Abstract
Background
Studies from high income countries report that HIV-positive people have an impaired systolic and diastolic cardiac function compared to HIV-negative people. It is unclear if results can be translated directly to the Sub-Saharan Africa context. This study assesses electro- and echocardiographic characteristics in an urban African population, comparing HIV-positive people (treated and not yet treated) with HIV-negative controls.
Methods
We conducted a cross-sectional study in Johannesburg, South Africa. We enrolled HIV-positive participants from three randomized controlled trials that had recruited participants from routine HIV testing programs. HIV-negative controls were recruited from the community. Data were collected on demographics, cardiovascular risk factors, medical history and electrocardiographic and echocardiographic characteristics.
Results
In total, 394 HIV-positive participants and 153 controls were enrolled. The mean age of HIV-positive participants was 40±9 years (controls: 35±10 years), and 34% were male (controls: 50%). Of HIV-positive participants 36% were overweight or obese (controls: 44%), 23% had hypertension (controls: 28%) and 12% were current smoker (controls: 37%). Median time since HIV diagnosis was 6.0 years (IQR 2.3–10.0) and median treatment duration was 4.0 years (IQR 0.0–8.0), 50% had undetectable viral load. The frequency of anatomical cardiac abnormalities was low and did not differ between people with and without HIV. We observed no relation between HIV or anti-retroviral therapy (ART) and systolic or diastolic heart function. There was an association between ART use and corrected QT interval: +11.8 ms compared to HIV-negative controls (p<0.01) and +18.9 ms compared to ART-naïve participants (p = 0.01). We also observed a higher left ventricular mass index in participants on ART (+7.8 g/m2, p<0.01), but this association disappeared after adjusting for CD4 cell count, viral load and HIV-duration.
Conclusion
The low number of major cardiac abnormalities in this relatively young, well managed urban African HIV-positive population is reassuring. The increase in corrected QT interval and left ventricular mass may contribute to higher cardiac mortality and morbidity in people living with HIV in the long term.
Introduction
Non-communicable diseases, such as cardiovascular disease (CVD), have emerged as a major cause of morbidity and mortality in the aging human immunodeficiency virus (HIV) positive population [1]. People living with HIV (PLHIV) have a higher prevalence of cardiac disease, such as myocardial infarction, as compared to HIV-negative people [2–4]. Although the exact mechanisms are still unclear, the increased CVD risk in PLHIV seems to have a multifactorial aetiology where HIV itself, immune activation, ART and conventional cardiovascular risk factors all play a part [5–7]. Furthermore, HIV-infection is associated with multiple cardiac disorders, like pericardial effusions, arrhythmias, HIV-related heart failure and HIV-associated pulmonary hypertension (PAH) [8].
A recent systematic review consisting of studies from Europe and the United States on the effect of HIV on cardiac structure and function in the era of ART, reported that PLHIV had an impaired systolic and diastolic function, and a higher left ventricular (LV) mass (LVM) index, than HIV-negative people [9]. This may predict a higher risk of heart failure and long-term mortality for PLHIV compared to the HIV-negative population [10–12]. It is unclear if results from studies conducted in high-income settings can be translated directly to PLHIV in Sub-Saharan Africa (SSA), where approximately 70% of the world’s HIV-positive population resides [13]. In Europe and the United States, the majority of PLHIV consist of white men who have sex with men and intravenous drug users [14], who have a higher cardiovascular risk than the general population [15, 16]. In SSA, the majority of PLHIV are black Africans from the general population, with a higher proportion of females than males being HIV-positive [14].
This study aims to assess the relation between HIV and electrocardiographic and echocardiographic characteristics in an urban African population. Furthermore, the association between ART and cardiac structure and function will be evaluated by comparing PLHIV on ART to PLHIV not yet on ART.
Materials and methods
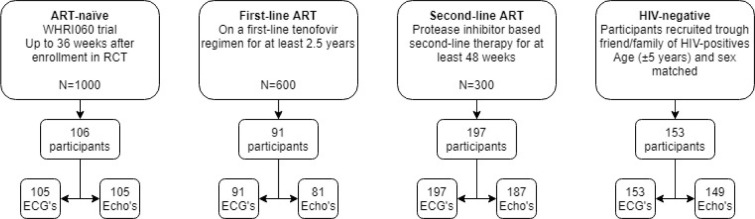
Between July 2016 and November 2017, we conducted a cross-sectional study in Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South-Africa. We included both HIV-positive participants as well as HIV-negative participants. All participants were aged 18 years and over. HIV-positive participants were recruited from three completed or ongoing randomized controlled trials (RCTs) comparing different first-and second line ART regimens. Participants in these RCTs were recruited from public HIV testing sites and HIV clinics in the inner city of Johannesburg. Exclusion that applied to these RCTs were, amongst others, abnormal kidney- and liver function, tuberculosis co-infection, pregnancy and hepatitis B co-infection. Details of the individual studies are referenced [17–19].
We divided HIV-positive participants into three groups, according to which RCT they were recruited from. The first group consisted of newly diagnosed HIV-positive participants without previous exposure to ART. Participants were recruited from an ongoing RCT comparing two new first-line ART regimens with the current standard of care first-line ART (trial WHRI060) [17]. Participants were eligible for inclusion in our study between the moment of enrolment in WRHI060 up to 36 weeks thereafter.
The second group consisted of participants on first-line ART. They were recruited from a RCT comparing two first-line ART regimens that had been completed in 2016 [18]. At the moment of inclusion in our study, participants were on a first-line tenofovir containing regimen for at least 2.5 years.
The third group consisted of participants on second-line ART. They were recruited from an open label randomized study to demonstrate non-inferiority of a low-dose boosted darunavir regimen compared to a boosted lopinavir-based regimen [19]. Inclusion criteria for this trial was second-line ART-use for at least 48 weeks. Participants of this trial were approached for enrolment in our study during the baseline visit or one of the follow-up visits.
The fourth group consisted of HIV-negative participants. They were recruited through HIV-positive participants to ensure they had the same socio-economic characteristics. HIV-positive participants were invited to refer a family member or friend of the same age (±5 years) and sex without a known seropositive HIV-status. These participants underwent HIV testing upon enrolment according to South African Department of Health HIV testing guideline [20]. Participants who tested HIV-negative, were included in the HIV-negative group. If participants had a positive HIV test results, they were assigned to one of the other groups depending on their ART-status (i.e. naïve, on first-line or on second-line treatment). Participants that tested HIV-positive and were not on ART were referred to a local clinic to initiate treatment. Fig 1 shows a flow diagram of participant recruitment.
Fig 1. Participant recruitment.
Study approval was obtained from the Medical Human Research Ethics Committee of the University of Witwatersrand, Johannesburg, South Africa (M160130). All participants provided written informed consent prior to enrolment.
