect of month of admission on death and this was not significant. The preponderance of evidence thus far indicates that HCQ does not have benefit for COVID-19 illness, and has been linked to significant side effects (30, 31), with the major HCQ randomized controlled trials (RECOVERY, ORCHID, SOLIDARITY) all terminated due to lack of benefit. Our center stopped using HCQ as a treatment modality for COVID-19 infection by April 4th, 2020 because concern for lack of efficacy for COVID-19 in the literature as mentioned above. It is plausible that there might be unmeasured confounding that stayed not captured by inverse probability treatment weighting propensity matching. 31,32
The impact of chronic immunosuppression on COVID-19 is not known but is highly relevant because host inflammatory responses constitute an important cause of associated organ injury. Prior studies have reported elevated biomarkers of inflammation among SOT recipients at presentation 4,21. Despite the chronic immunosuppression, SOT recipients were able to mount a higher inflammatory response than controls and had similar degree of lymphopenia at presentation in our study. This may explain similar incidence of severe disease. The majority of our patients were on calcineurin inhibitors-based regimen which were continued through their treatment course on the premise that T-cell migration and signaling are needed for cytokine activation 33 and therefore, inhibiting T-cell function would mitigate progression to severe disease. We only had 2 patients in whom calcineurin inhibitor was discontinued because of hyperkalemia and progressive renal failure. We held mycophenolate in almost all of the patients during the course of COVID-19 infection.
The limitation of our is a retrospective study design from a single center which could result in potential bias due to unmeasured patient characteristics and patient selection, Contrarily, our case-control study design provided with matched controls allowed us to compare the COVID-19 outcomes between SOT recipients and non-SOT matched controls with COVID-19. The drug treatment of COVID-19 rapidly evolved during the study period with new clinical trials available to the patients during the latter half of the study period. Although our study did not account for those changes in practices, the management was similar for the cases as well as control given the single center study. Furthermore, we found no significant difference in survival based on admission month.
In conclusion, SOT recipients with COVID-19 are more likely to be males and Blacks. Despite higher baseline and peak inflammatory response and higher need for RRT, the odds of severe COVID-19 and short-term risk of death in SOT recipients with COVID-19 were similar to that of matched nontransplant controls. HCQ use among SOT recipients with COVID-19 was associated with higher risk of death compared to nonuse, calling further into question its role in COVID-19 management.
Supplementary Material
Figure 4b.
Covariate adjusted survival among HCQ treated SOT vs HCQ untreated SOT (Controls)
Acknowledgments
Drs. Sharma, Doshi and Colvin are supported by University of Michigan Taubman Innovator Award. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIH, CDC or the Department of Health and Human Services. Dr. Fung was supported by the National Institutes of Health (NIH/NHLBI 1K12HL133304). Efforts for data collection for University of Michigan COVID-19 Rapid Registry was supported by the Centers for Disease Control and Prevention (CDC U01IP000974; NIH/NCATS UL1TR002240); Dr. Golob was supported by American Society of Transplantation and Cellular Therapy New Investigator Award.
Funding: Drs. Sharma, Doshi and Colvin are supported by University of Michigan Taubman Innovator Award. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIH, CDC or the Department of Health and Human Services. Dr. Fung was supported by the National Institutes of Health (NIH/NHLBI 1K12HL133304). Efforts for data collection for University of Michigan COVID-19 Rapid Registry was supported by the Centers for Disease Control and Prevention (NIH/NCATS UL1TR002240; CDC U01IP000974); Dr. Golob was supported by American Society of Transplantation and Cellular Therapy New Investigator Award.