Data was collected on demographics, life-style, conventional cardiovascular risk factors, medical history and pharmacologic therapy. Information on cardiovascular risk factors was collected using a modified version of WHO-STEPs instrument [21]. Information on physical activity was assessed using the International Physical Activity Questionnaire and accordingly categorised in low, intermediate and high physical activity [22]. Students, retirees, disabled and volunteers were considered unemployed. Participants who stated to have quit smoking less than one month ago were considered current smokers. Pack-years were calculated for current smokers only. Twenty cigarettes, cigars or pipes smoked per day for one year was considered one pack-year. A positive family history of CVD was defined as a history of stroke and/or heart attack of a first-degree family member (parent or sibling) before the age of 60. A self-reported history of stroke, myocardial infarction or angina pectoris was considered a positive history for CVD.
A physical examination included measurement of height, weight, waist circumference, blood pressure and heart rate. Blood pressure was measured with an electronic device in a seated position after a five minutes’ rest at the left arm and the right arm, and repeated at the arm with the highest value. The average of the second and third measurement was reported. Waist circumference was measured halfway between the lower rib and the iliac crest during expiration, in standing position. For the HIV-negative controls, blood was drawn for the analysis of total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, random glucose, and for HIV viral load and CD4 cell count for HIV-positive participants. For the HIV-positive participants’ laboratory data of the RCT visit closest to the visit at our study site was used.
Hypertension, abdominal obesity, dyslipidaemia and metabolic syndrome was defined according to the National Cholesterol Education Program, Adult Treatment Panel III (NCEP ATP III) [23]. Accordingly, hypertension was defined as systolic blood pressure (SBP) of >130 mmHg, diastolic blood pressure (DBP) of >85 mmHg or use of antihypertensive medication. Abdominal obesity was defined as a waist circumference ≥102 cm for men and ≥88 cm for women. Diabetes mellitus was defined as random glucose of >11 mmol/L or the use of blood glucose lowering medication. Dyslipidaemia was defined as elevated triglycerides (≥1.7 mmol/L) and/or reduced HDL cholesterol (<1.0 mmol/L for men and <1.3 mmol/L for women). Metabolic syndrome was defined as ≥ 3 out of: diabetes, hypertension, elevated triglycerides, lowered HDL cholesterol or abdominal obesity.
For all participants the Framingham 10-years CVD risk score [24], and the 5-years CVD risk score from the Data-collection on Adverse Effects of Anti-HIV Drugs Study (D:A:D) [25] has been calculated.
A standard 12-lead electrocardiogram (ECG) was recorded (using a laptop-based ECG device, SE-1515 DP12, EDAN) according to standardized procedure. All ECGs were visually assessed by a physician for atrial fibrillation, atrial flutter, atrioventricular block, bundle branch blocks and intraventricular conduction delays (IVCD). An experienced cardiologist was consulted in cases of any doubts about the findings. Heart rate, PR interval, corrected QT interval (QTc) (according to Bazett’s formula), and R and S-wave were calculated automatically by the ECG software. Left ventricular hypertrophy (LVH) according to the Solokow-Lyon Voltage was defined as SV1 + RV5 ≥35 mV. LVH according to the Cornell Voltage was defined as RaVL +SV3 ≥28 mV for men and ≥20 mV for women. LVH according to the Cornell Product was defined as (RaVL + SV3) x QRS duration ≥2440 mmxms [26].
Transthoracic echocardiography was performed using the Siemens Acuson P500 with a 4–2 MHz phased array transducer for cardiac imaging. Dimensions, volumes and other echocardiographic parameters were measured offline according to the recommendations of the American Society of Echocardiography by two experienced ultrasonographers who were blinded for the HIV-status of the participant [27]. Cardiac chamber dimensions and volumes were indexed by body surface area, calculated according to the Mosteller formula [28]. Left atrial and left ventricular volumes were calculated using the biplane Simpsons method. LVM was calculated using the intraventricular septal thickness (IVS), LV end-diastolic diameter (LVED) and the left ventricular posterior wall thickness (LVPW) with the following formula: LVM = 0.8 x 1.04 x [(IVS + LVED + LVPW)3—LVED3] + 0.6g. LVH was defined as a LVM index >95 g/m2 for women and >115 g/m2 for men. Relative wall thickness (RWT) was calculated with the following formula: RWT = 2 x LVPW / LVED. Geometry of the left ventricle (LV) was categorised as follows: normal (defined as no LVH and RWT ≤0.42), concentric remodelling (defined as no LVH and RWT >0.42), eccentric hypertrophy (defined as LVH and RWT ≤0.42), concentric hypertrophy (defined as LVH and RWT >0.42) [27]. Diastolic function was assessed using the ratio of mitral inflow in early diastole and atrial contraction (E/A ratio), mitral inflow deceleration time (DT) and left atrium (LA) volume index. Diastolic dysfunction was defined as: E/A ratio <0.8 and DT >280 ms or LA volume index >34 mL/m2 [29]. PAH was defined as a pulmonary artery pressure >35 mmHg.
Statistical analysis
Continuous parametric variables were reported as means with standard deviations (SD), continuous non-parametric variables as median with interquartile range (IQR). Categorical variables were reported as frequency counts with percentages. For the comparison of means, an analysis of variance (ANOVA) was used. For categorical variables, a chi-squared test was used. A Fischer’s exact test was used when criteria for a chi-square were not met.
For the comparison of means, and analysis of co-variance (ANCOVA) was used. For categorical variables with a binary outcome, a binary logistic regression was used. For categorical variables with a multinominal outcome, a multinominal logistic regression was used. All analyses were adjusted for age and sex. Multivariable linear regression analysis with dummy coding was used to obtain p-values for every ART group individually (supplements).
To assess the relation between HIV and ART and QTc, multivariable linear regression models with dummy coding for ART-status were performed, using the HIV-negative group as reference group. Three models were created. Model 1 is adjusted for age and sex, model 2 is additionally adjusted for conventional cardiovascular risk factors (ever smoked, body mass index (BMI), systolic blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, and glucose) that had a relevant association (p<0.2) with the dependent variable in univariable analysis. Model 3 is the same as Model 2, but only includes the HIV-positive group using the ART-naïve group as reference group. Model 3 is additionally adjusted for HIV-related factors (CD4 cell count, log10 viral load, and HIV-duration) with a relevant association (p<0.2) with the dependant variable in univariable analysis.
In a similar way, the relation between HIV and ART and EF, LVM index, E/A ratio, and LA volume index was assessed. Here, the group on first-line and second-line ART were combined to form the ‘On ART group’, due to the low number of participants on first-line ART with complete echocardiograms (n = 49).
A two-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics software, version 25 (IBM, Armonk, New York, United States).
Results
A total of 547 participants were recruited. Socio-demographic, cardiovascular and HIV-related characteristics of the study population are shown in Table 1. The HIV-negative group included 153 participants (35±11 years old, 50% male), the ART-naïve group consisted of 106 participants (36±18 years old, 38% male), the group on first-line ART of 91 participants (37±7 years old, 40% male), and the group on second-line ART of 197 participants (43±8 years old, 27% male). The majority of participants were moderately to highly physically active and did not smoke. Hypertension and overweight/obesity was frequently observed in all groups, although less often in the ART-naïve group.
Table 1. Socio-demographic, cardiovascular and HIV related characteristics of study population.
| HIV-negative | ART-naïve | 1st line ART | 2nd line ART | p | ||
|---|---|---|---|---|---|---|
| (n = 153) | (n = 106) | (n = 91) | (n = 197) | |||
| Demographics, n (%) | ||||||
| Male sex | 77 (50.3) | 40 (37.7) | 36 (39.6) | 54 (27.4) | <0.01 | |
| Age, mean (SD), years | 35.1 (10.5) | 35.5 (8.4) | 37.0 (6.6) | 43.4 (8.0) | <0.001 | |
| Age in categories, years | <0.001 | |||||
| 18–29 | 57 (37.3) | 40 (37.7) | 8 (8.7) | 5 (2.5) | ||
| 30–49 | 80 (52.3) | 60 (56.6) | 79 (86.8) | 152 (77.2) | ||
| ≥50 | 16 (10.5) | 6 (5.7) | 4 (4.4) | 40 (20.3) | ||
| Partnership status: single | 119 (78.3) | 87 (82.9) | 53 (58.9) | 111 (56.3) | <0.001 | |
| No or only primary education | 12 (7.9) | 13 (12.5) | 10 (11.1) | 27 (13.8) | 0.37 | |
| Employment status: unemployed | 102 (67.1) | 50 (47.6) | 14 (15.6) | 65 (33.0) | <0.001 | |
| Lifestyle, n (%) | ||||||
| Physical activity | 0.47 | |||||
| Low | 76 (49.7) | 47 (44.8) | 32 (35.2) | 86 (43.7) | ||
| Moderate | 57 (37.3) | 42 (40.0) | 40 (44.0) | 79 (40.1) | ||
| High | 20 (13.1) | 16 (15.2) | 19 (20.7) | 32 (16.2) | ||
| Smoking | <0.001 | |||||
| Current smoker | 56 (36.6) | 30 (28.3) | 11 (12.1) | 17 (8.6) | ||
| Pack-years, median [IQR], years | 3.5 [2.4–6.2] | 5.8 [1.2–9.9] | 2.5 [1.4–5.6] | 4.0 [1.5–6.4] | 0.38 | |
| Previous smoker | 6 (3.9) | 5 (4.7) | 8 (8.8) | 14 (7.1) | ||
| Never smoked | 91 (59.5) | 71 (67.0) | 72 (79.1) | 166 (84.3) | ||
| Heavy alcohol user | 16 (10.5) | 4 (3.8) | 3 (3.3) | 0 (0.0) | <0.001 | |
| Chronic medication use | ||||||
| Anti-hypertensive | 6 (3.9) | 6 (5.7) | 3 (3.3) | 20 (10.2) | 0.05 | |
| Anti-diabetic | 0 (0.0) | 1 (0.9) | 0 (0.0) | 5 (2.5) | 0.10 | |
| Lipid lowering | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (5.1) | <0.001 | |
| HIV-related factors, median [IQR] | ||||||
| Known HIV-duration, years | - | 0.0 [0.0–0.1] | 4.0 [3.2–6.1] | 9.0 [7.0–12.3] | <0.001 | |
| ART-duration, years | - | 0.0 [0.0–0.0] | 3.3 [3.0–4.0] | 8.0 [6.0–10.5] | <0.001 | |
| 2nd line ART-duration years | - | - | - | 1.0 [0.3–4.0] | N/A | |
| Last CD4 count, cells/mm3 | - | 281 [194–401] | 411 [276–576] | 619 [430–797] | <0.001 | |
| Last CD4 count, n (%), <200 cells/mm3 | - | 21 (27.3) | 6 (7.2) | 7 (3.7) | <0.001 | |
| Last viral load, copies/mL | - | 13735 [2082–46539] | 39 [39–39] | 1 [1–1] | <0.001 | |
| Last viral load, categorized, n (%), copies/mL | ||||||
| <50 | - | 13 (12.6) | 79 (90.8) | 180 (95.2) | ||
| 50–1000 | - | 10 (9.7) | 3 (3.4) | 4 (2.1) | ||
| >1000 | - | 80 (77.7) | 5 (5.7) | 5 (2.6) | ||
| Anthropometric measurements, n (%) | ||||||
| BMI, mean (SD), kg/m2 | 26.0 (6.5) | 24.6 (5.4) | 25.7 (5.5) | 27.9 (6.5) | <0.001 | |
| BMI in categories, kg/m2 | <0.01 | |||||
| Underweight; <18.5 | 8 (5.2) | 7 (6.6) | 3 (3.3) | 3 (1.5) | ||
| Normal; 18.5–25 | 77 (50.3) | 58 (54.7) | 47 (51.6) | 77 (39.1) | ||
| Overweight; >25–30 | 34 (22.2) | 26 (24.5) | 19 (20.9) | 50 (25.4) | ||
| Obesity; >30 | 34 (22.2) | 15 (14.2) | 22 (24.2) | 67 (34.0) | ||
| Abdominal obesity | 59 (38.6) | 38 (36.2) | 31 (34.1) | 110 (55.8) | <0.001 | |
| Cardiovascular measurements, mean (SD) | ||||||
| Systolic blood pressure, mmHg | 124 (18) | 119 (15) | 124 (17) | 122 (19) | 0.18 | |
| Diastolic blood pressure, mmHg | 78 (13) | 75 (9) | 81 (10) | 78 (12) | <0.01 | |
| Heart rate, beats/minute | 70 (11) | 72 (10) | 74 (12) | 68 (11) | <0.01 | |
| Biochemical measurements, mean (SD) | ||||||
| Total cholesterol, mmol/L | 4.1 (0.9) | 3.9 (0.8) | 4.4 (1.1) | 4.8 (0.8) | <0.001 | |
| HDL cholesterol, mmol/L | 1.3 (0.5) | 1.2 (0.5) | 1.4 (0.4) | 1.4 (0.4) | 0.01 | |
| LDL cholesterol, mmol/L | 2.2 (0.7) | 2.3 (0.7) | 2.5 (0.9) | 3.1 (0.8) | <0.001 | |
| Triglycerides, mmol/L | 1.1 (0.6) | 1.0 (0.4) | 1.3 (0.8) | 1.4 (0.9) | <0.001 | |
| Random glucose, mmol/L | 4.9 (1.0) | 4.5 (0.9) | 5.0 (0.9) | 4.8 (1.6) | 0.03 | |
| Cardiovascular risk factors, n (%) | ||||||
| Hypertension | 42 (27.5) | 20 (19.0) | 36 (40.0) | 65 (33.0) | 0.01 | |
| Diabetes | 0 (0.0) | 1 (1.0) | 0 (0.0) | 6 (3.3) | 0.05 | |
| Dyslipidaemia | 54 (37.0) | 55 (55.0) | 32 (38.1) | 95 (49.7) | 0.01 | |
| Metabolic syndrome | 11 (7.6) | 10 (10.0) | 11 (13.1) | 43 (22.8) | <0.001 | |
| Positive family history | 7 (4.6) | 2 (1.9) | 4 (4.4) | 21 (10.7) | 0.01 | |
| History of cardiovascular disease | 3 (2.0) | 0 (0.0) | 3 (3.3) | 5 (2.6) | 0.32 | |
| CVD prediction models, median [IQR] | ||||||
| Framingham, 10-year CVD risk, % | 1.8 [0.9–3.4] | 1.6 [0.9–2.8] | 2.0 [1.3–3.0] | 3.0 [1.5–5.3] | <0.001 | |
| D:A:D, 5-year CVD risk, % | - | 0.3 [0.2–0.6] | 0.5 [0.3–0.8] | 1.0 [0.6–2.0] | <0.001 | |
Abbreviations: ART = antiretroviral therapy; BMI = body mass index; CVD = cardiovascular disease; D:A:D: = Data Collection of Adverse Events on Anti-HIV Drugs; HDL = high-density lipoprotein; HIV = human immune deficiency virus; IQR = inter quartile ranges; LDL = low-density lipoprotein; SD = standard deviation.