Abbreviations:
- BMI
Body Mass Index
- CKD
Chronic Kidney Disease
- COPD
chronic obstructive pulmonary Disease
- CRP
High-Sensitivity C-reactive Protein
- CVD
Cardiovascular Disease
- HCQ
Hydroxychloroquine
- IPTW
Inverse probability Treatment Weighting
- RRT
Renal Replacement Therapy
- SOT
Solid Organ Transplant
Glossary
- PS
Participated in the research design, contributed to data collection, interpretation of data analysis and writing of the paper
- VC
Participated in the research design, contributed to data collection, interpretation of data analysis and editing of the paper
- CMF
Participated in the research design, contributed to data collection, interpretation of data analysis and editing of the paper
- JPT
Participated in the research design, performed the statistical analysis, interpretation of data analysis and editing of the paper
- VNP
Participated in the research design, contributed to data collection, interpretation of data analysis and editing of the paper
- MC
Participated in the research design, contributed to data collection, interpretation of data analysis and editing of the paper
- Sn
Participated in the research design, contributed to data collection and editing of the paper
- PG
Participated in the research design, contributed to data collection and editing of the paper
- MC
Participated in the research design and editing of the paper
- KA
Participated in the research design and editing of the paper
- Christopher J Sonnenday
Content expert and editing of the paper
- JLG
Participated in the research design, interpretation of data analysis and editing of the paper
- ECS
Participated in the research design, interpretation of data analysis and editing of the paper
- MMD
Participated in the research design, contributed to data collection, interpretation of data analysis and writing of the paper
Footnotes
Disclosures: None
References:
- 1.Organization WH. Archived: WHO Timeline - COVID-19. 2020; https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19. Accessed July 4, 2020.
- 2.Control CfD. CDC COVID cases tracker. 2020; https://www.cdc.gov/covid-data-tracker/#cases. Accessed July 4, 2020.
- 3.Cholankeril G, Podboy A, Alshuwaykh OS, et al. Early Impact of COVID-19 on Solid Organ Transplantation in the United States. Transplantation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nacif LS, Zanini LY, Waisberg DR, et al. COVID-19 in solid organ transplantation patients: A systematic review. Clinics (Sao Paulo). 2020;75:e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi SG, Rogers AW, Saharia A, et al. Early Experience With COVID-19 and Solid Organ Transplantation at a US High-volume Transplant Center. Transplantation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siracusano G, Pastori C, Lopalco L. Humoral Immune Responses in COVID-19 Patients: A Window on the State of the Art. Front Immunol. 2020;11:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingraham NE, Lotfi-Emran S, Thielen BK, et al. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8(6):544–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsan M, Stantcheva S, Yang D, et al. Disparities in Coronavirus 2019 Reported Incidence, Knowledge, and Behavior Among US Adults. JAMA Netw Open. 2020;3(6):e2012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicastro E, Di Giorgio A, Zambelli M, et al. Impact of the SARS-CoV-2 outbreak on pediatric liver transplant recipients residing in Lombardy, Northern Italy. Liver Transpl. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Dai H, Xie X. Solid Organ Transplantation During the COVID-19 Pandemic. Front Immunol. 2020;11:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group ICC. Global outbreak research: harmony not hegemony. Lancet Infect Dis. 2020;20(7):770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009(113):S1–130. [DOI] [PubMed] [Google Scholar]
- 15.Arshad S KP, Chaudhry ZS, Oneill W, et al. Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19. International Journal of Infectious Diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers EC EG, Troost JP, Golob JL, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clinical Infectious Diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.JR. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model 1986;7:1393–1512. [Google Scholar]
- 18.Sharma P, Shu X, Schaubel DE, et al. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transpl. 2016;22(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: A case-control study. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and Kidney Transplantation. N Engl J Med. 2020;382(25):2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adapa S, Chenna A, Balla M, et al. COVID-19 Pandemic Causing Acute Kidney Injury and Impact on Patients With Chronic Kidney Disease and Renal Transplantation. J Clin Med Res. 2020;12(6):352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–940. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P, Schaubel DE, Guidinger MK, et al. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11(11):2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed MMB LI, Torres-Ortiz AE, Walker JB, et al. Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans. Kidney 360. 2020;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd R, Lindo EG, Weeks LD, et al. On Racism: A New Standard For Publishing On Racial Health Inequities. Health Affairs Blog. 2020. [Google Scholar]
- 27.Tanner K. Map: Detroit releases coronavirus cases by ZIP code. Detroit Free Press2020. [Google Scholar]
- 28.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartz D, Chitnis T, Kaiser UB, et al. Clinical Advances in Sex- and Gender-Informed Medicine to Improve the Health of All: A Review. JAMA Intern Med. 2020. [DOI] [PubMed] [Google Scholar]
- 30.La Vignera S, Cannarella R, Condorelli RA, et al. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int J Mol Sci. 2020;21(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59(1–3):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.