The group on first-line ART had a median duration of known HIV-infection of four years, with an approximate median treatment duration of three years. The group on second-line ART had a median HIV-duration of nine years, with median of eight years of treatment (of which one year of second-line treatment). The majority of the participants on first and second-line ART had an undetectable viral load (91% and 95% respectively).
ECG was available for 545 of 547 participants (99.6%). Rhythm, blocks and IVCD could not be assessed for 10 of 545 ECGs (2%) because of missing files. These incomplete ECGs were spread across groups as follows: 3 in the HIV-negative group (2%), 1 in the ART-naïve group (1%), 2 in the group on first-line ART (2%), and 4 in the group on second-line ART (2%). ECG characteristics are shown in Table 2 and S1 Table. Rhythm, rate and conduction system abnormality did not differ significantly between groups when adjusting for age and sex. Compared to the HIV-negative controls, the group on 1st line ART had a significantly longer QTc (402±27 ms vs. 416±26 ms respectively, p<0.01)), when adjusting for age and sex (see supplements for individual p-values). The group on second line ART had more cases with intraventricular conduction delay than the other groups (p <0.01) and a higher percentage of first degree AV-block, although this was not significant.
Table 2. ECG.
| ECG finding, n (%) | HIV-negative | ART-naïve | 1st line ART | 2nd line ART | pa |
|---|---|---|---|---|---|
| (n = 153) | (n = 106) | (n = 91) | (n = 195) | ||
| Rhythm | 0.99 | ||||
| Sinus rhythm | 148 (98.7) | 104 (99.0) | 89 (100) | 191 (100) | |
| Ectopic rhythm | 2 (1.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | |
| Sinus tachycardia (>100 bpm) | 3 (2.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0.98 |
| Sinus bradycardia (<60 bpm) | 48 (31.4) | 27 (25.5) | 27 (29.7) | 71 (36.4) | <0.01 |
| Shortened PR-interval (<120 ms) | 13 (8.5) | 10 (9.4) | 8 (8.8) | 7 (3.6) | 0.27 |
| QTc, ms, mean (SD) | 402 (27) | 407 (24) | 416 (26) | 413 (33) | 0.02 |
| QTc prolongation (males: >440 ms, females: >460 ms) | 4 (2.6) | 1 (0.9) | 7 (7.7) | 18 (9.2) | 0.08 |
| AV-block | 0.19 | ||||
| First degree | 2 (1.3) | 2 (1.9) | 1 (1.1) | 12 (6.3) | |
| Second degree | 1 (0.7) | 1 (1.0) | 1 (1.1) | 0 (0.0) | |
| Third degree | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Intraventricular conduction delay | 48 (32.0) | 32 (30.5) | 8 (9.0) | 53 (27.9) | <0.01 |
| LBBB | 0.93 | ||||
| Incomplete (QRS 100–120 ms) | 4 (2.7) | 1 (1.0) | 2 (2.2) | 3 (1.6) | |
| Complete (QRS >120 ms) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | |
| RBBB | 0.97 | ||||
| Incomplete (QRS 100–120 ms) | 0 (0.0) | 3 (2.9) | 2 (2.2) | 7 (3.7) | |
| Complete (QRS >120 ms) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| LVH acc. Solokow-Lyon voltage | 38 (24.8) | 32 (30.2) | 16 (17.6) | 29 (14.9) | 0.19 |
| LVH acc. Cornell voltage | 15 (9.8) | 7 (6.6) | 7 (7.7) | 20 (10.3) | 0.56 |
| LVH acc. Cornell product | 8 (5.2) | 1 (0.9) | 1 (1.1) | 6 (3.1) | 0.28 |
aAdjusted for age and sex.
Abbreviations: AV-block = atrioventricular block; ART = anti-retroviral therapy; ECG = electrocardiogram; HIV = human immune deficiency virus; LBBB = left bundle branch block; LVH = left ventricular hypertrophy; QTc = corrected QT interval; RBBB = right bundle branch block; SD = standard deviation.
Echocardiogram was available for 522 of 547 participants (95%). Dimensions and volumes could not be measured for 50 of 522 (10%) echocardiograms as the scans could only be assessed visually because of a technical error. These incomplete echocardiograms were spread across groups as follows: 6 in the HIV-negative group (4%), 5 in the ART-naïve group (5%), 32 in the group on first-line ART (40%), and 7 in the group on second-line ART (4%).
Echocardiographic characteristics are shown in Table 3 and S2 Table. There were no significant differences in LV, LA or right ventricular dilation, diastolic dysfunction, right ventricular function, valve dysfunction or pericardial effusion between groups when adjusting for age and sex. None of the participants had PAH. Compared to the HIV-negative controls, the group on first-line ART had significantly more participants with concentric remodelling (20% vs. 45% respectively, p = 0.03), and the group on second-line ART had significantly more participants with eccentric hypertrophy (5% vs. 13% respectively, p = 0.03) (see supplements for individual p-values). The group on second line ART were more likely to have left ventricular dilatation, LVH and diastolic dysfunction as measured from E:A ratio, although differences were non-significant.
Table 3. Echocardiography.
| HIV-negative | ART-naïve | 1st line ART | 2nd line ART | pa | |
|---|---|---|---|---|---|
| (n = 149) | (n = 105) | (n = 81) | (n = 187) | ||
| Left Ventricle, mean (SD) | |||||
| LVED index, mm/m2 | 26 (3) | 26 (3) | 26 (3) | 26 (3) | 0.29 |
| LV dilatation, n (%) | 0.13 | ||||
| Normal (≤31 mm/m2) | 138 (96.5) | 99 (99.0) | 49 (100) | 173 (96.1) | |
| Mild (32–34 mm/m2) | 4 (2.8) | 1 (1.0) | 0 (0.0) | 5 (2.8) | |
| Moderate—severe (≥35 mm/m2) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 2 (1.1) | |
| LVESD index, mm/m2 | 16 (3) | 17 (3) | 15 (4) | 16 (3) | 0.01 |
| LV EDV index, mL/m2 | 57 (15) | 58 (15) | 56 (11) | 53 (15) | 0.51 |
| LV ESV index, mL/m2 | 24 (8) | 25 (9) | 24 (7) | 22 (8) | 0.05 |
| LVM index, g/m2 | 76 (21) | 73 (18) | 80 (19) | 83 (21) | 0.03 |
| LVH, n (%) | 10 (7.0) | 6 (6.1) | 6 (12.2) | 33 (18.3) | 0.09 |
| Geometry, n (%) | 0.03 | ||||
| Normal | 105 (73.4) | 79 (79.0) | 26 (53.1) | 94 (52.2) | |
| Concentric remodelling | 28 (19.6) | 15 (15.0) | 17 (34.7) | 53 (29.4) | |
| Eccentric hypertrophy | 7 (4.9) | 5 (5.0) | 5 (10.2) | 23 (12.8) | |
| Concentric hypertrophy | 3 (2.1) | 1 (1.0) | 1 (2.0) | 10 (5.6) | |
| Left atrium, mean (SD) | |||||
| LA index, mm/m2 | 18 (2) | 19 (2) | 19 (2) | 19 (2) | 0.09 |
| LA volume index, mL/m2 | 18 (6) | 19 (5) | 17 (4) | 18 (6) | 0.15 |
| LA dilatation (>34 mL/m2), n (%) | 2 (1.4) | 0 (0.0) | 0 (0.0) | 3 (1.7) | 0.99 |
| Collapse IVC >50%, n (%) | 143 (100) | 101 (98.1) | 78 (98.7) | 176 (100) | 0.98 |
| Systolic and diastolic function, mean (SD) | |||||
| Simpsons EF, % | 59 (6) | 58 (6) | 57 (10) | 60 (6) | 0.06 |
| Depressed EF (<55%), n (%) | 35 (26.1) | 27 (27.8) | 20 (43.5) | 36 (20.7) | 0.04 |
| Mitral inflow E/A ratio | 1.62 (0.48) | 1.63 (0.43) | 1.48 (0.31) | 1.40 (0.39) | 0.74 |
| Mitral inflow deceleration time, ms | 188 (45) | 180 (40) | 188 (36) | 191 (38) | 0.79 |
| Diastolic dysfunction, n (%) | 6 (4.2) | 1 (1.0) | 0 (0.0) | 11 (6.3) | 0.81 |
| Right ventricle, mean (SD) | |||||
| RV base index, mm/m2 | 20 (3) | 21 (3) | 20 (3) | 20 (3) | 0.26 |
| RV dilated (eyeball), n (%) | 0.14 | ||||
| Normal | 133 (89.9) | 92 (87.6) | 74 (92.5) | 159 (85.9) | |
| Mild | 14 (9.5) | 8 (7.6) | 6 (7.5) | 25 (13.5) | |
| Moderate | 1 (0.7) | 5 (4.8) | 0 (0.0) | 1 (0.5) | |
| Decreased systolic RV function (TAPSE <16 mm), n (%) | 3 (2.3) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0.31 |
| Pulmonary artery hypertension (PAP >35 mmHg), n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Valves, mean (SD) | |||||
| Mitral valve, n (%) | 0.06 | ||||
| Normal | 127 (85.2) | 82 (78.1) | 54 (66.7) | 152 (82.2) | |
| Trivial—mild MR | 20 (13.4) | 23 (21.9) | 25 (30.9) | 30 (16.2) | |
| Moderate–severe MR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | |
| Other pathology | 2 (1.3) | 0 (0.0) | 2 (2.5) | 2 (1.1) | |
| Aortic valve, n (%) | 0.45 | ||||
| Normal | 148 (99.3) | 105 (100) | 79 (98.8) | 179 (96.2) | |
| Trivial—mild AR | 1 (0.7) | 0 (0.0) | 1 (1.2) | 6 (3.2) | |
| Moderate—severe AR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | |
| Aortic valve stenosis (PG >20 mmHg), n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| Tricuspid valve, n (%) | 0.77 | ||||
| Normal | 115 (77.2) | 79 (75.2) | 63 (77.8) | 142 (77.2) | |
| Trivial—mild TR | 34 (22.7) | 26 (24.8) | 18 (22.2) | 40 (21.7) | |
| Moderate—severe TR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.1) | |
| Pulmonary valve, n (%) | 0.18 | ||||
| Normal | 124 (83.8) | 79 (75.2) | 62 (76.5) | 142 (76.8) | |
| Trivial—mild PR | 24 (16.2) | 26 (24.8) | 19 (23.5) | 43 (23.2) | |
| Pulmonary valve stenosis (PG >36 mmHg), n (%) | 0 (0.0 | 0 (0.0 | 0 (0.0 | 0 (0.0 | N/A |
| Other, n (%) | |||||
| Pericardial effusion | 0 (0.0) | 3 (2.9) | 2 (2.5) | 5 (2.7) | 1.00 |
aAdjusted for age and sex.
Abbreviations: AR = aortic valve regurgitation; EDV = end-diastolic volume, E/A = early diastole/atrial contraction; EF = ejection fraction; ESV = end-systolic volume; HIV = human immune deficiency virus; IVC = inferior vena cava; LA = left atrium; LV = left ventricle; LVED = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; LVH = left ventricular hypertrophy; LVM = left ventricular mass; MR = mitral valve regurgitation; PG = peak gradient; PR = pulmonary valve regurgitation; RV = right ventricle; RVSP = Right Ventricular Systolic Pressure; SD = standard deviation; TAPSE = tricuspid annular plane excursion; TR = tricuspid valve regurgitation.
Table 4 shows the relation between HIV, ART and QTc. Compared to the HIV-negative controls, the group on first-line ART had longer QTc (+11.8 ms, p<0.01) than the HIV-negative controls, independent of cardiovascular risk factors. An even stronger effect was found when taking the ART-naïve group as reference group, comparing with the group on first-line ART. The group on first-line ART had a longer QTc (+19.9ms, p = 0.01), independent of cardiovascular risk factors and HIV-related factors. Infection with HIV on its own or second-line ART use were not associated with a longer QTc.
Table 4. The relation between HIV, ART and QTc.
| HIV-negative | ART-naïve | p | 1st line ART | p | 2nd line ART | p | |
|---|---|---|---|---|---|---|---|
| QTc, β (95% CI), ms | n = 153 | n = 106 | n = 91 | n = 195 | |||
| Model 1 (n = 544) | REF | 3.1 (-3.7, 9.8) | 0.37 | 11.6 (4.5, 18.7) | <0.01 | 3.4 (-2.9, 9.6) | 0.29 |
| Model 2 (n = 518) | REF | 3.7 (-3.4, 10.8) | 0.31 | 11.8 (4.3, 19.2) | <0.01 | 4.3 (-2.5, 11.2) | 0.22 |
| Model 3 (n = 342) | - | REF | 18.9 (4.9, 32.8) | 0.01 | 17.5 (-1.4, 36.4) | 0.07 |
Significant results (p<0.05) are shown in bold font.
Model 1 is adjusted for age and sex.
Model 2 is model 1, additionally adjusted for cardiovascular risk factors with a relevant association in univariate analysis (p<0.2).
Model 3 is model 2, additionally adjusted for HIV-related factors with a relevant association in univariate analysis (p<0.2).
Abbreviations: ART = anti-retroviral therapy; CI = confidence interval; HIV = human immune deficiency virus; QTc = corrected QT interval; REF = reference value.
Table 5 shows the relation between HIV, ART and EF, LVM index, E/A ratio, and LA volume index. ART-use was associated with a higher LVM index (+7.8 g/m2, p<0.01), independent of cardiovascular risk factors. However, this association disappeared after adjusting for HIV-related factors like CD4 cell count, log10 viral load and HIV-duration. We observed no association between HIV or ART and EF, E/A ratio or LA volume index following adjustment.
Table 5. The relation of HIV and ART, and EF, LVM index, E/A ratio and LA volume index.
| HIV-negative | ART-naïve | p | On ART | p | |
|---|---|---|---|---|---|
| Simpsons EF, β (95% CI), % | n = 134 | n = 98 | n = 220 | ||
| Model 1 (n = 452) | REF | -1.8 (-3.5. -0.1) | 0.04 | -0.5 (-2.0, 1.0) | 0.48 |
| Model 2 (n = 440) | REF | -1.6 (-3.3, 0.2) | 0.08 | -0.5 (-2.1, 1.0) | 0.51 |
| Model 3 (n = 279) | - | REF | 0.7 (2.9, 4.3) | 0.70 | |
| LVM index, β (95% CI), g/m2 | n = 143 | n = 100 | n = 229 | ||
| Model 1 (n = 472) | REF | -0.7 (-5.8, 4.4) | 0.79 | 5.5 (1.0, 9.9) | 0.02 |
| Model 2 (n = 440) | REF | 1.3 (-4.2, 6.7) | 0.65 | 7.8 (2.7, 12.9) | <0.01 |
| Model 3 (n = 280) | - | REF | 0.9 (-10.9, 12.6) | 0.89 | |
| E/A ratio, β (95% CI) | n = 144 | n = 102 | n = 221 | ||
| Model 1 (n = 467) | REF | 0.0 (-0.1, 0.1) | 0.91 | -0.0 (-0.1, 0.1) | 0.45 |
| Model 2 (n = 435) | REF | 0.0 (-0.1, 0.1) | 0.85 | -0.0 (-0.1, 0.1) | 0.46 |
| Model 3 (n = 274) | - | REF | -0.1 (-0.3, 0.1) | 0.34 | |
| LA volume index, β (95% CI), mL/m2 | n = 138 | n = 102 | n = 222 | ||
| Model 1 (n = 462) | REF | 0.8 (-0.7, 2.3) | 0.28 | -0.7 (-2.0, 0.6) | 0.27 |
| Model 2 (n = 462) | REF | 1.0 (-0.4, 2.5) | 0.16 | -0.6 (-1.9, 0.6) | 0.33 |
| Model 3 (n = 322) | - | REF | -1.4 (3.1, 0.40) | 0.13 |
The group ‘On ART’ is a combination of the group on first-line ART and second-line ART.
Significant results (p<0.05) are shown in bold font.
Model 1 is adjusted for age and sex.
Model 2 is model 1, additionally adjusted for cardiovascular risk factors with a relevant association in univariate analysis (p<0.2).
Model 3 is model 2, additionally adjusted for HIV-related factors with a relevant association in univariate analysis (p<0.2).
Abbreviations: ART = anti-retroviral therapy; CI = confidence interval; E/A = early diastole/atrial contraction; EF = ejection fraction; HIV = human immune deficiency virus; LA = left atrium; LVM = left ventricular mass; REF = reference value.
Discussion
In this large, relatively young, urban African group of well-managed PLHIV on ART, not yet on ART and HIV-negative controls, the prevalence of major cardiac conduction disorders was low and there were no clinically relevant arrhythmias. First-line ART use was independently associated with longer QTc. Basic echocardiographic characteristics did not differ between PLHIV and HIV-negative controls. We did not observe an association between ART use and cardiac structure or function. We did, however, observe that participants on ART (first and second-line combined) had a higher LVM index than HIV-negative controls.
HIV-positive participants did not have more major ECG abnormalities than HIV-negative controls, which is a reassuring finding. However, we did observe an independent association between first-line ART and QTc prolongation. Two previous studies also observed longer QTc in PLHIV compared to HIV-negative controls, of +4.5 and +12.5 ms respectively [30, 31]. They did not, however, observe an independent relation between ART and QTc but this might be explained by a low number of ART-naïve participants in these studies and might hence be a lack of statistical power. Efavirenz, the most commonly used non-nucleoside reverse transcriptase inhibitor in first-line ART in South Africa, has been associated with QTc prolongation and might be an explanation of our finding [32]. QTc is a predictor of both overall and CVD mortality and prolonged QTc may predispose individuals to sudden cardiac death [33]. The higher frequency of prolonged QTc we found in participants on first-line ART may be a predictor of cardiac death in this group at a later age. Studies with follow-up are needed to asses if this is truly the case.
The low frequency of dilated cardiomyopathy in our study is reassuring. LV dilatation was present in only a few participants on second-line ART and this was not significantly different from the HIV-negative control group. In addition, we did not find an association between HIV or ART and EF. Prior to the introduction of ART, HIV-related heart failure, a disease that is related to severe immune compromise, was regularly reported in SSA [34, 35]. Our findings support the observation that HIV-related heart failure is hardly seen any more in SSA in the context of well treated HIV [36]. The timely diagnosis of HIV, probably explains why we do not find more heart failure and dilated cardiomyopathy in our HIV-positive, ART-naïve group than in our HIV-negative controls.
Multiple studies, both in high-income countries and SSA, have reported higher rates of diastolic dysfunction in PLHIV compared to HIV-negative people [37–39], and this has been associated with HIV-related factors like nadir CD4 cell count [40]. Diastolic function, as evaluated with E/A ratio and LA volume, did not differ between the groups in our study following adjustment for age and sex. However, our results are limited by the lack of tissue Doppler imaging data and may hence be an underestimation of diastolic dysfunction.
We did not observe PAH in any of the participants. The prevalence of HIV-associated PAH in an high-income setting is 0.5% [41]. Our results might be an underestimation of PAH, because participants did not undergo invasive catheterization to assed pulmonary artery pressure. The ongoing multinational Pan African Pulmonary hypertension Cohort (PAPUCO) study, will give more insight in the epidemiology of HIV-associated PAH in SSA [42].
Another reassuring negative result of this study is the low frequency of valve dysfunction. A study conducted in Germany, among 803 HIV-infected individuals (44±10 years old, 84% male), diagnosed valve dysfunction (mostly regurgitation) in 77% of the participants, of which 5% was clinically relevant [43]. We did not observe such a high frequency of valve problems, and this is surprising given that rheumatic heart disease is still prevalent in Africa. The high percentage of participants with valve problems in the German study possibly reflects the older age of participants (the mean age is approximately 6 years older than in our study) and the high number of intravenous drug users (9%) [43].
Participants on ART (first and second-line combined) had a higher LVM index than HIV-negative controls, independent of cardiovascular risk factors. However, there was no difference in LVM index when comparing between participants on ART and ART-naïve participants, when correcting for HIV-related factors. This indicates that this association depends on HIV-related factors, rather than ART use. This is in accordance to previous studies in both high-income settings and SSA that found a higher LVM index in PLHIV compared to HIV-negative controls [9, 38, 39, 44, 45]. Both higher immune activation and increased atherosclerotic processes have been proposed as a possible cause of the increase in LVM in PLHIV, in addition to conventional risk factors like obesity and hypertension [44, 46]. This would mean that inhibition of viral activity does help in counteracting the process of LVM increase, thus emphasizing the importance of effective ART administration.
A major strength of our study is that it is the largest echocardiographic study conducted in PLHIV and HIV-negative controls in SSA. Another strength is the fact that we enrolled HIV-positive participants on ART and not yet on ART, which enabled us to gain insight into the relation of ART and cardiac function.
The cross-sectional design of our study, however, precluded evaluation of causal relations. Another limitation is the relatively young age of our participants, a relatively short duration since HIV diagnosis and high percentage of participants on ART who were virally suppressed. This may partly explain the relatively low prevalence of pathology that we observed and results might not be generalizable to an older population with less viral control. Third, HIV-positive participants recruited from RCTs were compared to controls from the general population. We acknowledge that this, at first sight, may results in a bias as participants in RCTs differ from the general population. However, HIV-positive participants were recruited at public HIV treatment centres. Study participation provides participants with benefits beyond the normal health care system like scheduled appointments (and so less waiting time), personal attention by dedicated study team members and a reimbursement per visit. As a result, we believe that participation in an RCT was attractive to the majority of participants in the HIV clinics and that participants represent the general HIV-positive population in the inner city of Johannesburg. HIV negative controls were recruited through HIV-positive participants to assure that they represent the same source population. There was a trend for PLHIV on second line ART to have more cardiac abnormalities then the other groups, although not significant. This may be the result of a lack of power as the HIV-negative group is smaller than the HIV-positive group. Finally, the missing echocardiograms for the group on first-line ART precluded the analysis for first- and second-line ART separately.
Our results demonstrate that the frequency of major cardiac abnormalities such as dilated cardiomyopathy, PAH or valvular disease is the same for PLHIV and HIV-negative people in this urban African setting. This is in contrast to what was observed before the introduction of ART, when cardiac abnormalities were frequently seen in PLHIV in SSA [47]. At the same time, both the QTc prolongation and the increase in LVM that we observed in PLHIV on first-line ART may contribute to higher cardiac morbidity and mortality in the long term. Our results emphasize the importance of effective and safe ART administration to ensure healthy aging in PLHIV. Follow-up studies are needed to evaluate if the QTc prolongation and increase in LVM does translate in adverse clinical outcomes.
Supporting information
aAdjusted for age and sex. Abbreviations: AV-block = atrioventricular block; ART = anti-retroviral therapy; ECG = electrocardiogram; HIV = human immune deficiency virus; LBBB = left bundle branch block; LVH = left ventricular hypertrophy; QTc = corrected QT interval; RBBB = right bundle branch block; REF = reference value; SD = standard deviation.
(DOCX)
aAdjusted for age and sex. Abbreviations: AR = aortic valve regurgitation; EDV = end-diastolic volume, E/A = early diastole/atrial contraction; EF = ejection fraction; ESV = end-systolic volume; HIV = human immune deficiency virus; IVC = inferior vena cava; LA = left atrium; LV = left ventricle; LVED = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; LVH = left ventricular hypertrophy; LVM = left ventricular mass; MR = mitral valve regurgitation; PG = peak gradient; PR = pulmonary valve regurgitation; RV = right ventricle; RVSP = Right Ventricular Systolic Pressure; REF = reference value; SD = standard deviation; TAPSE = tricuspid annular plane excursion; TR = tricuspid valve regurgitation.
(DOCX)
(XLSX)
Acknowledgments
We thank Mrs C. Morisset, cardiac ultrasonographer, for her role in the analysis of the echocardiographic data.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. 10.1016/S0140-6736(14)60604-8 [DOI] [PubMed] [Google Scholar]
- 2.Islam FM, Wu J, Jansson J, et al. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):453–68. 10.1111/j.1468-1293.2012.00996.x [DOI] [PubMed] [Google Scholar]
- 3.Friis-Møller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. 10.1056/NEJMoa030218 [DOI] [PubMed] [Google Scholar]
- 4.Butt AA, Chang C-C, Kuller L, Goetz MB, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–43. 10.1001/archinternmed.2011.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in participants with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318–30. 10.1086/649897 [DOI] [PubMed] [Google Scholar]
- 6.Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35(21):1373–81. 10.1093/eurheartj/eht528 [DOI] [PubMed] [Google Scholar]
- 7.Lang S, Mary-Krause M, Simon A, et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55(4):600–7. 10.1093/cid/cis489 [DOI] [PubMed] [Google Scholar]
- 8.Manga P, McCutcheon K, Tsabedze N, et al. HIV and Nonischemic Heart Disease. J Am Coll Cardiol. 2017;69(1):83–91. 10.1016/j.jacc.2016.09.977 [DOI] [PubMed] [Google Scholar]
- 9.Reimer Jensen AM, Ramalho SHR, Claggett B, et al. P5419Influence of HIV infection on cardiac structure and function in the era of HAART: a systematic review and meta-analysis of case-control studies. Eur Heart J. 2018;39(suppl_1):1116. [Google Scholar]
- 10.Haider AW, Larson MG, Benjamin EJ, et al. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32(5):1454–9. 10.1016/s0735-1097(98)00407-0 [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1–20. 10.1016/j.jchf.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 12.Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105(16):1928–33. 10.1161/01.cir.0000015076.37047.d9 [DOI] [PubMed] [Google Scholar]
- 13.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS DATA 2018, http://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf. (2018, accessed 1 February 2019)
- 14.Fettig J, Swaminathan M, Murrill CS, et al. Global epidemiology of HIV. Infect Dis Clin North Am. 2014;28(3):323–37. 10.1016/j.idc.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among participants with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. 10.1210/jc.2006-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–44. 10.7326/M14-0954 [DOI] [PubMed] [Google Scholar]
- 17.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019;381(9):803–15. 10.1056/NEJMoa1902824 [DOI] [PubMed] [Google Scholar]
- 18.Venter WDF, Kambugu A, Chersich MF, et al. Efficacy and safety of tenofovir disoproxil fumarate versus low-dose stavudine over 96 weeks: a multi-country randomised, non-inferiority trial. J Acquir Immune Defic Syndr. 2018;80(2):224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venter W, Moorhouse M, Sokhela S, et al. Non-inferior efficacy for darunavir/ritonavir 400/100 mg once daily versus lopinavir/ritonavir, for participants with HIV RNA below 50 copies/mL in South Africa: The 48-week WRHI 052 study. J Int AIDS Soc. 2018;21:156–7. [Google Scholar]
- 20.Department of Health South Africa. NATIONAL HIV TESTING SERVICES: POLICY 2016. https://sahivsoc.org/Files/HTSPolicy 28Jul finalcopy.pdf. (2016, accessed 14 March 2019)
- 21.World Health Organization (WHO). The STEPS Instrument and Support Materials v.3.2. http://www.who.int/chp/steps/instrument/en/index.html. (accessed 1 February 2019)
- 22.International Physical Activity Questionnaire (IPAQ). IPAQ scoring protocol. https://sites.google.com/site/theipaq/scoring-protocol. (2005, accessed 1 February 2019)
- 23.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation. 2009;120(16):1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 25.Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–23. 10.1177/2047487315579291 [DOI] [PubMed] [Google Scholar]
- 26.Alfakih K, Walters K, Jones T. New Gender-Specific Partition Values for ECG Criteria of Left Ventricular Hypertrophy. Hypertension. 2004;44(2):175–9. 10.1161/01.HYP.0000135249.66192.30 [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 28.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098 10.1056/NEJM198710223171717 [DOI] [PubMed] [Google Scholar]
- 29.Isasti G, Pérez I, Moreno T. Echocardiographic Abnormalities and Associated Factors in a Cohort of Asymptomatic HIV-Infected Participants. AIDS Res Hum Retroviruses. 2013;29(1):20–4. 10.1089/AID.2012.0096 [DOI] [PubMed] [Google Scholar]
- 30.Wu KC, Zhang L, Haberlen SA, et al. Predictors of electrocardiographic QT interval prolongation in men with HIV. Heart. 2018;1–7. 10.1136/heartjnl-2018-313667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinsch N, Arendt M, Geisel MH, S et al. Prolongation of the QTc interval in HIV-infected individuals compared to the general population. Infection. 2017;45(5):659–67. 10.1007/s15010-017-1053-9 [DOI] [PubMed] [Google Scholar]
- 32.Chinello P, Lisena FP, Angeletti C. Role of antiretroviral treatment in prolonging QTc interval in HIV-positive participants. J Infect. 2007;54(6):597–602. 10.1016/j.jinf.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Post WS, Blasco-Colmenares E, et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22(5):660–70. 10.1097/EDE.0b013e318225768b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nzuobontane D, Blackett KN, Kuaban C. Cardiac involvement in HIV infected people in Yaounde, Cameroon. Postgrad Med J. 2002;78(925):678–81. 10.1136/pmj.78.925.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longo-Mbenza B, Seghers K V, Phuati M, et al. Heart involvement and HIV infection in African participants: determinants of survival. Int J Cardiol. 1998;64(1):63–73. 10.1016/s0167-5273(97)00321-5 [DOI] [PubMed] [Google Scholar]
- 36.Bloomfield GS, Alenezi F, Barasa FA, et al. Human Immunodeficiency Virus and Heart Failure in Low- and Middle-Income Countries. JACC Hear Fail. 2015;3(8):579–90. 10.1016/j.jchf.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontes-Carvalho R, Mancio J, Marcos A, et al. HIV Participants Have Impaired Diastolic Function that is Not Aggravated by Anti-Retroviral Treatment. Cardiovasc Drugs Ther. 2015;29(1):31–9. 10.1007/s10557-015-6573-x [DOI] [PubMed] [Google Scholar]
- 38.Ogunmodede JA, Kolo PM, Katibi IA, et al. Structural echocardiographic abnormalities seen in HIV/AIDS participants are independent of cd4 count. Niger J Clin Pract. 2017;20(6):716–23. 10.4103/1119-3077.208954 [DOI] [PubMed] [Google Scholar]
- 39.Chesa Mankwe A, James Odia O. Association between CD4+ Lymphocyte Count and Left Ventricular Diastolic Function and Geometry in Newly Diagnosed Highly Active Antiretroviral Therapy (HAART) Naive HIV/AIDS Participants Seen at University of Port Harcourt Teaching Hospital, Port Harcourt, Ri. J Cardiovasc Dis Diagnosis. 2017;05(05):1–8. [Google Scholar]
- 40.Okeke NL, Alenezi F, Bloomfield GS, et al. Determinants of Left Ventricular Hypertrophy and Diastolic Dysfunction in an HIV Clinical Cohort. J Card Fail. 2018;24(8):496–503. 10.1016/j.cardfail.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sitbon O, Lascoux-Combe C, Delfraissy J-F, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–13. 10.1164/rccm.200704-541OC [DOI] [PubMed] [Google Scholar]
- 42.Thienemann F, Dzudie A, Mocumbi AO, et al. Rationale and design of the Pan African Pulmonary hypertension Cohort (PAPUCO) study: implementing a contemporary registry on pulmonary hypertension in Africa. BMJ Open. 2014;4(10):e005950 10.1136/bmjopen-2014-005950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinsch N, Esser S, Gelbrich G, et al. Valvular manifestations of human immunodeficiency virus infection—results from the prospective, multicenter HIV-HEART study. J Cardiovasc Med. 2013;14(10):733–9. 10.2459/JCM.0b013e32835dc953 [DOI] [PubMed] [Google Scholar]
- 44.Hsue PY, Hunt PW, Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3(1):132–9. 10.1161/CIRCHEARTFAILURE.109.854943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mansoor A, Golub ET, Dehovitz J. The Association of HIV Infection with Left Ventricular Mass/Hypertrophy. AIDS Res Hum Retroviruses. 2009;25(5):475–81. 10.1089/aid.2008.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy D, Anderson KM, Savage DD, et al. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108(1):7–13. 10.7326/0003-4819-108-1-7 [DOI] [PubMed] [Google Scholar]
- 47.Ntsekhe M, Hakim J. Impact of human immunodeficiency virus infection on cardiovascular disease in Africa. Circulation. 2005;112(23):3602–7. 10.1161/CIRCULATIONAHA.105.549220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
aAdjusted for age and sex. Abbreviations: AV-block = atrioventricular block; ART = anti-retroviral therapy; ECG = electrocardiogram; HIV = human immune deficiency virus; LBBB = left bundle branch block; LVH = left ventricular hypertrophy; QTc = corrected QT interval; RBBB = right bundle branch block; REF = reference value; SD = standard deviation.
(DOCX)
aAdjusted for age and sex. Abbreviations: AR = aortic valve regurgitation; EDV = end-diastolic volume, E/A = early diastole/atrial contraction; EF = ejection fraction; ESV = end-systolic volume; HIV = human immune deficiency virus; IVC = inferior vena cava; LA = left atrium; LV = left ventricle; LVED = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; LVH = left ventricular hypertrophy; LVM = left ventricular mass; MR = mitral valve regurgitation; PG = peak gradient; PR = pulmonary valve regurgitation; RV = right ventricle; RVSP = Right Ventricular Systolic Pressure; REF = reference value; SD = standard deviation; TAPSE = tricuspid annular plane excursion; TR = tricuspid valve regurgitation.
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.